Abstract
In order to efficiently remove phosphorus, thermodynamic equilibrium diagrams of the P-H2O system and P-M-H2O system (M stands for Fe, Al, Ca, Mg) were analyzed by software from Visual MINTEQ to identify the existence of phosphorus ions and metal ions as pH ranged from 1 to 14. The results showed that the phosphorus ions existed in the form of H3PO4, H2PO4−, HPO42−, and PO43−. Among them, H2PO4− and HPO42− were the main species in the acidic medium (99% at pH = 5) and alkaline medium (97.9% at pH = 10). In the P-Fe-H2O system ((P) = 0.01 mol/L, (Fe3+) = 0.01 mol/L), H2PO4− was transformed to FeHPO4+ at pH = 0–7 due to the existence of Fe3+ and then transformed to HPO42− at pH > 6 as the Fe3+ was mostly precipitated. In the P-Ca-H2O system ((P) = 0.01 mol/L, (Ca2+) = 0.015 mol/L), the main species in the acidic medium was CaH2PO4+ and HPO42−, and then transformed to CaPO4− at pH > 7. In the P-Mg-H2O system ((P) = 0.01 mol/L, (Mg2+) = 0.015 mol/L), the main species in the acidic medium was H2PO4− and then transformed to MgHPO4 at pH = 5–10, and finally transformed to MgPO4− as pH increased. The verification experiments (precipitation experiments) with single metal ions confirmed that the theoretical analysis could be used to guide the actual experiments.
1. Introduction
Phosphorus plays an important role in living organisms as it is one of the main components of cell structure [1]. Usually, phosphorus exists in three species (orthophosphate, polyphosphate, and organic phosphorus) in solution. Furthermore, the primary phosphorus compounds are generally orthophosphates [2]. The overuse and inefficient use of phosphorus lead to the eutrophication problem in natural water, which is a serious global problem that needs to be treated [3,4].
Phosphorus mitigation is expensive and difficult in the waste stream. Many methods have been developed for phosphorus removal. The conventional biological methods have difficulty in removing phosphorus due to the inherent limitations of the activated sludge method. The removal efficiency of phosphorus is less than 30%, and the residual phosphorus concentration is still over the wastewater discharge guideline [5]. As a result of catastrophic environmental implications, including eutrophication and red tide, the governments have established some biological wastewater treatment systems to treat the wastewater containing phosphorus and limited the emission standard for residual phosphorus concentration (0.5–1 mg TP/L in the USA, <0.2 mg TP/L in South Korea, and 1–2 mg TP/L in France) [6]. The conventional biological treatment processes can only achieve low removal efficiency of phosphorus due to large volume requirements and long hydraulic retention time. Thus, the development of advanced technology to improve phosphorus removal efficiency is needed, including easy installation, short intention time, little space, low operation cost, and low capital investment [2,7,8,9,10]. Enhanced biological phosphorus removal processes have attracted much more attention [11,12,13,14,15]. The key factor in the enhanced biological phosphorus removal process is the use of phosphorus accumulating organisms. Under anaerobic conditions, the stored poly-phosphate (poly-P) is hydrolyzed to supply energy for the polyhydroxyalkanoates uptake. During the subsequent aerobic process, phosphorus accumulating organisms absorb the excessive amounts of phosphorus for poly-P. Finally, the phosphorus is removed when the waste-activated sludge is separated from the treated wastewater at the end of the aerobic stage. In the whole process, stable phosphorus removal efficiency is hard to maintain. Thus, some physicochemical treatments have come to the forefront [16,17,18]. Among them, chemical precipitation methods are widely used for phosphorus removal with metal ion salts. Calcium salts, such as CaCl2, Ca (NO3)2, are often added for the removal of phosphorus as calcium and phosphorus have a strong affinity and could form insoluble Ca3(PO4)2 [19]. The concentration of phosphorus can be reduced from 15.1 ppmw to 0.2 ppmw with the addition of magnesium in acidic conditions [20]. Commonly, magnesium is used with ammonium for phosphorus removal called the magnesium ammonium phosphate method [21,22,23,24], but it is limited by co-precipitation, low separation efficiency caused by the separation equipment, serious ammonia wastewater, and ammonia gas pollution [25]. Ferric and aluminum are also used for phosphorus removal as they can generate co-precipitates with phosphate, and the hydroxyl complexes formed by hydrolyzing of Fe3+ and Al3+ show great adsorption performance for orthophosphate [26,27,28].
From the above analysis, the existing form of phosphorus and the metal ion in the solution has a great influence on phosphorus removal efficiency. Thus, the present work focuses on the existence of phosphorus ions and metal ions in solution at different pH ranges by employing the Visual MINTEQ software. This work aims to provide a theoretical basis for efficient phosphorus removal from wastewater.
2. Results
2.1. Thermodynamic Analysis of the P-H2O System
In the P-H2O system, the phosphorus existed in the form of HPO42−, H2PO4−, H3PO4, and PO43−. These four species were interchangeable with changed pH according to Equations (1)–(3).
Based on the above equations, thermodynamic studies of the P-H2O system were performed to determine the chemical state of ions in the wastewater. A mole fraction of different phosphorus species in the P-H2O system was plotted as the pH ranged from 1 to 14 and (P) = 0.01 mol/L to 0.09 mol/L. The results are shown in Figure 1.
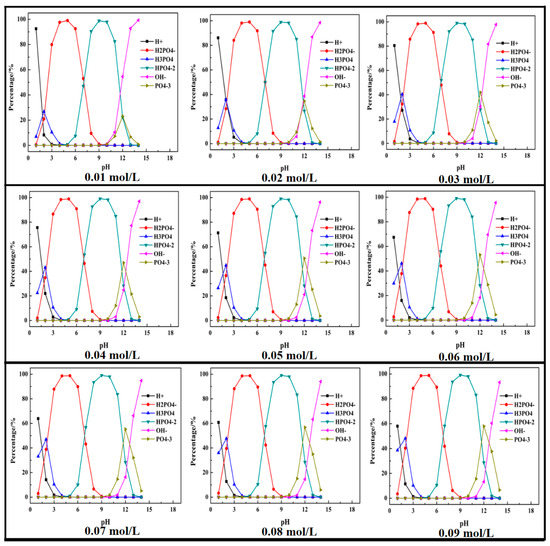
Figure 1.
Mole fraction diagram of phosphorus species in the P-H2O system at 298 K at various pH ((P) = 0.01 to 0.90 mol/L).
It can be seen that the phosphorus mainly existed in the form of H3PO4, H2PO4−, HPO42−, and PO43−. With the increase in pH value, the phosphorus transformed to H2PO4−, HPO42− and PO43−. The maximum percentage of H3PO4, H2PO4−, HPO42−, PO43− was, respectively, 26.8% (at pH = 2), 99% (at pH = 5), 97.9% (when pH = 10), and 23.1% (at pH = 12). With the increase in phosphorus concentration, the percentage of H2PO4− and HPO42− had no obvious change, while the maximum percentage of H3PO4 and PO43− increased with the increase in phosphorus concentration, but the pH was kept constant.
2.2. Thermodynamic Analysis of M-P-H2O System
2.2.1. Fe (Ca, Mg)-P-H2O System
In this section, a single metal salt was used to remove phosphorus from the wastewater, and Fe3+, Ca2+, and Mg2+ were selected. The composition in the phosphorus solution with single metal salt was simulated by Visual MINTEQ software. The results are shown in Figure 2, Figure 3 and Figure 4.
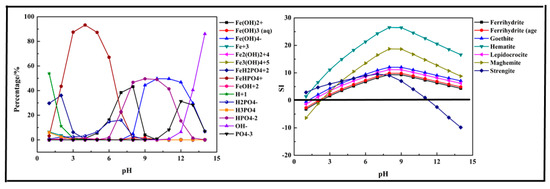
Figure 2.
Percentage of species in the Fe-P-H2O system at 298 K at ranged pH ((P) = 0.01 mol/L, (Fe3+) = 0.01 mol/L).
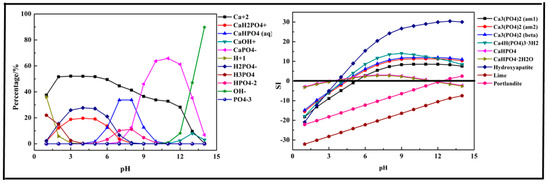
Figure 3.
Percentage of species in the Ca-P-H2O system at 298 K at ranged pH ((P) = 0.01 mol/L, (Ca2+) = 0.015 mol/L).
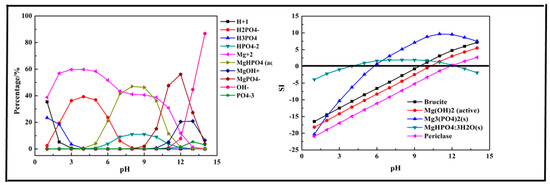
Figure 4.
Percentage of species in the Mg-P-H2O system at 298 K at ranged pH ((P) = 0.01 mol/L, (Mg2+) = 0.015 mol/L).
The results shown in Figure 2 indicated that the main species in the Fe-P-H2O system were Fe (OH)2+, Fe (OH)3 (aq), Fe (OH)4−, Fe3+, Fe2 (OH)24+, Fe3 (OH)45+, FeH2PO42+, FeHPO4+, FeOH2+, H+, H2PO4−, H3PO4, HPO42−, OH−, and PO43−. Due to the existence of Fe3+, phosphorus first transformed into FeH2PO42+ at pH < 2 and then transformed into FeHPO4+ at pH = 2–7. In an alkaline medium (pH > 7), the phosphorus and Fe3+ mainly existed in the form of HPO42− and Fe (OH)4−. The results shown in Figure 2 also display the predicted precipitation products. The main products of the reaction were Ferrihydrite (Fe5HO8·4H2O), Goethite (HFeO2), Hematite (Fe2O3), Lepidocrocite (FeO (OH)), Maghemite (γ-Fe2O3), and Strengite (FePO4·2H2O). Almost all the precipitation products were hydrolyzed Fe3+, but only Strengite is our aim. The suitable pH for precipitation of phosphorus with Fe3+ was below 11.
The results displayed in Figure 3 show that the main species in the Ca-P-H2O system were quite different from the Fe-P system. In the acidic medium (pH < 7), the phosphorus existed in the form of HPO42−, H2PO4−. As pH value increased, it transformed into CaHPO4 (aq) and CaH2PO4+. When pH>7, it mainly existed in the form of CaPO4−. During the phosphorus removal process, there was no precipitation formed at pH < 4 because the SI of all predicted products was below 0. The first precipitation product, also the main product, was Hydroxyapatite (Ca5(PO4)3(OH)). As pH value increased, some other products might be generated, such as Ca3(PO4)2, Ca4H(PO4)3·3H2O, CaHPO4, and CaHPO4·2H2O. But CaHPO4 and CaHPO4·2H2O dissolved again at pH > 11. Above all, Ca2+ salts were used efficiently for phosphorus removal.
The results shown in Figure 4 indicate that the main species in the Mg-P-H2O system were H2PO4−, H3PO4, HPO42−, Mg2+, MgHPO4 (aq), MgOH+, MgPO4−, OH−, and PO43−. Phosphorus existing as H3PO4 first transformed into H2PO4− at pH < 7 and then transformed into MgHPO4 (aq) and HPO42− at pH = 4–12, owing to the existence of Mg2+. As pH value increased to 9, MgPO4− was the main form of phosphorus and up to 56.1% at pH = 12. The main products of the reaction were predicted as brucite (Mg(OH)2), Mg(OH)2, Mg3(PO4)2, MgHPO4·3H2O, and Periclase (MgO). The removal of phosphorus with Mg2+ should be conducted at pH > 6 and especially at pH > 10 as there was no precipitation generated at pH < 5 and only Mg3(PO4)2, MgHPO4·3H2O generated after pH = 5. After pH = 11, almost all the predicted products generated except MgHPO4·3H2O, which dissolved again. The optimal pH ranged from 7 to 14.
In conclusion, the removal of phosphorus could achieve high efficiency in an alkaline medium. Using Fe3+ salts and Ca2+ salts more easily generated phosphorus precipitation and were more efficient in removing phosphorus.
2.2.2. Two Salts System
The results shown in Figure 5 indicate that the main species in the Fe-Ca-P-H2O system could be divided into three sections. At pH =1–6, CaH2PO4+, H2PO4−, FeHPO4+, and Ca2+ were the main species in the solution. As pH value increased, the above ions transformed into CaHPO4 (aq), HPO42−, and Fe(OH)2+ at pH = 6–9. At pH > 9, the main species ions were CaPO4−, Fe (OH)4−, HPO42−, OH−, and PO43−. Strengite was the first predicted precipitation product generated in the precipitation process, and it dissolved again after pH > 11. Other precipitation products were generated after pH > 3, and the main phosphorus precipitation products were Hydroxyapatite, three kinds of Ca3(PO4)2 and Ca4H(PO4)3·3H2O. CaHPO4, CaHPO4 generated at pH = 5–10 and then dissolved after pH > 11.
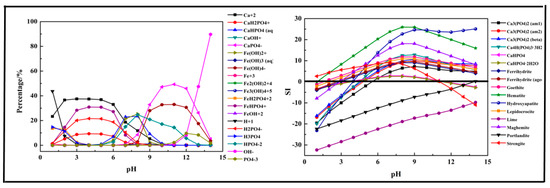
Figure 5.
Percentage of species in the Fe-Ca-P-H2O system at 298 K at ranged pH ((P) = 0.01 mol/L, (Fe3+) = 0.005 mol/L, (Ca2+) = 0.0075 mol/L).
The results shown in Figure 6 indicate that the main species in Ca-Mg-P-H2O system could be divided into three sections. At pH =1–6, H2PO4−, Ca2+, CaH2PO4+, and Mg2+ were the main species in the solution. As pH value increased, above ions transformed into CaHPO4 (aq), MgHPO4 (aq), HPO42− at pH = 6–9. At pH > 9, the main species ions were CaPO4−, MgOH+, and MgPO4−, Fe(OH)4−, OH−, and PO43−. Hydroxyapatite (Ca5(PO4)3(OH)) was first precipitated at pH > 4, then Ca4H(PO4)3·3H2O, Ca3(PO4)2 followed. The precipitation containing Mg, such as Mg3(PO4)2, was formed after pH > 7. For the Ca-Mg-P system, the suitable pH for phosphorus removal was after 5.
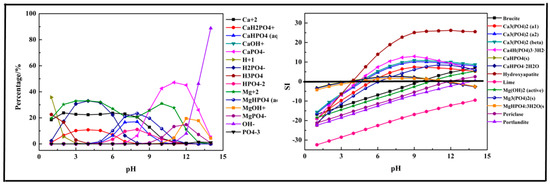
Figure 6.
Percentage of species in the Ca-Mg-P-H2O system at 298 K at ranged pH ((P) = 0.01 mol/L, (Ca2+) = 0.0075 mol/L, (Mg2+) = 0.0075 mol/L).
The results shown in Figure 7 indicate that the main species in the Fe-Mg-P-H2O system could be divided into three sections. At pH = 1–6, H2PO4−, FeHPO4+, and Mg2+ were the main species in the solution. As pH value increased, the above ions transformed into MgHPO4 (aq) (pH = 6–11), HPO42− (pH = 6–13), Fe(OH)4− (pH > 8), and MgPO4− (pH > 9). Strengite was the first predicted precipitation product generated in the precipitation process, and it dissolved again after pH > 11. Mg3(PO4)2, MgHPO4·3H2O generated after pH = 6. MgHPO4·3H2O was formed at pH = 5 and then dissolved after pH > 12.
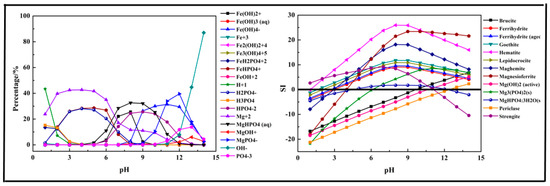
Figure 7.
Percentage of species in the Fe-Mg-P-H2O system at 298 K at ranged pH ((P) = 0.01 mol/L, (Fe3+) = 0.003 mol/L, (Mg2+) = 0.0075 mol/L).
2.2.3. Fe-Ca-Mg-P-H2O System
The results shown in Figure 8 indicate that the main species in the Fe-Ca-Mg-P system could be divided into three sections. At pH = 1–6, CaH2PO4+, H2PO4−, Mg2+, FeHPO4+, and Ca2+ were the main species in the solution. As pH value increased, above ions transformed into MgHPO4 (aq) (pH = 5–13), CaHPO4 (aq) (pH = 5–10), HPO42− (5–13), CaPO4− (pH = 8–14), Fe(OH)2+ (pH = 5–12), and MgPO4− (pH = 9–14). The first precipitation containing phosphorus was Strengite after pH > 2, while it occurred at pH = 0 in a single-salt system and two-salts system. Then other precipitations formed continually. The composition in the three salts system was more complex, and the precipitation was no more stable than the single salt system.
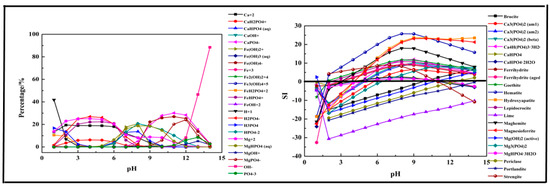
Figure 8.
Percentage of species in the Fe-Ca-Mg-P-H2O system at 298 K at ranged pH ((P) = 0.01 mol/L, (Fe3+) = 0.004 mol/L, (Ca2+) = 0.0045 mol/L, (Mg2+) = 0.0045 mol/L).
2.3. Phosphorous Removal Experiments
The phosphorous removal experiments were conducted with Ca2+, Al3+, and Fe3+. The results are shown in Figure 9. The phosphorus ion was precipitated under the selected reaction conditions. The dosage of metal ions had a significant effect on the removal efficiency of phosphorous. For Ca2+ salts, the high removal efficiency was 81.25% and achieved at n(Ca2+)/n(P) = 0.6. A further increase in Ca had no obvious effect on the removal efficiency. While for Al3+ salts, the removal efficiency was a step increase, and the highest was 80.13%. Great removal performance was achieved by Fe3+ at n(Fe)/n(P) = 1 with a removal efficiency of 91.54%. These actual experimental results are consistent with the analysis above, indicating that the theoretical analysis could be used to guide the actual experiments.
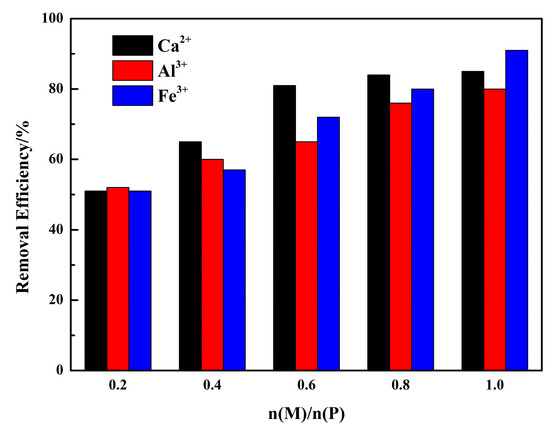
Figure 9.
Phosphorous removal experiments ((P) = 0.01 mol/L, n(Ca)/n(P) = 0.2–1, n(Al)/n(P) = 0.2–1, n(Fe)/n(P) = 0.2–1).
3. Materials and Methods
The thermodynamic equilibrium diagrams of P-H2O system and P-M-H2O system (M stands for Fe, Al, Ca, Mg) were simulated by the VISUAL MINTEQ software. The concentration of P and M are detailed in Table 1. For the P-H2O system, the concentration of phosphorus ranged from 0.01 mol/L to 0.09 mol/L. In the Fe-P-H2O system, the concentration was (P) = 0.01 mol/L, (Fe3+) = 0.01 mol/L. For theCa-P-H2O system, the concentration was (P) = 0.01 mol/L, (Ca2+) = 0.015 mol/L. For the Mg-P-H2O system, the concentration was (P) = 0.01 mol/L, (Mg2+) = 0.015 mol/L. For the Fe-Mg-P-H2O system, the concentration was (P) = 0.01 mol/L, (Fe3+) = 0.005 mol/L, (Mg2+) = 0.0075 mol/L. For the Ca-Mg-P-H2O system, the concentration was (P) = 0.01 mol/L, (Ca2+) = 0.0075 mol/L, (Mg2+) = 0.0075 mol/L. For the Fe-Ca-P-H2O system, the concentration was (P) = 0.01 mol/L, (Fe3+) = 0.003 mol/L, (Ca2+) = 0.0075 mol/L. For Fe-Ca-Mg-P-H2O system, the concentration was (P) = 0.01 mol/L, (Fe3+) = 0.004 mol/L, (Ca2+) = 0.0045 mol/L, (Mg2+) = 0.0045 mol/L. The activity coefficients of the charged materials were calculated with the Davies equation. Meanwhile, the saturation index (SI) was used to predict the trend of precipitated and dissolved species and was calculated based on Equation (1). If SI > 0, the content was in oversaturation and might precipitate; SI = 0, the content was in an equilibrium state; SI < 0, the content existed in the form of ions in the solution.
where IAP is the selected ion activity in the Visual MINTEQ software, and Ks is the solubility product constant.

Table 1.
The concentration for simulating process.
4. Conclusions
Based on the results obtained in this study, the following conclusions can be obtained:
- (1)
- The phosphorus ions existed in the form of H3PO4, H2PO4−, HPO42−, and PO43−. Among them, H2PO4− and HPO42− were the main species in the acidic medium (99% at pH = 5) and alkaline medium (97.9% at pH = 10). In the P-Fe-H2O System ((P) = 0.01 mol/L, (Fe3+) = 0.01 mol/L), H2PO4− was transformed to FeHPO4+ at pH = 0–7 due to the existence of Fe3+ and then transformed into HPO42− at pH > 6 as the Fe3+ was mostly precipitated. In the P-Ca-H2O System ((P) = 0.01 mol/L, (Ca2+) = 0.015 mol/L), the main species in the acidic medium were CaH2PO4+ and HPO42−, and then transformed into CaPO4− at pH > 7. In the P-Mg-H2O System ((P) = 0.01 mol/L, (Mg3+) = 0.015 mol/L), the main species in the acidic medium was H2PO4− and then transformed into MgHPO4 at pH = 5–10, and finally transformed into MgPO4− as pH increased.
- (2)
- The phosphorus was more easily precipitated in the P-Fe-H2O system than the P-Ca-H2O system and P-Mg-H2O system. The suitable pH of the solution for phosphorus precipitation was about 5–10 in all precipitation systems.
- (3)
- The verification experiments (precipitation experiments) with single metal ions confirm that the theoretical analysis can be used to guide the actual experiments.
Author Contributions
Conceptualization, H.P.; methodology, H.P.; software, H.P.; validation, H.P.; formal analysis, H.P.; investigation, H.Q., C.W. and J.G.; resources, H.P.; data curation, Z.H., C.Z., Y.G., Y.R. and J.G.; writing—original draft preparation, H.P.; writing—review and editing, H.P.; visualization, H.P.; supervision, H.P.; project administration, H.P.; funding acquisition, H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Research Program of Chongqing Municipal Education Commission (No. KJQN201901403 and No. CXQT20026) and Chongqing Science and Technology Commission (No. cstc2018jcyjAX0018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Okano, K.; Yamamoto, Y.; Takano, H.; Aketo, T.; Honda, K.; Ohtake, H. A simple technology for phosphorus recovery using acid-treated concrete sludge. Sep. Purif. Technol. 2016, 165, 173–178. [Google Scholar] [CrossRef]
- Tran, N.; Drogui, P.; Blais, J.-F.; Mercier, G. Phosphorus removal from spiked municipal wastewater using either electrochemical coagulation or chemical coagulation as tertiary treatment. Sep. Purif. Technol. 2012, 95, 16–25. [Google Scholar] [CrossRef]
- Okano, K.; Uemoto, M.; Kagami, J.; Miura, K.; Aketo, T.; Toda, M.; Honda, K.; Ohtake, H. Novel technique for phosphorus recovery from aqueous solutions using amorphous calcium silicate hydrates (A-CSHs). Water Res. 2013, 47, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Weidler, P.G.; Berg, U.; Nüesch, R.; Donnert, D. Calcite-seeded crystallization of calcium phosphate for phosphorus recovery. Chemosphere 2006, 63, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Hosni, K.; Ben Moussa, S.; Ben Amor, M. Conditions influencing the removal of phosphate from synthetic wastewater: Influence of the ionic composition. Desalination 2007, 206, 279–285. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Ngo, H.H.; Guo, W.; Nguyen, T.T.; Chang, S.W.; Jang, A.; Yoon, Y.S. Can electrocoagulation process be an appropriate technology for phosphorus removal from municipal wastewater? Sci. Total Environ. 2016, 563–564, 549–556. [Google Scholar] [CrossRef]
- Asselin, M.; Drogui, P.; Benmoussa, H.; Blais, J.-F. Effectiveness of electrocoagulation process in removing organic compounds from slaughterhouse wastewater using monopolar and bipolar electrolytic cells. Chemosphere 2008, 72, 1727–1733. [Google Scholar] [CrossRef]
- Gatsios, E.; Hahladakis, J.N.; Gidarakos, E. Optimization of electrocoagulation (EC) process for the purification of a real industrial wastewater from toxic metals. J. Environ. Manag. 2015, 154, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.A.; Hassine, R.B.; Jellali, S. Removal of phosphorus from aqueous solution by Posidonia oceanica fibers using continuous stirring tank reactor. J. Hazard. Mater. 2011, 189, 577–585. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Ngo, H.H.; Yoon, Y.S. A new hybrid treatment system of bioreactors and electrocoagulation for superior removal of organic and nutrient pollutants from municipal wastewater. Bioresour. Technol. 2014, 153, 116–125. [Google Scholar] [CrossRef]
- Mandel, A.; Zekker, I.; Jaagura, M.; Tenno, T. Enhancement of anoxic phosphorus uptake of denitrifying phosphorus removal process by biomass adaption. Int. J. Environ. Sci. Technol. 2019, 16, 5965–5978. [Google Scholar] [CrossRef]
- Torresi, E.; Tang, K.; Deng, J.; Sund, C.; Smets, B.F.; Christensson, M.; Andersen, H.R. Removal of micropollutants during biological phosphorus removal: Impact of redox conditions in MBBR. Sci. Total Environ. 2019, 663, 496–506. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, Y.; Huang, W.; Wang, J.; Chen, L.; Zhou, J.; He, Q. Single-stage denitrifying phosphorus removal biofilter utilizing intracellular carbon source for advanced nutrient removal and phosphorus recovery. Bioresour. Technol. 2019, 277, 27–36. [Google Scholar] [CrossRef]
- Ge, Y.; Lan, J.; Zhan, C.; Zhou, Y.; Ma, C.; Zhao, L. Biological removal of phosphorus and diversity analysis of microbial community in the enhanced biological phosphorus removal (EBPR) system. Water Environ. J. 2019, 34, 563–574. [Google Scholar] [CrossRef]
- Zou, H.; Wang, Y. Phosphorus removal and recovery from domestic wastewater in a novel process of enhanced biological phosphorus removal coupled with crystallization. Bioresour. Technol. 2016, 211, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Shober, A.L.; Scheckel, K.G.; Penn, C.J.; Turner, K.C. Mechanisms of Phosphorus Removal by Phosphorus Sorbing Materials. J. Environ. Qual. 2018, 47, 1232–1241. [Google Scholar] [CrossRef]
- Shi, J.; Yin, D.; Xu, Z.; Song, D.; Cao, F. Fosfomycin removal and phosphorus recovery in a schorl/H2O2 system. RSC Adv. 2016, 6, 68185–68192. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, S.; Shao, D.; Jin, R.; Zhou, J. Simultaneous nitrogen and phosphorus removal by combined anammox and denitrifying phosphorus removal process. J. Chem. Technol. Biotechnol. 2018, 93, 94–104. [Google Scholar] [CrossRef]
- Shimpo, T.; Yoshikawa, T.; Morita, K. Thermodynamic study of the effect of calcium on removal of phosphorus from silicon by acid leaching treatment. Metall. Mater. Trans. B 2004, 35, 277–284. [Google Scholar] [CrossRef]
- Zhu, M.; Azarov, A.; Monakhov, E.; Tang, K.; Safarian, J. Phosphorus separation from metallurgical-grade silicon by magnesium alloying and acid leaching. Sep. Purif. Technol. 2020, 240, 116614. [Google Scholar] [CrossRef]
- Shu, J.; Wu, H.; Chen, M.; Peng, H.; Li, B.; Liu, R.; Liu, Z.; Wang, B.; Huang, T.; Hu, Z. Fractional removal of manganese and ammonia nitrogen from electrolytic metal manganese residue leachate using carbonate and struvite precipitation. Water Res. 2019, 153, 229–238. [Google Scholar] [CrossRef]
- Shu, J.; Liu, R.; Liu, Z.; Chen, H.; Tao, C. Simultaneous removal of ammonia and manganese from electrolytic metal manganese residue leachate using phosphate salt. J. Clean. Prod. 2016, 135, 468–475. [Google Scholar] [CrossRef]
- Wang, R.; Shu, J.; Chen, M.; Wang, R.; He, D.; Wang, J.; Tang, C.; Han, Y.; Luo, Z. An innovative method for fractionally removing high concentrations of Ni2+, PO43−, TP, COD, and NH4+-N from printed-circuit-board nickel plating wastewater. Sep. Purif. Technol. 2021, 260, 118241. [Google Scholar] [CrossRef]
- Shu, J.; Chen, M.; Wu, H.; Li, B.; Wang, B.; Li, B.; Liu, R.; Liu, Z. An innovative method for synergistic stabilization/solidification of Mn2+, NH4+-N, PO43− and F− in electrolytic manganese residue and phosphogypsum. J. Hazard. Mater. 2019, 376, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, Y.; Huang, D.; Xiong, T.; Li, M.; Balogun, M.S.; Tong, Y. Efficient hydrogen and oxygen evolution electrocatalysis by cobalt and phosphorus dual-doped vanadium nitride nanowires. Mater. Today Chem. 2019, 11, 1–7. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, Y.; Bao, S.; Huang, J. Precipitation of vanadium using ammonium salt in alkaline and acidic media and the effect of sodium and phosphorus. Hydrometallurgy 2018, 180, 113–120. [Google Scholar] [CrossRef]
- Yang, L.; Liang, X.; Han, Y.; Cai, Y.; Zhao, H.; Sheng, M.; Cao, G. The coupling use of advanced oxidation processes and sequencing batch reactor to reduce nitrification inhibition of industry wastewater: Characterization and optimization. Chem. Eng. J. 2019, 360, 1577–1586. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Yu, P.; Pan, S.; Xu, Y.; Sun, Y.; Pan, S.-Y.; Yu, Y.; Zheng, H. Enhanced removal of tris(2-chloroethyl) phosphate using a resin-based nanocomposite hydrated iron oxide through a Fenton-like process: Capacity evaluation and pathways. Water Res. 2020, 175, 115655. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).