Fabrication of Highly Textured 2D SnSe Layers with Tunable Electronic Properties for Hydrogen Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Materials
2.2. Computation Details
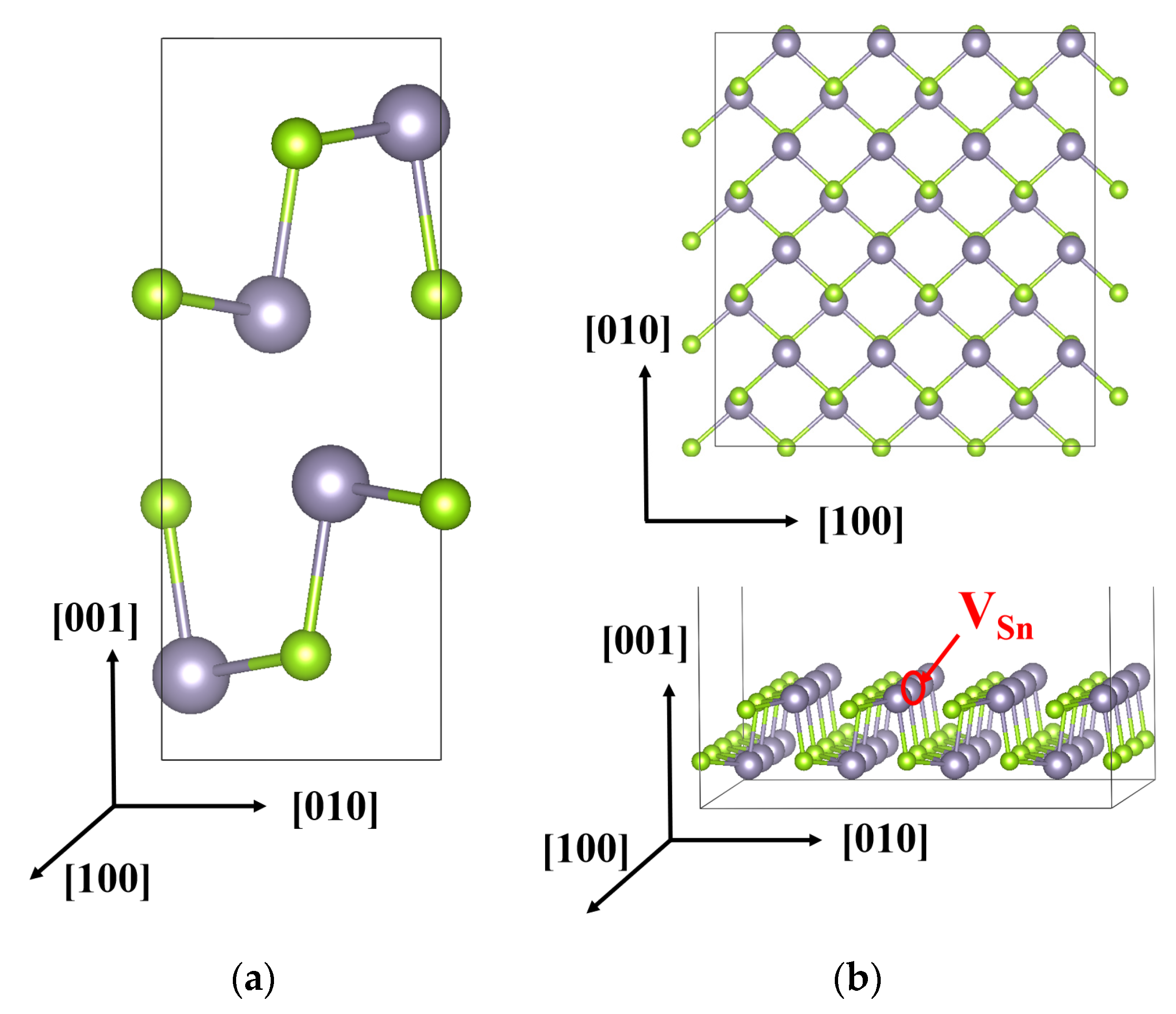
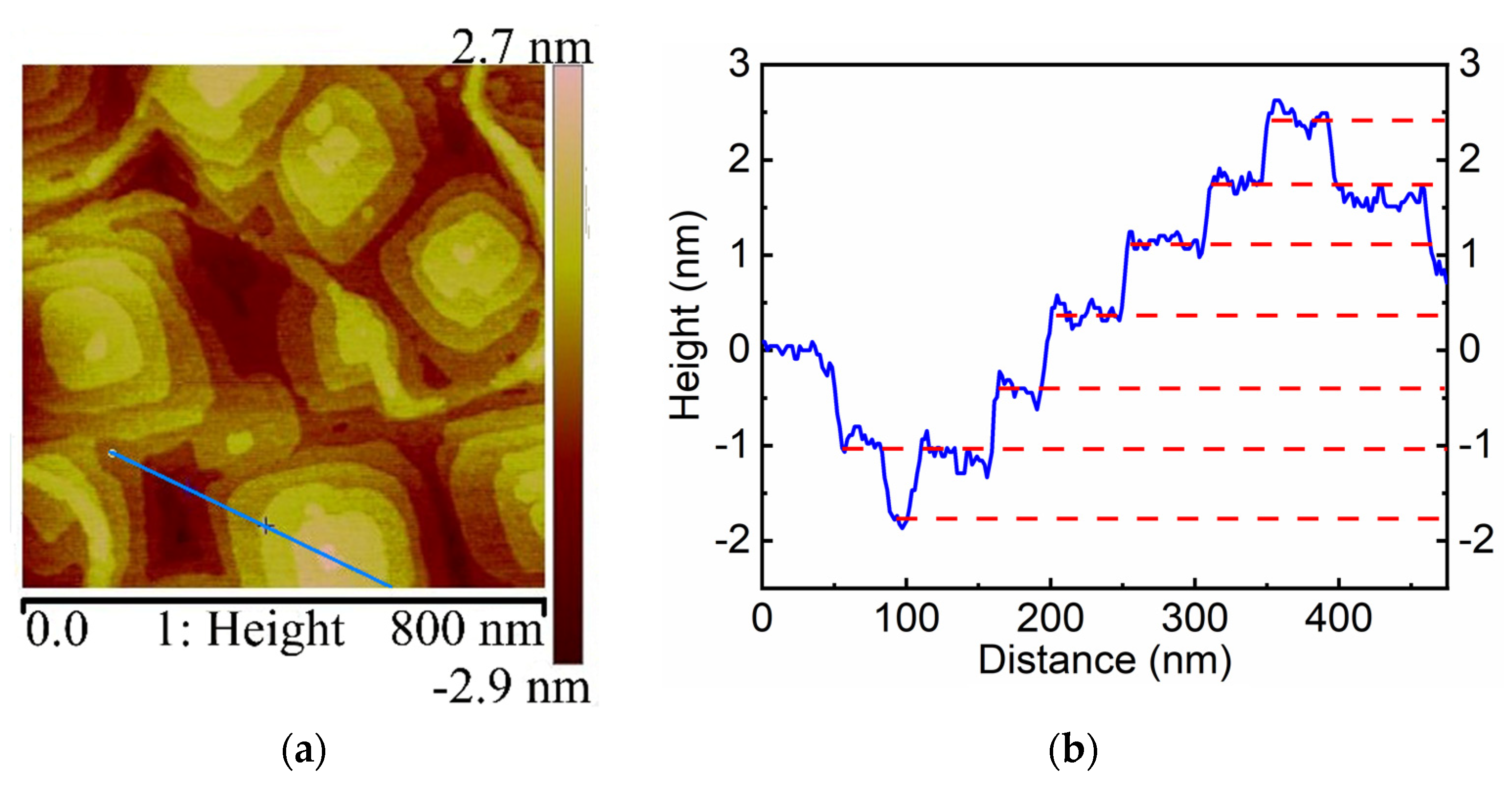
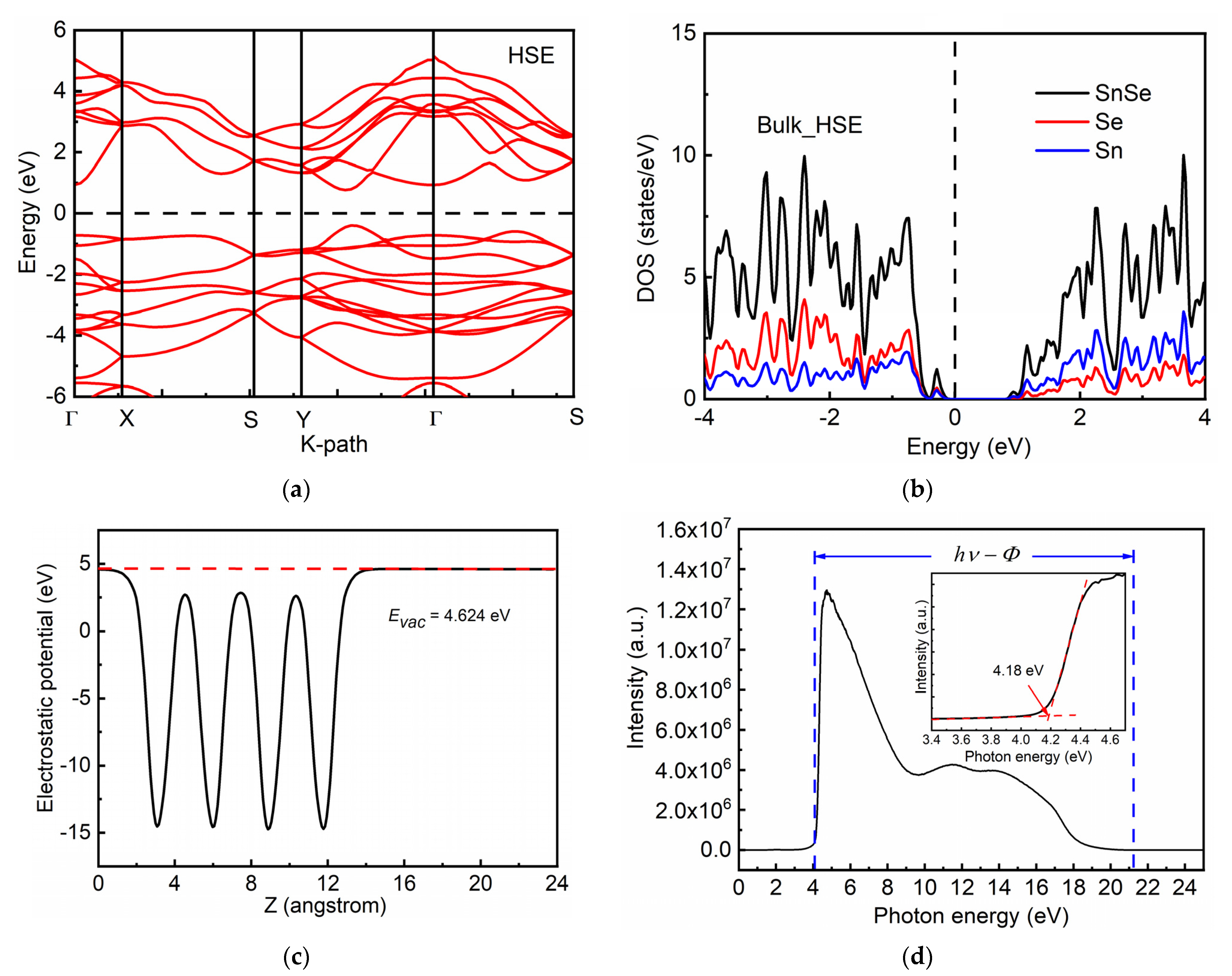
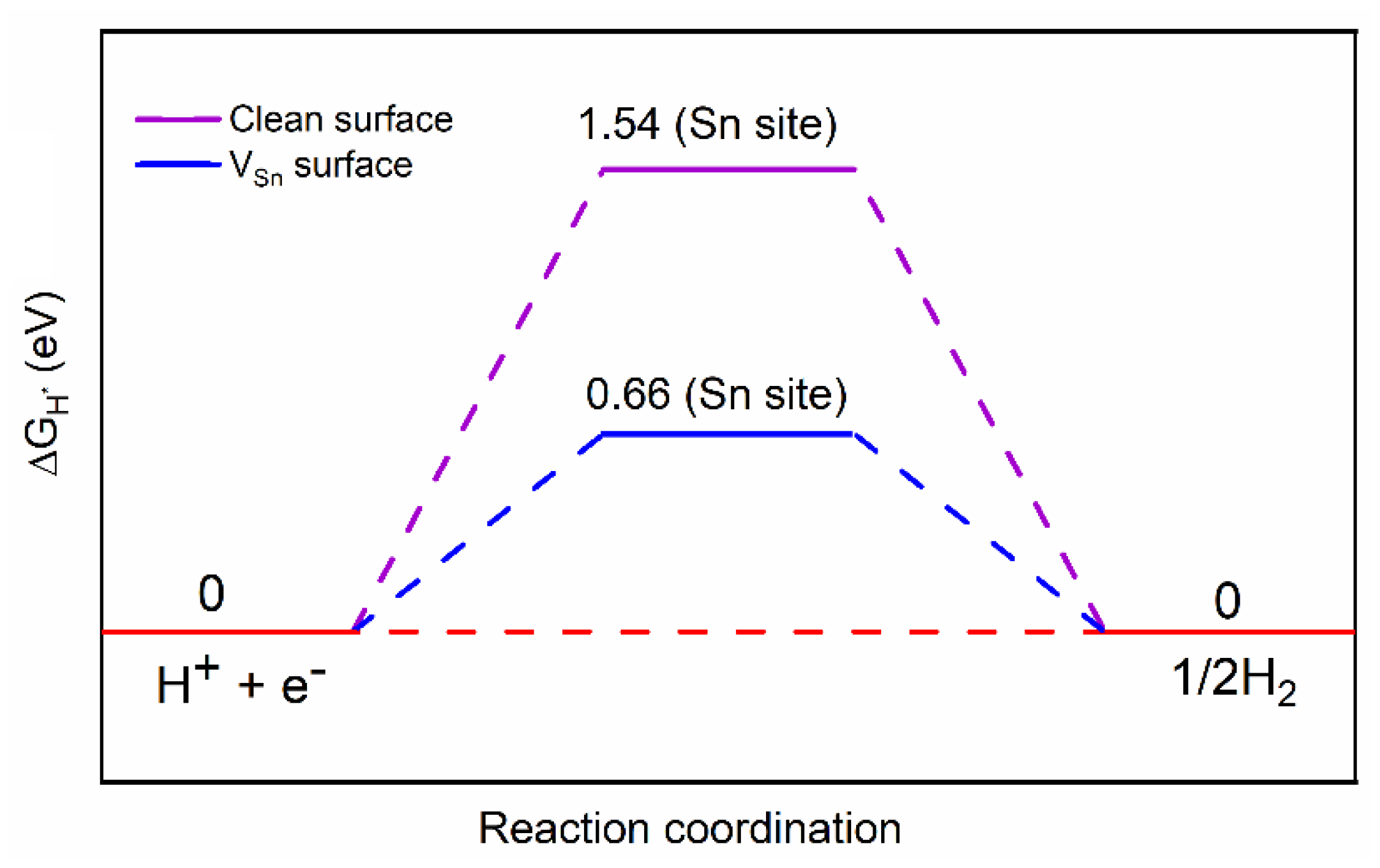
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muradov, N.Z.; Veziroğlu, T.N. “Green” path from fossil-based to hydrogen economy: An overview of carbon-neutral technologies. Int. J. Hydrogen Energy 2008, 33, 6804–6839. [Google Scholar] [CrossRef]
- Mallapaty, S. How China could be carbon neutral by mid-century. Nature 2020, 586, 482–483. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Cao, R. Solar-to-hydrogen energy conversion based on water splitting. Adv. Energy Mater. 2017, 1701620, 1–16. [Google Scholar] [CrossRef]
- Razek, S.A.; Popeil, M.R.; Wangoh, L.; Rana, J.; Suwandaratne, N.; Andrews, J.L.; Watson, D.F.; Banerjee, S.; Piper, L.F.J. Designing catalysts for water splitting based on electronic structure considerations. Electron. Struct. 2020, 2, 023001. [Google Scholar] [CrossRef]
- Li, X.; Zuo, X.; Jiang, X.; Li, D.; Cui, B.; Liu, D. Enhanced photocatalysis for water splitting in layered tin chalcogenides with high carrier mobility. Phys. Chem. Chem. Phys. 2019, 21, 7559–7566. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, L.; Johansson, E.M.J.; Wang, Y.; Jin, P. Photocatalytic activity and mechanism of bisphenol a removal over TiO2−x/rGO nanocomposite driven by visible light. Chem. Eng. J. 2018, 350, 1043–1055. [Google Scholar] [CrossRef]
- Miyoshi, A.; Nishioka, S.; Maeda, K. Water Splitting on Rutile TiO2-Based Photocatalysts. Chem. Eur. J. 2018, 24, 18204–18219. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Ma, S.S.K.; Li, Y.; Domen, K. Core/shell structured La- and Rh-codoped SrTiO3 as a hydrogen evolution photocatalyst in Z-scheme overall water splitting under visible light irradiation. Chem. Mater. 2014, 26, 4144–4150. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Jia, Q.; Tokudome, H.; Zhong, M.; Wang, C.; Pan, Z.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 2016, 15, 611–615. [Google Scholar] [CrossRef]
- Berglund, S.P.; Flaherty, D.W.; Hahn, N.T.; Bard, A.J.; Mullins, C.B. Photoelectrochemical oxidation of water using nanostructured BiVO4 films. J. Phys. Chem. C 2011, 115, 3794–3802. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Liu, Y.; Kanhere, P.D.; Wong, C.L.; Tian, Y.; Feng, Y.; Boey, F.; Wu, T.; Chen, H.; White, T.J.; Chen, Z.; et al. Hydrazine-hydrothermal method to synthesize three-dimensional chalcogenide framework for photocatalytic hydrogen generation. J. Solid State Chem. 2010, 183, 2644–2649. [Google Scholar] [CrossRef]
- Nie, L.; Zhang, Q. Recent progress in crystalline metal chalcogenides as efficient photocatalysts for organic pollutant degradation. Inorg. Chem. Front. 2017, 4, 1953–1962. [Google Scholar] [CrossRef]
- Yuan, Y.P.; Ruan, L.W.; Barber, J.; Joachim Loo, S.C.; Xue, C. Hetero-nanostructured suspended photocatalysts for solar-to-fuel conversion. Energy Environ. Sci. 2014, 7, 3934–3951. [Google Scholar] [CrossRef]
- Maeda, K. Z-scheme water splitting using two different semiconductor photocatalysts. ACS Catal. 2013, 3, 1486–1503. [Google Scholar] [CrossRef]
- Sasaki, Y.; Nemoto, H.; Saito, K.; Kudo, A. Solar water splitting using powdered photocatalysts driven by Z-schematic interparticle electron transfer without an electron mediator. J. Phys. Chem. C 2009, 113, 17536–17542. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Hisatomi, T.; Nakabayashi, M.; Shibata, N.; Kubota, J.; Domen, K. Z-scheme water splitting using particulate semiconductors immobilized onto metal layers for efficient electron relay. J. Catal. 2015, 328, 308–315. [Google Scholar] [CrossRef]
- An, L.; Zang, X.; Ma, L.; Guo, J.; Liu, Q.; Zhang, X. Graphene layer encapsulated MoNi4-NiMoO4 for electrocatalytic water splitting. Appl. Surf. Sci. 2020, 504, 144390. [Google Scholar] [CrossRef]
- Yang, L.; Wang, P.; Yin, J.; Wang, C.; Dong, G.; Wang, Y.; Ho, W. Engineering of reduced graphene oxide on nanosheet–g-C3N4/perylene imide heterojunction for enhanced photocatalytic redox performance. Appl. Catal. B Environ. 2019, 250, 42–51. [Google Scholar] [CrossRef]
- Jian, W.; Cheng, X.; Huang, Y.; You, Y.; Zhou, R.; Sun, T.; Xu, J. Arrays of ZnO/MoS2 nanocables and MoS2 nanotubes with phase engineering for bifunctional photoelectrochemical and electrochemical water splitting. Chem. Eng. J. 2017, 328, 474–483. [Google Scholar] [CrossRef]
- Najafi, L.; Bellani, S.; Oropesa-Nuñez, R.; Prato, M.; Martín-García, B.; Brescia, R.; Bonaccorso, F. Carbon Nanotube-Supported MoSe2 Holey Flake: Mo2C Ball Hybrids for Bifunctional pH-Universal Water Splitting. ACS Nano 2019, 13, 3162–3176. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Wang, Y.; Laleyan, D.A.; Wu, Y.; Ouyang, B.; Ou, P.; Song, J.; Mi, Z. Wafer-scale synthesis of monolayer WSe2: A multi-functional photocatalyst for efficient overall pure water splitting. Nano Energy 2018, 51, 54–60. [Google Scholar] [CrossRef]
- Gao, J.; Tay, Q.; Li, P.Z.; Xiong, W.W.; Zhao, Y.; Chen, Z.; Zhang, Q. Surfactant–Thermal method to synthesize a novel two-Dimensional Oxochalcogenide. Chem. Asian J. 2014, 9, 131–134. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Y.; Zhang, L.; Yin, Q. Preparation and photoelectrochemical properties of SnS/SnSe and SnSe/SnS bilayer structures fabricated via electrodeposition. Appl. Surf. Sci. 2019, 484, 560–567. [Google Scholar] [CrossRef]
- Wang, S.F.; Fong, W.K.; Wang, W.; Surya, C. Growth of highly textured SnS van der Waals epitaxy on mica using a novel SnSe buffer layer. Thin Solid Film 2014, 564, 206–212. [Google Scholar] [CrossRef]
- Wang, S.F.; Wang, W.; Fong, P.W.K.; Yu, Y.; Surya, C. Tin compensation for the SnS based optoelectronic devices. Sci. Rep. 2017, 7, 39704. [Google Scholar] [CrossRef] [PubMed]
- Do, T.N.; Idrees, M.; Amin, B.; Hieu, N.N.; Phuc, H.V.; Hoa, L.T.; Nguyen, C.V. First principles study of structural, optoelectronic and photocatalytic properties of SnS, SnSe monolayers and their van der Waals heterostructure. Chem. Phys. 2020, 539, 110939. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, W.; Fu, X.; Qiao, C.; Xia, C.; Jia, Y. Electronic and magnetic properties of SnSe monolayers doped by Ga, In, As, and Sb: A first-principles study. Phys. Chem. Chem. Phys. 2016, 18, 8158–8164. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Xu, Y.; Shen, Y. Magnetic properties of SnSe monolayer doped by transition-metal atoms: A first-principle calculation. Results Phys. 2020, 17, 103126. [Google Scholar] [CrossRef]
- Tang, Y.; Cheng, F.; Li, D.; Deng, S.; Chen, Z.; Sun, L.; Liu, W.; Shen, L.; Deng, S. Contrastive thermoelectric properties of strained SnSe crystals from the first-principles calculations. Phys. B 2018, 539, 8–13. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, X.; Song, L.; Wu, X. Computational screening toward hydrogen evolution reaction by the introduction of point defects at the edges of group IVA monochalcogenides: A first-principles study. J. Phys. Chem. Lett. 2020, 11, 7664–7671. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yuan, L.; Liu, X.; Zhou, W.; Song, X.; Zhang, S. First-principles study of SO2 sensors based on phosphorene and its isoelectronic counterparts: GeS, GeSe, SnS, SnSe. Chem. Phys. Lett. 2017, 686, 83–87. [Google Scholar] [CrossRef]
- Noerskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. Phys. Inorg. Chem. 2005, 36, 12154. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, J.; Wang, T.; Xiao, W.; Tao, K.; Xue, D.; Ding, J. Metallic Ni3N nanosheets with exposed active surface sites for efficient hydrogen evolution. J. Mater. Chem. A 2016, 4, 17363–17369. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Wang, M.; Li, Y.; Liu, Y.; Chen, Y.; Wu, Q.; Wang, S. Fabrication of Highly Textured 2D SnSe Layers with Tunable Electronic Properties for Hydrogen Evolution. Molecules 2021, 26, 3319. https://doi.org/10.3390/molecules26113319
Zhou Q, Wang M, Li Y, Liu Y, Chen Y, Wu Q, Wang S. Fabrication of Highly Textured 2D SnSe Layers with Tunable Electronic Properties for Hydrogen Evolution. Molecules. 2021; 26(11):3319. https://doi.org/10.3390/molecules26113319
Chicago/Turabian StyleZhou, Qianyu, Mengya Wang, Yong Li, Yanfang Liu, Yuanfu Chen, Qi Wu, and Shifeng Wang. 2021. "Fabrication of Highly Textured 2D SnSe Layers with Tunable Electronic Properties for Hydrogen Evolution" Molecules 26, no. 11: 3319. https://doi.org/10.3390/molecules26113319
APA StyleZhou, Q., Wang, M., Li, Y., Liu, Y., Chen, Y., Wu, Q., & Wang, S. (2021). Fabrication of Highly Textured 2D SnSe Layers with Tunable Electronic Properties for Hydrogen Evolution. Molecules, 26(11), 3319. https://doi.org/10.3390/molecules26113319






