Photosynthetic Light-Harvesting (Antenna) Complexes—Structures and Functions
Abstract
1. Introduction
2. The Photosynthetic Apparatus of Plants (and Algae)
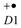 [22]. Alternative and complementary pathways are being discussed for the primary charge separation in PSII [23]. Analogously, also in PSI, Chl a was shown to act as the primary electron donor, which transfers an electron onto another Chl a (denoted A0) as the acceptor. Subsequently, the “special pair” P700 (actually being a heterodimer of Chl a and Chl a’, its C13 epimer) is rapidly oxidized [19,24].
[22]. Alternative and complementary pathways are being discussed for the primary charge separation in PSII [23]. Analogously, also in PSI, Chl a was shown to act as the primary electron donor, which transfers an electron onto another Chl a (denoted A0) as the acceptor. Subsequently, the “special pair” P700 (actually being a heterodimer of Chl a and Chl a’, its C13 epimer) is rapidly oxidized [19,24].2.1. Plant (and Algal) Peripheral Light-Harvesting Complexes
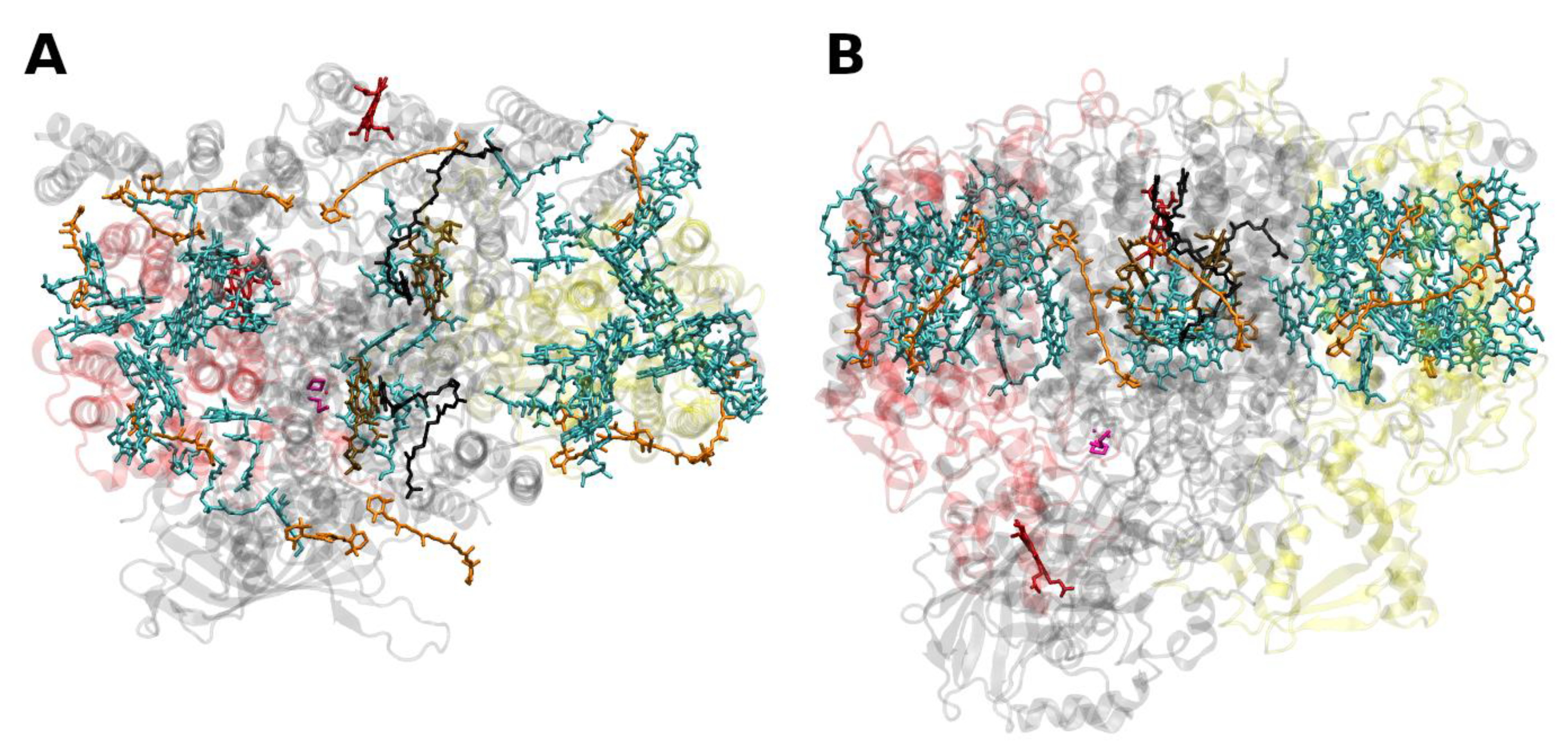
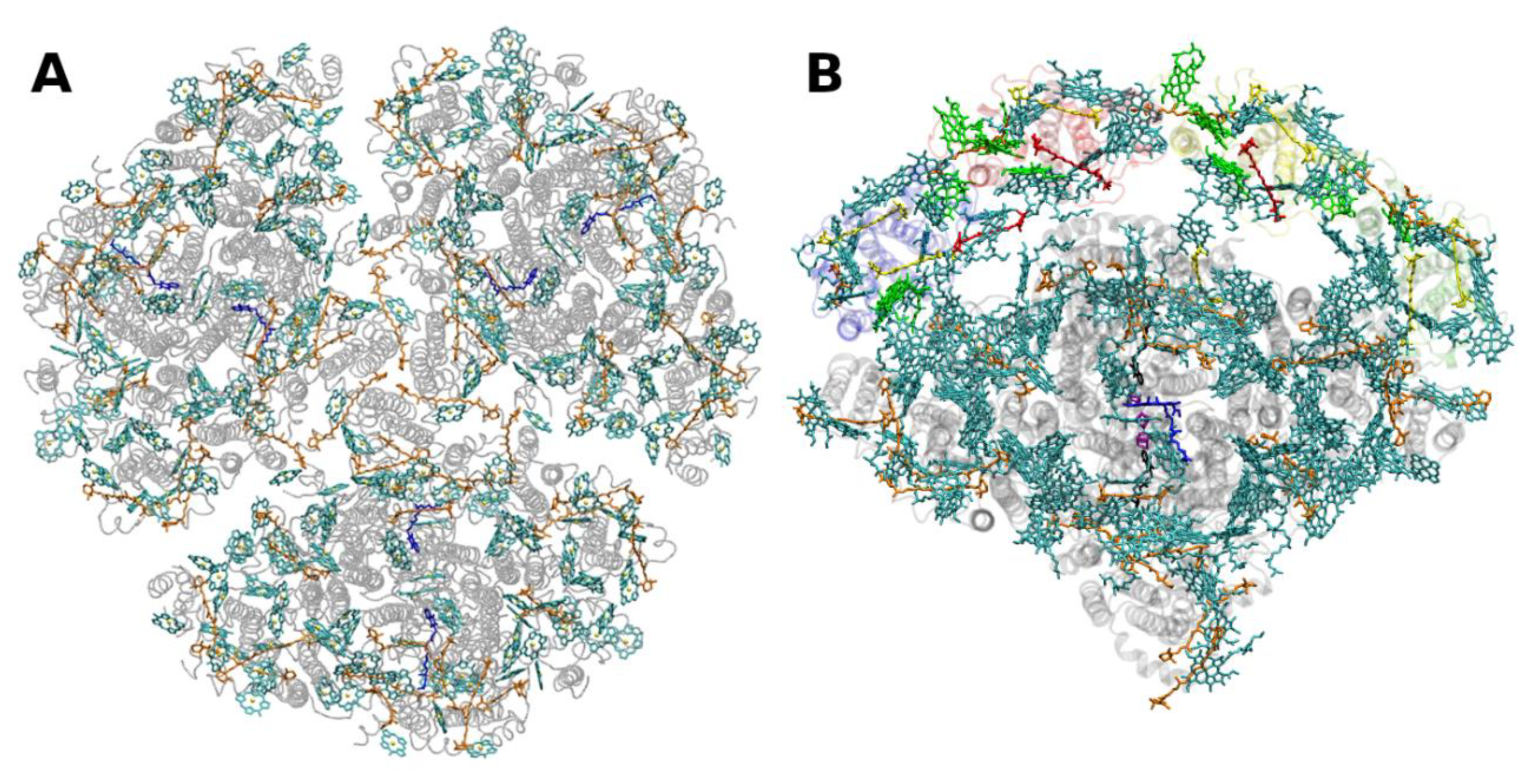
2.2. Core Antenna Complexes
2.3. Peridinin-Chlorophyll a-Protein (PCP)
3. Antenna Systems of Photosynthetic Prokaryotes
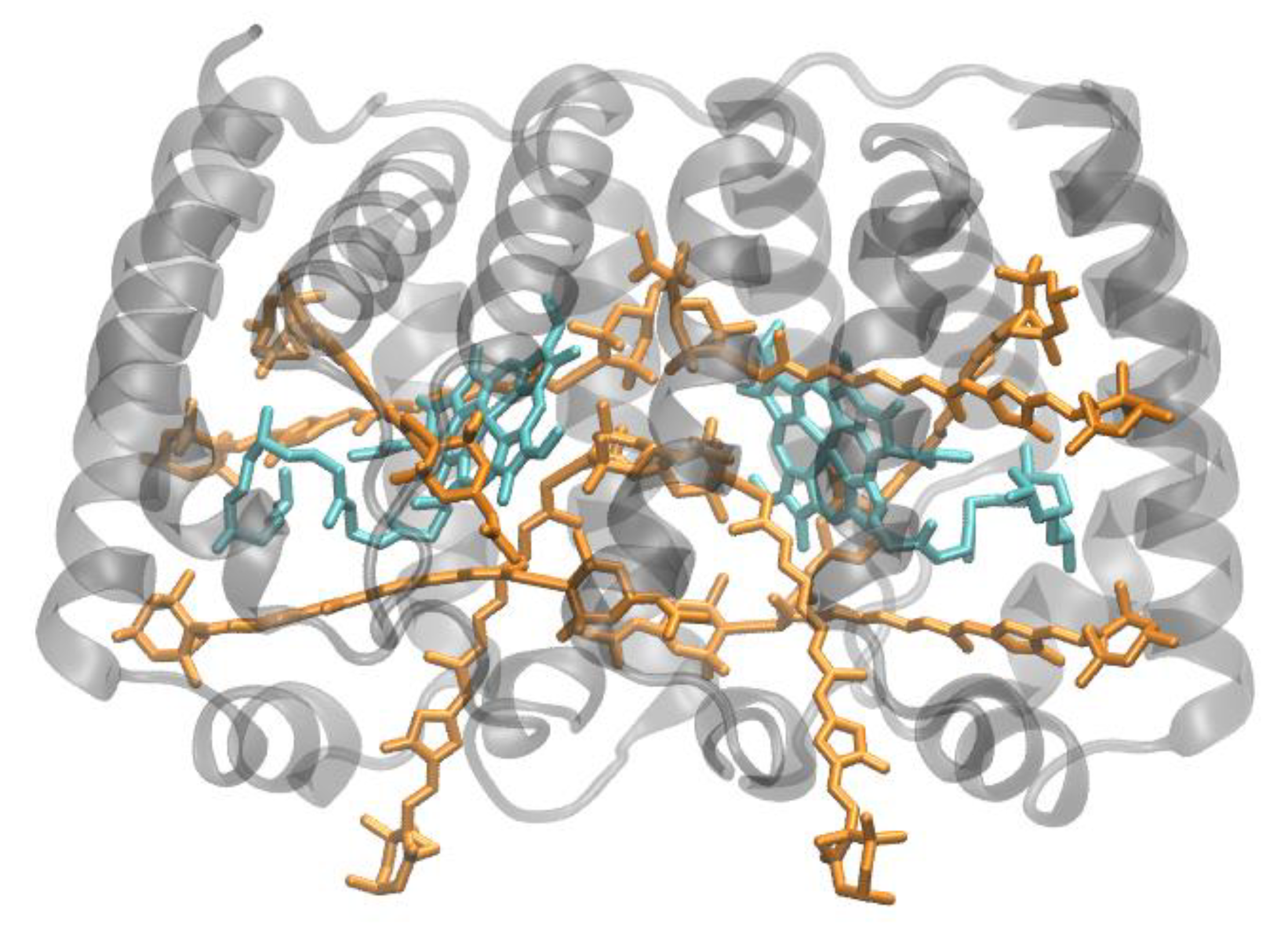
3.1. Purple Bacterial Antenna Complexes
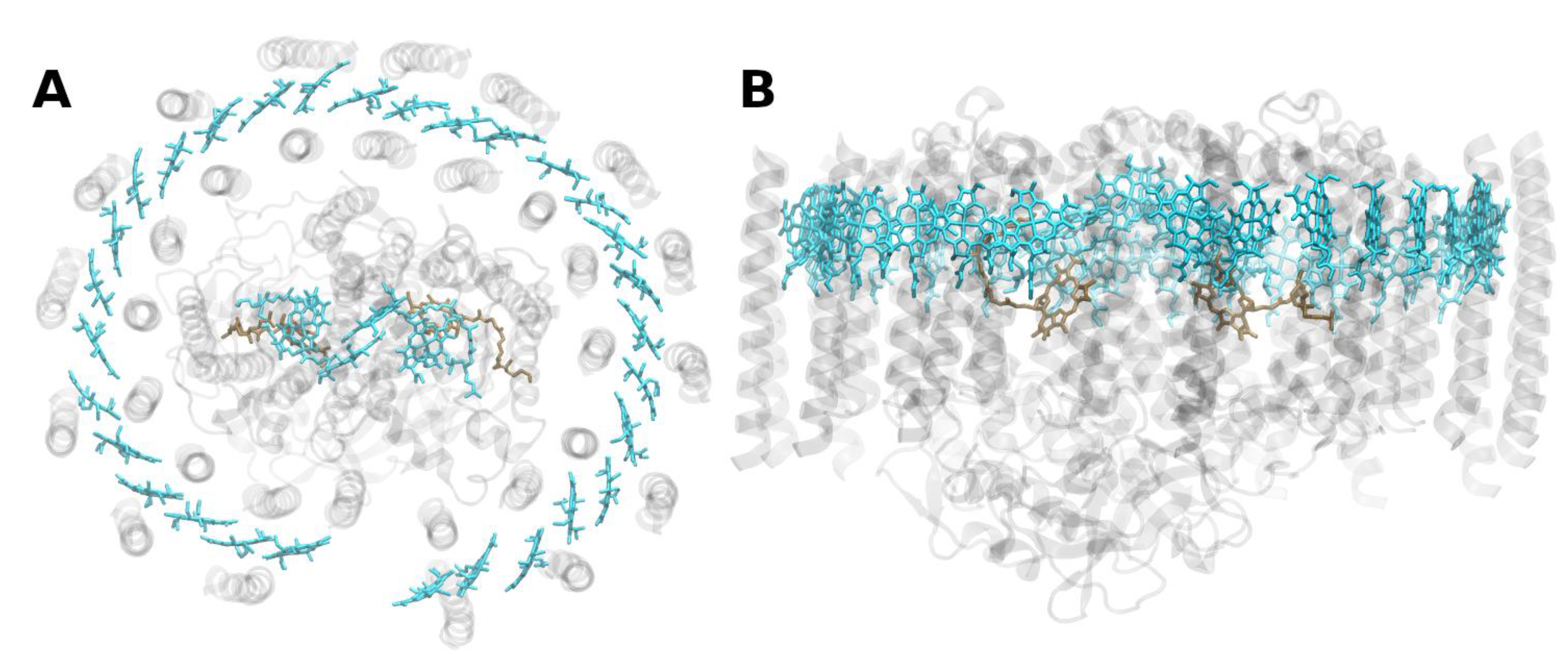
3.2. Chlorosomes and Other Antenna System Components of Photosynthetic Green Bacteria
3.3. Cyanobacterial Antenna Systems
3.4. IsiA and Pcbs
4. In Vitro Reconstitution of LHCs
5. Photodamage, Photoprotection and Regulation of Functional Antenna Size
6. Highlights
- We review the wide variety of naturally occurring photosynthetic light-harvesting complexes (LHCs) from a structure-function relationship perspective.
- A common feature of natural light-harnessing systems is a trimeric (or multiples thereof) organization.
- We provide a simple explanation of this—so far unaccounted for—phenomenon.
- Natural light-harnessing systems can be used as inspirations for biomimetic assemblies and/or attached to biohybrid devices for sustainable solar energy conversion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Renger, G. (Ed.) Primary Processes of Photosynthesis, Part 2: Principles and Apparatus; Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Green, B.R.; Parson, W.W. (Eds.) Light-Harvesting Antennas in Photosynthesis; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar] [CrossRef]
- Tomo, T.; Allakhverdiev, S.I.; Mimuro, M. Constitution and energetics of photosystem I and photosystem II in the chlorophyll d-dominated cyanobacterium Acaryochloris marina. J. Photochem. Photobiol. B Biol. 2011, 104, 333–340. [Google Scholar] [CrossRef]
- Murray, J.; Duncan, J.; Barber, J. CP43-like chlorophyll binding proteins: Structural and evolutionary implications. Trends Plant Sci. 2006, 11, 152–158. [Google Scholar] [CrossRef]
- Hoffman, G.E.; Sanchez-Puerta, M.V.; Delwiche, C.F. Evolution of light-harvesting complex proteins from Chl c-containing algae. BMC Evol. Biol. 2011, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.-L.; Neilan, B.A.; Scheer, H. A Red-Shifted Chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Ikemoto, H.; Kurano, N.; Adachi, K.; Chihara, M.; Miyachi, S. Chlorophyll d as a major pigment. Nature 1996, 383, 402. [Google Scholar] [CrossRef]
- Loughlin, P.; Lin, Y.; Chen, M. Chlorophyll d and Acaryochloris marina: Current status. Photosynth. Res. 2013, 116, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Gan, F.; Zhang, S.; Rockwell, N.C.; Martin, S.S.; Lagarias, J.C.; Bryant, D.A. Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science 2014, 345, 1312–1317. [Google Scholar] [CrossRef]
- Nürnberg, D.J.; Morton, J.; Santabarbara, S.; Telfer, A.; Joliot, P.; Antonaru, L.A.; Ruban, A.V.; Cardona, T.; Krausz, E.; Boussac, A.; et al. Photochemistry beyond the red limit in chlorophyll f–containing photosystems. Science 2018, 360, 1210–1213. [Google Scholar] [CrossRef]
- Cherepanov, D.A.; Shelaev, I.V.; Gostev, F.E.; Aybush, A.V.; Mamedov, M.D.; Shen, G.; Nadtochenko, V.A.; Bryant, D.A.; Semenov, A.Y.; Golbeck, J.H. Evidence that chlorophyll f functions solely as an antenna pigment in far-red-light photosystem I from Fischerella thermalis PCC 7521. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148184. [Google Scholar] [CrossRef]
- Hashimoto, H.; Sugai, Y.; Uragami, C.; Gardiner, A.T.; Cogdell, R.J. Natural and artificial light-harvesting systems utilizing the functions of carotenoids. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 46–70. [Google Scholar] [CrossRef]
- Fiedor, L.; Dudkowiak, A.; Pilch, M. The origin of the dark S1 state in carotenoids: A comprehensive model. J. R. Soc. Interface 2019, 16. [Google Scholar] [CrossRef]
- Arvidsson, P.-O.; Sundby, C. A model for the topology of the chloroplast thylakoid membrane. Funct. Plant Biol. 1999, 26, 687–694. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Krupinska, K. New Insights in Thylakoid Membrane Organization. Plant Cell Physiol. 2005, 46, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Broess, K.; Trinkunas, G.; Van Hoek, A.; Croce, R.; van Amerongen, H. Determination of the excitation migration time in Photosystem II. Biochim. Biophys. Acta Bioenerg. 2008, 1777, 404–409. [Google Scholar] [CrossRef]
- Renger, G. The light reactions of photosynthesis. Curr. Sci. 2010, 98, 1305–1319. [Google Scholar]
- Groot, M.L.; Pawlowicz, N.P.; van Wilderen, L.J.G.W.; Breton, J.; van Stokkum, I.H.M.; van Grondelle, R. Initial electron donor and acceptor in isolated Photosystem II reaction centers identified with femtosecond mid-IR spectroscopy. Proc. Natl. Acad. Sci. USA 2005, 102, 13087–13092. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.G.; Niklas, J.; Lubitz, W.; Holzwarth, A.R. Ultrafast Transient Absorption Studies on Photosystem I Reaction Centers from Chlamydomonas reinhardtii. 1. A New Interpretation of the Energy Trapping and Early Electron Transfer Steps in Photosystem I. Biophys. J. 2003, 85, 3899–3922. [Google Scholar] [CrossRef]
- Holzwarth, A.R.; Müller, M.G.; Niklas, J.; Lubitz, W. Ultrafast Transient Absorption Studies on Photosystem I Reaction Centers from Chlamydomonas reinhardtii. 2: Mutations near the P700 Reaction Center Chlorophylls Provide New Insight into the Nature of the Primary Electron Donor. Biophys. J. 2006, 90, 552–565. [Google Scholar] [CrossRef]
- Holzwarth, A.R.; Müller, M.G.; Reus, M.; Nowaczyk, M.; Sander, J.; Rogner, M. Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: Pheophytin is the primary electron acceptor. Proc. Natl. Acad. Sci. USA 2006, 103, 6895–6900. [Google Scholar] [CrossRef] [PubMed]
- Renger, G.; Renger, T. Photosystem II: The machinery of photosynthetic water splitting. Photosynth. Res. 2008, 98, 53–80. [Google Scholar] [CrossRef] [PubMed]
- Novoderezhkin, V.I.; Romero, E.; Dekker, J.P.; van Grondelle, R. Multiple Charge-Separation Pathways in Photosystem II: Modeling of Transient Absorption Kinetics. ChemPhysChem 2011, 12, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Giera, W.; Ramesh, V.; Webber, A.N.; van Stokkum, I.; van Grondelle, R.; Gibasiewicz, K. Effect of the P700 pre-oxidation and point mutations near A0 on the reversibility of the primary charge separation in Photosystem I from Chlamydomonas reinhardtii. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 106–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitchell, P. Conduction of protons through membranes of mitochondria and bacteria by uncouplers of oxidative phosphorylation. Biochem. J. 1961, 81, 24. [Google Scholar]
- Mitchell, P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. 1966, 41, 445–501. [Google Scholar] [CrossRef]
- Junge, W. Photophosphorylation. In Primary Processes of Photosynthesis, Part 2: Principles and Apparatus; Renger, G., Ed.; Royal Society of Chemistry: Cambridge, UK, 2007; pp. 447–487. [Google Scholar] [CrossRef]
- Jansson, S. The light-harvesting chlorophyll ab-binding proteins. Biochim. Biophys. Acta Bioenerg. 1994, 1184, 1–19. [Google Scholar] [CrossRef]
- Pan, X.; Ma, J.; Su, X.; Cao, P.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Structure of the maize photosystem I supercomplex with light-harvesting complexes I and II. Science 2018, 360, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Su, X.; Cao, P.; Liu, X.; Chang, W.; Li, M.; Zhang, X.; Liu, Z. Structure of spinach photosystem II–LHCII supercomplex at 3.2 Å resolution. Nature 2016, 534, 69–74. [Google Scholar] [CrossRef]
- Ben-Shem, A.; Frolow, F.; Nelson, N. Crystal structure of plant photosystem I. Nature 2003, 426, 630–635. [Google Scholar] [CrossRef]
- Croce, R.; van Amerongen, H. Light-harvesting in photosystem I. Photosynth. Res. 2013, 116, 153–166. [Google Scholar] [CrossRef]
- Mazor, Y.; Borovikova, A.; Caspy, I.; Nelson, N. Structure of the plant photosystem I supercomplex at 2.6 Å resolution. Nat. Plants 2017, 3, 17014. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, H.; Wang, K.; Kuang, T.; Zhang, J.; Gui, L.; An, X.; Chang, W. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 2004, 428, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Standfuss, J.; Van Scheltinga, A.C.T.; Lamborghini, M.; Kühlbrandt, W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J. 2005, 24, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, M.; Wan, T.; Wang, L.; Jia, C.; Hou, Z.; Zhao, X.; Zhang, J.; Chang, W. Structural insights into energy regulation of light-harvesting complex CP29 from spinach. Nat. Struct. Mol. Biol. 2011, 18, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Schubert, A.; Beenken, W.J.; Stiel, H.; Voigt, B.; Leupold, D.; Lokstein, H. Excitonic Coupling of Chlorophylls in the Plant Light-Harvesting Complex LHC-II. Biophys. J. 2002, 82, 1030–1039. [Google Scholar] [CrossRef][Green Version]
- Kühlbrandt, W.; Wang, D.N.; Fujiyoshi, Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature 1994, 367, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Rogl, H.; Schödel, R.; Lokstein, H.; Kühlbrandt, W.; Schubert, A. Assignment of Spectral Substructures to Pigment-Binding Sites in Higher Plant Light-Harvesting Complex LHC-II. Biochemistry 2002, 41, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Krikunova, M.; Voigt, B.; Lokstein, H. Direct evidence for excitonically coupled chlorophylls a and b in LHC II of higher plants by nonlinear polarization spectroscopy in the frequency domain. Biochim. Biophys. Acta Bioenerg. 2002, 1556, 1–5. [Google Scholar] [CrossRef][Green Version]
- Pieper, J.; Irrgang, K.-D. Nature of low-energy exciton levels in light-harvesting complex II of green plants as revealed by satellite hole structure. Photosynth. Res. 2020, 146, 279–285. [Google Scholar] [CrossRef]
- Lokstein, H.; Leupold, D.; Voigt, B.; Nowak, F.; Ehlert, J.; Hoffmann, P.; Garab, G. Nonlinear polarization spectroscopy in the frequency domain of light-harvesting complex II: Absorption band substructure and exciton dynamics. Biophys. J. 1995, 69, 1536–1543. [Google Scholar] [CrossRef][Green Version]
- Voigt, B.; Krikunova, M.; Lokstein, H. Influence of detergent concentration on aggregation and spectroscopic properties of light-harvesting complex II. Photosynth. Res. 2007, 95, 317–325. [Google Scholar] [CrossRef]
- Ginsberg, N.S.; Davis, J.; Ballottari, M.; Cheng, Y.-C.; Bassi, R.; Fleming, G.R. Solving structure in the CP29 light harvesting complex with polarization-phased 2D electronic spectroscopy. Proc. Natl. Acad. Sci. USA 2011, 108, 3848–3853. [Google Scholar] [CrossRef]
- Jurinovich, S.; Viani, L.; Prandi, I.G.; Renger, T.; Mennucci, B. Towards an ab initio description of the optical spectra of light-harvesting antennae: Application to the CP29 complex of photosystem II. Phys. Chem. Chem. Phys. 2015, 17, 14405–14416. [Google Scholar] [CrossRef]
- König, C.; Neugebauer, J. First-principles calculation of electronic spectra of light-harvesting complex II. Phys. Chem. Chem. Phys. 2011, 13, 10475–10490. [Google Scholar] [CrossRef]
- Jang, S.J.; Mennucci, B. Delocalized excitons in natural light-harvesting complexes. Rev. Mod. Phys. 2018, 90, 035003. [Google Scholar] [CrossRef]
- Dreuw, A.; Harbach, P.H.P.; Mewes, J.M.; Wormit, M. Quantum chemical excited state calculations on pigment–protein complexes require thorough geometry re-optimization of experimental crystal structures. Theor. Chem. Acc. 2009, 125, 419–426. [Google Scholar] [CrossRef]
- Götze, J.P.; Thiel, W. TD-DFT and DFT/MRCI study of electronic excitations in Violaxanthin and Zeaxanthin. Chem. Phys. 2013, 415, 247–255. [Google Scholar] [CrossRef]
- König, C.; Neugebauer, J. Quantum Chemical Description of Absorption Properties and Excited-State Processes in Photosynthetic Systems. ChemPhysChem 2011, 13, 386–425. [Google Scholar] [CrossRef] [PubMed]
- Curutchet, C.; Mennucci, B. Quantum Chemical Studies of Light Harvesting. Chem. Rev. 2016, 117, 294–343. [Google Scholar] [CrossRef] [PubMed]
- Liguori, N.; Croce, R.; Marrink, S.J.; Thallmair, S. Molecular dynamics simulations in photosynthesis. Photosynth. Res. 2020, 144, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Cupellini, L.; Bondanza, M.; Nottoli, M.; Mennucci, B. Successes & challenges in the atomistic modeling of light-harvesting and its photoregulation. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148049. [Google Scholar] [CrossRef] [PubMed]
- Renger, T.; Müh, F. Understanding photosynthetic light-harvesting: A bottom up theoretical approach. Phys. Chem. Chem. Phys. 2013, 15, 3348–3371. [Google Scholar] [CrossRef]
- Sláma, V.; Cupellini, L.; Mennucci, B. Exciton properties and optical spectra of light harvesting complex II from a fully atomistic description. Phys. Chem. Chem. Phys. 2020, 22, 16783–16795. [Google Scholar] [CrossRef]
- Zouni, A.; Witt, H.-T.; Kern, J.; Fromme, P.; Krauss, N.; Saenger, W.; Orth, P.P. Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature 2001, 409, 739–743. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Fromme, P.; Grotjohann, I. Structure of Cyanobacterial Photosystems I and II. In Bioenergetic Processes of Cyanobacteria; Springer: Dordrecht, The Netherlands, 2011; pp. 285–335. [Google Scholar] [CrossRef]
- Malavath, T.; Caspy, I.; Netzer-El, S.Y.; Klaiman, D.; Nelson, N. Structure and function of wild-type and subunit-depleted photosystem I in Synechocystis. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 645–654. [Google Scholar] [CrossRef]
- Hatazaki, S.; Sharma, D.K.; Hirata, S.; Nose, K.; Iyoda, T.; Kölsch, A.; Lokstein, H.; Vacha, M. Identification of Short- and Long-Wavelength Emitting Chlorophylls in Cyanobacterial Photosystem I by Plasmon-Enhanced Single-Particle Spectroscopy at Room Temperature. J. Phys. Chem. Lett. 2018, 9, 6669–6675. [Google Scholar] [CrossRef] [PubMed]
- Kerfeld, C.A. Structure and Function of the Water-Soluble Carotenoid-Binding Proteins of Cyanobacteria. Photosynth. Res. 2004, 81, 215–225. [Google Scholar] [CrossRef]
- Hofmann, E.; Wrench, P.M.; Sharples, F.P.; Hiller, R.G.; Welte, W.; Diederichs, K. Structural Basis of Light Harvesting by Carotenoids: Peridinin-Chlorophyll-Protein from Amphidinium carterae. Science 1996, 272, 1788–1791. [Google Scholar] [CrossRef]
- Krikunova, M.; Lokstein, H.; Leupold, D.; Hiller, R.G.; Voigt, B. Pigment-Pigment Interactions in PCP of Amphidinium carterae Investigated by Nonlinear Polarization Spectroscopy in the Frequency Domain. Biophys. J. 2006, 90, 261–271. [Google Scholar] [CrossRef][Green Version]
- Leupold, D.; Lokstein, H.; Scheer, H. Excitation Energy Transfer between (Bacterio) Chlorophylls—The Role of Excitonic Coupling. In Chlorophylls and Bacteriochlorophylls; Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 413–430. [Google Scholar] [CrossRef]
- Damjanović, A.; Ritz, T.; Schulten, K. Excitation Transfer in the Peridinin-Chlorophyll-Protein of Amphidinium carterae. Biophys. J. 2000, 79, 1695–1705. [Google Scholar] [CrossRef]
- Krueger, B.P.; Lampoura, S.S.; Van Stokkum, I.H.; Papagiannakis, E.; Salverda, J.M.; Gradinaru, C.; Rutkauskas, D.; Hiller, R.G.; Van Grondelle, R. Energy Transfer in the Peridinin Chlorophyll-a Protein of Amphidinium carterae Studied by Polarized Transient Absorption and Target Analysis. Biophys. J. 2001, 80, 2843–2855. [Google Scholar] [CrossRef]
- Zigmantas, D.; Polivka, T.; Hiller, R.G.; Yartsev, A.; Sundström, V. Spectroscopic and Dynamic Properties of the Peridinin Lowest Singlet Excited States. J. Phys. Chem. A 2001, 105, 10296–10306. [Google Scholar] [CrossRef]
- Zigmantas, D.; Hiller, R.G.; Sundstrom, V.; Polivka, T. Carotenoid to chlorophyll energy transfer in the peridinin-chlorophyll-a-protein complex involves an intramolecular charge transfer state. Proc. Natl. Acad. Sci. USA 2002, 99, 16760–16765. [Google Scholar] [CrossRef] [PubMed]
- Zigmantas, D.; Hiller, R.G.; Sharples, F.P.; Frank, H.A.; Sundström, V.; Polivka, T. Effect of a conjugated carbonyl group on the photophysical properties of carotenoids. Phys. Chem. Chem. Phys. 2004, 6, 3009–3016. [Google Scholar] [CrossRef]
- Shima, S.; Ilagan, R.P.; Gillespie, N.; Sommer, B.J.; Hiller, R.G.; Sharples, F.P.; Frank, H.A.; Birge, R.R. Two-Photon and Fluorescence Spectroscopy and the Effect of Environment on the Photochemical Properties of Peridinin in Solution and in the Peridinin-Chlorophyll-Protein from Amphidinium carterae. J. Phys. Chem. A 2003, 107, 8052–8066. [Google Scholar] [CrossRef]
- Polívka, T.; Sundström, V. Ultrafast Dynamics of Carotenoid Excited States—From Solution to Natural and Artificial Systems. Chem. Rev. 2004, 104, 2021–2072. [Google Scholar] [CrossRef]
- Kleima, F.J.; Wendling, M.; Hofmann, E.; Peterman, E.J.G.; van Grondelle, A.R.; van Amerongen, H. Peridinin Chlorophyll a Protein: Relating Structure and Steady-State Spectroscopy. Biochemistry 2000, 39, 5184–5195. [Google Scholar] [CrossRef]
- Ilagan, R.P.; Shima, S.; Melkozernov, A.; Lin, S.; Blankenship, R.E.; Sharples, F.P.; Hiller, R.G.; Birge, R.R.; Frank, H.A. Spectroscopic Properties of the Main-Form and High-Salt Peridinin—Chlorophyll a Proteins from Amphidinium carterae. Biochemistry 2004, 43, 1478–1487. [Google Scholar] [CrossRef]
- Wörmke, S.; Mackowski, S.; Brotosudarmo, T.; Jung, C.; Zumbusch, A.; Ehrl, M.; Scheer, H.; Hofmann, E.; Hiller, R.; Bräuchle, C. Monitoring fluorescence of individual chromophores in peridinin–chlorophyll–protein complex using single molecule spectroscopy. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 956–964. [Google Scholar] [CrossRef]
- Vinklárek, I.S.; Bornemann, T.L.V.; Lokstein, H.; Hofmann, E.; Alster, J.; Pšenčík, J. Temperature Dependence of Chlorophyll Triplet Quenching in Two Photosynthetic Light-Harvesting Complexes from Higher Plants and Dinoflagellates. J. Phys. Chem. B 2018, 122, 8834–8845. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.L.; Greco, J.A.; Enriquez, M.M.; Frank, H.A.; Birge, R.R. The Nature of the Intramolecular Charge Transfer State in Peridinin. Biophys. J. 2013, 104, 1314–1325. [Google Scholar] [CrossRef]
- Götze, J.P.; Karasulu, B.; Patil, M.; Thiel, W. Vibrational relaxation as the driving force for wavelength conversion in the peridinin–chlorophyll a-protein. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 1509–1517. [Google Scholar] [CrossRef]
- Knecht, S.; Marian, C.M.; Kongsted, J.; Mennucci, B. On the Photophysics of Carotenoids: A Multireference DFT Study of Peridinin. J. Phys. Chem. B 2013, 117, 13808–13815. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bishop, M.M.; Roscioli, J.D.; LaFountain, A.M.; Frank, H.A.; Beck, W.F. Excitation Energy Transfer by Coherent and Incoherent Mechanisms in the Peridinin–Chlorophyll a Protein. J. Phys. Chem. Lett. 2017, 8, 463–469. [Google Scholar] [CrossRef]
- Parson, W.W. Functional Patterns of Reaction Centers in Anoxygenic Photosynthetic Bacteria. In Primary Processes of Photosynthesis, Part 2: Principles and Apparatus; Renger, G., Ed.; Royal Society of Chemistry: Cambridge, UK, 2007; pp. 57–109. [Google Scholar] [CrossRef]
- Peschek, G.A.; Bernroitner, M.; Sari, S.; Pairer, M.; Obinger, C. Life Implies Work: A Holistic Account of Our Microbial Biosphere Focussing on the Bioenergetic Processes of Cyanobacteria, the Ecologically Most Successful Organisms on Our Earth. In Bioenergetic Processes of Cyanobacteria; Peschek, G.A., Obinger, C., Renger, G., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 3–70. [Google Scholar] [CrossRef]
- Cogdell, R.J.; Gall, A.; Köhler, J. The architecture and function of the light-harvesting apparatus of purple bacteria: From single molecules to in vivo membranes. Q. Rev. Biophys. 2006, 39, 227–324. [Google Scholar] [CrossRef]
- Hunter, C.N.; Daldal, F.; Thurnauer, M.C.; Beatty, J.T. (Eds.) The Purple Phototrophic Bacteria; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Gabrielsen, M.; Gardiner, A.T.; Cogdell, R.J. Peripheral Complexes of Purple Bacteria. In The Purple Phototrophic Bacteria; Hunter, C.N., Daldal, F., Thurnauer, M.C., Beatty, J.T., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 135–153. [Google Scholar] [CrossRef]
- Law, C.J.; Cogdell, R.J. The Light-Harvesting System of Purple Anoxygenic Photosynthetic Bacteria. In Primary Processes of Photosynthesis, Part 1: Principles and Apparatus; Renger, G., Ed.; Royal Society of Chemistry: Cambridge, UK, 2007; pp. 205–259. [Google Scholar] [CrossRef]
- Roszak, A.W.; Howard, T.D.; Southall, J.; Gardiner, A.T.; Law, C.J.; Isaacs, N.W.; Cogdell, R.J. Crystal Structure of the RC-LH1 Core Complex from Rhodopseudomonas palustris. Science 2003, 302, 1969–1972. [Google Scholar] [CrossRef]
- Cherezov, V.; Clogston, J.; Papiz, M.Z.; Caffrey, M. Room to Move: Crystallizing Membrane Proteins in Swollen Lipidic Mesophases. J. Mol. Biol. 2006, 357, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Leupold, D.; Stiel, H.; Teuchner, K.; Nowak, F.; Sandner, W.; Ücker, B.; Scheer, H. Size Enhancement of Transition Dipoles to One- and Two-Exciton Bands in a Photosynthetic Antenna. Phys. Rev. Lett. 1996, 77, 4675–4678. [Google Scholar] [CrossRef] [PubMed]
- Leupold, D.; Voigt, B.; Beenken, W.; Stiel, H. Pigment-protein architecture in the light-harvesting antenna complexes of purple bacteria: Does the crystal structure reflect the native pigment-protein arrangement? FEBS Lett. 2000, 480, 73–78. [Google Scholar] [CrossRef]
- Leupold, D.; Stiel, H.; Ehlert, J.; Nowak, F.; Teuchner, K.; Voigt, B.; Bandilla, M.; Ücker, B.; Scheer, H. Photophysical characterization of the B800-depleted light harvesting complex B850 of Rhodobacter sphaeroides. Chem. Phys. Lett. 1999, 301, 537–545. [Google Scholar] [CrossRef]
- Krikunova, M.; Kummrow, A.; Voigt, B.; Rini, M.; Lokstein, H.; Moskalenko, A.; Scheer, H.; Razjivin, A.; Leupold, D. Fluorescence of native and carotenoid-depleted LH2 from Chromatium minutissimum, originating from simultaneous two-photon absorption in the spectral range of the presumed (optically ’dark’) S(1) state of carotenoids. FEBS Lett. 2002, 528, 227–229. [Google Scholar] [CrossRef]
- Stepanenko, I.; Kompanetz, V.; Makhneva, Z.; Chekalin, S.; Moskalenko, A.; Razjivin, A. Two-Photon Excitation Spectroscopy of Carotenoid-Containing and Carotenoid-Depleted LH2 Complexes from Purple Bacteria. J. Phys. Chem. B 2009, 113, 11720–11723. [Google Scholar] [CrossRef]
- Theiss, C.; Leupold, D.; Moskalenko, A.A.; Razjivin, A.P.; Eichler, H.J.; Lokstein, H. Femtosecond Spectroscopy of Native and Carotenoidless Purple-Bacterial LH2 Clarifies Functions of Carotenoids. Biophys. J. 2008, 94, 4808–4811. [Google Scholar] [CrossRef] [PubMed]
- Shibl, M.F.; Schulze, J.; Al-Marri, M.J.; Kühn, O. Multilayer-MCTDH approach to the energy transfer dynamics in the LH2 antenna complex. J. Phys. B At. Mol. Opt. Phys. 2017, 50, 184001. [Google Scholar] [CrossRef]
- Ramos, F.C.; Nottoli, M.; Cupellini, L.; Mennucci, B. The molecular mechanisms of light adaption in light-harvesting complexes of purple bacteria revealed by a multiscale modeling. Chem. Sci. 2019, 10, 9650–9662. [Google Scholar] [CrossRef]
- Cupellini, L.; Jurinovich, S.; Campetella, M.; Caprasecca, S.; Guido, C.A.; Kelly, S.M.; Gardiner, A.T.; Cogdell, R.; Mennucci, B. An Ab Initio Description of the Excitonic Properties of LH2 and Their Temperature Dependence. J. Phys. Chem. B 2016, 120, 11348–11359. [Google Scholar] [CrossRef] [PubMed]
- Nottoli, M.; Jurinovich, S.; Cupellini, L.; Gardiner, A.T.; Cogdell, R.; Mennucci, B. The role of charge-transfer states in the spectral tuning of antenna complexes of purple bacteria. Photosynth. Res. 2018, 137, 215–226. [Google Scholar] [CrossRef]
- Pšenčík, J.; Ikonen, T.; Laurinmäki, P.; Merckel, M.; Butcher, S.; Serimaa, R.; Tuma, R. Lamellar Organization of Pigments in Chlorosomes, the Light Harvesting Complexes of Green Photosynthetic Bacteria. Biophys. J. 2004, 87, 1165–1172. [Google Scholar] [CrossRef]
- Griebenow, K.; Holzwarth, A.R.; van Mourik, F.; van Grondelle, R. Pigment organization and energy transfer in green bacteria. 2. Circular and linear dichroism spectra of protein-containing and protein-free chlorosomes isolated from Chloroflexus aurantiacus strain Ok-70-fl. Biochim. Biophys. Acta Bioenerg. 1991, 1058, 194–202. [Google Scholar] [CrossRef]
- Dostál, J.; Vácha, F.; Pšenčík, J.; Zigmantas, D. 2D Electronic Spectroscopy Reveals Excitonic Structure in the Baseplate of a Chlorosome. J. Phys. Chem. Lett. 2014, 5, 1743–1747. [Google Scholar] [CrossRef]
- Matthews, B.; Fenna, R.; Bolognesi, M.; Schmid, M.; Olson, J. Structure of a bacteriochlorophyll a-protein from the green photosynthetic bacterium Prosthecochloris aestuarii. J. Mol. Biol. 1979, 131, 259–285. [Google Scholar] [CrossRef]
- Olson, J.M. The FMO Protein. Photosynth. Res. 2004, 80, 181–187. [Google Scholar] [CrossRef]
- Camara-Artigas, A.; Blankenship, R.E.; Allen, J.P. The structure of the FMO protein from Chlorobium tepidum at 2.2 Å resolution. Photosynth. Res. 2003, 75, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Tronrud, D.; Wen, J.; Gay, L.; Blankenship, R.E. The structural basis for the difference in absorbance spectra for the FMO antenna protein from various green sulfur bacteria. Photosynth. Res. 2009, 100, 79–87. [Google Scholar] [CrossRef]
- Alster, J.; Kabeláč, M.; Tuma, R.; Pšenčík, J.; Burda, J. Computational study of short-range interactions in bacteriochlorophyll aggregates. Comput. Theor. Chem. 2012, 998, 87–97. [Google Scholar] [CrossRef]
- Busch, M.S.A.; Müh, F.; Madjet, M.E.-A.; Renger, T. The Eighth Bacteriochlorophyll Completes the Excitation Energy Funnel in the FMO Protein. J. Phys. Chem. Lett. 2010, 2, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Mühlbacher, L.; Kleinekathöfer, U. Preparational Effects on the Excitation Energy Transfer in the FMO Complex. J. Phys. Chem. B 2012, 116, 3900–3906. [Google Scholar] [CrossRef]
- Jurinovich, S.; Curutchet, C.; Mennucci, B. The Fenna-Matthews-Olson Protein Revisited: A Fully Polarizable (TD)DFT/MM Description. ChemPhysChem 2014, 15, 3194–3204. [Google Scholar] [CrossRef]
- Bold, B.M.; Sokolov, M.; Maity, S.; Wanko, M.; Dohmen, P.M.; Kranz, J.J.; Kleinekathöfer, U.; Höfener, S.; Elstner, M. Benchmark and performance of long-range corrected time-dependent density functional tight binding (LC-TD-DFTB) on rhodopsins and light-harvesting complexes. Phys. Chem. Chem. Phys. 2020, 22, 10500–10518. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kuhn, O. Vibrational and vibronic coherences in the dynamics of the FMO complex. Chem. Phys. 2016, 481, 272–280. [Google Scholar] [CrossRef]
- Dostál, J.; Pšenčík, J.; Zigmantas, D. In situ mapping of the energy flow through the entire photosynthetic apparatus. Nat. Chem. 2016, 8, 705–710. [Google Scholar] [CrossRef] [PubMed]
- MacColl, R. Cyanobacterial Phycobilisomes. J. Struct. Biol. 1998, 124, 311–334. [Google Scholar] [CrossRef]
- Adir, N. Elucidation of the molecular structures of components of the phycobilisome: Reconstructing a giant. Photosynth. Res. 2005, 85, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Adir, N.; Bar-Zvi, S.; Harris, D. The amazing phycobilisome. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148047. [Google Scholar] [CrossRef] [PubMed]
- Fromme, R.; Brune, D.; Fromme, P. High resolution structure of C-Phycocyanin from Thermosynechococcus elongatus. 2010. to be published. [Google Scholar]
- Kehoe, D.M.; Gutu, A. Responding to Color: The Regulation of Complementary Chromatic Adaptation. Annu. Rev. Plant Biol. 2006, 57, 127–150. [Google Scholar] [CrossRef]
- Dolganov, N.A.; Bhaya, D.; Grossman, A.R. Cyanobacterial protein with similarity to the chlorophyll a/b binding proteins of higher plants: Evolution and regulation. Proc. Natl. Acad. Sci. USA 1995, 92, 636–640. [Google Scholar] [CrossRef]
- Montané, M.-H.; Kloppstech, K. The family of light-harvesting-related proteins (LHCs, ELIPs, HLIPs): Was the harvesting of light their primary function? Gene 2000, 258, 1–8. [Google Scholar] [CrossRef]
- Engelken, J.; Brinkmann, H.; Adamska, I. Taxonomic distribution and origins of the extended LHC (light-harvesting complex) antenna protein superfamily. BMC Evol. Biol. 2010, 10, 233. [Google Scholar] [CrossRef]
- Psencik, J.; Hey, D.; Grimm, B.; Lokstein, H. Photoprotection of Photosynthetic Pigments in Plant One-Helix Protein 1/2 Heterodimers. J. Phys. Chem. Lett. 2020, 11, 9387–9392. [Google Scholar] [CrossRef]
- Pakrasi, H.B.; Riethman, H.C.; Sherman, L.A. Organization of pigment proteins in the photosystem II complex of the cyanobacterium Anacystis nidulans R2. Proc. Natl. Acad. Sci. USA 1985, 82, 6903–6907. [Google Scholar] [CrossRef]
- Burnap, R.L.; Troyan, T.; Sherman, L.A. The Highly Abundant Chlorophyll-Protein Complex of Iron-Deficient Synechococcus sp. PCC7942 (CP43′) Is Encoded by the isiA Gene. Plant Physiol. 1993, 103, 893–902. [Google Scholar] [CrossRef]
- Bibby, T.S.; Nield, J.; Barber, J. Iron deficiency induces the formation of an antenna ring around trimeric photosystem I in cyanobacteria. Nature 2001, 412, 743–745. [Google Scholar] [CrossRef]
- Sandström, S.; Park, Y.-I.; Öquist, G.; Gustafsson, P. CP43′, the isiA Gene Product, Functions as an Excitation Energy Dissipator in the Cyanobacterium Synechococcus sp. PCC 7942. Photochem. Photobiol. 2001, 74, 431–437. [Google Scholar] [CrossRef]
- Ihalainen, J.A.; D’Haene, S.; Yeremenko, N.; Van Roon, H.; Arteni, A.A.; Boekema, E.J.; Van Grondelle, R.; Matthijs, H.C.P.; Dekker, J.P. Aggregates of the Chlorophyll-Binding Protein IsiA (CP43′) Dissipate Energy in Cyanobacteria. Biochemistry 2005, 44, 10846–10853. [Google Scholar] [CrossRef]
- Bibby, T.S.; Nield, J.; Partensky, F.; Barber, J. Antenna ring around photosystem I. Nature 2001, 413, 590. [Google Scholar] [CrossRef] [PubMed]
- Bibby, T.S.; Nield, J.; Chen, M.; Larkum, A.W.D.; Barber, J. Structure of a photosystem II supercomplex isolated from Prochloron didemni retaining its chlorophyll a/b light-harvesting system. Proc. Natl. Acad. Sci. USA 2003, 100, 9050–9054. [Google Scholar] [CrossRef] [PubMed]
- Plumley, F.G.; Schmidt, G.W. Reconstitution of chlorophyll a/b light-harvesting complexes: Xanthophyll-dependent assembly and energy transfer. Proc. Natl. Acad. Sci. USA 1987, 84, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, H.; Rümler, U.; Rüdiger, W. Reconstitution of pigment-containing complexes from light-harvesting chlorophyll a/b-binding protein overexpressed in Escherichia coli. Planta 1990, 181, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Hobe, S.; Prytulla, S.; Kühlbrandt, W.; Paulsen, H. Trimerization and crystallization of reconstituted light-harvesting chlorophyll a/b complex. EMBO J. 1994, 13, 3423–3429. [Google Scholar] [CrossRef]
- Paulsen, H. Pigment ligation to proteins of the photosynthetic apparatus in higher plants. Physiol. Plant. 1997, 100, 760–768. [Google Scholar] [CrossRef]
- Lokstein, H.; Tian, L.; Polle, J.E.; DellaPenna, D. Xanthophyll biosynthetic mutants of Arabidopsis thaliana: Altered nonphotochemical quenching of chlorophyll fluorescence is due to changes in Photosystem II antenna size and stability. Biochim. Biophys. Acta Bioenerg. 2002, 1553, 309–319. [Google Scholar] [CrossRef]
- Pawlak, K.; Paul, S.; Liu, C.; Reus, M.; Yang, C.; Holzwarth, A.R. On the PsbS-induced quenching in the plant major light-harvesting complex LHCII studied in proteoliposomes. Photosynth. Res. 2020, 144, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Ostroumov, E.E.; Götze, J.P.; Reus, M.; Lambrev, P.H.; Holzwarth, A.R. Characterization of fluorescent chlorophyll charge-transfer states as intermediates in the excited state quenching of light-harvesting complex II. Photosynth. Res. 2020, 144, 171–193. [Google Scholar] [CrossRef] [PubMed]
- Lokstein, H.; Härtel, H.; Hoffmann, P.; Woitke, P.; Renger, G. The role of light-harvesting complex II in excess excitation energy dissipation: An in-vivo fluorescence study on the origin of high-energy quenching. J. Photochem. Photobiol. B Biol. 1994, 26, 175–184. [Google Scholar] [CrossRef]
- Takahashi, H.; Iwai, M.; Minagawa, J. Identification of the mobile light-harvesting complex II polypeptides for state transitions in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2006, 103, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Yokono, M.; Inada, N.; Minagawa, J. Live-cell imaging of photosystem II antenna dissociation during state transitions. Proc. Natl. Acad. Sci. USA 2010, 107, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Oxborough, K.; Lee, P.; Horton, P. Regulation of thylakoid protein phosphorylation by high-energy-state quenching. FEBS Lett. 1987, 221, 211–214. [Google Scholar] [CrossRef]
- Barber, J.; Andersson, B. Too much of a good thing: Light can be bad for photosynthesis. Trends Biochem. Sci. 1992, 17, 61–66. [Google Scholar] [CrossRef]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef] [PubMed]
- Schödel, R.; Irrgang, K.-D.; Voigt, J.; Renger, G. Rate of Carotenoid Triplet Formation in Solubilized Light-Harvesting Complex II (LHCII) from Spinach. Biophys. J. 1998, 75, 3143–3153. [Google Scholar] [CrossRef][Green Version]
- You, Z.-Q.; Hsu, C.-P. Ab Inito Study on Triplet Excitation Energy Transfer in Photosynthetic Light-Harvesting Complexes. J. Phys. Chem. A 2011, 115, 4092–4100. [Google Scholar] [CrossRef]
- Frank, H.A.; Bautista, J.A.; Josue, J.S.; Young, A.J. Mechanism of nonphotochemical quenching in green plants: Energies of the lowest excited singlet states of violaxanthin and zeaxanthin. Biochemistry 2000, 39, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Härtel, H.; Lokstein, H.; Grimm, B.; Rank, B. Kinetic Studies on the Xanthophyll Cycle in Barley Leaves (Influence of Antenna Size and Relations to Nonphotochemical Chlorophyll Fluorescence Quenching). Plant Physiol. 1996, 110, 471–482. [Google Scholar] [CrossRef]
- Frank, H.A.; Cua, A.; Chynwat, V.; Young, A.; Gosztola, D.; Wasielewski, M.R. Photophysics of the carotenoids associated with the xanthophyll cycle in photosynthesis. Photosynth. Res. 1994, 41, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Kröner, D.; Götze, J.P. Modeling of a violaxanthin–chlorophyll b chromophore pair in its LHCII environment using CAM-B3LYP. J. Photochem. Photobiol. B Biol. 2012, 109, 12–19. [Google Scholar] [CrossRef]
- Dreuw, A.; Wormit, M. Simple replacement of violaxanthin by zeaxanthin in LHC-II does not cause chlorophyll fluorescence quenching. J. Inorg. Biochem. 2008, 102, 458–465. [Google Scholar] [CrossRef]
- Götze, J.P.; Kröner, D.; Banerjee, S.; Karasulu, B.; Thiel, W. Carotenoids as a Shortcut for Chlorophyll Soret-to-Q Band Energy Flow. ChemPhysChem 2014, 15, 3392–3401. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Wentworth, M.; Ruban, A. Control of the light harvesting function of chloroplast membranes: The LHCII-aggregation model for non-photochemical quenching. FEBS Lett. 2005, 579, 4201–4206. [Google Scholar] [CrossRef]
- Li, X.-P.; Björkman, O.; Shih, C.; Grossman, A.R.; Rosenquist, M.; Jansson, S.; Niyogi, K.K. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 2000, 403, 391–395. [Google Scholar] [CrossRef]
- Peers, G.; Truong, T.B.; Ostendorf, E.; Busch, A.E.; Elrad, D.; Grossman, A.R.; Hippler, M.; Niyogi, K.K. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 2009, 462, 518–521. [Google Scholar] [CrossRef]
- Daskalakis, V. Protein–protein interactions within photosystem II under photoprotection: The synergy between CP29 minor antenna, subunit S (PsbS) and zeaxanthin at all-atom resolution. Phys. Chem. Chem. Phys. 2018, 20, 11843–11855. [Google Scholar] [CrossRef]
- Miloslavina, Y.; Wehner, A.; Lambrev, P.H.; Wientjes, E.; Reus, M.; Garab, G.; Croce, R.; Holzwarth, A.R. Far-red fluorescence: A direct spectroscopic marker for LHCII oligomer formation in non-photochemical quenching. FEBS Lett. 2008, 582, 3625–3631. [Google Scholar] [CrossRef] [PubMed]
- Kell, A.; Feng, X.; Lin, C.; Yang, Y.; Li, J.; Reus, M.; Holzwarth, A.R.; Jankowiak, R. Charge-Transfer Character of the Low-Energy Chl a Qy Absorption Band in Aggregated Light Harvesting Complexes II. J. Phys. Chem. B 2014, 118, 6086–6091. [Google Scholar] [CrossRef]
- Razjivin, A.; Solov’Ev, A.; Kompanets, V.; Chekalin, S.; Moskalenko, A.; Lokstein, H. The origin of the “dark” absorption band near 675 nm in the purple bacterial core light-harvesting complex LH1: Two-photon measurements of LH1 and its subunit B820. Photosynth. Res. 2019, 140, 207–213. [Google Scholar] [CrossRef]
- Holt, N.E.; Zigmantas, D.; Valkunas, L.; Li, X.-P.; Niyogi, K.K.; Fleming, G.R. Carotenoid Cation Formation and the Regulation of Photosynthetic Light Harvesting. Science 2005, 307, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Wormit, M.; Harbach, P.H.P.; Mewes, J.-M.; Amarie, S.; Wachtveitl, J.; Dreuw, A. Excitation energy transfer and carotenoid radical cation formation in light harvesting complexes—A theoretical perspective. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 738–746. [Google Scholar] [CrossRef]
- Bode, S.; Quentmeier, C.C.; Liao, P.-N.; Hafi, N.; Barros, T.; Wilk, L.; Bittner, F.; Walla, P.J. On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proc. Natl. Acad. Sci. USA 2009, 106, 12311–12316. [Google Scholar] [CrossRef]
- Betke, A.; Lokstein, H. Two-photon excitation spectroscopy of photosynthetic light-harvesting complexes and pigments. Faraday Discuss. 2019, 216, 494–506. [Google Scholar] [CrossRef]
- Šebelík, V.; Kuznetsova, V.; Lokstein, H.; Polívka, T. Transient Absorption of Chlorophylls and Carotenoids after Two-Photon Excitation of LHCII. J. Phys. Chem. Lett. 2021, 12, 3176–3181. [Google Scholar] [CrossRef] [PubMed]
- Gacek, D.A.; Betke, A.; Nowak, J.; Lokstein, H.; Walla, P.J. Two-photon absorption and excitation spectroscopy of carotenoids, chlorophylls and pigment-protein complexes. Phys. Chem. Chem. Phys. 2021, 23, 8731–8738. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Ajlani, G.; Verbavatz, J.-M.; Vass, I.; Kerfeld, C.A.; Kirilovsky, D. A Soluble Carotenoid Protein Involved in Phycobilisome-Related Energy Dissipation in Cyanobacteria. Plant Cell 2006, 18, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Kirilovsky, D.; Kerfeld, C.A. Cyanobacterial photoprotection by the orange carotenoid protein. Nat. Plants 2016, 2, 16180. [Google Scholar] [CrossRef] [PubMed]
- Leverenz, R.L.; Sutter, M.; Wilson, A.; Gupta, S.; Thurotte, A.; De Carbon, C.B.; Petzold, C.; Ralston, C.; Perreau, F.; Kirilovsky, D.; et al. A 12 A carotenoid translocation in a photoswitch associated with cyanobacterial photoprotection. Science 2015, 348, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Bondanza, M.; Cupellini, L.; Lipparini, F.; Mennucci, B. The Multiple Roles of the Protein in the Photoactivation of Orange Carotenoid Protein. Chem 2020, 6, 187–203. [Google Scholar] [CrossRef]
- Boulay, C.; Wilson, A.; D’Haene, S.; Kirilovsky, D. Identification of a protein required for recovery of full antenna capacity in OCP-related photoprotective mechanism in cyanobacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 11620–11625. [Google Scholar] [CrossRef]
- Reimers, J.R.; Biczysko, M.; Bruce, D.; Coker, D.F.; Frankcombe, T.J.; Hashimoto, H.; Hauer, J.; Jankowiak, R.; Kramer, T.; Linnanto, J.; et al. Challenges facing an understanding of the nature of low-energy excited states in photosynthesis. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1627–1640. [Google Scholar] [CrossRef]
- Lambert, N.; Chen, Y.-N.; Cheng, Y.-C.; Li, C.-M.; Chen, G.-Y.; Nori, F. Quantum biology. Nat. Phys. 2012, 9, 10–18. [Google Scholar] [CrossRef]
- Cao, J.; Cogdell, R.J.; Coker, D.F.; Duan, H.-G.; Hauer, J.; Kleinekathöfer, U.; Jansen, T.L.C.; Mančal, T.; Miller, R.J.D.; Ogilvie, J.P.; et al. Quantum biology revisited. Sci. Adv. 2020, 6, eaaz4888. [Google Scholar] [CrossRef]
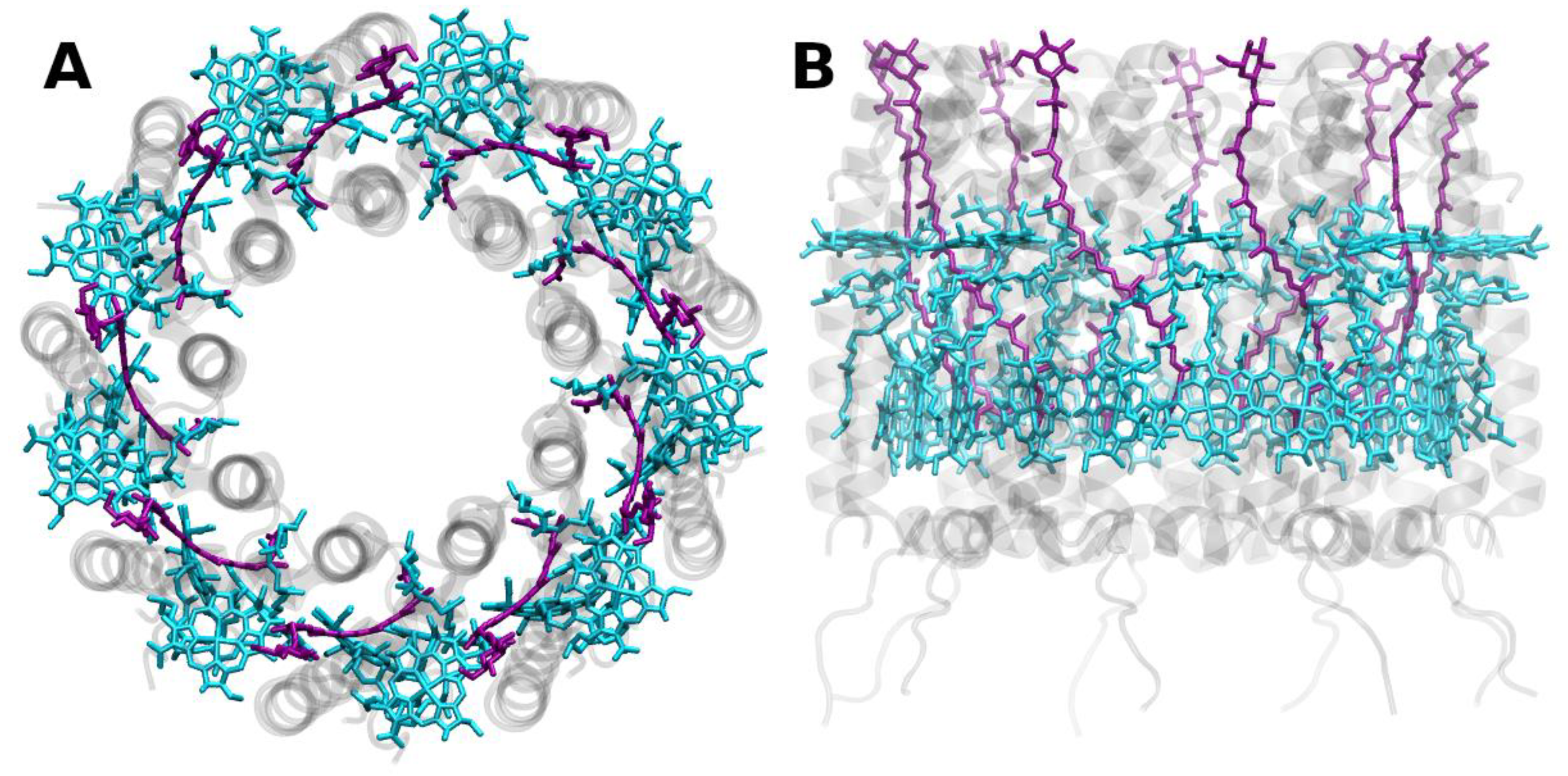

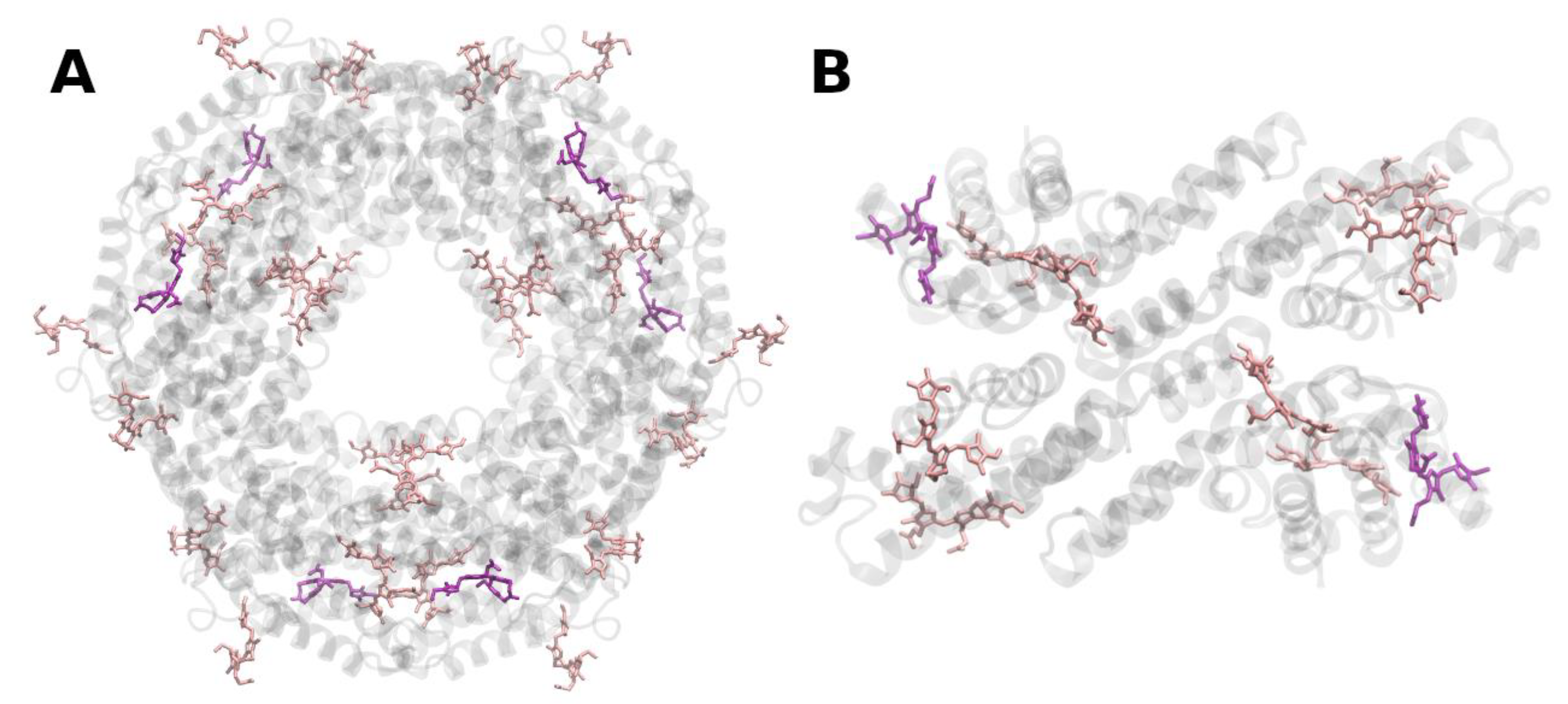
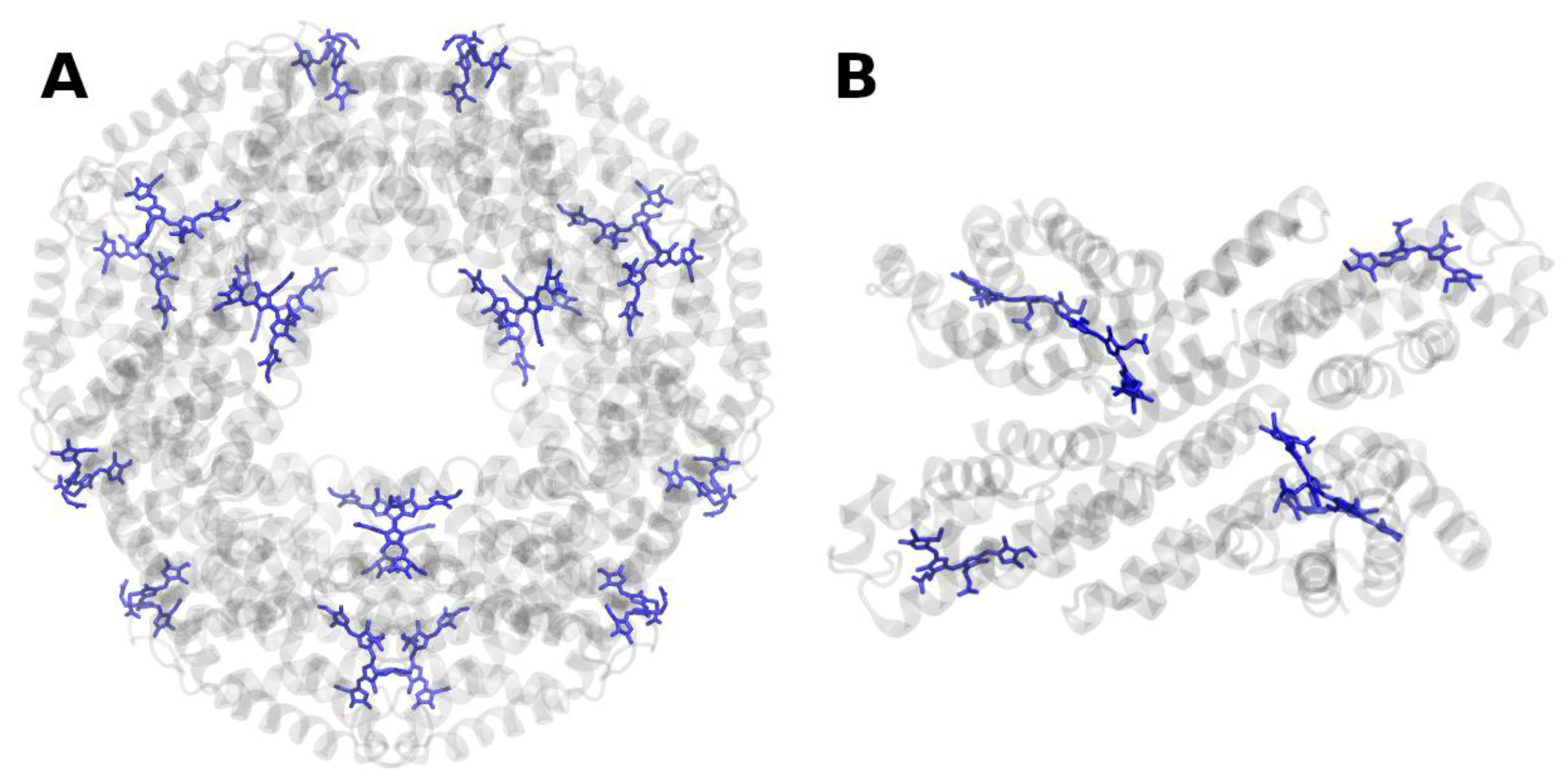
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lokstein, H.; Renger, G.; Götze, J.P. Photosynthetic Light-Harvesting (Antenna) Complexes—Structures and Functions. Molecules 2021, 26, 3378. https://doi.org/10.3390/molecules26113378
Lokstein H, Renger G, Götze JP. Photosynthetic Light-Harvesting (Antenna) Complexes—Structures and Functions. Molecules. 2021; 26(11):3378. https://doi.org/10.3390/molecules26113378
Chicago/Turabian StyleLokstein, Heiko, Gernot Renger, and Jan P. Götze. 2021. "Photosynthetic Light-Harvesting (Antenna) Complexes—Structures and Functions" Molecules 26, no. 11: 3378. https://doi.org/10.3390/molecules26113378
APA StyleLokstein, H., Renger, G., & Götze, J. P. (2021). Photosynthetic Light-Harvesting (Antenna) Complexes—Structures and Functions. Molecules, 26(11), 3378. https://doi.org/10.3390/molecules26113378





