Characterizing the Volatile and Sensory Profiles, and Sugar Content of Beeswax, Beebread, Bee Pollen, and Honey
Abstract
1. Introduction
2. Results and Discussion
2.1. The Profile of Volatiles Compounds in Bee Products
2.2. Sugar Contents in Bee Products
2.3. QDA Profile of Bee Products in the Aspect of Color and Odor Quality
2.4. The PLS Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Research Material
3.3. Determination of Volatile Composition of Bee Products
3.4. Determination of Sugar Content in Bee Products
3.5. Quantitative Descriptive Analysis (QDA) of Bee Products
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kacániová, M.; Vuković, N.; Chlebo, R.; Haščik, P.; Cubon, J.; Dżugan, M.; Pasternakiewicz, A. The antimicrobial activity of honey, bee pollen loads and beeswax from Slovakia. Arch. Biol. Sci. 2012, 64, 927–934. [Google Scholar] [CrossRef]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as a new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Waheed, M.; Hussain, M.B.; Javed, A.; Mushtaq, Z.; Hassan, S.; Shariati, M.A.; Khan, M.U.; Majeed, M.; Nigam, M.; Mishra, A.P.; et al. Honey and cancer: A mechanistic review. Clin. Nutr. 2019, 38, 2499–2503. [Google Scholar] [CrossRef]
- Kaškoniene, V.; Venskutonis, P.R. Floral markers in honey of various botanical and geographic origins: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Kopała, E.; Kuźnicka, E.; Balcerak, M. Survey of consumer preferences on the bee product market. Part 1. Honey. Ann. Warsaw Univ. Life Sci. SGGW Anim. Sci. 2019, 58, 153–158. [Google Scholar] [CrossRef]
- Aparna, A.R.; Rajalakshmi, D. Honey—Its characteristics, sensory aspects, and applications. Food Rev. Int. 1999, 15, 455–471. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee pollen: Chemical composition and therapeutic application. Evid. Based Complement. Alternat. Med. 2015, 6, 297425. [Google Scholar] [CrossRef]
- Bobis, O.; Marghitas, L.A.; Dezmirean, D.; Morar, O.; Bonta, V.; Chirila, F. Quality parameters and nutritional value of different commercial bee products. Bul. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2010, 67, 1–2. [Google Scholar]
- Bogdanov, S. Beeswax: Production, properties composition and control. In Beeswax Book; Muehlethurnen, Switzerland, 2009; Volume 1, pp. 1–17. Available online: citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.567.4441&rep=rep1&type=pdf (accessed on 12 May 2021).
- Regulation EC 1333/2008 (2008). Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives; Official Journal of the European Union: 16-33 (L 354). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1333 (accessed on 12 May 2021).
- EFSA. Beeswax (E901) as a glazing agent and a carrier for flavours. Scientific opinion of the pane on food additives, flavourings, processing aids and materials in contact with food (AFC). EFSA J. 2007, 615, 1–28. [Google Scholar]
- Escudero, O.; Dobre, I.; Fernandez-Gonzalez, M.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar]
- Kaškoniene, V.; Venskutonis, P.R.; Čeksterytė, V. Carbohydrate composition and electrical conductivity of different origin honeys from Lithuania. LWT Food Sci. Technol. 2010, 43, 801–807. [Google Scholar] [CrossRef]
- Tomczyk, M.; Zaguła, G.; Dżugan, M. A simple method of enrichment of honey powder with phytochemicals and its potential application in isotonic drink industry. LWT Food Sci. Technol. 2020, 125, 109204. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Barać, M.B.; Ali Shariati, M.; Tešić, Ž.L.; Pešić, M.B. The Application of Pollen as a Functional Food and Feed Ingredient—The Present and Perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef]
- Amores-Arrocha, A.; Roldán, A.; Jiménez-Cantizano, A.; Caro, I.; Palacios, V. Evaluation of the use of multiflora bee pollen on the volatile compounds and sensorial profile of Palomino fino and Riesling white young wines. Food Res. Int. 2018, 105, 197–209. [Google Scholar] [CrossRef]
- Rodriguez-Flores, M.S.; Falcão, S.I.; Escuredo, O.; Seijo, M.C.; Vilas-Boas, M. Description of volatile fraction of Erica honey from the northwest of the Iberian Peninsula. Food Chem. 2021, 336, 127758. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Conte, P.; Del Caro, A.; Urgeghe, P.P.; Petretto, G.L.; Montanari, L.; Piga, A.; Fedda, C. Nutritional and aroma improvement of gluten-free bread: Is bee pollen effective? LWT Food Sci. Technol. 2020, 118, 108711. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Use of multiflora bee pollen as a flor velum yeast growth activator in biological aging wines. Molecules 2019, 24, 1763. [Google Scholar] [CrossRef]
- Keskin, M.; Özkök, A. Effects of drying techniques on chemical composition and volatile constituents of bee pollen. Czech J. Food Sci. 2020, 38, 203–208. [Google Scholar] [CrossRef]
- Carpes, S.T.; de Alencar, S.M.; Cabral, I.S.R.; Oldoni, T.L.C.; Mourao, G.B.; Haminiuk, C.W.I.; da Luz, C.F.P.; Masson, M.L. Polyphenols and palynological origin of bee pollen of Apis mellifera L. from Brazil. Characterization of polyphenols of bee pollen. CYTA J. Food 2013, 11, 150–161. [Google Scholar] [CrossRef]
- Lima Neto, J.S.; Lopes, J.A.D.; Moita Neto, J.M.; Lima, S.G.; Luz, C.F.P.; Citró, A.M.G.L. Volatile compounds and palynological analysis from pollen pots of stingless bees from the mid-north region of Brazil. Braz. J. Pharm. Sci. 2017, 53, e14093. [Google Scholar] [CrossRef]
- Kaškoniene, V.; Kaškonas, P.; Maruška, A. Volatile compounds composition and antioxidant activity of bee pollen collected in Lithuania. Chem. Pap. 2015, 69, 291–299. [Google Scholar] [CrossRef]
- Pilevar, Z.; Bahrami, A.; Beikzadeh, S.; Hosseini, H.; Jafari, S.M. Migration of styrene monomer from polystyrene packaging into foods: Characterization and safety evaluation. Trends Food Sci. Technol. 2019, 91, 248–261. [Google Scholar] [CrossRef]
- Schwarz, K.J.; Boitz, L.I.; Methner, F.J. Enzymatic formation of styrene during wheat beer fermentation is dependent on pitching rate and cinnamic acid content. J. Inst. Brew. 2012, 118, 280–284. [Google Scholar] [CrossRef]
- Sawicki, T.; Bączek, N.; Starowicz, M. Characterisation of the total phenolic, Vitamins C and E content and antioxidant properties of the beebread and honey from the same batch. Czech J. Food Sci. 2020, 38, 158–163. [Google Scholar] [CrossRef]
- Kaškoniene, V.; Venskutonis, P.R.; Čeksterytė, V. Composition of volatile compounds of honey of various floral origin and beebread collected in Lithuania. Food Chem. 2008, 111, 988–997. [Google Scholar] [CrossRef]
- Srivastava, R.; Bousquieres, J.; Cepeda-Vazquez, M.; Roux, S.; Bonazzi, C.; Rega, B. Kinetic study of furan and furfural generation during baking of cake models. Food Chem. 2018, 267, 329–336. [Google Scholar] [CrossRef]
- Ivanišová, E.; Kačániová, M.; Frančáková, H.; Petrová, J.; Hutková, J.; Brovarskyi, V.; Velychko, S.; Adamchuk, L.; Schubertová, Z.; Musilová, J. Bee bread—Perspective source of bioactive compounds for future. Potravináestvo 2015, 9, 592–598. [Google Scholar] [CrossRef]
- Kim, J.M.; To, T.K.; Matsui, A.; Tanoi, K.; Kobayashi, N.I.; Matsuda, F.; Seki, M. Acetate-mediated novel survival strategy against drought in plants. Nat. Plants 2017, 3, 17097. [Google Scholar] [CrossRef]
- Wang, X.; Rogers, K.M.; Li, Y.; Yang, S.; Chen, L.; Zhou, J. Untargeted and targeted discrimination of honey collected by Apis cerana and Apis mellifera based on volatiles using HS-GC-IMS and HS-SPME-GC–MS. J. Agric. Food Chem. 2019, 67, 43, 12144–12152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Si, J.-J.; Li, S.-S.; Zhang, G.-Z.; Wang, S.; Zheng, H.-Q.; Hu, F.-L. Chemical Analyses and Antimicrobial Activity of Nine Kinds of Unifloral Chinese Honeys Compared to Manuka Honey (12+ and 20+). Molecules 2021, 26, 2778. [Google Scholar] [CrossRef] [PubMed]
- Ferber, C.E.M.; Nursten, H.E. The aroma of beeswax. J. Sci. Food Agric. 1977, 28, 511–518. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef]
- Szczęsna, T. Study on the sugar composition of honeybee collected pollen. J. Apic. Sci. 2007, 51, 15–21. [Google Scholar]
- Kirs, E.; Pall, R.; Martverk, K.; Laos, K. Physicochemical and melissopalynological characterization of Estonian summer honeys. Procedia Food Sci. 2011, 1, 616–624. [Google Scholar] [CrossRef]
- Borawska, M.; Arciuch, L.; Puścian-Jakubik, A.; Lewoc, D. Content of sugars (fructose, glucose, sucrose) and proline in different varieties of natural bee honey. Probl. Hig. Epidemiol. 2015, 96, 816–820. [Google Scholar]
- European Union Directive (EU). Council Directive 2001/110/EC Relating to Honey; Official Journal of the European Communities: 2002. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2014:164:FULL&from=IT (accessed on 12 May 2021).
- Bertoncelj, J.; Polak, T.; Pucinar, T.; Lilek, N.; Borovšak, A.K.; Korošec, M. Carbohydrate composition of Slovenian bee pollens. Int. J. Food Sci. Tech. 2018, 53, 1880–1888. [Google Scholar] [CrossRef]
- Martins, M.C.T.; Morgano, A.M.; Vicente, E.; Baggio, S.R.; Rodriguez-Amaya, D.B. Physicochemical composition of bee pollen from eleven Brazilian states. J. Apic. Sci. 2011, 55, 107–116. [Google Scholar]
- Kalaycıoglu, Z.; Kaygusuz, H.; Doker, S.; Kolaylı, S.; Bedia Erim, F. Characterization of Turkish honeybee pollens by principal component analysis based on their individual organic acids, sugars, minerals, and antioxidant activities. LWT Food Sci. Technol. 2017, 84, 402–408. [Google Scholar] [CrossRef]
- Szczęsna, T.; Rybak-Chmielewska, H.; Waś, E.; Kachaniuk, K.; Teper, D. Characteristics of polish unifloral honeys. I. Rape honey (Brassica napus L. var. Oleifera Metzger). J. Apic. Sci. 2011, 55, 111–119. [Google Scholar]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; González-Viñas, M.A.; Pérez-Coello, M.S. Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme and heather honeys based on volatile composition and sensory descriptive analysis. Food Chem. 2009, 112, 1022–1030. [Google Scholar] [CrossRef]
- Marcazzan, G.L.; Mucignal-Caretta, C.; Marchese, C.M.; Piano, M.L. A review of methods for honey sensory analysis. J. Apic. Res. 2018, 57, 75–87. [Google Scholar] [CrossRef]
- Sipos, L.; Végh, R.; Bodor, Z.; Zinia Zaukuu, J.-L.; Hitka, G.; Bázár, G.; Kovacs, Z. Classification of bee pollen and prediction of sensory and colorimetric attributes—a sensometric fusion approach by e-Nose, e-Tongue and NIR. Sensors 2020, 20, 6768. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M.; Lelujka, E.; Ciska, E.; Lamparski, G.; Sawicki, T.; Wronkowska, M. The application of Lamiaceae Lindl. promotes aroma compounds formation, sensory properties, and antioxidant activity of oat and buckwheat-based cookies. Molecules 2020, 25, 5626. [Google Scholar] [CrossRef]
- Plutowska, B.; Chmiel, T.; Dymerski, T.; Wardencki, W. A headspace solid-phase microextraction method development and its application in the determination of volatiles in honeys by gas chromatography. Food Chem. 2011, 126, 1288–1298. [Google Scholar] [CrossRef]
- Brummer, Y.; Cui, S.W. Understanding Carbohydrate Analysis. In Food Carbohydrates; Cui, S.W., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; Volume 2, pp. 67–104. [Google Scholar]
- Saeeduddin, M.; Abid, M.; Jabbar, S.; Hu, B.; Hashim, M.M.; Khan, M.A.; Xie, M.; Wu, T.; Zeng, X. Physicochemical parameters, bioactive compounds and microbial quality of sonicated pear juice. Int. J. Food Sci. Technol. 2016, 51, 1552–1559. [Google Scholar] [CrossRef]
- ISO. PN-EN ISO 13299:2016-05. In Sensory Analysis- Methodology- General Guidance for Establishing a Sensory Profile; ISO: Warsaw, Poland, 2016. [Google Scholar]
- ISO. PN-EN ISO 8586:2014. In Sensory Analysis: General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors; ISO: Warsaw, Poland, 2014. [Google Scholar]
- ISO. ISO 8589: 2010. In Sensory Analysis: General Guidance for the Design of Test Rooms; ISO: Warsaw, Poland, 2010. [Google Scholar]
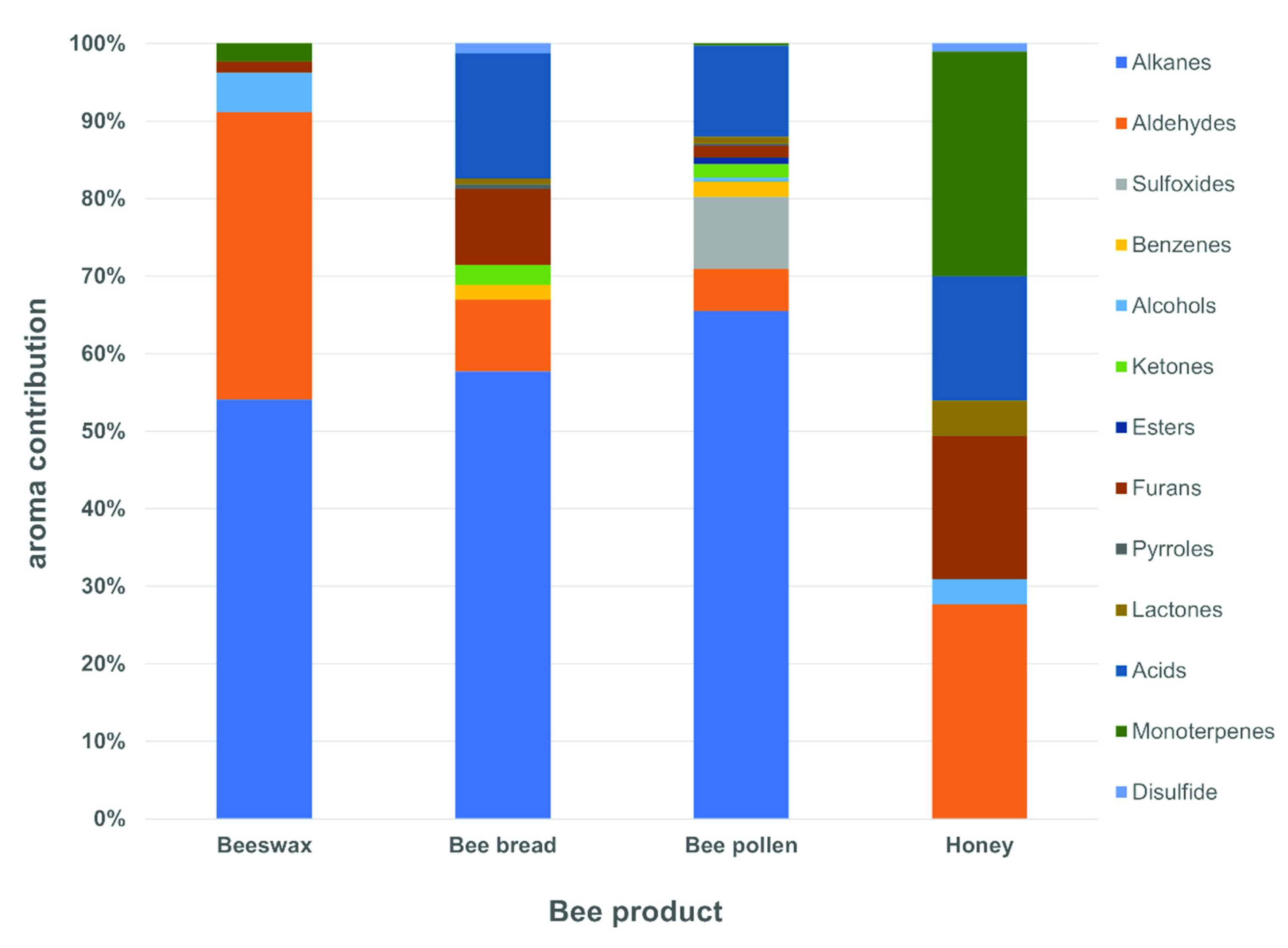
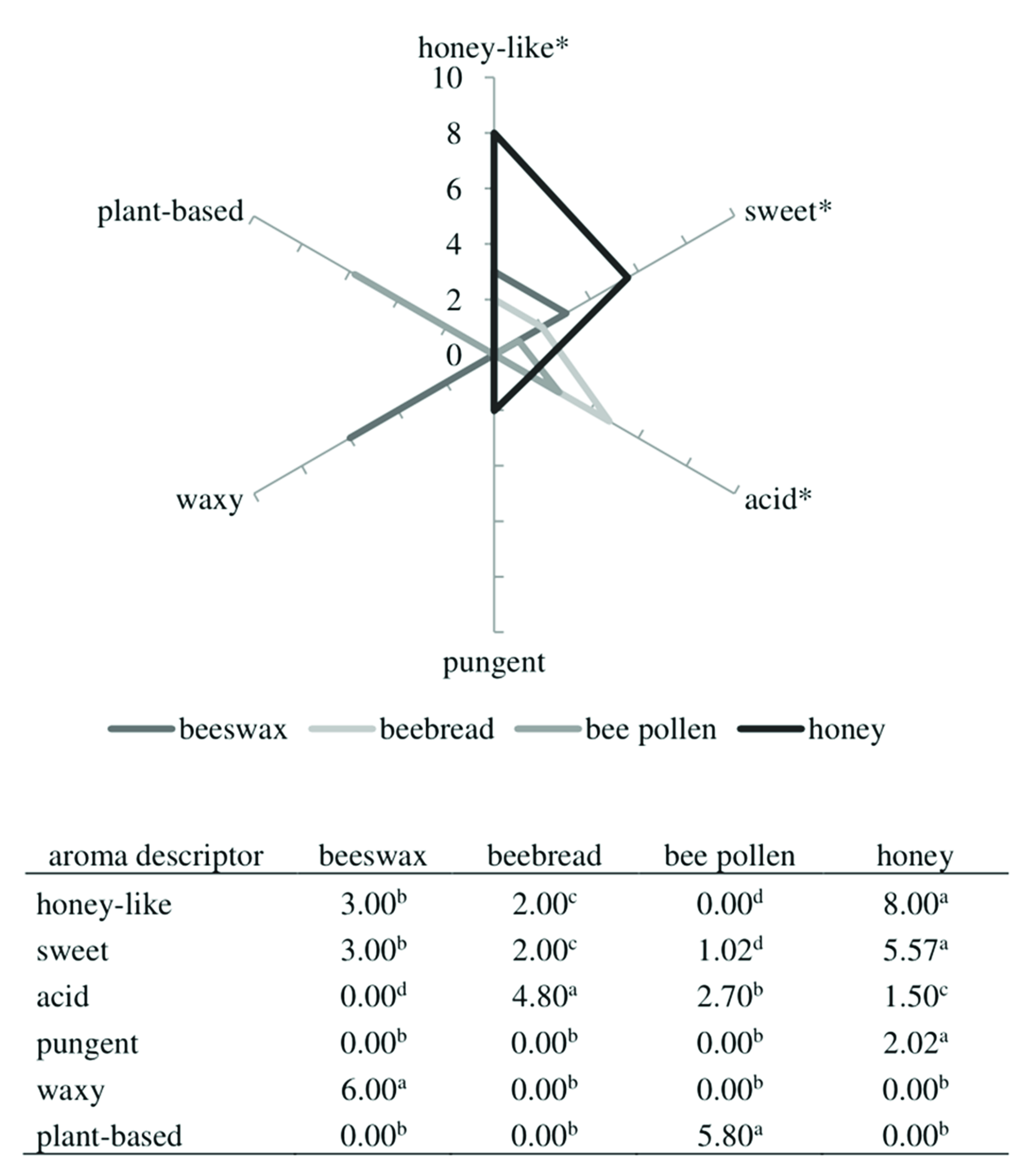
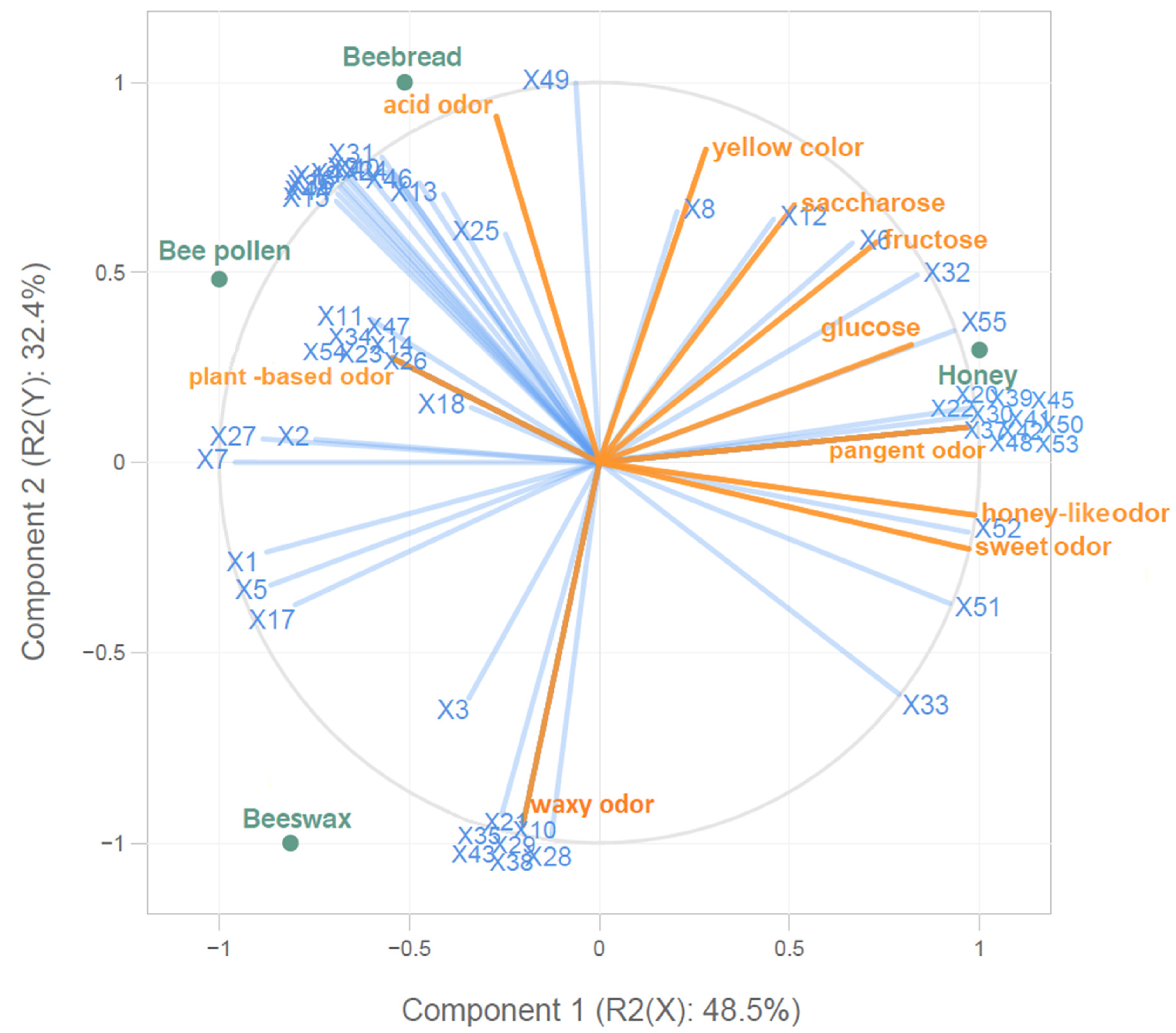
| No. | Compound | Aroma Description | LRI exp. | LRI lit. | Bee Products | |||
|---|---|---|---|---|---|---|---|---|
| Beeswax | Beebread | Bee Pollen | Honey | |||||
| Peak Areas [ppm] | ||||||||
| X1 | 2,4-dimethyl-heptane | - | 789 | 797 | 26.07 ± 2.47 a B | 12.29 ± 0.06 d C | 36.73 ± 2.94 a A | - |
| X2 | 4-methyl-octane | - | 822 | 823 | 12.56 ± 0.19 fg BC | 6.42 ± 0.64 efg C | 36.98 ± 4.42 a A | - |
| X3 | nonane | Alkane * | 899 | 900 | 7.60 ± 0.45 e A | 4.50 ± 0.38 ghjk B | - | - |
| X4 | 3,8-dimethyl-decane | - | 948 | MS | - | 5.35 ± 0.47 fghi A | 5.02 ± 0.04 f A | - |
| X5 | 2-methyl-nonane | - | 954 | 960 | 9.75 ± 0.17 de A | 8.07 ± 0.57 ef B | 6.59 ± 0.47 f B | - |
| X6 | decane | - | 1000 | 1000 | - | 22.55 ± 0.34a A | 23.49 ± 3.40 bc A | - |
| X7 | 4-methyl-decane | - | 1001 | 1005 | 3.99 ± 0.20 fg A | 4.57 ± 0.35 ghij A | 4.11 ± 0.59 f A | - |
| X8 | 2,6-dimethyl-nonane | - | 1010 | 1014 | - | 15.05 ± 0.82 bc A | 6.50 ± 0.08 f B | - |
| X9 | 2,6,11-trimethyl-dodecane | - | 1012 | 1015 | - | 23.92 ± 0.63 a A | 17.95 ± 3.10 cd A | - |
| X10 | α-pinene | pine, turpentine * | 1029 | 1030 | 3.14 ± 0.32 fgh | - | - | - |
| X11 | 5-methyl-decane | - | 1059 | MS | - | 2.95 ± 0.08 hijkl B | 19.61 ± 0.47 bc A | - |
| X12 | dimethyl disulfide | sulfurous * | 1087 | 1094 | - | 2.92 ± 0.44 hijkl A | - | 2.08 ± 0.24 h A |
| X13 | hexanal | green, fruity ** | 1090 | 1093 | - | 13.71 ± 1.46 cd A | 4.03 ± 0.00 f B | |
| X14 | benzyl methyl sulfoxide | - | 1102 | MS | - | - | 24.94 ± 3.28 b | - |
| X15 | 1-(3,3-dimethylbutyl)benzene | - | 1120 | MS | - | 1.83 ± 0.07 jkl A | 2.48 ± 0.35 f A | - |
| X16 | 2,6,10-trimethyl-tetradecane | - | 1190 | MS | - | 2.57 ± 0.05 jkl A | 2.77 ± 0.07 f B | - |
| X17 | dodecane | alkane * | 1225 | 1227 | 10.14 ± 0.44 de B | 2.79 ± 0.20 ijkl C | 12.79 ± 0.16 de A | - |
| X18 | nonadecane | alkane * | 1265 | 1263 | 2.81 ± 0.24 fgh B | 5.98 ± 0.66 efg A | - | - |
| X19 | styrene | pungent ** | 1270 | 1273 | - | 2.75 ± 0.05 ijkl A | 2.72 ± 0.10 f A | - |
| X20 | o-cymene | - | 1275 | 1276 | - | - | - | 2.39 ± 0.29 gh |
| X21 | octanal | aldehydic * | 1291 | 1291 | 18.60 ± 0.41 b A | 1.90 ± 0.21 jkl B | 0.70 ± 0.01 f C | - |
| X22 | p-cymene | sweet | 1293 | 1292 | - | - | - | 13.67 ± 2.10 d |
| X23 | 2-penten-1-ol | fruity | 1315 | 1314 | - | - | 1.42 ± 0.01 f | - |
| X24 | 6-methyl-5-hepten-2-one | green, citrus | 1342 | 1341 | - | 4.40 ± 0.18 ghijk A | 2.38 ± 0.08 f B | - |
| X25 | 1-tridecene | pleasant | 1344 | 1342 | - | 22.09 ± 0.45 a A | 0.72 ± 0.02 f B | - |
| X26 | 2-propenyl 2-propenoic acid, ester | - | 1456 | MS | - | - | 1.52 ± 0.02f | - |
| X27 | tetradecane | alkane * | 1399 | 1400 | 1.91 ± 0.14 fgh A | 2.51 ± 0.37 jkl A | 1.76 ± 0.04 f A | - |
| X28 | nonanal | aldehydic * | 1416 | 1418 | 15.18 ± 1.75 c A | 1.63 ± 0.18 l B | 2.78 ± 0.04 f B | 3.44 ± 0.12 gh B |
| X29 | 1-heptanol | green * | 1458 | 1459 | 1.55 ± 0.01 fgh | - | - | - |
| X30 | cis-linalool oxide | flower, woody | 1462 | 1461 | - | - | - | 2.43 ± 0.10 gh |
| X31 | acetic acid | sour, pungent | 1468 | 1470 | - | 17.37 ± 1.34 b A | 20.08 ± 3.41 bc A | 3.57 ± 0.02 gh B |
| X32 | furfural | bready * | 1477 | 1476 | 2.08 ± 0.15 fgh C | 24.07 ± 2.06a B | 3.87 ± 0.04 f C | 37.19 ± 1.51 b A |
| X33 | 2-ethyl-1-hexanol | citrus * | 1490 | 1493 | 1.54 ± 0.02 fgh B | - | - | 2.02 ± 0.01 h A |
| X34 | (E,E)-2,4-heptadienal | - | 1497 | MS | - | - | 0.81 ± 0.06 f | - |
| X35 | decanal | aldehydic * | 1499 | 1500 | 13.18 ± 1.01 c | - | - | - |
| X36 | (E,E)-3,5-octadien-2-one | - | 1545 | MS | - | 1.92 ± 0.11 jkl B | 2.19 ± 0.04 f A | - |
| X37 | benzaldehyde | almond-like | 1558 | 1562 | 4.32 ± 0.13 f B | 5.55 ± 0.96 fgh B | 6.05 ± 0.88 f B | 52.39 ± 1.74 a A |
| X38 | 1-octanol | waxy * | 1561 | 1561 | 1.35 ± 0.04 fgh | - | - | - |
| X39 | linalool | floral, sweet | 1566 | 1565 | - | - | - | 28.79 ± 1.09 c |
| X40 | 1-ethyl-1H-pyrrole-2-carbaldehyde | burnt, roasted | 1612 | 1616 | - | 1.58 ± 0.01 l A | 0.93 ± 0.07 f B | - |
| X41 | hotrienol | sweet tropical * | 1633 | 1632 | - | - | - | 8.45 ± 0.14 ef |
| X42 | dihydro-4-methyl-2(3H)-furanone | - | 1649 | 1653 | - | - | - | 9.17 ± 0.09 e |
| X43 | 1- nonanol | floral * | 1660 | 1668 | 0.85 ± 0.01 h | - | - | - |
| X44 | butyrolactone | sweet, caramel ** | 1677 | 1673 | - | 1.81 ± 0.39 kl A | 2.24 ± 0.26 f A | - |
| X45 | 2/3-methyl-butanoic acid | sweaty | 1682 | 1684 | - | 1.20 ± 0.14 l B | - | 14.91 ± 1.15 d A |
| X46 | 2-methyl-hexanoic acid | fruity | 1755 | 1757 | - | 11.43 ± 1.05 d A | 4.67 ± 0.23 f B | - |
| X47 | verbenone (I) | minty, spicy ** | 1732 | 1733 | - | - | 0.66 ± 0.03 f | - |
| X48 | 3-methylvaleric acid | animal * | 1823 | 1826 | - | - | - | 2.91 ± 0.01 gh |
| X49 | hexanoic acid | fatty * | 1844 | 1842 | - | 8.51 ± 0.13 e A | 6.70 ± 0.02 ef B | 5.27 ± 0.02 fg C |
| X50 | p-cymen-8-ol | floral, sweet ** | 1879 | 1872 | - | - | - | 2.59 ± 0.06 gh |
| X51 | benzyl alcohol | sweet, floral * | 1900 | 1906 | 0.98 ± 0.01 gh B | - | - | 2.09 ± 0.01 gh A |
| X52 | phenylethyl alcohol | floral * | 1923 | 1931 | 0.69 ± 0.01 h B | - | - | 2.47 ± 0.01 gh A |
| X53 | heptanoic acid | cheesy * | 1991 | 1990 | - | - | - | 2.84 ± 0.01 gh |
| X54 | 2-propenoic acid, 3-phenyl-, methyl ester | fruity | 2056 | 2065 | - | - | 0.92 ± 0.11 f | - |
| X55 | nonanoic acid | waxy * | 2200 | 2202 | - | 1.21 ± 0.25 l B | - | 3.02 ± 0.02 gh A |
| sum | 138.29 ± 5.73 C | 245.40 ± 1.70 A | 267.13 ± 8.32 A | 201.70 ± 3.39 B | ||||
| Bee Products | Sugar [g/100 g] | ||||
|---|---|---|---|---|---|
| Fructose | Glucose | Sucrose | TOTAL Amount of Sugars | F/G Ratio | |
| Beeswax | 0.06 ± 0.00 d | 2.03 ± 0.14 d | - | 2.09 | 0.03 |
| Beebread | 11.58 ± 0.12 c | 5.12 ± 0.33 c | 0.11 ± 0.00 a | 16.81 | 2.26 |
| Bee pollen | 14.06 ± 0.01 b | 11.56 ± 0.43 b | 0.01 ± 0.00 b | 25.63 | 1.22 |
| Honey | 27.60 ± 0.48 a | 26.32 ± 0.49 a | 0.10 ± 0.01 a | 54.02 | 1.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starowicz, M.; Hanus, P.; Lamparski, G.; Sawicki, T. Characterizing the Volatile and Sensory Profiles, and Sugar Content of Beeswax, Beebread, Bee Pollen, and Honey. Molecules 2021, 26, 3410. https://doi.org/10.3390/molecules26113410
Starowicz M, Hanus P, Lamparski G, Sawicki T. Characterizing the Volatile and Sensory Profiles, and Sugar Content of Beeswax, Beebread, Bee Pollen, and Honey. Molecules. 2021; 26(11):3410. https://doi.org/10.3390/molecules26113410
Chicago/Turabian StyleStarowicz, Małgorzata, Paweł Hanus, Grzegorz Lamparski, and Tomasz Sawicki. 2021. "Characterizing the Volatile and Sensory Profiles, and Sugar Content of Beeswax, Beebread, Bee Pollen, and Honey" Molecules 26, no. 11: 3410. https://doi.org/10.3390/molecules26113410
APA StyleStarowicz, M., Hanus, P., Lamparski, G., & Sawicki, T. (2021). Characterizing the Volatile and Sensory Profiles, and Sugar Content of Beeswax, Beebread, Bee Pollen, and Honey. Molecules, 26(11), 3410. https://doi.org/10.3390/molecules26113410







