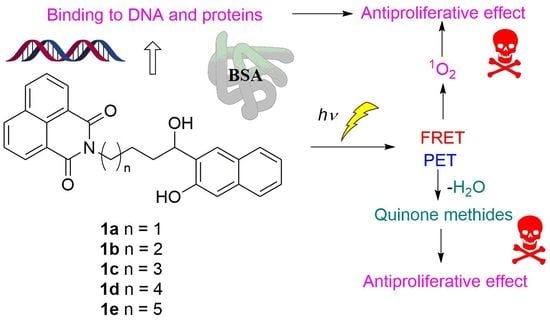
Photochemical Reactivity of Naphthol-Naphthalimide Conjugates and Their Biological Activity
Abstract
1. Introduction
2. Results and Discussion
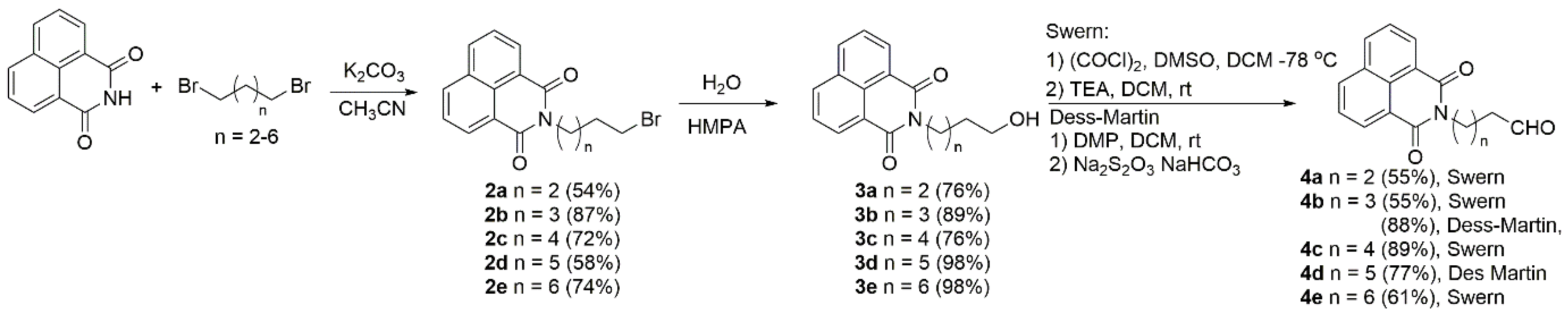
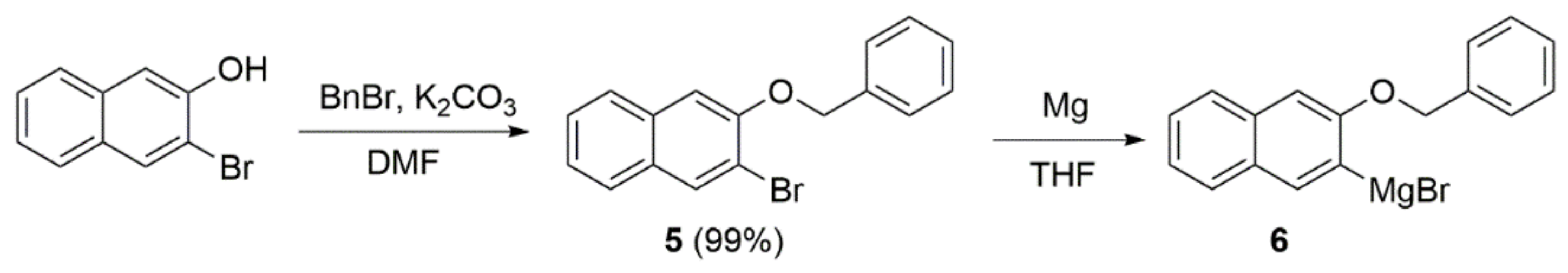
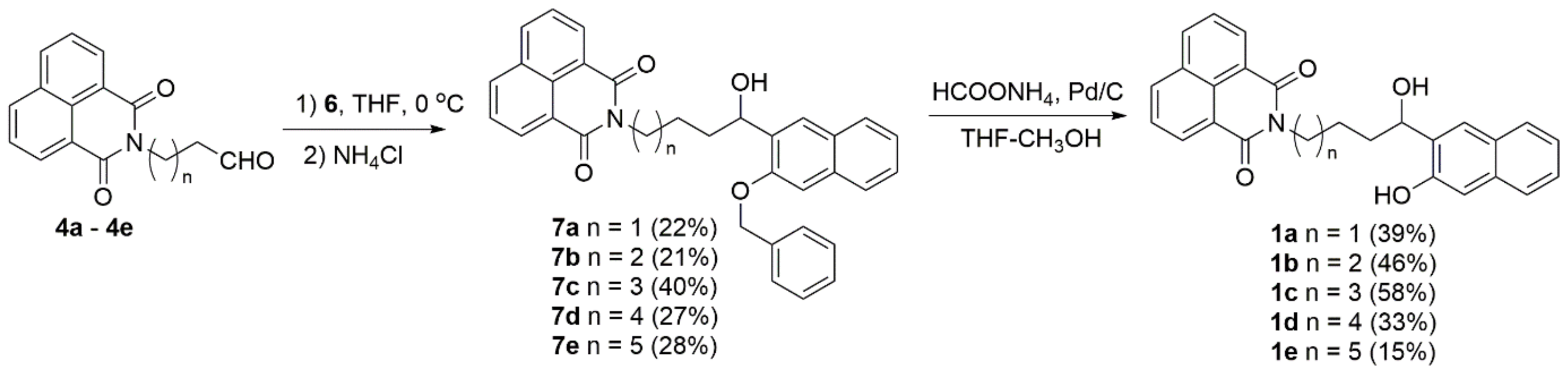
2.1. Synthesis
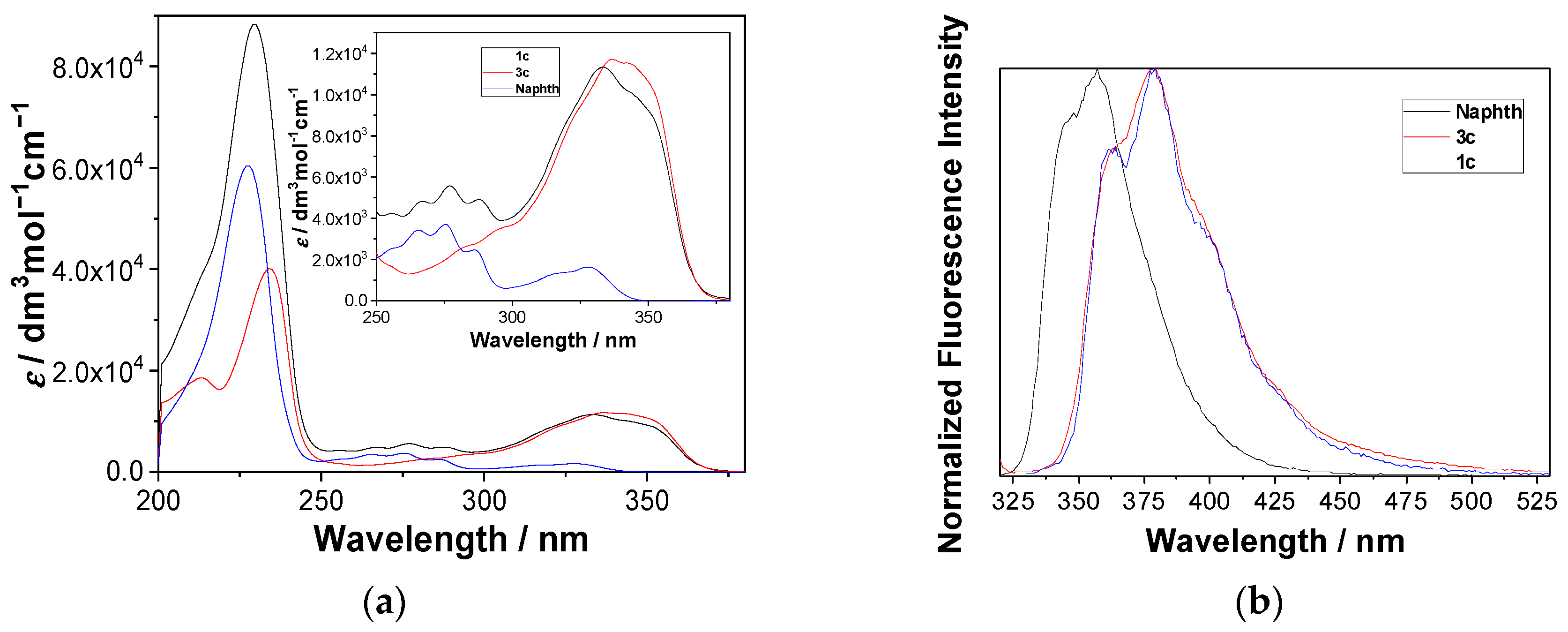
2.2. Photophysical Properties
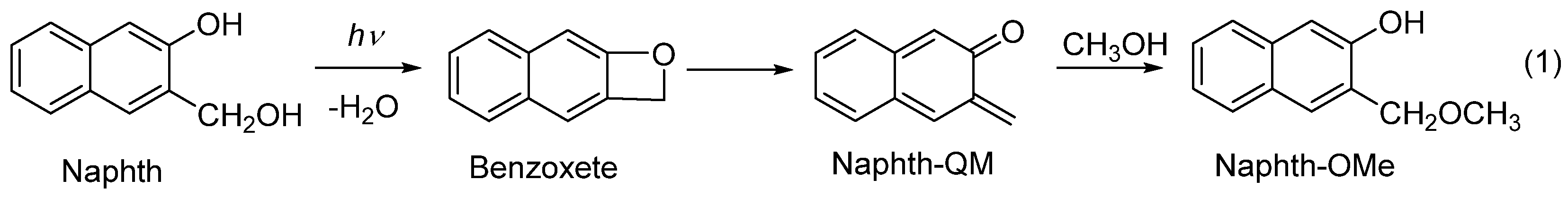
2.3. Photochemistry
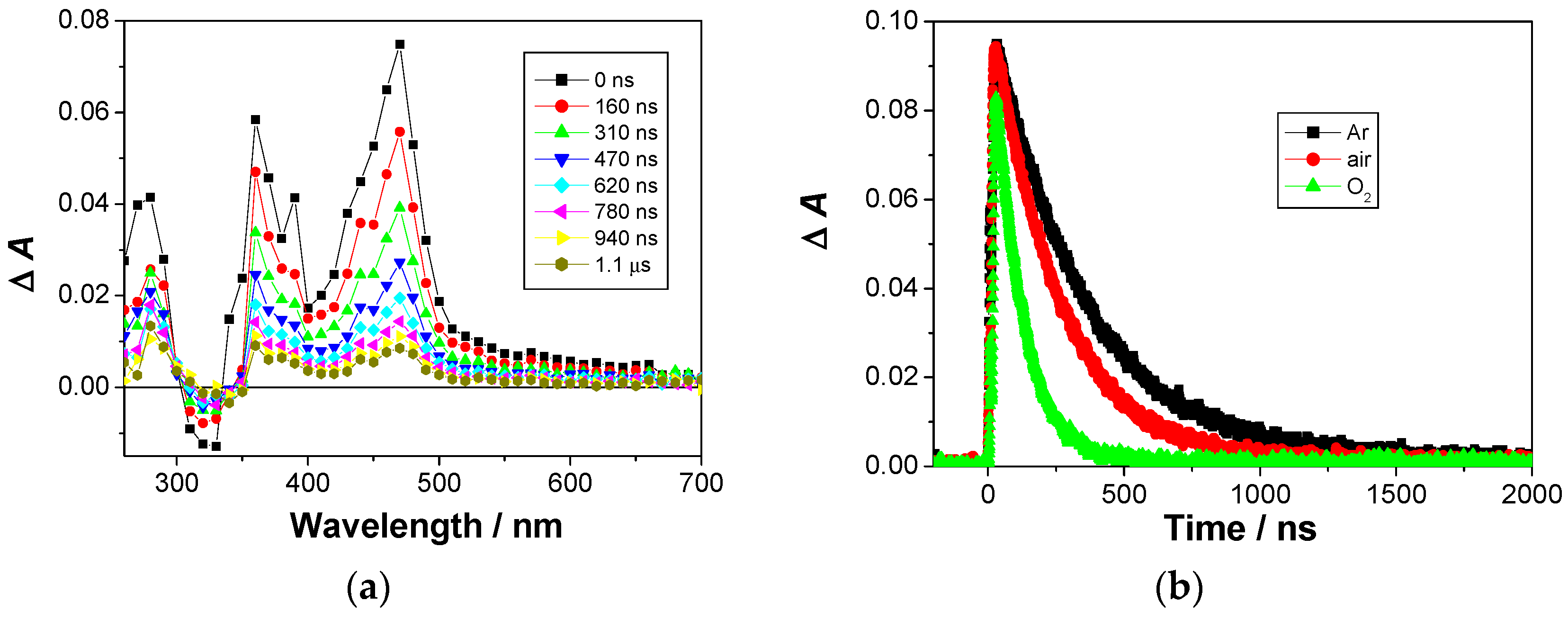
2.4. Laser Flash Photolysis (LFP)
2.5. Photochemical Reaction Mechanism
2.6. Noncovalent Binding to ct-DNA
2.6.1. Thermal Denaturation
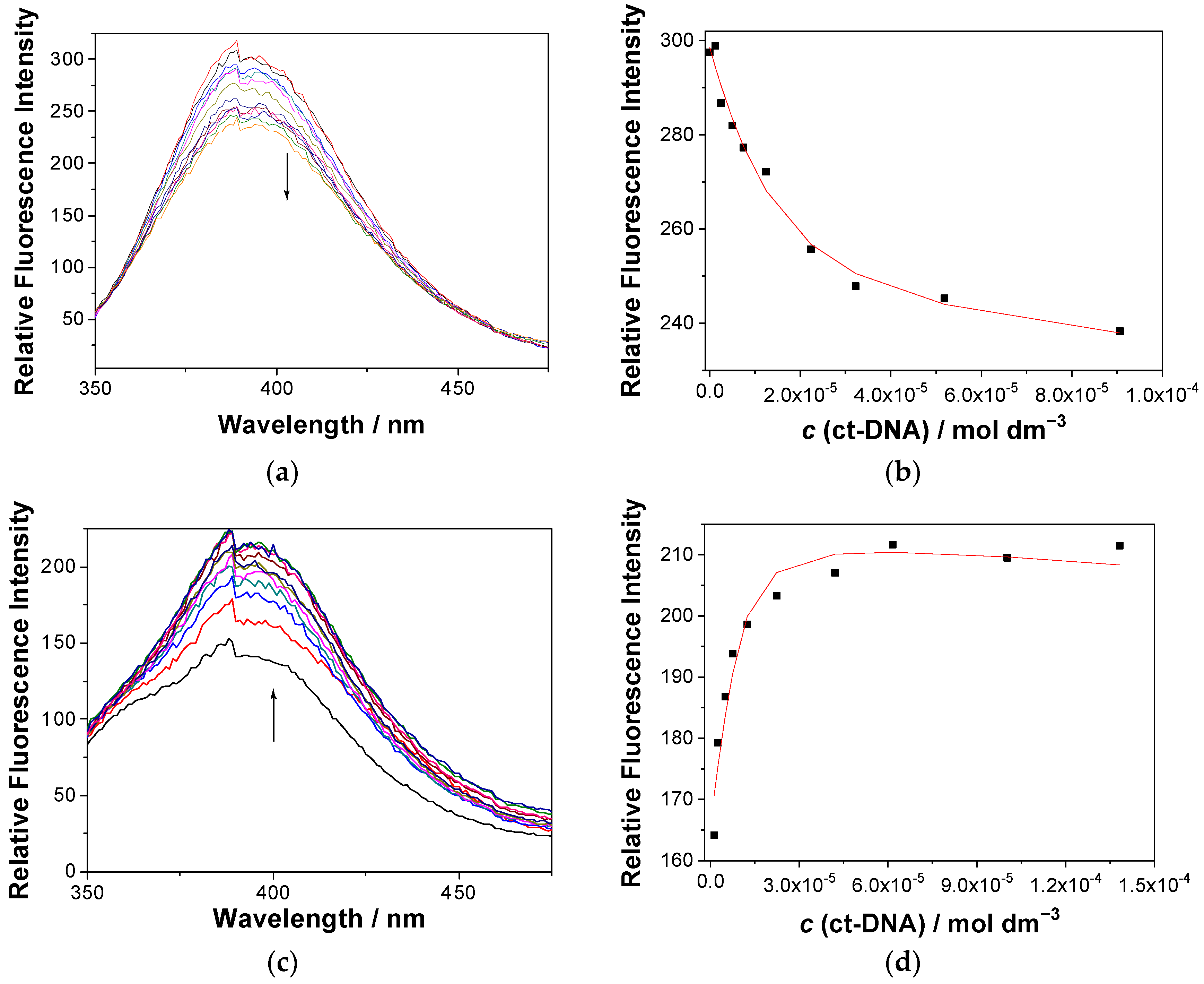
2.6.2. UV-Vis and Fluorescence Titrations
2.6.3. CD Experiments
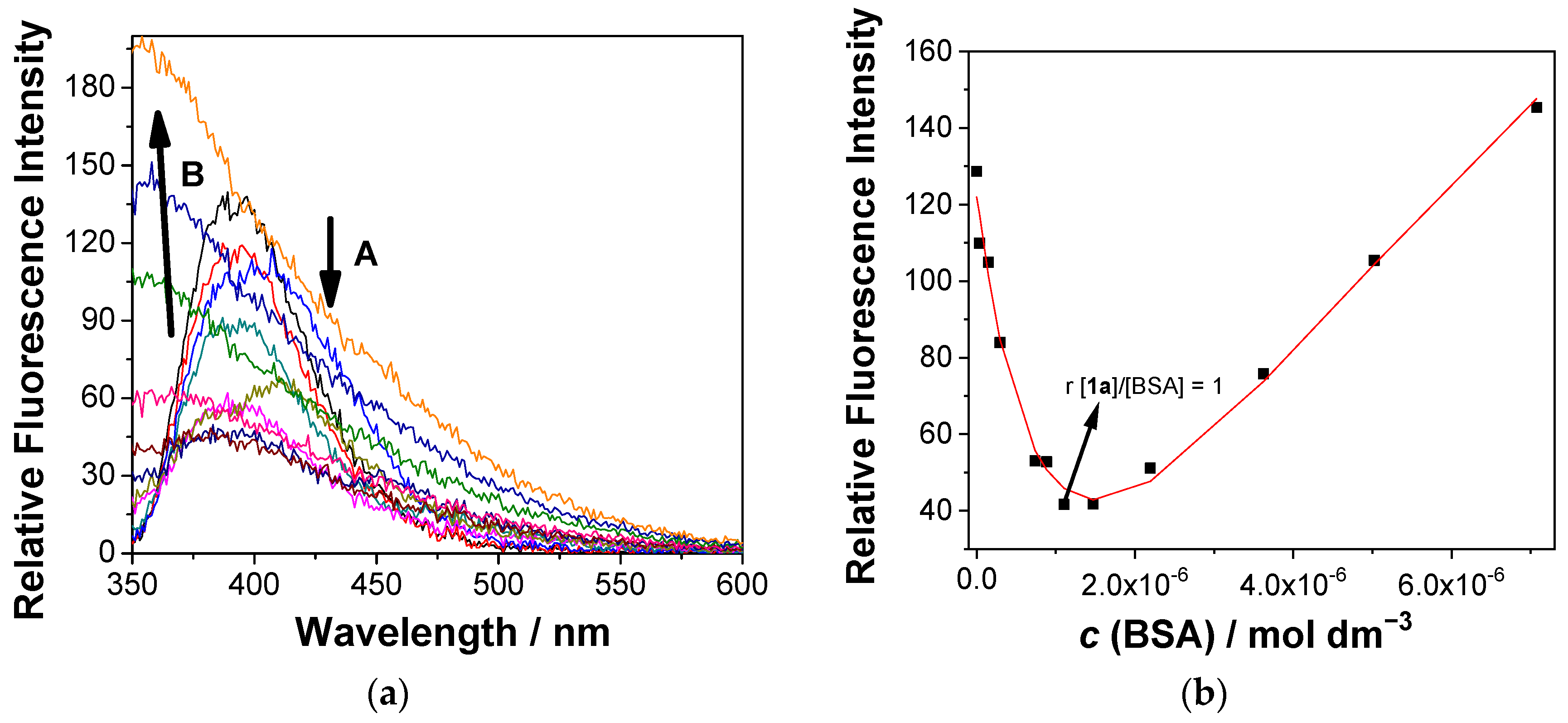
2.7. Noncovalent and Covalent Binding to BSA Protein
2.8. Antiproliferative Activity
3. Conclusions
4. Materials and Methods
- General procedure for the preparation of N-(ω-formylalkyl)-1,8-naphthalimide 4—Swern oxidation
- General procedure for the preparation of N-(ω-formylalkyl)-1,8-naphthalimide—Dess–Martin oxidation [95]
- Grignard reaction of N-(ω-formylalkyl)-1,8-naphthalimide 4 and 6—general procedure
- Removal of the benzyl group—general procedure
- General procedure for the preparation of 1-OMe ethers using the acid-catalyzed thermal method
- Irradiation experiments—general
- Irradiation of 1a
- Irradiation of 1c
- Irradiation of 1e
- Quantum yield of methanolysis
- Absorption and fluorescence measurements
- Thermal denaturation experiments with ct-DNA [86]
- Fluorescence titrations with ct-DNA
- Circular dichroism spectroscopy with ct-DNA
- Fluorescence titrations with BSA
- Photochemical alkylation of BSA
- Laser flash photolysis (LFP)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rajski, S.R.; Williams, R.M. DNA Cross-Linking Agents as Antitumor Drugs. Chem. Rev. 1998, 98, 2723–2796. [Google Scholar] [CrossRef]
- Rokita, S.E. (Ed.) Quinone Methides; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Frecero, M. Quinone Methides as Alkylating and Cross-Linking Agents. Mini Rev. Org. Chem. 2004, 1, 403–415. [Google Scholar] [CrossRef]
- Wang, P.; Song, Y.; Zhang, L.; He, H.; Zhou, X. Quinone Methide Derivatives: Important Intermediates to DNA Alkylating and DNA Cross-linking Actions. Curr. Med. Chem. 2005, 12, 2893–2913. [Google Scholar] [CrossRef]
- McCracken, P.G.; Bolton, J.L.; Thatcher, G.R.J. Covalent Modification of Proteins and Peptides by the Quinone Methide from 2-tert-Butyl-4,6-dimethylphenol: Selectivity and Reactivity with Respect to Competitive Hydration. J. Org. Chem. 1997, 62, 1820–1825. [Google Scholar] [CrossRef]
- Arumugam, S.; Guo, J.; Mbua, N.E.; Friscourt, F.; Lin, N.; Nekongo, E.; Boons, G.-J.; Popik, V.V. Selective and reversible photochemical derivatization of cysteine residues in peptides and proteins. Chem. Sci. 2014, 5, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruiz, R.; Molins-Molina, O.; Lence, E.; González-Bello, C.; Miranda, M.A.; Jiménez, M.C. Photogeneration of Quinone Methides as Latent Electrophiles for Lysine Targeting. J. Org. Chem. 2018, 83, 13019–13029. [Google Scholar] [CrossRef] [PubMed]
- Pande, P.; Shearer, J.; Yang, J.; Greenberg, W.A.; Rokita, S.E. Alkylation of Nucleic Acids by a Model Quinone Methide. J. Am. Chem. Soc. 1999, 121, 6773–6779. [Google Scholar] [CrossRef]
- Veldhuyzen, W.F.; Shallop, A.J.; Jones, R.A.; Rokita, S.E. Thermodynamic versus Kinetic Products of DNA Alkylation as Modeled by Reaction of Deoxyadenosine. J. Am. Chem. Soc. 2001, 123, 11126–11132. [Google Scholar] [CrossRef]
- Veldhuyzen, W.F.; Pande, P.; Rokita, S.E. A Transient Productof DNA Alkylation Can Be Stabilized by Binding Localization. J. Am. Chem. Soc. 2003, 125, 14005–14013. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.N.; Maggi, S.; Mels, S.C.; Palumbo, M.; Freccero, M. Binol Quinone Methides as Bisalkylating and DNA Cross-Linking Agents. J. Am. Chem. Soc. 2004, 126, 13973–13979. [Google Scholar] [CrossRef]
- Verga, D.; Nadai, M.; Doria, F.; Percivalle, C.; Di Antonio, M.; Palumbo, M.; Richter, S.N.; Freccero, M. Photogeneration and Reactivity of Naphthoquinone Methides as Purine Selective DNA Alkylating Agents. J. Am. Chem. Soc. 2010, 132, 14625–14637. [Google Scholar] [CrossRef] [PubMed]
- Di Antonio, M.; Doria, F.; Richter, S.N.; Bertipaglia, C.; Mella, M.; Sissi, C.; Palumbo, M.; Freccero, M. Quinone Methides Tethered to Naphthalene Diimides as Selective G-Quadruplex Alkylating Agents. J. Am. Chem. Soc. 2009, 131, 13132–13141. [Google Scholar] [CrossRef] [PubMed]
- Nadai, M.; Doria, F.; Di Antonio, M.; Sattin, G.; Germani, L.; Percivalle, C.; Palumbo, M.; Richter, S.N.; Freccero, M. Naph-thalene Diimide Scaffolds with Dual Reversible and Covalent Interaction Properties towards G-Quadruplex. Biochimie 2011, 93, 1328–1340. [Google Scholar] [CrossRef]
- Doria, F.; Nadai, M.; Folini, M.; Di Antonio, M.; Germani, L.; Percivalle, C.; Sissi, C.; Zaffaroni, N.; Alcaro, S.; Artese, A.; et al. Hybrid ligand–alkylating agents targeting telomeric G-quadruplex structures. Org. Biomol. Chem. 2012, 10, 2798–2806. [Google Scholar] [CrossRef] [PubMed]
- Doria, F.; Nadai, M.; Folini, M.; Scalabrin, M.; Germani, L.; Sattin, G.; Mella, M.; Palumbo, M.; Zaffaroni, N.; Fabris, D.; et al. Targeting Loop Adeninesin G-Quadruplexby a Selective Oxirane. Chem. Eur. J. 2013, 19, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Li, V.S.; Kohn, H. Studies on the bonding specificity for mitomycin C-DNA monoalkylation processes. J. Am. Chem. Soc. 1991, 113, 275–283. [Google Scholar] [CrossRef]
- Han, I.; Russell, D.J.; Kohn, H. Studies on the Mechanism of Mitomycin C(1) Electrophilic Transformations: Structure-Reactivity Relationships. J. Org. Chem. 1992, 57, 1799–1807. [Google Scholar] [CrossRef]
- Tomasz, M.; Das, A.; Tang, K.S.; Ford, M.G.J.; Minnock, A.; Musser, S.M.; Waring, M.J. The Purine 2-Amino Group as the Critical Recognition Element for Sequence-Specific Alkylation and Cross-Linking of DNA by Mitomycin C. J. Am. Chem. Soc. 1998, 120, 11581–11593. [Google Scholar] [CrossRef]
- Wang, H.; Wahi, M.S.; Rokita, S.E. Immortalizing a Transient Electrophile for DNA Cross-Linking. Angew. Chem. Int. Ed. 2008, 47, 1291–1293. [Google Scholar] [CrossRef]
- Wang, H.; Rokita, S.E. Dynamic Cross-Linking is Retained in Duplex DNA after Multiple Exchange of Strands. Angew. Chem. Int. Ed. 2010, 49, 5957–5960. [Google Scholar] [CrossRef]
- Rossiter, C.S.; Modica, E.; Kumar, D.; Rokita, S.E. Few Constraints Limit the Design of Quinone Methide-Oligonucleotide Self-Adducts for Directing DNA Alkylation. Chem. Commun. 2010, 47, 1476–1478. [Google Scholar] [CrossRef]
- Basarić, N.; Mlinarić-Majerski, K.; Kralj, M. Quinone Methides: Photochemical Generation and its Application in Biomedicine. Curr. Org. Chem. 2014, 18, 3–18. [Google Scholar] [CrossRef]
- Percivalle, C.; Doria, F.; Freccero, M. Quinone Methides as DNA Alkylating Agents: An Overview on Efficient Activation Protocols for Enhanced Target Selectivity. Curr. Org. Chem. 2014, 18, 19–43. [Google Scholar] [CrossRef]
- Chiang, Y.; Kresge, A.J.; Zhu, Y. Kinetics and Mechanisms of Hydration of o-Quinone Methides in Aqueous Solution. J. Am. Chem. Soc. 2000, 122, 9854–9855. [Google Scholar] [CrossRef]
- Chiang, Y.; Kresge, A.J.; Zhu, Y. Flash photolytic generation of ortho-quinone methide in aqueous solution and study of its chemistry in that medium. J. Am. Chem. Soc. 2001, 123, 8089–8094. [Google Scholar] [CrossRef]
- Toteva, M.M.; Richard, J.P. The Generation and Reactions of Quinone Methides. Adv. Phys. Org. Chem. 2011, 45, 39–91. [Google Scholar] [CrossRef] [PubMed]
- Basarić, N.; Cindro, N.; Bobinac, D.; Mlinarić-Majerski, K.; Uzelac, L.; Kralj, M.; Wan, P. Sterically Congested Quinone Methides in Photodehydration Reactions of 4-Hydroxybiphenyl Derivatives and Investigation of their Antiproliferative Activity. Photochem. Photobiol. Sci. 2011, 10, 1910–1925. [Google Scholar] [CrossRef]
- Basarić, N.; Cindro, N.; Bobinac, D.; Uzelac, L.; Mlinarić-Majerski, K.; Kralj, M.; Wan, P. Zwitterionic Biphenyl Quinone Methides in Photodehydration Reactions of 3-Hydroxybiphenyl Derivatives: Laser Flash Photolysis and Antiproliferation Study. Photochem. Photobiol. Sci. 2012, 11, 381–396. [Google Scholar] [CrossRef]
- Veljković, J.; Uzelac, L.; Molčanov, K.; Mlinarić-Majerski, K.; Kralj, M.; Wan, P.; Basarić, N. Sterically Congested Adamantyl-naphthalene Quinone Methides. J. Org. Chem. 2012, 77, 4596–4610. [Google Scholar] [CrossRef]
- Sambol, M.; Ester, K.; Landgraf, S.; Mihaljević, B.; Cindrić, M.; Kralj, M.; Basarić, N. Competing Photochemical Reactions of bis-Naphthols and Their Photoinduced Antiproliferative Activity. Photochem. Photobiol. Sci. 2019, 18, 1197–1211. [Google Scholar] [CrossRef]
- Škalamera, Đ.; Mlinarić-Majerski, K.; Martin Kleiner, I.; Kralj, M.; Oake, J.; Wan, P.; Bohne, C.; Basarić, N. Photochemical Formation of Anthracene Quinone Methide Derivatives. J. Org. Chem. 2017, 82, 6006–6021. [Google Scholar] [CrossRef]
- Uzelac, L.; Škalamera, Đ.; Mlinarić-Majerski, K.; Basarić, N.; Kralj, M. Selective Photocytotoxicity of Anthrols on Cancer Stem-like Cells: The Effect of Quinone Methides or Reactive Oxygen Species. Eur. J. Med. Chem. 2017, 137, 558–574. [Google Scholar] [CrossRef] [PubMed]
- Basarić, N.; Kralj, M.; Mikecin, A.M.; Cindrić, M. Quinone Methide Precursors with BODIPY Chromophore, Method of Preparation, Biological Activity and Application in Fluorescent Labeling. PCT/HR2017/000005, 15 May 2017. [Google Scholar]
- Erben, A.; Matić, J.; Basarić, N.; Piantanida, I. The Phenanthridine-modified Tyrosine Dipeptide: Synthesis and Non-covalent Binding to DNA and RNA. Croat. Chem. Acta. 2019, 92, 249–258. [Google Scholar] [CrossRef]
- Arumugam, S.; Popik, V.V. Photochemical Generation and the Reactivity of o-Naphthoquinone Methides in Aqueous Solu-tions. J. Am. Chem. Soc. 2009, 131, 11892–11899. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Popik, V.V. Attach, Remove, or Replace: Reversible Surface Functionalization Using Thiol–Quinone Methide Photoclick Chemistry. J. Am. Chem. Soc. 2012, 134, 8408–8411. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Popik, V.V. Light-Induced Hetero-DielsAlder Cycloaddition: A Facile and Selective Photoclick Reaction. J. Am. Chem. Soc. 2011, 133, 5573–5579. [Google Scholar] [CrossRef]
- Arumugam, S.; Popik, V.V. Patterned Surface Derivatization Using Diels–Alder Photoclick Reaction. J. Am. Chem. Soc. 2011, 133, 15730–15736. [Google Scholar] [CrossRef]
- Arumugam, S.; Orski, S.V.; Locklin, J.; Popik, V.V. Photoreactive Polymer Brushes for High-Density Patterned Surface Derivatization Using a Diels–Alder Photoclick Reaction. J. Am. Chem. Soc. 2012, 134, 179–182. [Google Scholar] [CrossRef]
- Chang, S.-C.; Archer, B.J.; Utecht, R.E.; Lewis, D.E.; Judy, M.M.; Matthews, J.L. 4-Alkylamino-3-bromo-N-alkyl-1,8-naphthalimides: New Photochemically Activatable Antiviral Compounds. Bioorganic Med. Chem. Lett. 1993, 3, 555–556. [Google Scholar] [CrossRef]
- Chanh, T.C.; Lewis, D.E.; Judy, M.M.; Sogandares-Bernal, F.; Michalek, G.R.; Utecht, R.E.; Skiles, H.; Chang, S.-C.; Matthews, J.L. Inhibition of Retrovirus-Induced Syncytium Formation by Photoproducts of a Brominated 1,8-Naphthalimide Compound. Antivir. Res. 1994, 25, 133–146. [Google Scholar] [CrossRef]
- Chanh, T.C.; Lewis, D.E.; Allan, J.S.; Sogandares-Bernal, F.; Judy, M.M.; Utecht, R.E.; Matthews, J.L. Neutralization of HIV-1 and Inhibition of HIV-1-Induced Syncytia by 1,8-Naphthalimide Photoactive Compound. Aids Res. Hum. Retrovir. 1993, 9, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Andricopulo, A.D.; A Müller, L.; Filho, V.C.; Cani, G.S.; Roos, J.F.; Corrêa, R.; Santos, A.R.S.; Nunes, R.J.; Yunes, R.A. Analgesic Activity of Cyclic Imides: 1,8-Naphthalimide and 1,4,5,8-Naphthalenediimide Derivatives. Il Farm. 2000, 55, 319–321. [Google Scholar] [CrossRef]
- Mattocks, A.M.; Hutchison, O.S. Local Anesthetics: N-Dialkylaminoalkylimides of Naphthalic and Diphenylmaleic Acids. J. Am. Chem. Soc. 1948, 70, 3474–3475. [Google Scholar] [CrossRef] [PubMed]
- Muth, M.; Hoerr, V.; Glaser, M.; Ponte-Sucre, A.; Moll, H.; Stich, A.; Holzgrabe, U. Antitrypanosomal Activity of Quaternary Naphthalimide Derivatives. Bioorganic Med. Chem. Lett. 2007, 17, 1590–1593. [Google Scholar] [CrossRef] [PubMed]
- Langlois, M.; Soulier, J.; Rampillon, V.; Gallais, C.; Brémont, B.; Shen, S.; Yang, D.; Giudice, A.; Sureau, F. Synthesis of Quinazoline-2,4-dione and Naphthalimide Derivatives as New 5-HT3 Receptor Antagonists. Eur. J. Med. Chem. 1994, 29, 925–940. [Google Scholar] [CrossRef]
- Langlois, M.; Soulier, J.L.; Brémont, B.; Shen, S.; Rampillon, V.; Giudice, A. Derivatives of Naphthalimide: New Potent Con-formationally Restricted Antagonists of 5-HT3 Receptors. Bioorg. Med. Chem. Lett. 1992, 2, 691–694. [Google Scholar] [CrossRef]
- Berque-Bestel, I.; Soulier, J.-L.; Giner, M.; Rivail, L.; Langlois, M.; Sicsic, S. Synthesis and Characterization of the First Fluo-rescent Antagonists for Human 5-HT4 Receptors. J. Med. Chem. 2003, 46, 2606–2620. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, Z.; Zang, F.; Wang, Y.; Wang, C. Spectroscopic Study on the Interaction Between Naphthalimide–Polyamine Conjugates and DNA. J. Photochem. Photobiol. B Biol. 2014, 138, 202–210. [Google Scholar] [CrossRef]
- Tian, Z.-Y.; Li, J.-H.; Li, Q.; Zang, F.-L.; Zhao, Z.-H.; Wang, C.-J. Study on the Synthesis, Biological Activity and Spectroscopy of Naphthalimide-Diamine Conjugates. Molecules 2014, 19, 7646–7668. [Google Scholar] [CrossRef]
- Braña, M.; Ramos, A. Naphthalimides as Anticancer Agents: Synthesis and Biological Activity. Curr. Med. Chem. Agents 2001, 1, 237–255. [Google Scholar] [CrossRef]
- Brana, M.F.; Cacho, M.; Gradillas, A.; De Pascual-Teresa, B.; Ramos, A. Intercalators as Anticancer Drugs. Curr. Pharm. Des. 2001, 7, 1745–1780. [Google Scholar] [CrossRef]
- Rosell, R.; Carles, J.; Abad, A.; Ribelles, N.; Barnadas, A.; Benavides, A. Phase I Study of Mitonafide in 120 Hour Continuous Infusion in Non-small Cell Lung Cancer. Investig. New Drugs 1992, 10, 171–175. [Google Scholar] [CrossRef]
- Llombart, M.; Forner, E.; Olmos, T.; Ruiz, A.; Soriano, V.; Benavides, A.; Martín, M.; Schlick, E.; Guillém, V. Phase I Study of Mitonafide in Solid Tumors. Investig. New Drugs 1992, 10, 177–181. [Google Scholar] [CrossRef]
- Gesme, D.H.; Jett, J.R.; Schreffler, D.D.; Su, J.Q.; Mailliard, J.A.; Foley, J.F.; Krook, J.E.; Maksymiuk, A.W.; Hatfield, A.K.; Ebbert, L.P.; et al. A Randomized Phase II Trial of Amonafide or Tri-metrexate in Patients with Advanced non-Small Cell Lung Cancer. A Trial of the North Central Cancer Treatment Group. Cancer 1993, 71, 2723–2726. [Google Scholar] [CrossRef]
- Kornek, G.; Raderer, M.; Depisch, D.; Haider, K.; Fazeny, B.; Dittrich, C.; Scheithauer, W. Amonafide as First-Line Chemo-therapy for Metastatic Breast Cancer. Eur. J. Cancer 1994, 30, 398–400. [Google Scholar] [CrossRef]
- Ratain, M.J.; Rosner, G.; Allen, S.L.; Costanza, M.; Van Echo, D.A.; Henderson, I.C.; Schilsky, R.L. Population Pharmaco-dynamic Study of Amonafide: A Cancer and Leukemia Group B Study. J. Clin. Oncol. 1995, 13, 741–747. [Google Scholar] [CrossRef]
- Rogers, J.E.; Weiss, S.J.; Kelly, L.A. Photoprocesses of Naphthalene Imide and Diimide Derivatives in Aqueous Solutions of DNA. J. Am. Chem. Soc. 2000, 122, 427–436. [Google Scholar] [CrossRef]
- Rogers, J.E.; Le, T.P.; Kelly, L.A. Nucleotide Oxidation Mediated by Naphthalimide Excited States with Covalently Attached Viologen Cosensitizers. Photochem. Photobiol. 2001, 73, 223–229. [Google Scholar] [CrossRef]
- Saito, I.; Takayama, M.; Sugiyama, H.; Nakatani, K. Photoinduced DNA Cleavage via Electron Transfer: Demonstration That Guanine Residues Located 5’ to Guanine Are the Most Electron-Donating Sites. J. Am. Chem. Soc. 1995, 117, 6406–6407. [Google Scholar] [CrossRef]
- Saito, I.; Saito, I.; Takayama, M. Photoactivatable DNA-Cleaving Amino Acids: Highly Sequence-Selective DNA Photocleavage by Novel L-Lysine Derivatives. J. Am. Chem. Soc. 1995, 117, 5590–5591. [Google Scholar] [CrossRef]
- Percivalle, C.; La Rosa, A.; Verga, D.; Doria, F.; Mella, M.; Palumbo, M.; Di Antonio, M.; Freccero, M. Quinone Methide Gen-eration via Photoinduced Electron Transfer. J. Org. Chem. 2011, 76, 3096–3106. [Google Scholar] [CrossRef]
- Ramchander, J.; Rameshwar, N.; Reddy, T.S.; Raju, G.; Reddy, A.R. Synthesis and Photophysical Properties of 1,4-Disubstituted Naphthyloxymethyl-N-alkyl Naphthimido-1,2,3-triazole. J. Chem. Sci. 2014, 126, 1063–1074. [Google Scholar] [CrossRef]
- Yaqiu, L.; Bin, C. Multi-substituted Amine Compound, as well as Preparation Method and Use Thereof. CN103570683, 30 July 2012. [Google Scholar]
- Spallarossa, M.; Wang, Q.; Riva, R.; Zhu, J. Synthesis of Vinyl Isocyanides and Development of a Convertible Isonitrile. Org. Lett. 2016, 18, 1622–1625. [Google Scholar] [CrossRef]
- Reggelin, M.; Junker, B.; Heinrich, T.; Slavik, S.; Bühle, P. Asymmetric Synthesis of Highly Substituted Azapolycyclic Com-pounds via 2-Alkenyl Sulfoximines: Potential Scaffolds for Peptide Mimetics. J. Am. Chem. Soc. 2006, 128, 4023–4034. [Google Scholar] [CrossRef]
- Mandal, P.K.; McMurray, J.S. Pd−C-Induced Catalytic Transfer Hydrogenation with Triethylsilane. J. Org. Chem. 2007, 72, 6599–6601. [Google Scholar] [CrossRef]
- Anantharamaiah, G.M.; Sivanandaiah, K.M. Transfer Hydrogenation; a Convenient Method for Removal of Some Commonly Used Protecting Groups in Peptide Synthesis. J. Chem. Soc. Perkin Trans. 1977, 1, 490–491. [Google Scholar] [CrossRef]
- Bieg, T.; Szeja, W. Removal of O-Benzyl Protective Groups by Catalytic Transfer Hydrogenation. Synthesis 1985, 1985, 76–77. [Google Scholar] [CrossRef]
- Lee, J.; Robinson, G.W.; Webb, S.P.; Philips, L.A.; Clark, J.H. Hydration Dynamics of Protons from Photon Initiated Acids. J. Am. Chem. Soc. 1986, 108, 6538–6542. [Google Scholar] [CrossRef]
- Robinson, G.W. Proton Charge Transfer Involving the Water Solvent. J. Phys. Chem. 1991, 95, 10386–10391. [Google Scholar] [CrossRef]
- Tolbert, L.M.; Haubrich, J.E. Photoexcited Proton Transfer from Enhanced Photoacids. J. Am. Chem. Soc. 1994, 116, 10593–10600. [Google Scholar] [CrossRef]
- Solntsev, K.M.; Huppert, D.; Agmon, A.N.; Tolbert, L.M. Photochemistry of “Super” Photoacids. 2. Excited-State Proton Transfer in Methanol/Water Mixtures. J. Phys. Chem. A 2000, 104, 4658–4669. [Google Scholar] [CrossRef]
- Laws, W.R.; Brand, L. Analysis of Two-State Excited-State Reactions. The Fluorescence Decay of 2-Naphthol. J. Phys. Chem. 1979, 83, 795–802. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry; CRC Taylor and Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Oelgemöller, M.; Kramer, W.H. Synthetic Photochemistry of Naphthalimides and Related Compounds. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 210–244. [Google Scholar] [CrossRef]
- Wintgens, V.; Valat, P.; Kossanyi, J.; Biczok, L.; Demeter, A.; Bérces, T. Spectroscopic Properties of Aromatic Dicarboximides. Part 1—N–H and N-methyl-substituted Naphthalimides. J. Chem. Soc. Faraday Trans. 1994, 90, 411–421. [Google Scholar] [CrossRef]
- Demeter, A.; Biczok, L.; Berces, T.; Wintgens, V.; Valat, P.; Kossanyi, J. Laser Photolysis Studies of Transient Processes in the Photoreduction of Naphthalimides by Aliphatic Amines. J. Phys. Chem. 1993, 97, 3217–3224. [Google Scholar] [CrossRef]
- Kubo, Y.; Imaoka, T.; Shiragami, T.; Araki, T. A Photoallylation of N-Methylarenedicarboximides by Allylsilanes. Chem. Lett. 1986, 15, 1749–1752. [Google Scholar] [CrossRef]
- Goldstein, S.; Rabani, J. The ferrioxalate and Iodide–Iodate Actinometers in the UV Region. J. Photochem. Photobiol. A Chem. 2008, 193, 50–55. [Google Scholar] [CrossRef]
- Škalamera, Đ.; Antol, I.; Mlinarić-Majerski, K.; Vančik, H.; Phillips, D.L.; Ma, J.; Basarić, N. Ultrafast Adiabatic Photodehy-dration of 2-Hydroxymethylphenol and the Formation of Quinone Methide. Chem. Eur. J. 2018, 24, 9426–9435. [Google Scholar] [CrossRef]
- Mohan, H.; Hermann, R.; Naumov, S.; Mittal, J.P.; Brede, O. Two Channels of Electron Transfer Observed for the Reaction of n-Butyl Chloride Parent Radical Cations with Naphthols and Hydroxybiphenyls. J. Phys. Chem. A. 1998, 102, 5754–5762. [Google Scholar] [CrossRef]
- Cho, D.W.; Fujitsuka, M.; Yoon, U.C.; Majima, T. Intermolecular Photoinduced Electron-Transfer of 1,8-Naphthalimides in Protic Polar Solvents. Phys. Chem. Chem. Phys. 2008, 10, 4393–4399. [Google Scholar] [CrossRef]
- Horvat, M.; Mlinarić-Majerski, K.; Basarić, N. Photochemistry of N-alkyl and N-aryl Substituted Phthalimides: H-Abstractions, Single Elelctron Transfer and Cycloadditions. Croat. Chem. Acta 2010, 83, 179–188. [Google Scholar]
- Mergny, J.-L.; Lacroix, L. Analysis of Thermal Melting Curves. Oligonucleotides 2003, 13, 515–537. [Google Scholar] [CrossRef]
- Wilson, W.D.; Ratmeyer, L.; Zhao, M.; Strekowski, L.; Boykin, D. The Search for Structure-Specific Nucleic Acid-Interactive Drugs: Effects of Compound Structure on RNA versus DNA Interaction Strength. Biochemistry 1993, 32, 4098–4104. [Google Scholar] [CrossRef]
- McGhee, J.D.; von Hippel, P.H. Theoretical Aspects of DNA-Protein Interactions: Co-operative and Non-co-operative Binding of Large Ligands to a one-Dimensional Homogeneous Lattice. J. Mol. Biol. 1974, 86, 469–489. [Google Scholar] [CrossRef]
- Steenken, S.; Jovanovic, S.V. How Easily Oxidizable Is DNA? One-Electron Reduction Potentials of Adenosine and Guanosine Radicals in Aqueous Solution. J. Am. Chem. Soc. 1997, 119, 617–618. [Google Scholar] [CrossRef]
- Rodger, A.; Norden, B. Circular Dichroism and Linear Dichroism; Oxford University Press: New York, NY, USA, 1997; Chapter 2. [Google Scholar]
- Šmidlehner, T.; Piantanida, I.; Pescitelli, G. Polarization Spectroscopy Methods in the Determination of Interactions of Small Molecules with Nucleic Acids—Tutorial. Beilstein J. Org. Chem. 2018, 14, 84–105. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Nordén, B. Linear and Circular Dichroism of Drug-Nucleic Acid Complexes. Met. Enzymol. 2001, 340, 68–98. [Google Scholar]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of Equilibria in Solution. Determination of Equilibrium Constants with the HYPERQUAD Suite of Programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Doria, F.; Richter, S.N.; Nadai, M.; Colloredo-Mels, S.; Mella, M.; Palumbo, M.; Freccero, M. BINOL−Amino Acid Conjugates as Triggerable Carriers of DNA-Targeted Potent Photocytotoxic Agents. J. Med. Chem. 2007, 50, 6570–6579. [Google Scholar] [CrossRef]
- Duncan, E.J.; Chao-Pin, L. Endhothelin Converting Enzyme Inhibitors. WO9513817 (A1) (1995), 16 November 1994. [Google Scholar]
- Hunter, D.J.; Markwell, R.E.; Ward, R.W. Phosphonopeptides with Collagenase Inhibiting Activity. WO9309136 (A1) (1993), 5 April 1991. [Google Scholar]
- Škalamera, Đ.; Mlinarić-Majerski, K.; Martin-Kleiner, I.; Kralj, M.; Wan, P.; Basarić, N. Near-Visible Light Generation of a Quinone Methide from 3-Hydroxymethyl-2-anthrol. J. Org. Chem. 2014, 79, 4390–4397. [Google Scholar] [CrossRef]

| Comp. | Φf (CH3CN) × 103 | ΦFRET (CH3CN) b | Φf (CH3CN-H2O) × 103 c |
|---|---|---|---|
| 1a | 12 ± 1 | 0.70 | 14.3 ± 0.7 |
| 1b | 15 ± 2 | 0.79 | 32.6 ± 0.5 |
| 1c | 16 ± 2 | 0.82 | 37.4 ± 0.7 |
| 1d | 17 ± 2 | 0.71 | 40 ± 1 |
| 1e | 18 ± 2 | 0.69 | 48.2 ± 0.9 |
| Comp. | ΦR × 105 |
|---|---|
| 1a | 3.0 ± 0.3 |
| 1b | 2.6 ± 0.8 |
| 1c | 2.8 ± 0.5 |
| 1d | 3.5 ± 0.9 |
| 1e | 3.6 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambol, M.; Benčić, P.; Erben, A.; Matković, M.; Mihaljević, B.; Piantanida, I.; Kralj, M.; Basarić, N. Photochemical Reactivity of Naphthol-Naphthalimide Conjugates and Their Biological Activity. Molecules 2021, 26, 3355. https://doi.org/10.3390/molecules26113355
Sambol M, Benčić P, Erben A, Matković M, Mihaljević B, Piantanida I, Kralj M, Basarić N. Photochemical Reactivity of Naphthol-Naphthalimide Conjugates and Their Biological Activity. Molecules. 2021; 26(11):3355. https://doi.org/10.3390/molecules26113355
Chicago/Turabian StyleSambol, Matija, Patricia Benčić, Antonija Erben, Marija Matković, Branka Mihaljević, Ivo Piantanida, Marijeta Kralj, and Nikola Basarić. 2021. "Photochemical Reactivity of Naphthol-Naphthalimide Conjugates and Their Biological Activity" Molecules 26, no. 11: 3355. https://doi.org/10.3390/molecules26113355
APA StyleSambol, M., Benčić, P., Erben, A., Matković, M., Mihaljević, B., Piantanida, I., Kralj, M., & Basarić, N. (2021). Photochemical Reactivity of Naphthol-Naphthalimide Conjugates and Their Biological Activity. Molecules, 26(11), 3355. https://doi.org/10.3390/molecules26113355