In Situ Decarboxylation-Pressurized Hot Water Extraction for Selective Extraction of Cannabinoids from Cannabis sativa. Chemometric Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Response Surface Methodology
2.4. Model Fitting and Accuracy
2.5. Pressurized Hot Water Extraction
2.6. Preparation of Standard Solutions and Calibration Curve
2.7. UHPLC-MS/MS Conditions
2.8. Quality Control Sample Preparation
3. Results and Discussion
3.1. Method Validation of UHPLC-MS/MS
3.2. Response Surface Methodology
3.3. Model Fitting and Accuracy
3.4. The Effect of Decarboxylation Conditions on the Extracted Amount of Cannabinoids
3.5. Optimal Extraction Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Aladic, K.; Jarni, K.; Barbir, T.; Vidovic, S.; Vladic, J.; Bilic, M.; Jokic, S. Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind. Crops Prod. 2015, 76, 472–478. [Google Scholar] [CrossRef]
- Rovetto, L.J.; Aieta, N.V. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Devi, V.; Khanam, S. Study of ω-6 linoleic and ω-3 α-linolenic acids of hemp (Cannabis sativa) seed oil extracted by supercritical CO2 extraction: CCD optimization. J. Environ. Chem. Eng. 2019, 7, 102818. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Koch, S. Interplay between chemistry and morphology in medical cannabis (Cannabis sativa L.). Ind. Crops Prod. 2019, 129, 185–194. [Google Scholar] [CrossRef]
- Moreno, T.; Montanes, F.; Tallon, S.J.; King, J. Extraction of cannabinoids from hemp (Cannabis sativa L.) using high pressure solvents: An overview of different processing options. J. Supercrit. Fluids 2020, 161, 104850. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premolia, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Rabgay, K.; Waranuch, N.; Dilokthornsakul, P. The effects of cannabis, cannabinoids, and their administration routes on pain control efficacy and safety: A systematic review and network meta-analysis. J. Am. Pharm. Assoc. 2020, 60, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, P.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Del Rio, A. Herbal Preparations of Medical Cannabis: A Vademecum for Prescribing Doctors. Medicina 2020, 15, 23. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; Antwerp, J.V.; Parcher, J.F.; ElSohly, M.A.; Khan, I.A. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. Pharmacological and therapeutic targets for Δ9-tetrahydrocannabinol and cannabidiol. Euphytica 2004, 140, 73–82. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Delgado-Provedano, M.M.; Callado, C.S.; Priego-Capote, F.; Ferreiro-Vera, C. Untargeted characterization of extracts from Cannabis sativa L. cultivars by gas and liquid chromatography coupled to mass spectrometry in high resolution mode. Talanta 2020, 208, 120384. [Google Scholar] [CrossRef]
- Brighentia, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, L.A.; Ranieri, T.L.; Taylor, A.M. Commercial cannabis consumer products part 1: GC–MS qualitative analysis of cannabis cannabinoids. Forensic Sci. Int. 2018, 289, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Fabianio-Tixier, A.S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325. [Google Scholar] [CrossRef] [Green Version]
- Roberta, P.; Emilia, M.; Francesco, S.; Luca, G.; Francesco, P.; Francesco, P.B.; Busardò, S.P. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2017, 28, 1555–1563. [Google Scholar] [CrossRef]
- Silveira, d.R.G.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 56, 102234. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.W.; Yen, C.C.; Wu, M.T.; Hsu, M.C.; Wu, Y.T. Microwave-Assisted Extraction of Cannabinoids in Hemp Nut Using Response Surface Methodology: Optimization and Comparative Study. Molecules 2017, 22, 1894. [Google Scholar] [CrossRef] [Green Version]
- Nuapia, Y.; Tutu, H.; Chimuka, L.; Cukrowska, E. Selective Extraction of Cannabinoid Compounds from Cannabis Seed Using Pressurized Hot Water Extraction. Molecules 2020, 25, 1335. [Google Scholar] [CrossRef] [Green Version]
- Plaza, M.; Marina, M.L. Pressurized hot water extraction of bioactives. Trends Anal. Chem. 2019, 116, 236–247. [Google Scholar] [CrossRef]
- Gbashi, S.; Njobeh, P.B.; De Saeger, S.; De Boevre, M.; Madala, N.E. Development, chemometric-assisted optimization and in-house validation of a modified pressurized hot water extraction methodology for multi-mycotoxins in maize. Food Chem. 2020, 1, 125526. [Google Scholar] [CrossRef] [PubMed]
- Dejaegher, B.; Heyden, Y.V. Experimental designs and their recent advances in set-up, data interpretation, and analytical applications. J. Pharm. Biomed. Anal. 2011, 56, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Pacchetti, B.; Vandelli, M.A.; Forni, F.; Cannazza, G. Analysis of cannabinoids in commercial hemp seed oil and decarboxylation kinetics studies of cannabidiolic acid (CBDA). J. Pharm. Biomed. Anal. 2018, 149, 532–540. [Google Scholar] [CrossRef] [PubMed]
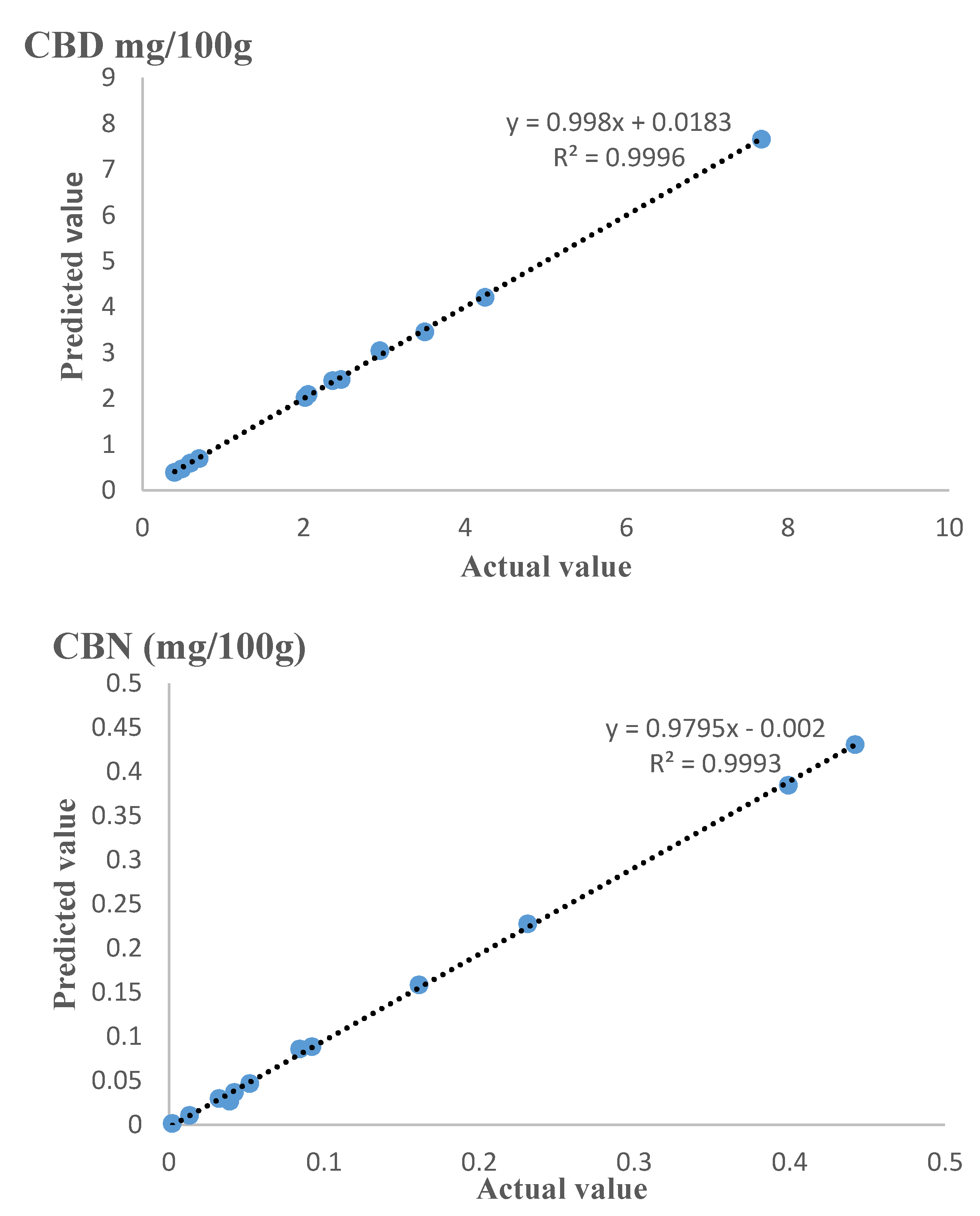

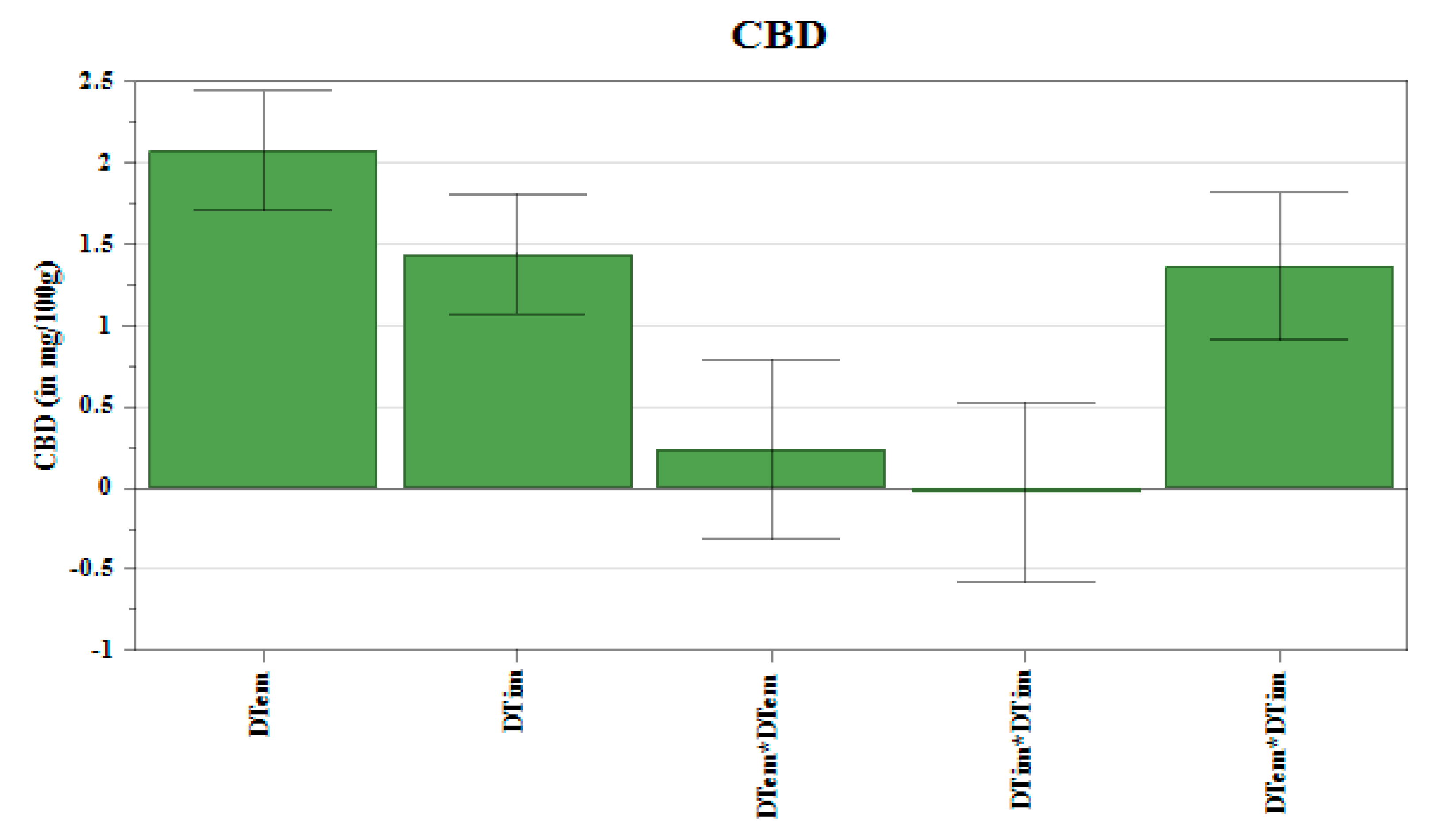
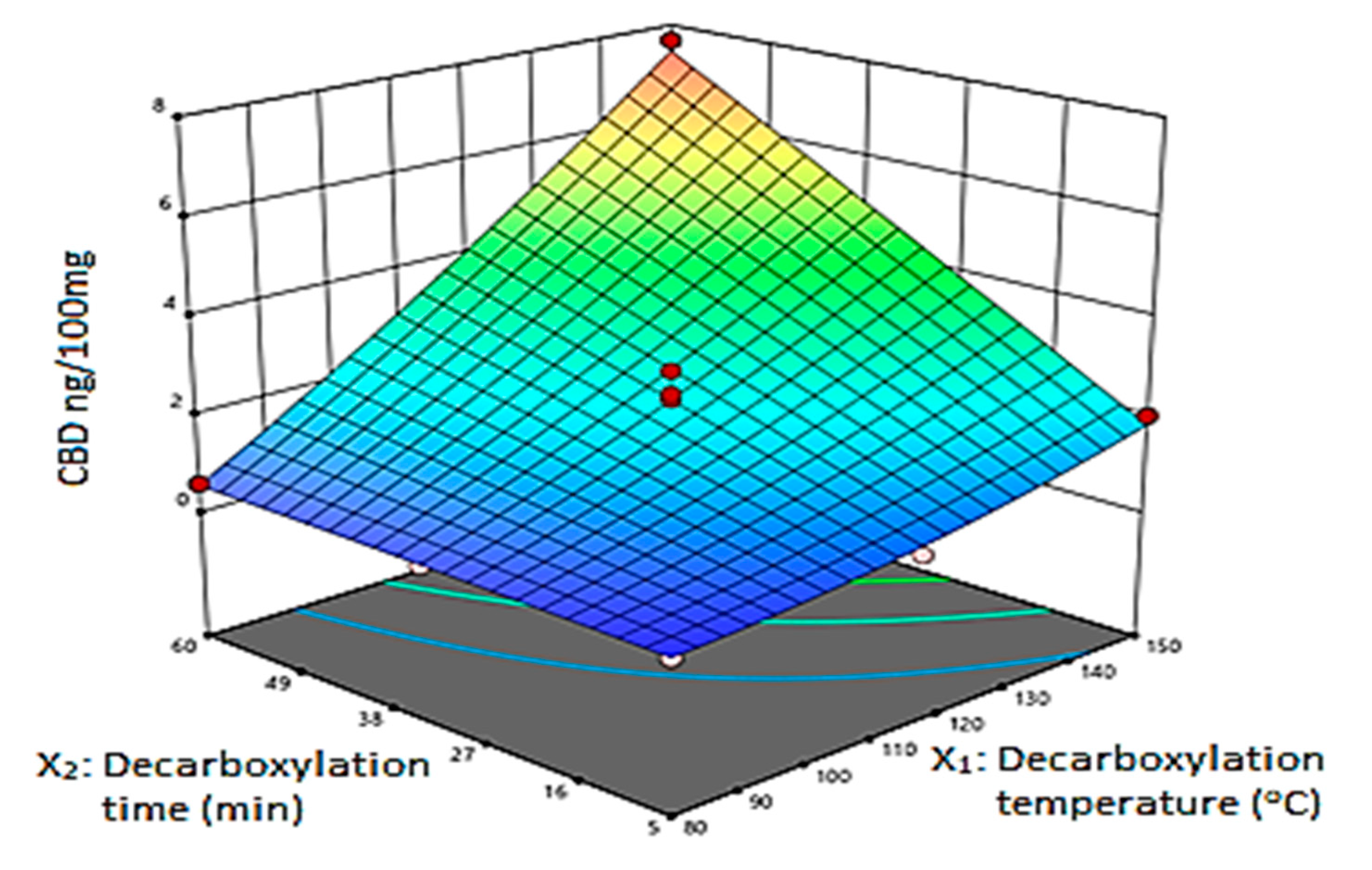
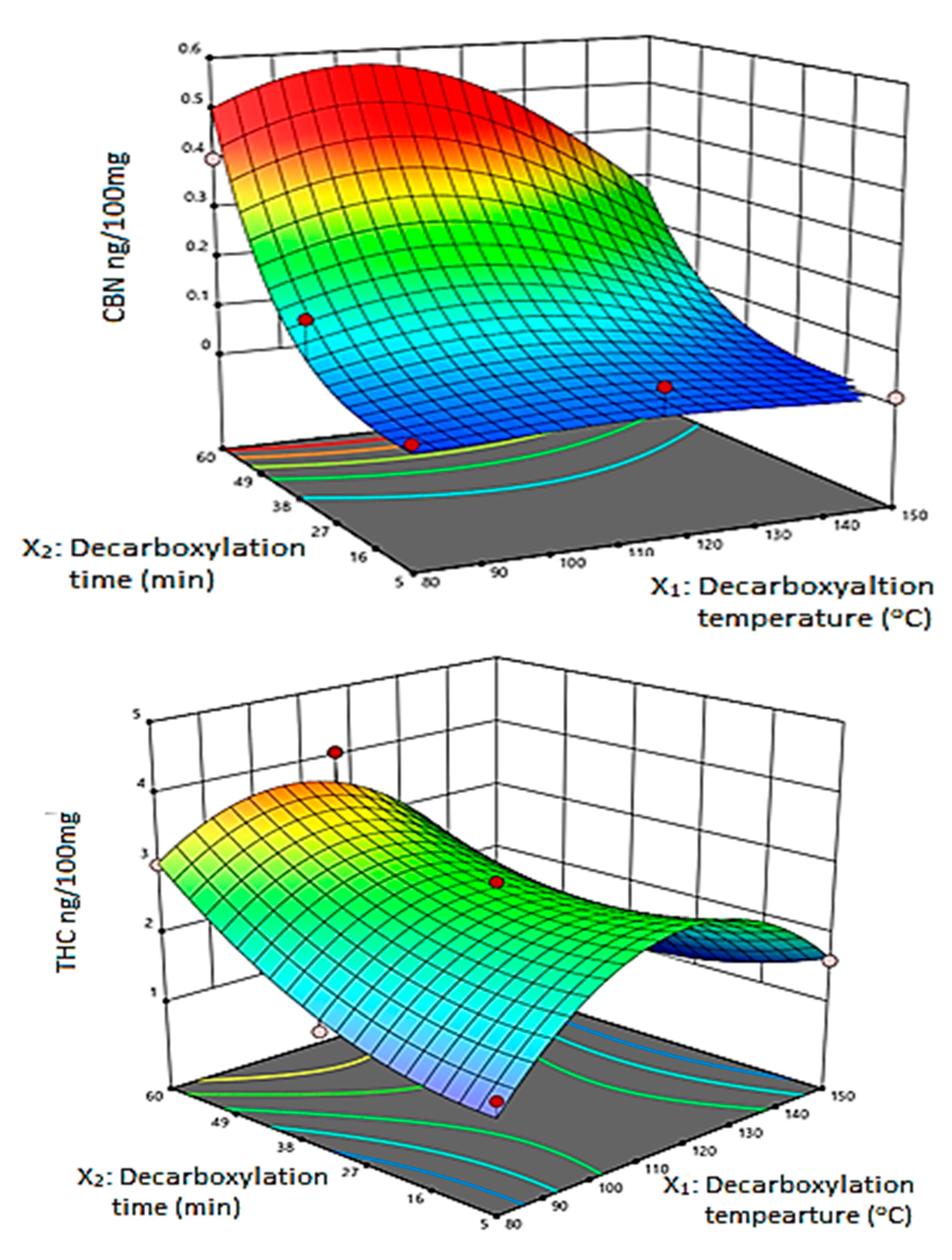
| Factors | THC | CBN | CBD | |||||
|---|---|---|---|---|---|---|---|---|
| Run Order | Decarboxylatio Temperature | Decarboxylation Time | Actual Value | Predicted Value | Actual Value | Predicted Value | Actual Value | Predicted Value |
| 8 | 80 | 5 | 1.239 | 1.036 | 0.032 | 0.03 | 0.395 | 0.404 |
| 5 | 115 | 5 | 2.529 | 2.547 | 0.084 | 0.086 | 0.696 | 0.703 |
| 9 | 150 | 5 | 1.597 | 1.469 | 0.013 | 0.011 | 2.012 | 2.031 |
| 3 | 80 | 32.5 | 1.347 | 1.368 | 0.161 | 0.159 | 0.486 | 0.479 |
| 12 | 115 | 32.5 | 2.749 | 2.628 | 0.052 | 0.047 | 2.458 | 2.427 |
| 6 | 150 | 32.5 | 1.409 | 1.357 | 0.002 | 0.002 | 4.246 | 4.219 |
| 4 | 80 | 60 | 2.997 | 3.061 | 0.399 | 0.385 | 0.589 | 0.601 |
| 11 | 115 | 60 | 4.055 | 3.987 | 0.442 | 0.431 | 3.499 | 3.468 |
| 10 | 150 | 60 | 1.066 | 0.958 | 0.231 | 0.228 | 7.672 | 7.672 |
| 7 | 115 | 32.5 | 2.749 | 2.538 | 0.092 | 0.089 | 2.052 | 2.098 |
| 1 | 115 | 32.5 | 2.549 | 2.348 | 0.039 | 0.027 | 2.355 | 2.401 |
| 2 | 115 | 32.5 | 2.349 | 2.245 | 0.042 | 0.037 | 2.939 | 3.056 |
| THC | CBN | CBD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Df | CE | F-Value | p-Value | CE | F-Value | p-Value | CE | F-Value | p-Value | |
| Model | 5 | 0.1824 | 12.15 | 0.0142 | 0.0524 | 549.89 | 0.0364 | 0.0524 | 67.87 | 0.0001 |
| a | 1 | 0.1520 | 3.57 | 0.1085 | 0.0047 | 143.32 | 0.057 | 0.0047 | 190.88 | 0.0001 |
| B | 1 | 0.1673 | 5.70 | 0.0819 | 0.0082 | 1389.17 | 0.0185 | 0.0082 | 92.14 | 0.0001 |
| aB | 1 | 0.2144 | 10.78 | 0.0168 | 0.0054 | 49.92 | 0.0948 | 0.0054 | 55.1 | 0.0003 |
| a2 | 1 | 0.2514 | 43 | 0.0010 | 0.0046 | 755.29 | 0.0264 | 0.0046 | 1.15 | 0.3239 |
| B2 | 1 | 0.2458 | 3.48 | 0.1253 | 0.0098 | 91.10 | 0.0706 | 0.0098 | 0.0136 | 0.9111 |
| PRESS | 2.56 | 2.12 | 3.12 | |||||||
| R2 | 0.9428 | 0.9993 | 0.9826 | |||||||
| Adj R2 | 0.8428 | 0.998 | 0.9522 | |||||||
| Pred | 0.9052 | 0.8956 | 0.9156 | |||||||
| Error | Equation |
|---|---|
| Absolute average deviation (ADD) | |
| Root mean square error (RSME) | |
| Mean absolute error (MAE) | |
| Standard error of predictions (SEP)% | |
| Model predictive error (MPE)% | |
| Chi-square () |
| Analyte | Quantification Range (ng mL−1) | Fitting Equation | Correlation Coefficient (R2) | LOD (ng mL−1) | LOQ (ng mL−1) |
|---|---|---|---|---|---|
| CBD | 0.5–1000 | 87.629x + 508.07 | 0.9971 | 0.199929 | 0.605847 |
| CBN | 0.5–1000 | 164.94x + 118.91 | 0.9999 | 0.031007 | 0.093959 |
| THC | 0.5–1000 | 93.594x + 416.33 | 0.9998 | 0.057157 | 0.173204 |
| Analyte | Concentration (ng mL−1) | Measured Concentration (ng mL−1) | Accuracy (%) | RSD (%) |
|---|---|---|---|---|
| 0.5 | 0.49 ± 0.02 | 98.00 | 5.40 | |
| CBD | 1 | 1.00 ± 0.03 | 99.67 | 5.71 |
| 10 | 9.50 ± 0.63 | 95.03 | 11.43 | |
| 50 | 49.69 ± 0.86 | 99.38 | 2.99 | |
| 100 | 100.55 ± 1.40 | 100.55 | 2.41 | |
| 500 | 496.55 ± 3.83 | 99.31 | 1.34 | |
| 0.5 | 0.46 ± 0.02 | 91.60 | 5.78 | |
| CBN | 1 | 0.84 ± 0.03 | 84.07 | 6.76 |
| 10 | 9.28 ± 0.39 | 92.80 | 7.28 | |
| 50 | 47.83 ± 0.86 | 95.66 | 3.11 | |
| 100 | 100.40 ± 3.70 | 100.40 | 6.38 | |
| 500 | 493.99 ± 4.90 | 98.80 | 1.72 | |
| 0.5 | 0.49 ± 0.01 | 97.33 | 5.17 | |
| THC | 1 | 1.02 ± 0.03 | 101.50 | 5.68 |
| 10 | 9.53 ± 0.20 | 95.30 | 3.68 | |
| 50 | 49.80 ± 0.72 | 99.61 | 2.50 | |
| 100 | 96.52 ± 2.06 | 96.52 | 3.69 | |
| 500 | 491.76 ± 6.38 | 98.35 | 2.25 |
| 0.5 ng/mL | 1 ng/mL | 10 ng/mL | 50 ng/mL | 100 ng/mL | 500 ng/mL | |
|---|---|---|---|---|---|---|
| CBD | 96.56 ± 10.60 | 97.54 ± 5.65 | 103.01 ± 20.22 | 99.33 ± 2.98 | 103.42 ± 6.22 | 100.25 ± 3.36 |
| CBN | 95.22 ± 12.16 | 96.52 ± 6.63 | 99.92 ± 6.50 | 99.70 ± 4.71 | 99.71 ± 1.66 | 99.95 ± 1.50 |
| THC | 99.62 ± 10.58 | 98.82 ± 0.94 | 98.21 ± 1.47 | 99.81 ± 2.52 | 99.53 ± 1.38 | 99.30 ± 2.48 |
| ADD | RMSE | MAE | SEP% | MPE% | ||
|---|---|---|---|---|---|---|
| THC | 5.724521 | 0.126433 | 0.10825 | 7.184042 | 5.724521 | 0.113183 |
| CBN | 7.342018 | 0.006739 | 0.005083 | 11.16061 | 8.991944 | 0.00804 |
| CBD | 1.555437 | 0.006739 | 0.029333 | 1.843651 | 1.530427 | 0.008005 |
| Response | Criterion | Predicted Value mg/100 g | Experimental Value mg/100 g | Error (%) |
|---|---|---|---|---|
| THC | Minimize | 1.047 | 1.030 ± 0.038 | 1.564 |
| CBN | Minimize | 0.051 | 0.493 ± 0.003 | 2.686 |
| CBD | Maximize | 5.717 | 5.780 ± 0.183 | 1.096 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuapia, Y.; Maraba, K.; Tutu, H.; Chimuka, L.; Cukrowska, E. In Situ Decarboxylation-Pressurized Hot Water Extraction for Selective Extraction of Cannabinoids from Cannabis sativa. Chemometric Approach. Molecules 2021, 26, 3343. https://doi.org/10.3390/molecules26113343
Nuapia Y, Maraba K, Tutu H, Chimuka L, Cukrowska E. In Situ Decarboxylation-Pressurized Hot Water Extraction for Selective Extraction of Cannabinoids from Cannabis sativa. Chemometric Approach. Molecules. 2021; 26(11):3343. https://doi.org/10.3390/molecules26113343
Chicago/Turabian StyleNuapia, Yannick, Kgomotso Maraba, Hlanganani Tutu, Luke Chimuka, and Ewa Cukrowska. 2021. "In Situ Decarboxylation-Pressurized Hot Water Extraction for Selective Extraction of Cannabinoids from Cannabis sativa. Chemometric Approach" Molecules 26, no. 11: 3343. https://doi.org/10.3390/molecules26113343
APA StyleNuapia, Y., Maraba, K., Tutu, H., Chimuka, L., & Cukrowska, E. (2021). In Situ Decarboxylation-Pressurized Hot Water Extraction for Selective Extraction of Cannabinoids from Cannabis sativa. Chemometric Approach. Molecules, 26(11), 3343. https://doi.org/10.3390/molecules26113343






