Chemical Conversion of Hardly Ionizable Rhenium Aryl Chlorocomplexes with p-Substituted Anilines
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Molecular Modeling
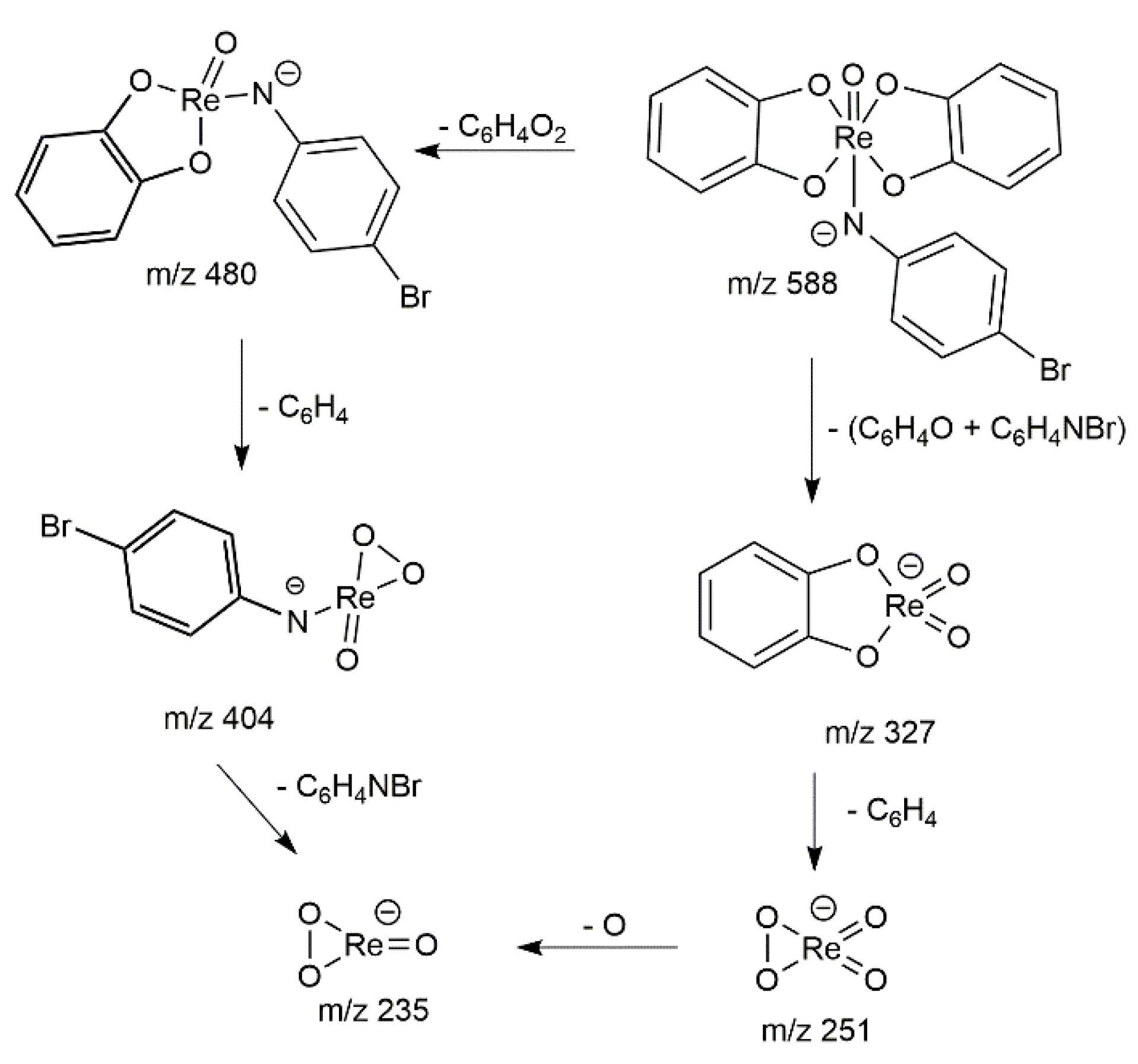
2.3. High-Resolution Mass Spectrometry Characterization
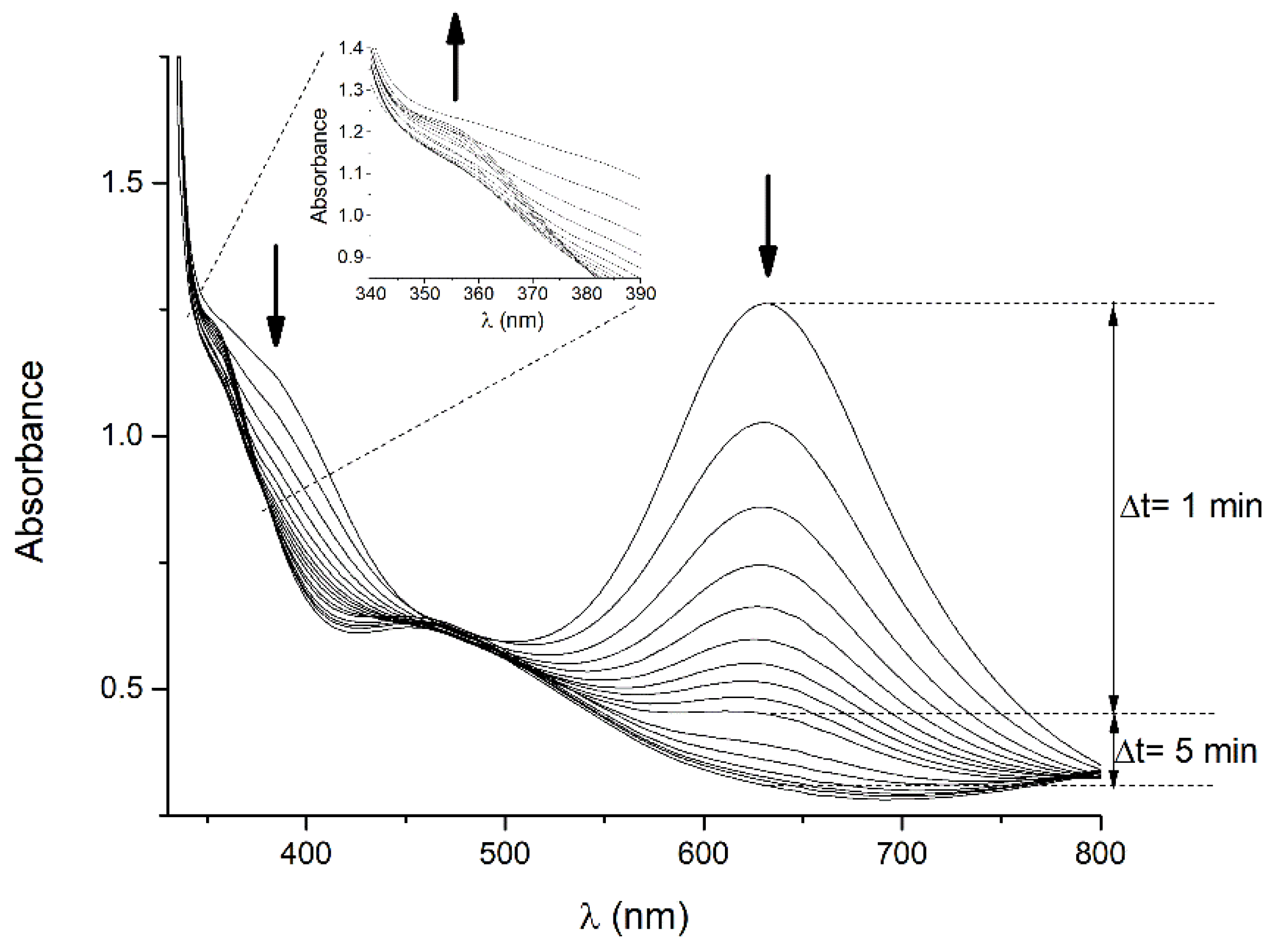
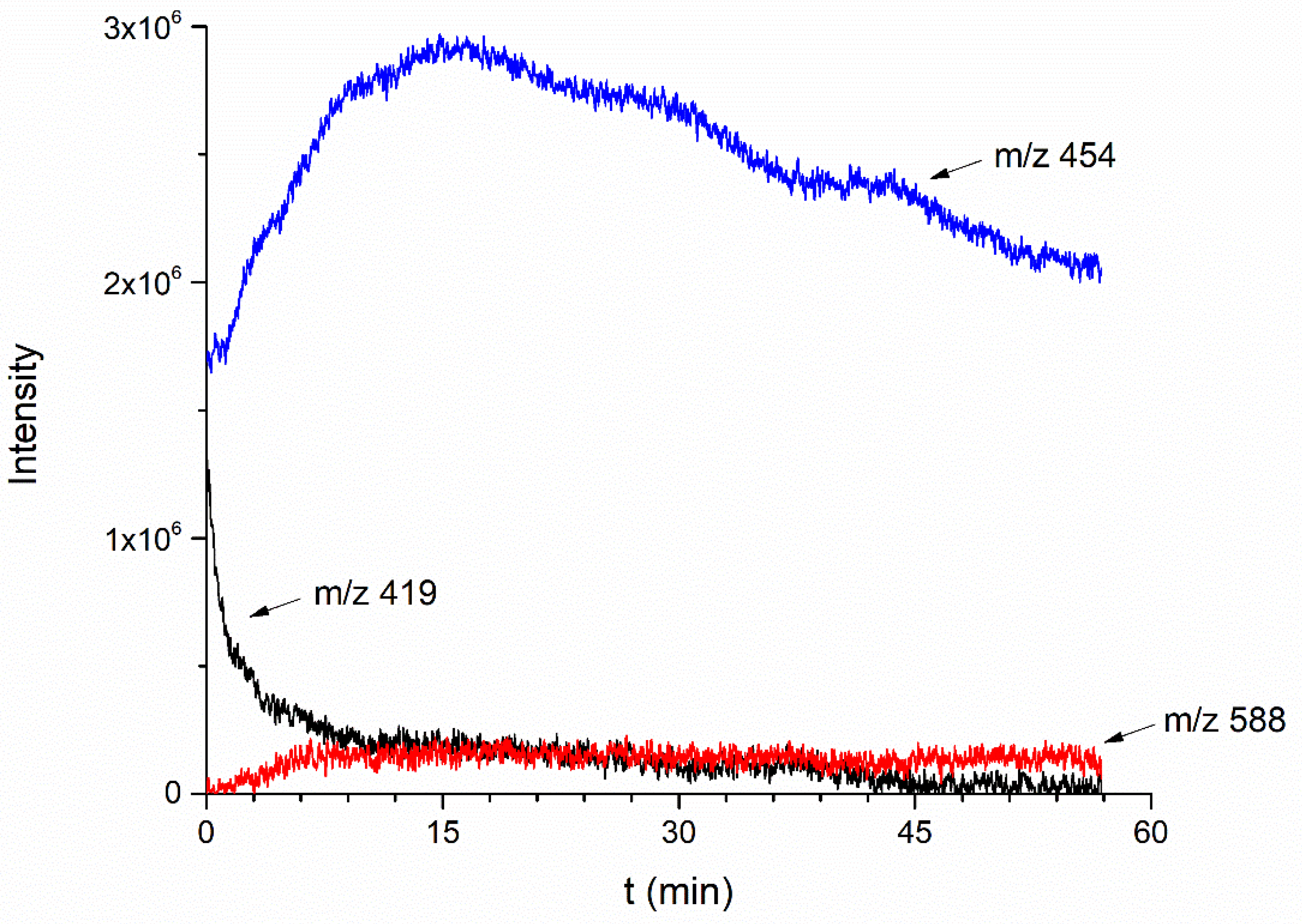
2.4. Reaction Time-Course
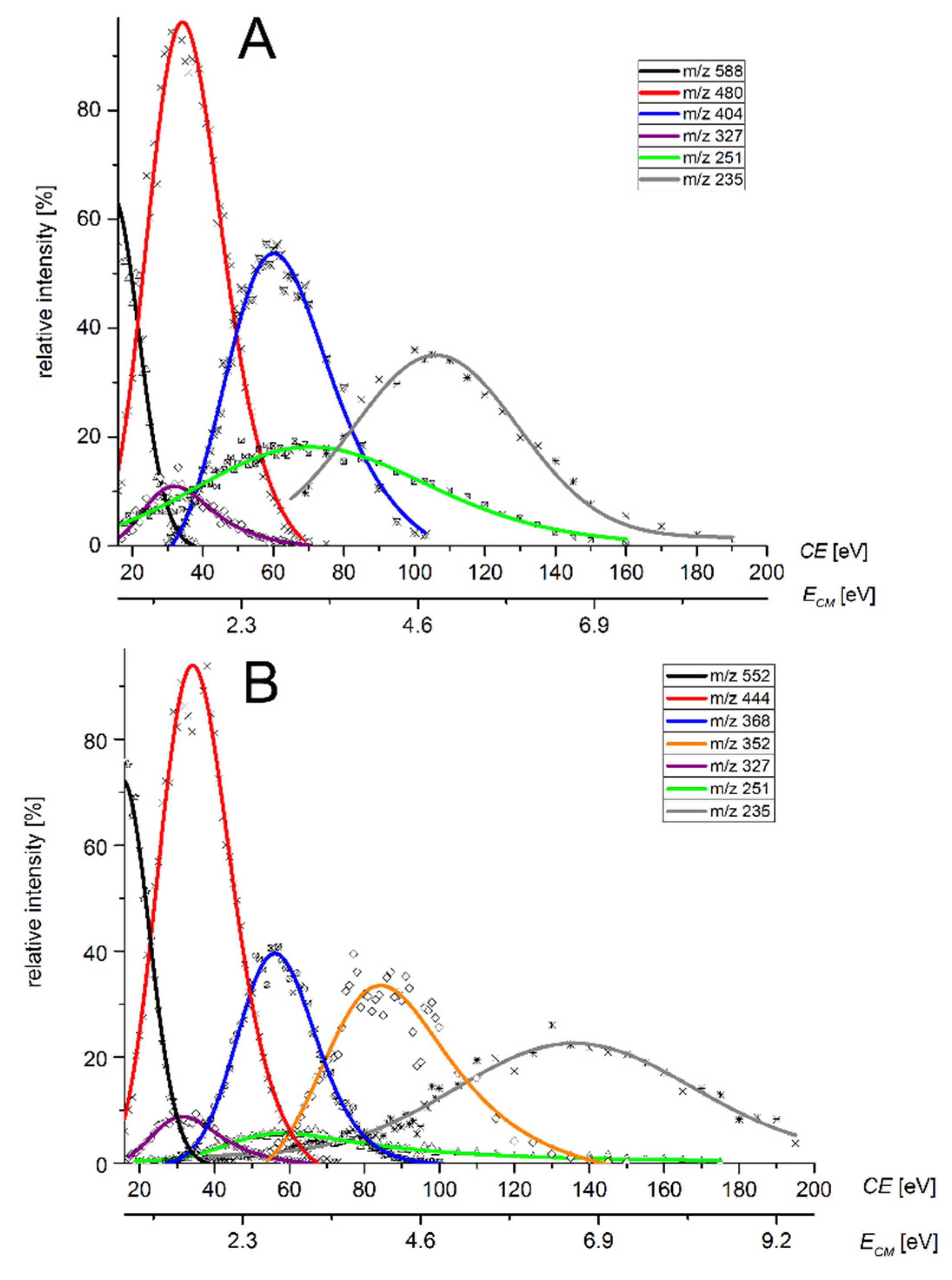
2.5. Collision Induced Dissociation (CID)
3. Materials and Methods
3.1. Materials and Reagents
3.2. Instrumentation and Software
3.3. DFT Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Anderegg, R.J. Derivatization in mass spectrometry: Strategies for controlling fragmentation. Mass Spectrom. Rev. 1988, 7, 395–424. [Google Scholar] [CrossRef]
- Eggink, M.; Wijtmans, M.; Ekkebus, R.; Lingeman, H.; De Esch, I.J.P.; Kool, J.; Niessen, W.M.A.; Irth, H. Development of a Selective ESI-MS Derivatization Reagent: Synthesis and optimization for the analysis of aldehydes in biological mixtures. Anal. Chem. 2008, 80, 9042–9051. [Google Scholar] [CrossRef]
- Blau, K.; King, G.S. Chapter 2–3. In Handbook of Derivatives for Chromatography; Heyden & Son Ltd.: London, UK, 1977. [Google Scholar]
- Niessen, W.M.A. State-of-the-art in liquid chromatography–mass spectrometry. J. Chromatogr. A 1999, 856, 179–197. [Google Scholar] [CrossRef]
- Zaikin, V.G.; Halket, J.M. Derivatization in mass spectrometry—7. On-line derivatization/degradation. Eur. J. Mass Spectrom. 2006, 12, 79–115. [Google Scholar] [CrossRef] [PubMed]
- Van Berkel, G.J.; Asano, K.G. Electrospray as a controlled current electrolytic cell—Electrochemical ionization of neutral analytes for detection by electrospray mass-spectrometry. Anal. Chem. 1994, 66, 2096–2102. [Google Scholar] [CrossRef]
- Quirke, J.M.E.; Van Berkel, G.J.; Adams, C.L. Chemical Derivatization for Electrospray Ionization Mass Spectrometry. 1. Alkyl Halides, Alcohols, Phenols, Thiols, and Amines. Anal. Chem. 1994, 66, 1302–1315. [Google Scholar] [CrossRef]
- Van Berkel, G.J.; Quirke, J.M.E.; Tigani, R.A.; Dilley, A.S.; Covey, T.R. Derivatization for electrospray ionization mass spectrometry. 3. Electrochemically ionizable derivatives. Anal. Chem. 1998, 70, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Niessen, W.M.A. Liquid Chromatography-Mass Spectrometry, 2nd ed.; Marcel Dekker: New York, NY, USA, 1999. [Google Scholar]
- Gao, S.; Zhang, Z.P.; Karnes, H.T. Sensitivity enhancement in liquid chromatography/atmospheric pressure ionization mass spectrometry using derivatization and mobile phase additives. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 825, 98–110. [Google Scholar] [CrossRef]
- Ming Ng, K.; Ling Ma, N.; Wai Tsang, C. Differentiation of isomeric polyaromatic hydrocarbons by electrospray Ag(I) cationization mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2082–2088. [Google Scholar] [CrossRef]
- Frenking, G.; Fröhlich, N. The Nature of the Bonding in Transition-Metal Compounds. Chem. Rev. 2000, 100, 717–774. [Google Scholar] [CrossRef]
- Nikolova-Damyanova, B. Retention of lipids in silver ion high-performance liquid chromatography: Facts and assumptions. J. Chromatogr. A 2009, 1216, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Nikolova-Damyanova, B.; Momchilova, S. Silver ion HPLC for the analysis of positionally isomeric fatty acids. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 1947–1965. [Google Scholar] [CrossRef]
- Momchilova, S.; Nikolova-Damyanova, B. Stationary phases for silver ion chromatography of lipids: Preparation and properties. J. Sep. Sci. 2003, 26, 261–270. [Google Scholar] [CrossRef]
- Bayer, E.; Gfrörer, P.; Rentel, C. Coordination-Ionspray-MS (CIS-MS), a Universal Detection and Characterization Method for Direct Coupling with Separation Techniques. Angew. Chem. Int. Ed. 1999, 38, 992–995. [Google Scholar] [CrossRef]
- Moriwaki, H. Electrospray ionization mass spectrometric detection of low polar compounds by adding NaAuCl4. J. Mass Spectrom. 2016, 51, 1096–1102. [Google Scholar] [CrossRef]
- Van Berkel, G.J.; Quirke, J.M.E.; Adams, C.L. Derivatization for electrospray ionization-mass spectrometry. 4. Alkenes and alkynes. Rapid Commun. Mass Spectrom. 2000, 14, 849–858. [Google Scholar] [CrossRef]
- Johnson, D.W. Contemporary clinical usage of LC/MS: Analysis of biologically important carboxylic acids. Clin. Biochem. 2005, 38, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Shimada, K. Derivatization of neutral steroids to enhance their detection characteristics in liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2004, 378, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Hayashi, S.; Hifumi, H.; Honma, Y.; Tanji, N.; Iwasawa, N.; Suzuki, Y.; Suzuki, K. MPAI (Mass Probes Aided Ionization) Method for Total Analysis of Biomolecules by Mass Spectrometry. Anal. Sci. 2007, 23, 11–15. [Google Scholar] [CrossRef]
- Van Berkel, G.J.; McLuckey, S.A.; Glish, G.L. Charge determination of product ions formed from collision-induced dissociation of multiply protonated molecules via ion molecule reactions. Anal. Chem. 1991, 63, 2064–2068. [Google Scholar] [CrossRef]
- Zaikin, V.G.; Halket, J.M. Derivatization in mass spectrometry—2. Acylation. Eur. J. Mass Spectrom. 2003, 9, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Hušek, P. Chloroformates in gas chromatography as general purpose derivatizing agents. J. Chromatogr. B 1998, 717, 57–91. [Google Scholar] [CrossRef]
- Kataoka, H. 2.1.2—Gas Chromatography of Amines as Various Derivatives. Journal of Chromatography Library 2005, 70, 364–404. [Google Scholar] [CrossRef]
- Štícha, M.; Jelínek, I.; Poláková, J.; Kaliba, D. Characterization of Rhenium(V) Complexes with Phenols Using Mass Spectrometry with Selected Soft Ionization Techniques. Anal. Lett. 2015, 48, 2329–2342. [Google Scholar] [CrossRef]
- Sticha, M.; Jelinek, I.; Kaliba, D.; Polakova, J. Analytical study of rhenium complexes with pyrogallol and catechol. Chem. Pap. 2017, 71, 819–830. [Google Scholar] [CrossRef]
- Williams, R. pKa Data Compiled by R. Williams. Available online: https://organicchemistrydata.org/hansreich/resources/pka/pka_data/pka-compilation-williams.pdf (accessed on 28 April 2021).
- Wan, K.X.; Vidavsky, I.; Gross, M.L. From similarity index to spectral contrast angle. J. Am. Soc. Mass Spectrom. 2002, 13, 85–88. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
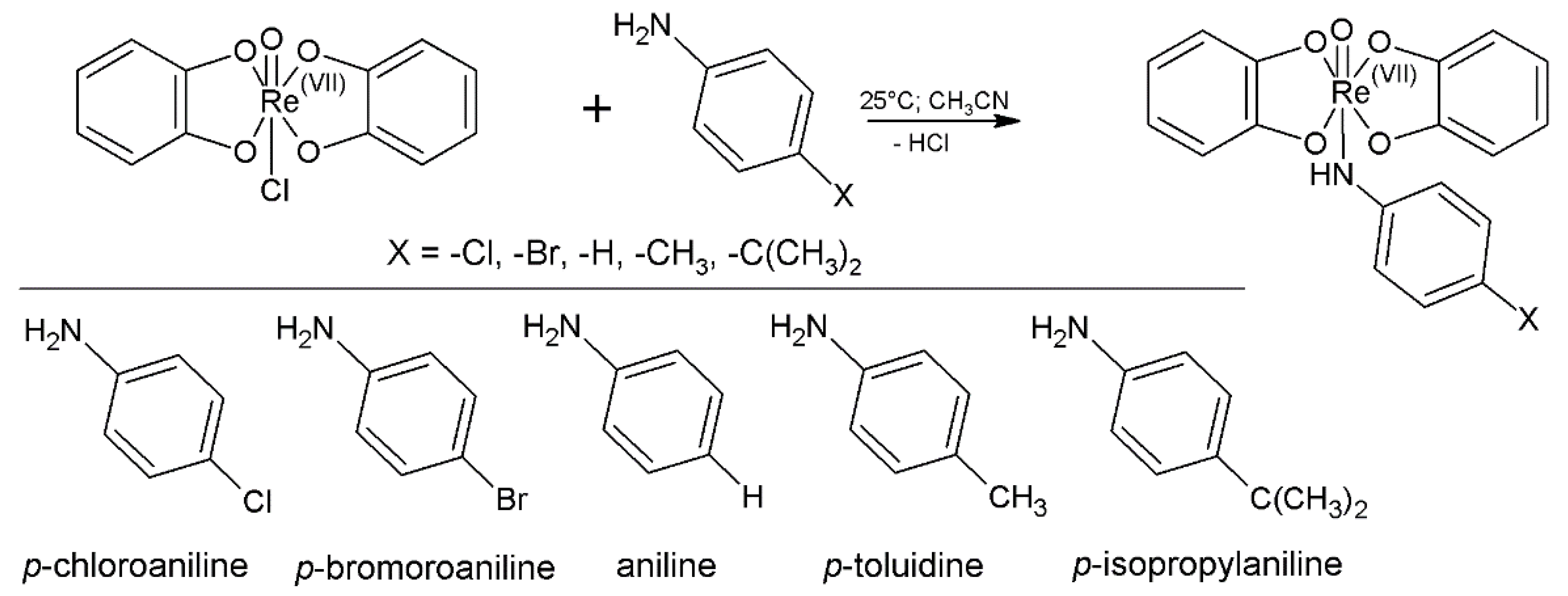
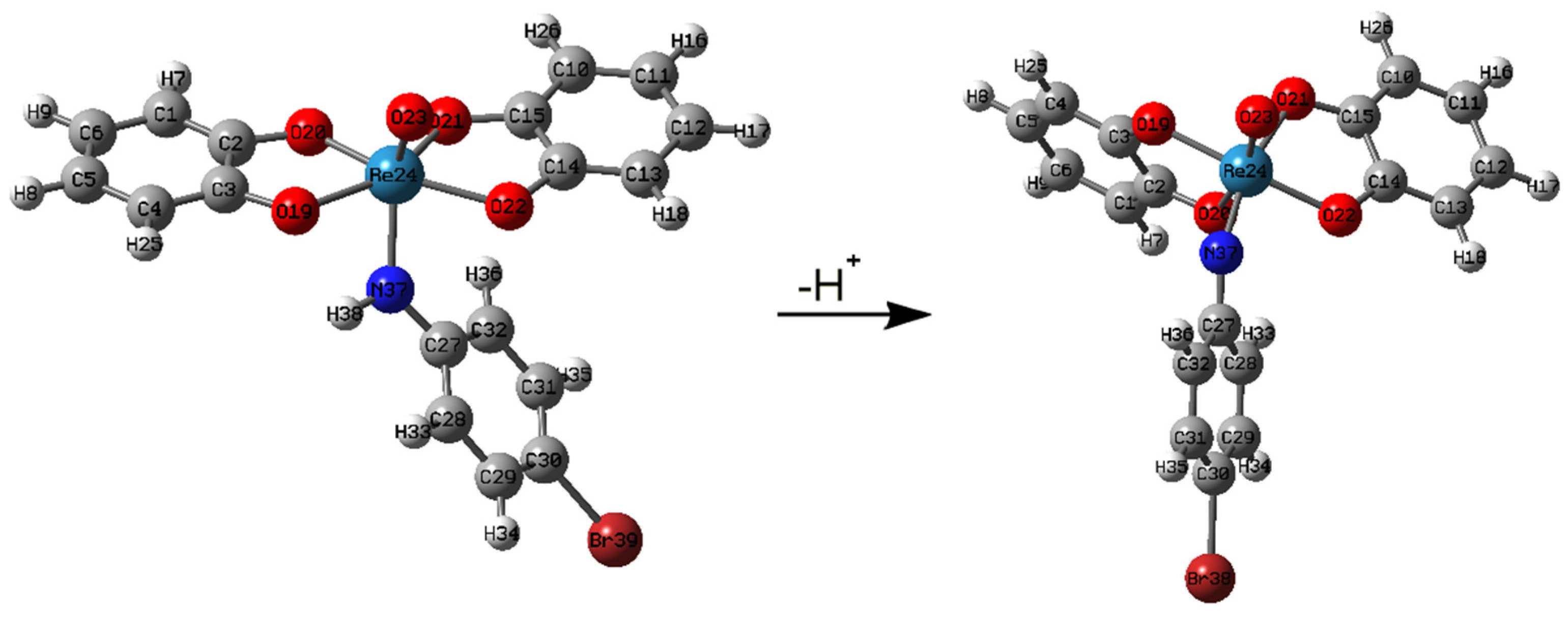
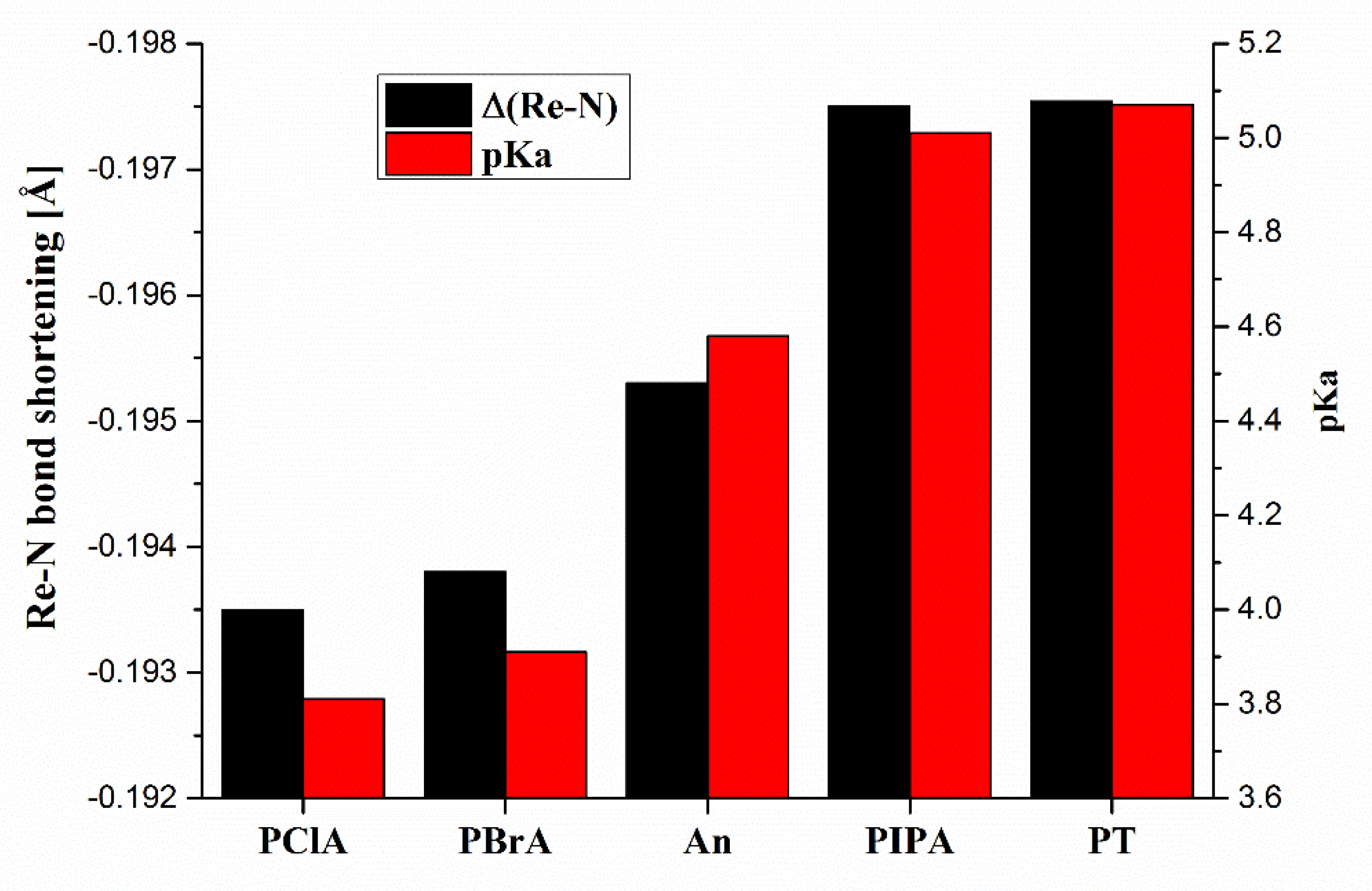
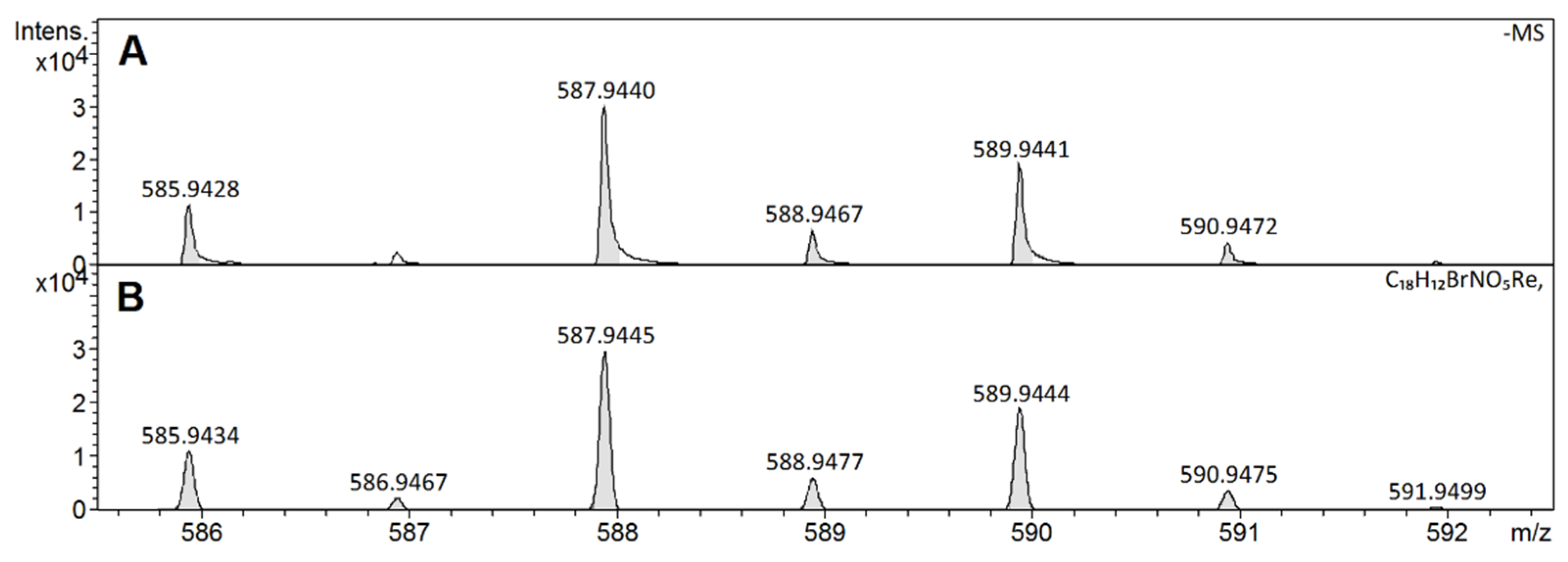

| Entry | X | Complex | Formula |
|---|---|---|---|
| 1 | Cl | [ReVII(O)(Cat)2PClA]− a | C18H12ClNO5Re |
| 2 | Br | [ReVII(O)(Cat)2PBrA]− a | C18H12BrNO5Re |
| 3 | H | [ReVII(O)(Cat)2An]− a | C18H13NO5Re |
| 4 | CH3 | [ReVII(O)(Cat)2PT]− a | C19H15NO5Re |
| 5 | C(CH3)2 | [ReVII(O)(Cat)2PIPA]− a | C21H19NO5Re |
| Entry | Molecular Formula | Theoretical m/z | Measured m/z | Error (mDa) | Error (ppm) | (1-SI) 100 (%) |
|---|---|---|---|---|---|---|
| [Re(O)(Cat)2PClA]− | C18H12ClNO5Re | 543.9958 | 543.9969 | −1.1 | −2.0 | 98.6 |
| [Re(O)(Cat)2PBrA]− | C18H12BrNO5Re | 587.9445 | 587.9440 | 0.4 | 0.8 | 94.2 |
| [Re(O)(Cat)2An]− | C18H13NO5Re | 510.0357 | 510.0360 | −0.3 | −0.6 | 98.1 |
| [Re(O)(Cat)2PT]− | C19H15NO5Re | 524.0514 | 524.0507 | 0.7 | 1.2 | 89.2 |
| [Re(O)(Cat)2PIPA]− | C21H19NO5Re | 552.0827 | 552.0828 | −0.2 | −0.3 | 98.1 |
| Nom. m/z | Ion Formula | Theoretical m/z | Measured m/z | Error (mDa) | Error (ppm) | Rel. Abundance (%) |
|---|---|---|---|---|---|---|
| 588 | C18H12BrNO5Re− | 587.9462 | 587.9459 | 0.5 | 0.3 | 0.3 |
| 480 | C12H8BrNO3Re− | 479.9233 | 479.9271 | −7.9 | −3.8 | −3.8 |
| 404 | C6H4BrNO3Re− | 403.892 | 403.8931 | −2.8 | −1.1 | −1.1 |
| 327 | C6H4O4Re− | 326.9673 | 326.9681 | −2.5 | −0.8 | −0.8 |
| 251 | ReO4− | 250.936 | 250.9354 | 2.3 | 0.6 | 0.6 |
| 235 | ReO3− | 234.9411 | 234.9383 | 11.7 | 2.8 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štícha, M.; Jelínek, I.; Vlk, M. Chemical Conversion of Hardly Ionizable Rhenium Aryl Chlorocomplexes with p-Substituted Anilines. Molecules 2021, 26, 3427. https://doi.org/10.3390/molecules26113427
Štícha M, Jelínek I, Vlk M. Chemical Conversion of Hardly Ionizable Rhenium Aryl Chlorocomplexes with p-Substituted Anilines. Molecules. 2021; 26(11):3427. https://doi.org/10.3390/molecules26113427
Chicago/Turabian StyleŠtícha, Martin, Ivan Jelínek, and Mikuláš Vlk. 2021. "Chemical Conversion of Hardly Ionizable Rhenium Aryl Chlorocomplexes with p-Substituted Anilines" Molecules 26, no. 11: 3427. https://doi.org/10.3390/molecules26113427
APA StyleŠtícha, M., Jelínek, I., & Vlk, M. (2021). Chemical Conversion of Hardly Ionizable Rhenium Aryl Chlorocomplexes with p-Substituted Anilines. Molecules, 26(11), 3427. https://doi.org/10.3390/molecules26113427








