In Vitro and In Vivo Trypanocidal Efficacy of Synthesized Nitrofurantoin Analogs
Abstract
1. Introduction
2. Results
2.1. In Vitro Experiment
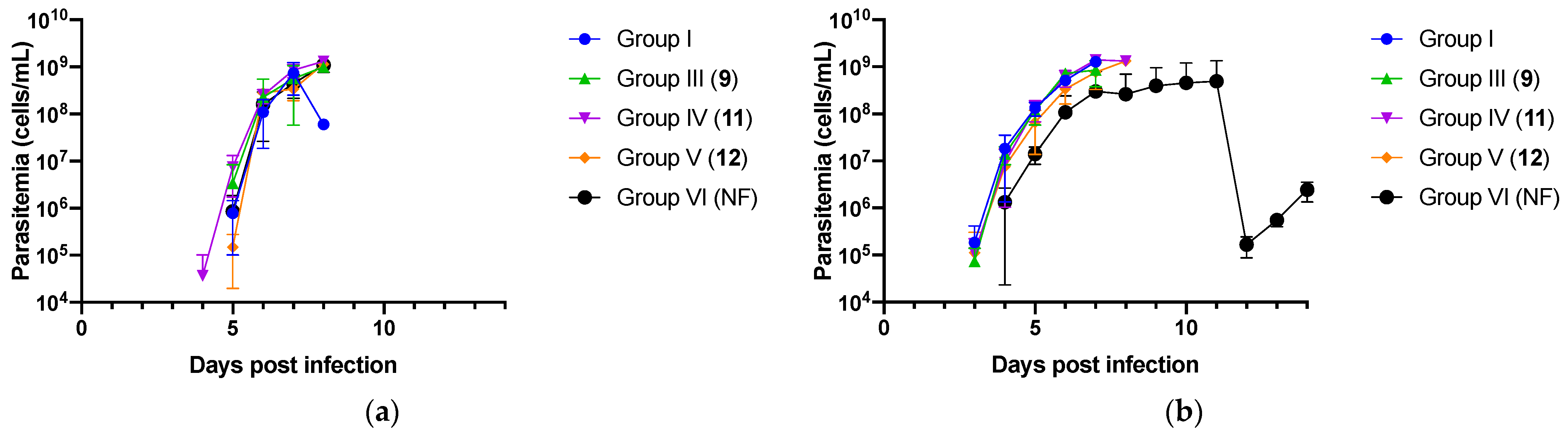
2.2. In Vivo Experiment
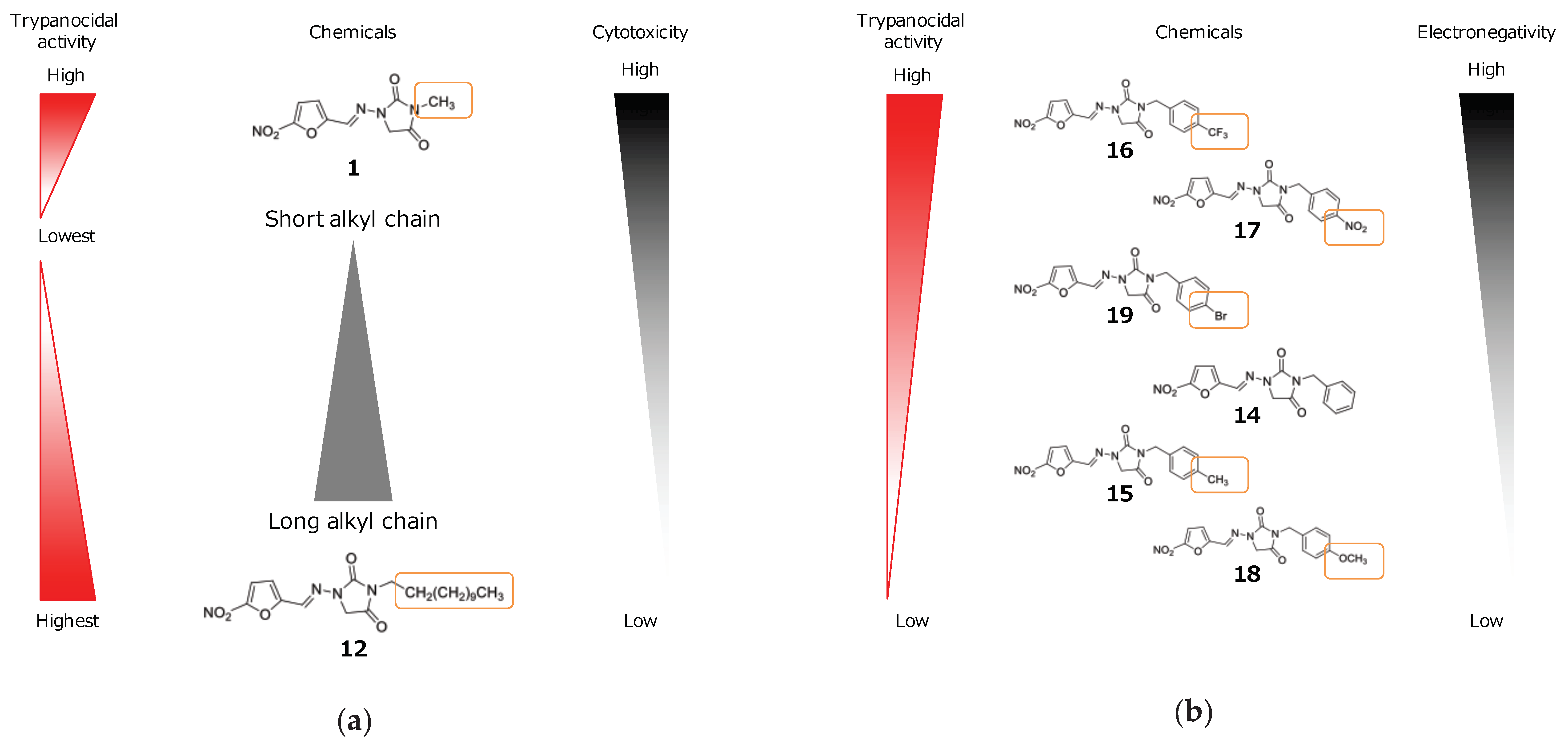
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. In Vitro Cultivation of Trypanosomes and Madin–Darby Bovine Kidney Cells
4.3. Evaluation of Trypanocidal Activity and Cytotoxicity of Nitrofurantoin Analogs In Vitro
4.4. In Vivo Evaluation of the Treatment Efficacy of Selected Nitrofurantoin Analogs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
- Mwiinde, A.M.; Simuunza, M.; Namangala, B.; Chama-Chiliba, C.M.; Machila, N.; Anderson, N.; Shaw, A.; Welburn, S.C. Estimating the economic and social consequences for patients diagnosed with human African trypanosomiasis in Muchinga, Lusaka and Eastern Provinces of Zambia (2004–2014). Infect. Dis. Poverty 2017, 6, 150. [Google Scholar] [CrossRef] [PubMed]
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; De Koning, H.P.; Barrett, M.P. The animal trypanosomiases and their chemotherapy: A review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.J.; Vezza, L.; Rowan, T.; Hope, J.C. Animal African trypanosomiasis: Time to increase focus on clinically relevant parasite and host species. Trends Parasitol. 2016, 32, 599–607. [Google Scholar] [CrossRef]
- Desquesnes, M.; Holzmuller, P.; Lai, D.H.; Dargantes, A.; Lun, Z.R.; Jittaplapong, S. Trypanosoma evansi and surra: A review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. BioMed Res. Int. 2013, 2013, 194176. [Google Scholar] [CrossRef]
- Fairlamb, A.H. Chemotherapy of human African trypanosomiasis: Current and future prospects. Trends Parasitol. 2003, 19, 488–494. [Google Scholar] [CrossRef]
- Wamwiri, F.N.; Changasi, R.E. Tsetse flies (Glossina) as vectors of human African trypanosomiasis: A review. BioMed Res. Int. 2016, 2016, 6201350. [Google Scholar] [CrossRef]
- World Health Organization. WHO Interim Guidelines for the Treatment of Gambiense Human African Trypanosomiasis; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Mesu, V.K.B.K.; Kalonji, W.M.; Bardonneau, C.; Mordt, O.V.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Kuziena, W.; Lubaki, J.-P.F. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: A pivotal multicentre, randomised, non-inferiority trial. Lancet 2018, 391, 144–154. [Google Scholar] [CrossRef]
- Wang, Y.; Gray, J.P.; Mishin, V.; Heck, D.E.; Laskin, D.L.; Laskin, J.D. Role of cytochrome P450 reductase in nitrofurantoin-induced redox cycling and cytotoxicity. Free Radic. Biol. Med. 2008, 44, 1169–1179. [Google Scholar] [CrossRef]
- Zuma, N.H.; Smit, F.J.; Seldon, R.; Aucamp, J.; Jordaan, A.; Warner, D.F.; David, D.D. Single-step synthesis and in vitro anti-mycobacterial activity of novel nitrofurantoin analogues. Bioorg. Chem. 2020, 96, 103587. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.S.; Wu, X.; Hu, L.; Wilkinson, S.R. Exploiting the drug-activating properties of a novel trypanosomal nitroreductase. Antimicrob. Agents Chemother. 2010, 54, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.D.; Pérez-Reinado, E.; Castillo, F.; Moreno-Vivián, C. Reduction of polynitroaromatic compounds: The bacterial nitroreductases. FEMS Microbiol. Rev. 2008, 32, 474–500. [Google Scholar] [CrossRef]
- Kaiser, M.; Mäser, P.; Tadoori, L.P.; Ioset, J.-R.; Brun, R. Antiprotozoal activity profiling of approved drugs: A starting point toward drug repositioning. PLoS ONE 2015, 10, e0135556. [Google Scholar] [CrossRef]
- Checchi, F.; Piola, P.; Ayikoru, H.; Thomas, F.; Legros, D.; Priotto, G. Nifurtimox plus eflornithine for late-stage sleeping sickness in Uganda: A case series. PLoS Negl. Trop. Dis. 2007, 1, e64. [Google Scholar] [CrossRef]
- Priotto, G.; Kasparian, S.; Ngouama, D.; Ghorashian, S.; Arnold, U.; Ghabri, S.; Karunakara, U. Nifurtimox-eflornithine combination therapy for second-stage Trypanosoma brucei gambiense sleeping sickness: A randomized clinical trial in Congo. Clin. Infect. Dis. 2007, 45, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D. The development of drugs for treatment of sleeping sickness: A historical review. Parasit. Vectors 2010, 3, 15. [Google Scholar] [CrossRef]
- Wermuth, C.G. The Practice of Medicinal Chemistry; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Jorge, S.D.; Palace-Berl, F.; Pasqualoto, K.F.M.; Ishii, M.; Ferreira, A.K.; Berra, C.M.; Bosch, R.V.; Maria, D.A.; Tavares, L.C. Ligand-based design, synthesis, and experimental evaluation of novel benzofuroxan derivatives as anti-Trypanosoma cruzi agents. Eur. J. Med. Chem. 2013, 64, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Palace-Berl, F.; Pasqualoto, K.F.M.; Jorge, S.D.; Zingales, B.; Zorzi, R.R.; Silva, M.N.; Ferreira, A.K.; de Azevedo, R.A.; Teixeira, S.F.; Tavares, L.C. Designing and exploring active N′-[(5-nitrofuran-2-yl) methylene] substituted hydrazides against three Trypanosoma cruzi strains more prevalent in Chagas disease patients. Eur. J. Med. Chem. 2015, 96, 330–339. [Google Scholar] [CrossRef]
- O’Shea, I.P.; Shahed, M.; Aguilera-Venegas, B.; Wilkinson, S.R. Evaluating 5-nitrothiazoles as trypanocidal agents. Antimicrob. Agents Chemother. 2016, 60, 1137–1140. [Google Scholar] [CrossRef]
- Palace-Berl, F.; Pasqualoto, K.F.M.; Zingales, B.; Moraes, C.B.; Bury, M.; Franco, C.H.; da Silva Neto, A.L.; Murayama, J.S.; Nunes, S.L.; Silva, M.N. Investigating the structure-activity relationships of N′-[(5-nitrofuran-2-yl) methylene] substituted hydrazides against Trypanosoma cruzi to design novel active compounds. Eur. J. Med. Chem. 2018, 144, 29–40. [Google Scholar] [CrossRef]
- Salas, C.O.; Faúndez, M.; Morello, A.; Diego Maya, J.; Tapia, R.A. Natural and synthetic naphthoquinones active against Trypanosoma cruzi: An initial step towards new drugs for Chagas disease. Cur. Med. Chem. 2011, 18, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Hernández, R.I.; Correa-Basurto, J.; Flores-Sandoval, C.A.; Padilla-Martínez, I.I.; Nogueda-Torres, B.; de Lourdes Villa-Tanaca, M.; Tamay-Cach, F.; Nolasco-Fidencio, J.J.; Trujillo-Ferrara, J.G. Fluorine-containing benzothiazole as a novel trypanocidal agent: Design, in silico study, synthesis and activity evaluation. Med. Chem. Res. 2016, 25, 211–224. [Google Scholar] [CrossRef]
- Vera, B.; Vázquez, K.; Mascayano, C.; Tapia, R.A.; Espinosa, V.; Soto-Delgado, J.; Salas, C.O.; Paulino, M. Structural analysis and molecular docking of trypanocidal aryloxy-quinones in trypanothione and glutathione reductases: A comparison with biochemical data. J. Biomol. Struct. Dyn. 2017, 35, 1785–1803. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, K.; Burrows, J.N.; Duncan, K.; Van Huijsduijnen, R.H.; Kaneko, T.; Kita, K.; Mowbray, C.E.; Schmatz, D.; Warner, P.; Slingsby, B. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Finiuk, N.; Hreniuh, V.; Ostapiuk, Y.V.; Matiychuk, V.; Frolov, D.; Obushak, M.; Stoika, R.; Babsky, A. Antineoplastic activity of novel thiazole derivatives. Вiopolymers Cell 2017, 33, 135–146. [Google Scholar] [CrossRef]
- Liu, S.; Su, M.; Song, S.-J.; Jung, J.H. Marine-derived Penicillium species as producers of cytotoxic metabolites. Mar. Drugs 2017, 15, 329. [Google Scholar] [CrossRef]
- Hall, B.S.; Bot, C.; Wilkinson, S.R. Nifurtimox activation by trypanosomal type I nitroreductases generates cytotoxic nitrile metabolites. J. Biol. Chem. 2011, 286, 13088–13095. [Google Scholar] [CrossRef]
- Tambosi, G.; Coelho, P.F.; Luciano, S.; Lenschow, I.C.S.; Zétola, M.; Stulzer, H.K.; Pezzini, B.R. Challenges to improve the biopharmaceutical properties of poorly water-soluble drugs and the application of the solid dispersion technology. Matéria (Rio Jan.) 2018, 23. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A. Chris Lipinski discusses life and chemistry after the Rule of Five. Drug Discov. Today 2003, 8, 12. [Google Scholar] [PubMed]
- Hirumi, H.; Hirumi, K. In vitro cultivation of Trypanosoma congolense bloodstream forms in the absence of feeder cell layers. Parasitology 1991, 102, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, K.; Allamanda, P.; Hakimi, H.; Zhou, M.; Angeles, J.M.; Kawazu, S.I.; Inoue, N. Establishment of ATP-based luciferase viability assay in 96-well plate for Trypanosoma congolense. J. Vet. Med. Sci. 2014, 76, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, K.; Yamasaki, S.; Molefe, N.I.; Musinguzi, P.S.; Davaasuren, B.; Mossaad, E.; Narantsatsral, S.; Battur, B.; Battsetseg, B.; Inoue, N. The establishment of in vitro culture and drug screening systems for a newly isolated strain of Trypanosoma equiperdum. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 200–205. [Google Scholar] [CrossRef] [PubMed]
| Cpd. | MW (g/mol) | Name | Structure |
|---|---|---|---|
| 1 | 252.18 | (E)-3-Methyl-1-([(5-nitrofuran-2-yl) methylene]amino)imidazolidine-2,4-dione | 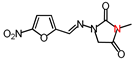 |
| 2 | 266.21 | (E)-3-Ethyl-1-([(5-nitrofuran-2-yl) methylene]amino)imidazolidine-2,4-dione |  |
| 3 | 280.24 | (E)-1-([(5-Nitrofuran-2-yl) methylene]amino)-3-propylimidazolidine-2,4-dione | 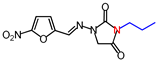 |
| 4 | 294.26 | (E)-3-Butyl-1-([(5-nitrofuran-2-yl) methylene]amino)imidazolidine-2,4-dione | 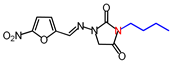 |
| 5 | 30,829 | (E)-1-([(5-Nitrofuran-2-yl)methylene] amino)-3-pentylimidazolidine-2,4-dione | 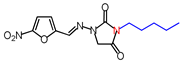 |
| 6 | 322.32 | (E)-3-Hexyl-1-([(5-nitrofuran-2-yl) methylene]amino)imidazolidine-2,4-dione |  |
| 7 | 336.34 | (E)-3-Heptyl-1-([(5-nitrofuran-2-yl) methylene]amino)imidazolidine-2,4-dione | 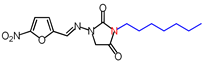 |
| 8 | 350.37 | (E)-1-([(5-Nitrofuran-2-yl)methylene] amino)-3-octylimidazolidine-2,4-dione | 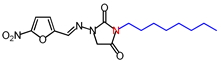 |
| 9 | 364.4 | (E)-1-([(5-Nitrofuran-2-yl)methylene] amino)-3-nonylimidazolidine-2,4-dione | 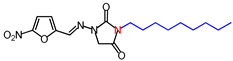 |
| 10 | 378.42 | (E)-3-Decyl-1-([(5-nitrofuran-2-yl)methylene] amino)imidazolidine-2,4-dione | 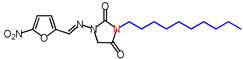 |
| 11 | 392.45 | (E)-1-([(5-Nitrofuran-2-yl)methylene] amino)-3-undecylimidazolidine-2,4-dione | 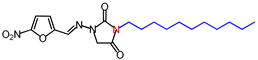 |
| 12 | 406.48 | (E)-3-Dodecyl-1-([(5-nitrofuran-2-yl) methylene]amino)imidazolidine-2,4-dione | 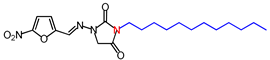 |
| 13 | 356.33 | (E)-1-([(5-Nitrofuran-2-yl)methylene] amino)-3-(3-phenylpropyl)imidazolidine-2,4-dione |  |
| 14 | 328.28 | (E)-3-Benzyl-1-([(5-nitrofuran-2-yl) methylene]amino)imidazolidine-2,4-dione |  |
| 15 | 342.31 | (E)-3-(p-Methylbenzyl)-1-([(5-nitrofuran-2-yl) methylene]amino)imidazolidine-2,4-dione | 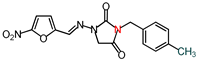 |
| 16 | 396.28 | (E)-1-([(5-Nitrofuran-2-yl)methylene] amino)-3-(p-(trifluoromethyl)benzyl) imidazolidine-2,4-dione | 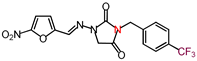 |
| 17 | 373.28 | (E)-3-(p-Nitrobenzyl)-1-([(5-nitrofuran-2-yl) methylene]amino)imidazolidine-2,4-dione | 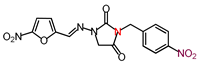 |
| 18 | 358.31 | (E)-3-(p-Methoxybenzyl)-1-([(5-nitrofuran-2-yl) methylene]amino)imidazolidine-2,4-dione | 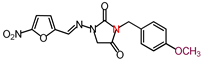 |
| 19 | 407.18 | (E)-3-(p-Bromobenzyl)-1-([(5-nitrofuran-2-yl) methylene]amino)imidazolidine-2,4-dione | 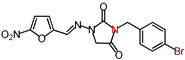 |
| Trypanocidal Activity IC50 ± SD (µM) c | Cytotoxicity IC50 ± SD (µM) c | Selectivity Index (IC50 of Trypanosomes/IC50 of MDBK) d | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | N a | clogP b | Tbb GUTat3.1 | Tbg IL1922 | Tbr IL1501 | Tbr Chirundu | Tc IL3000 | Tev Tansui | MDBK | Tbb GUTat3.1 | Tbg IL1922 | Tbr IL1501 | Tbr Chirundu | Tc IL3000 | Tev Tansui |
| 1 | 1 | −0.04 | 3.61 ± 0.40 | 3.60 ± 0.26 | 7.02 ± 0.094 | 0.73 ± 0.17 | 0.19 ± 0.076 | 1.61 ± 0.098 | 65.21 ± 12.78 | 18 | 18 | 9 | 89 | 342 | 41 |
| 2 | 2 | 0.36 | 10.81 ± 4.84 | 11.31 ± 4.80 | 11.40 ± 5.24 | 0.51 ± 0.25 | 0.42 ± 0.16 | 5.39 ± 0.53 | 44.79 ± 1.69 | 4 | 4 | 4 | 87 | 108 | 8 |
| 3 | 3 | 0.88 | 14.13 ± 0.34 | 14.73 ± 1.32 | 14.02 ± 1.42 | 0.81 ± 0.24 | 0.33 ± 0.18 | 6.53 ± 0.61 | 39.29 ± 1.09 | 3 | 3 | 3 | 49 | 120 | 6 |
| 4 | 4 | 1.33 | 33.29 ± 24.29 | 17.25 ± 2.23 | 32.74 ± 0.82 | 1.38 ± 0.34 | 0.31 ± 0.20 | 7.89 ± 0.53 | 35.78 ± 3.49 | 1 | 2 | 1 | 26 | 117 | 5 |
| 5 | 5 | 1.77 | 4.24 ± 1.99 | 3.42 ± 1.47 | 5.69 ± 1.66 | NA | 0.35 ± 0.15 | 5.44 ± 0.17 | 39.07 ± 5.86 | 9 | 11 | 7 | NA | 112 | 7 |
| 6 | 6 | 2.22 | 5.76 ± 4.01 | 2.59 ± 0.28 | 2.61 ± 0.12 | 0.17 ± 0.11 | 0.08 ± 0.047 | 3.76 ± 0.47 | 25.18 ± 3.52 | 4 | 10 | 10 | 146 | 321 | 7 |
| 7 | 7 | 2.66 | 2.06 ± 0.75 | 1.99 ± 0.75 | 1.45 ± 0.86 | 0.056 ± 0.026 | 0.040 ± 0.011 | 1.02 ± 0.10 | 92.28 ± 6.52 | 45 | 46 | 64 | 1654 | 2323 | 90 |
| 8 | 8 | 3.10 | 1.89 ± 0.69 | 1.40 ± 0.73 | 1.38 ± 0.82 | 0.039 ± 0.0084 | 0.030 ± 0.0050 | 0.81 ± 0.082 | 84.74 ± 8.99 | 45 | 61 | 62 | 2195 | 2809 | 105 |
| 9 | 9 | 3.55 | 0.57 ± 0.22 | 0.44 ± 0.17 | 0.60 ± 0.54 | 0.017 ± 0.0067 | 0.010 ± 0.0035 | 0.27 ± 0.025 | 251.18 ± 58.27 | 443 | 567 | 420 | 14,645 | 25,639 | 918 |
| 10 | 10 | 3.99 | 0.65 ± 0.56 | 0.62 ± 0.70 | 0.61 ± 0.06 | 0.017 ± 0.0044 | 0.011 ± 0.0044 | 0.28 ± 0.025 | 110.45 ± 14.38 | 170 | 177 | 180 | 6645 | 10,502 | 402 |
| 11 | 11 | 4.44 | 0.33 ± 0.12 | 0.33 ± 0.12 | 0.24 ± 0.13 | 0.021 ± 0.0094 | 0.011 ± 0.0035 | 0.16 ± 0.014 | >254.81 | 764 | 771 | 1052 | 11,933 | 21,978 | 1602 |
| 12 | 12 | 4.88 | 0.34 ± 0.12 | 0.23 ± 0.13 | 0.26 ± 0.11 | 0.031 ± 0.011 | 0.012 ± 0.0045 | 0.20 ± 0.017 | >246.02 | 715 | 1095 | 934 | 7937 | 21,322 | 1253 |
| 13 | 2.46 | 7.29 ± 3.16 | 4.83 ± 3.22 | 5.90 ± 3.75 | 0.14 ± 0.067 | 0.14 ± 0.030 | 3.11 ± 0.37 | >280.64 | 39 | 58 | 48 | 2,041 | 2058 | 90 | |
| 14 | 1.73 | 2.88 ± 0.26 | 2.34 ± 0.64 | 2.83 ± 0.61 | 0.27 ± 0.12 | 0.18 ± 0.017 | 1.26 ± 0.10 | >304.62 | 106 | 130 | 108 | 1142 | 1702 | 241 | |
| 15 | 2.24 | 8.24 ± 3.11 | 2.89 ± 0.51 | 6.10 ± 0.047 | 0.15 ± 0.068 | 0.13 ± 0.044 | 4.23 ± 0.40 | 34.28 ± 11.5 | 4 | 12 | 6 | 231 | 271 | 8 | |
| 16 | 2.60 | 0.52 ± 0.081 | 0.61 ± 0.26 | 0.96 ± 0.0087 | 0.02 ± 0.012 | 0.02 ± 0.073 | 0.44 ± 0.046 | 56.62 ± 40.76 | 108 | 93 | 59 | 3121 | 3802 | 128 | |
| 17 | 1.67 | 1.07 ± 0.74 | 1.05 ± 0.70 | 0.50 ± 0.012 | 0.04 ± 0.094 | 0.04 ± 0.017 | 0.61 ± 0.050 | 20.10 ± 1.02 | 19 | 19 | 45 | 497 | 469 | 33 | |
| 18 | 1.57 | 9.91 ± 0.092 | 9.78 ± 0.31 | 10.14 ± 0.62 | 0.33 ± 0.22 | 0.14 ± 0.030 | 2.40 ± 0.19 | 80.09 ± 36.89 | 8 | 8 | 8 | 244 | 585 | 33 | |
| 19 | 2.50 | 2.13 ± 0.23 | 2.04 ± 0.091 | 2.47 ± 0.69 | 0.041 ± 0.025 | 0.049 ± 0.033 | 1.15 ± 0.11 | 28.74 ± 5.69 | 14 | 14 | 12 | 700 | 587 | 25 | |
| Nitrofurantoin | −0.22 | 1.42 ± 0.15 | 1.62 ± 0.28 | 1.42 ± 0.25 | NA | 0.36 ± 0.043 | 1.96 ± 0.50 | ||||||||
| Nifurtimox | 4.66 ± 19.7 | 4.58 ± 2.38 | 4.35 ± 1.59 | 1.46 ± 0.35 | 1.06 ± 0.22 | 2.62 ± 1.40 | |||||||||
| Eflornithine | 38.56 ± 9.88 | 36.66 ± 12.87 | 45.99 ± 17.07 | 35.65 ± 10.16 | 16.13 ± 2.93 | 57.21 ± 17.56 | |||||||||
| Pentamidine | 0.041 ± 0.0023 | 0.014 ± 0.0031 | 0.029 ± 0.0062 | 0.050 ± 0.012 | 0.33 ± 0.054 | 0.00097 ± 0.00019 | |||||||||
| Suramin | 0.066 ± 0.0052 | 0.064 ± 0.0018 | 0.076 ± 0.011 | 0.066 ± 0.071 | 7.17 ± 0.87 | 0.38 ± 0.058 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munsimbwe, L.; Seetsi, A.; Namangala, B.; N’Da, D.D.; Inoue, N.; Suganuma, K. In Vitro and In Vivo Trypanocidal Efficacy of Synthesized Nitrofurantoin Analogs. Molecules 2021, 26, 3372. https://doi.org/10.3390/molecules26113372
Munsimbwe L, Seetsi A, Namangala B, N’Da DD, Inoue N, Suganuma K. In Vitro and In Vivo Trypanocidal Efficacy of Synthesized Nitrofurantoin Analogs. Molecules. 2021; 26(11):3372. https://doi.org/10.3390/molecules26113372
Chicago/Turabian StyleMunsimbwe, Linous, Anna Seetsi, Boniface Namangala, David D. N’Da, Noboru Inoue, and Keisuke Suganuma. 2021. "In Vitro and In Vivo Trypanocidal Efficacy of Synthesized Nitrofurantoin Analogs" Molecules 26, no. 11: 3372. https://doi.org/10.3390/molecules26113372
APA StyleMunsimbwe, L., Seetsi, A., Namangala, B., N’Da, D. D., Inoue, N., & Suganuma, K. (2021). In Vitro and In Vivo Trypanocidal Efficacy of Synthesized Nitrofurantoin Analogs. Molecules, 26(11), 3372. https://doi.org/10.3390/molecules26113372







