Sustainable Development of Chitosan/Calotropis procera-Based Hydrogels to Stimulate Formation of Granulation Tissue and Angiogenesis in Wound Healing Applications
Abstract
1. Introduction
2. Results
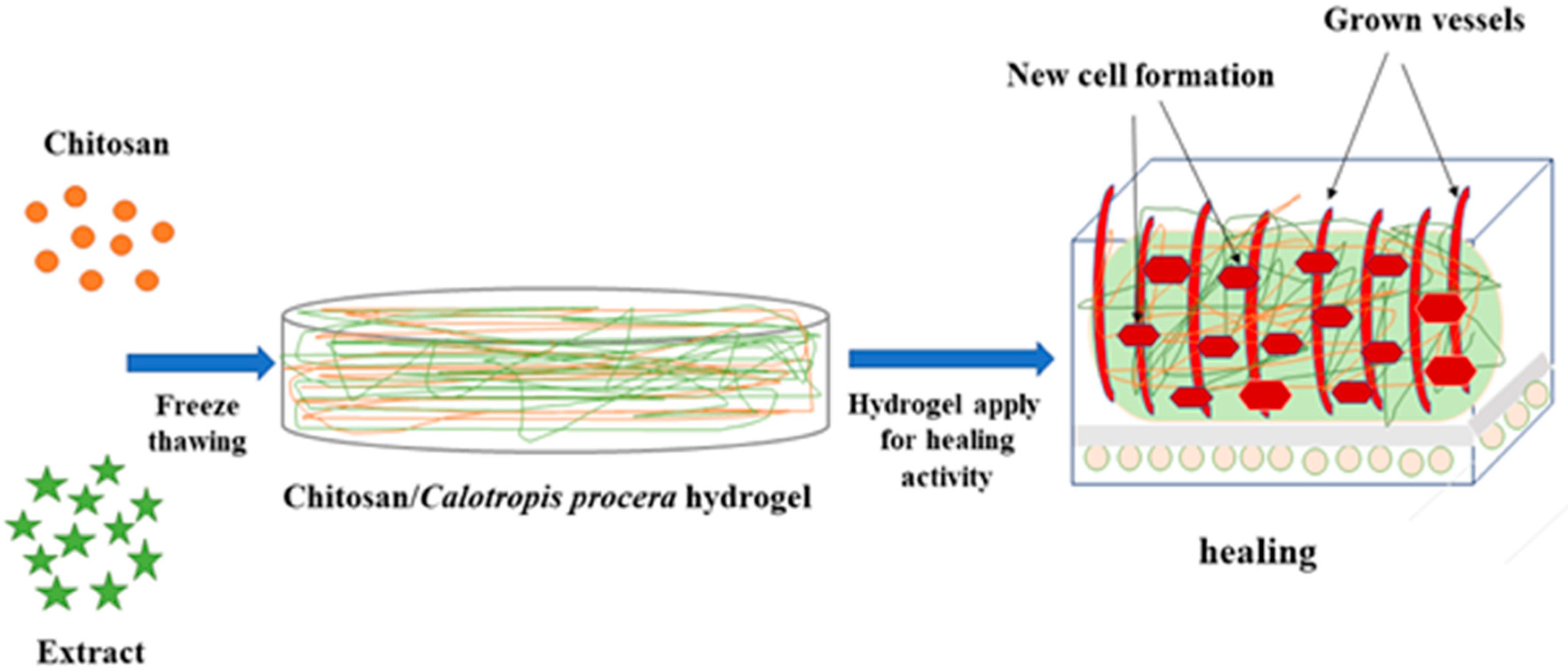
2.1. Preparation of Hydrogels
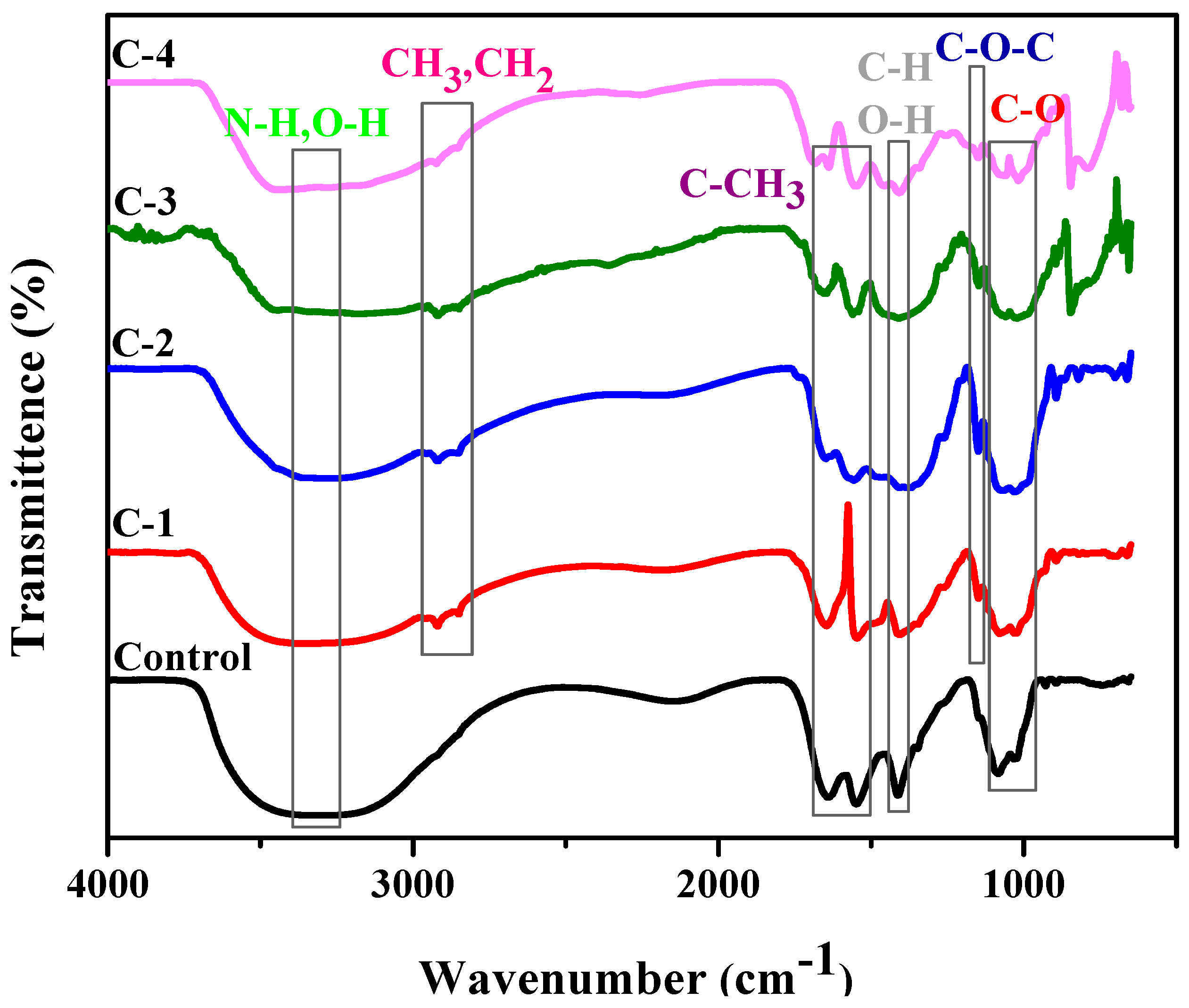
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
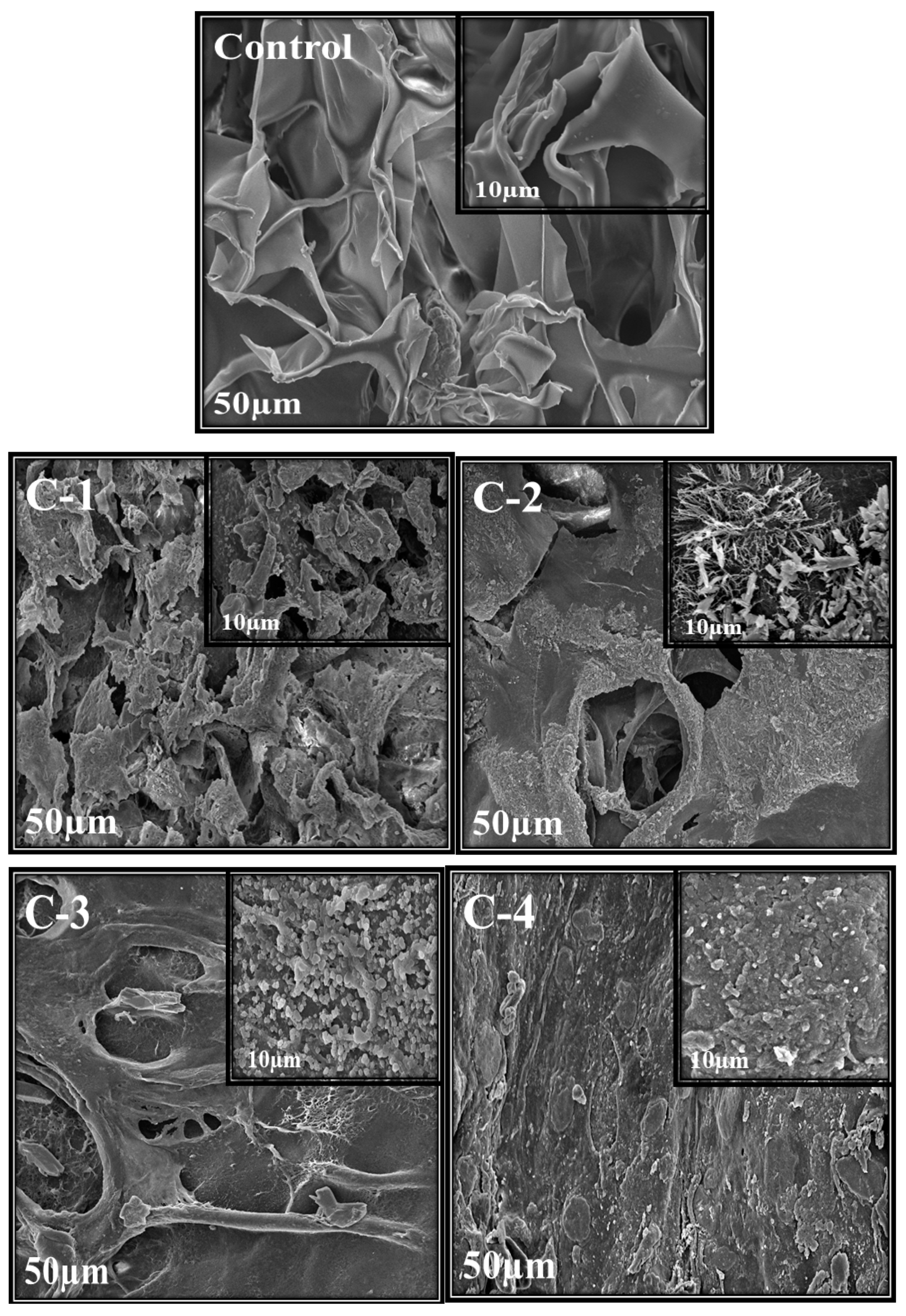
2.3. Scanning Electron Microscopy (SEM)
2.4. Swelling Analysis
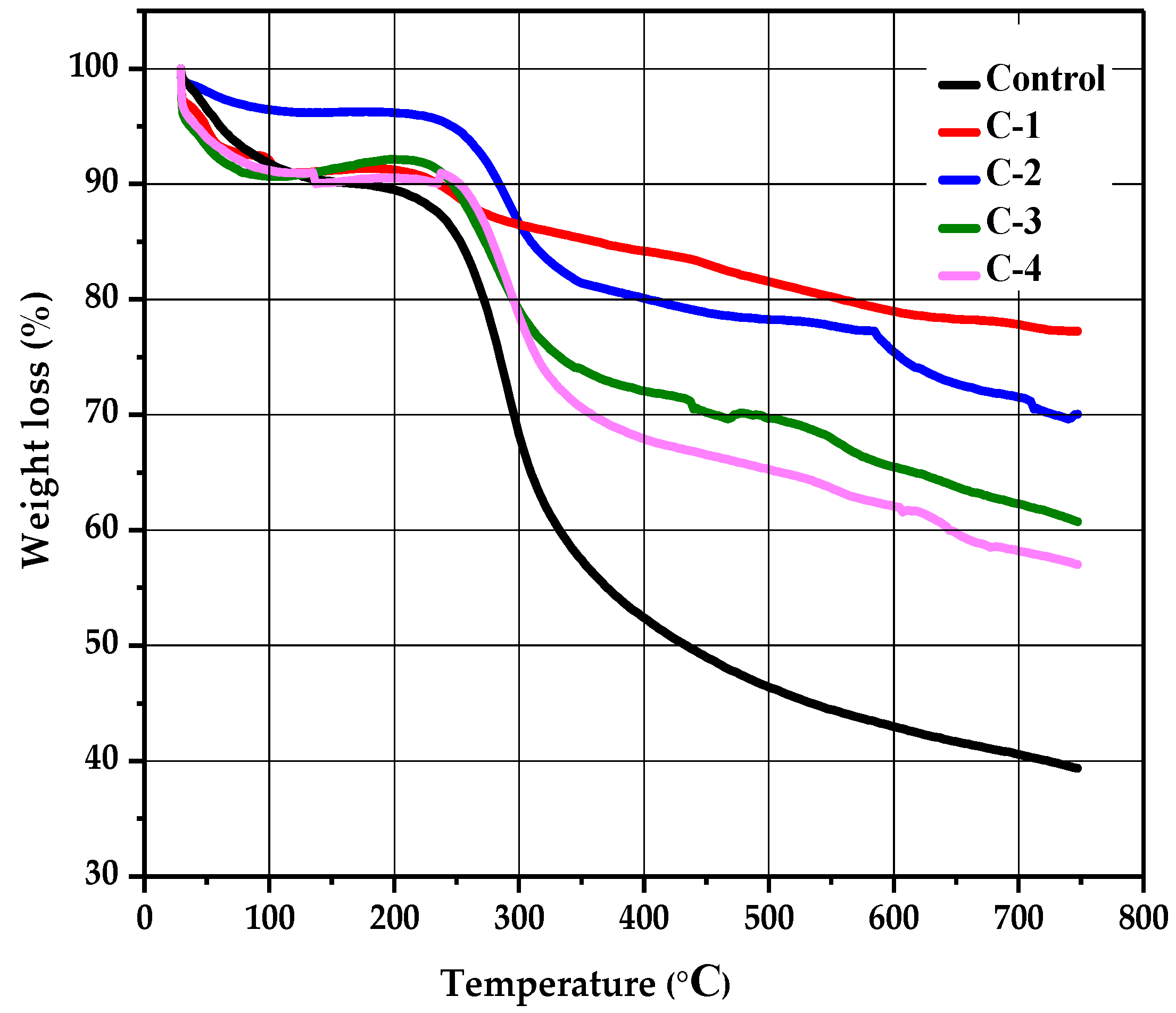
2.5. Thermogravimetric Analysis
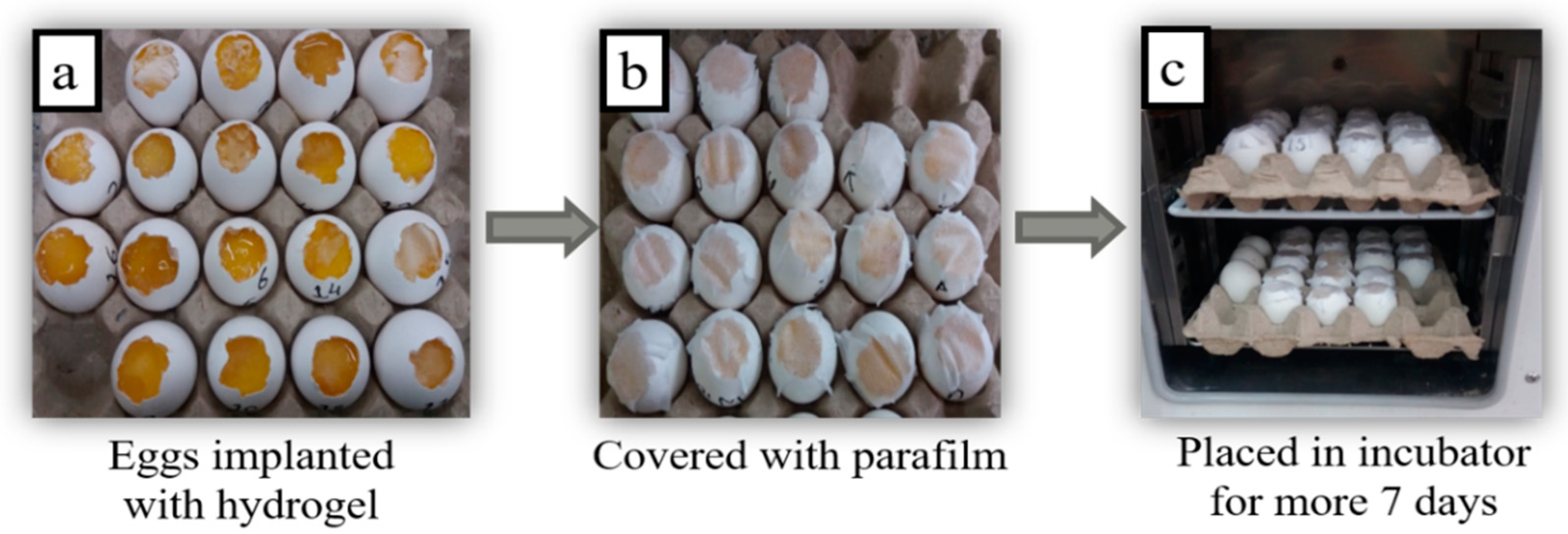
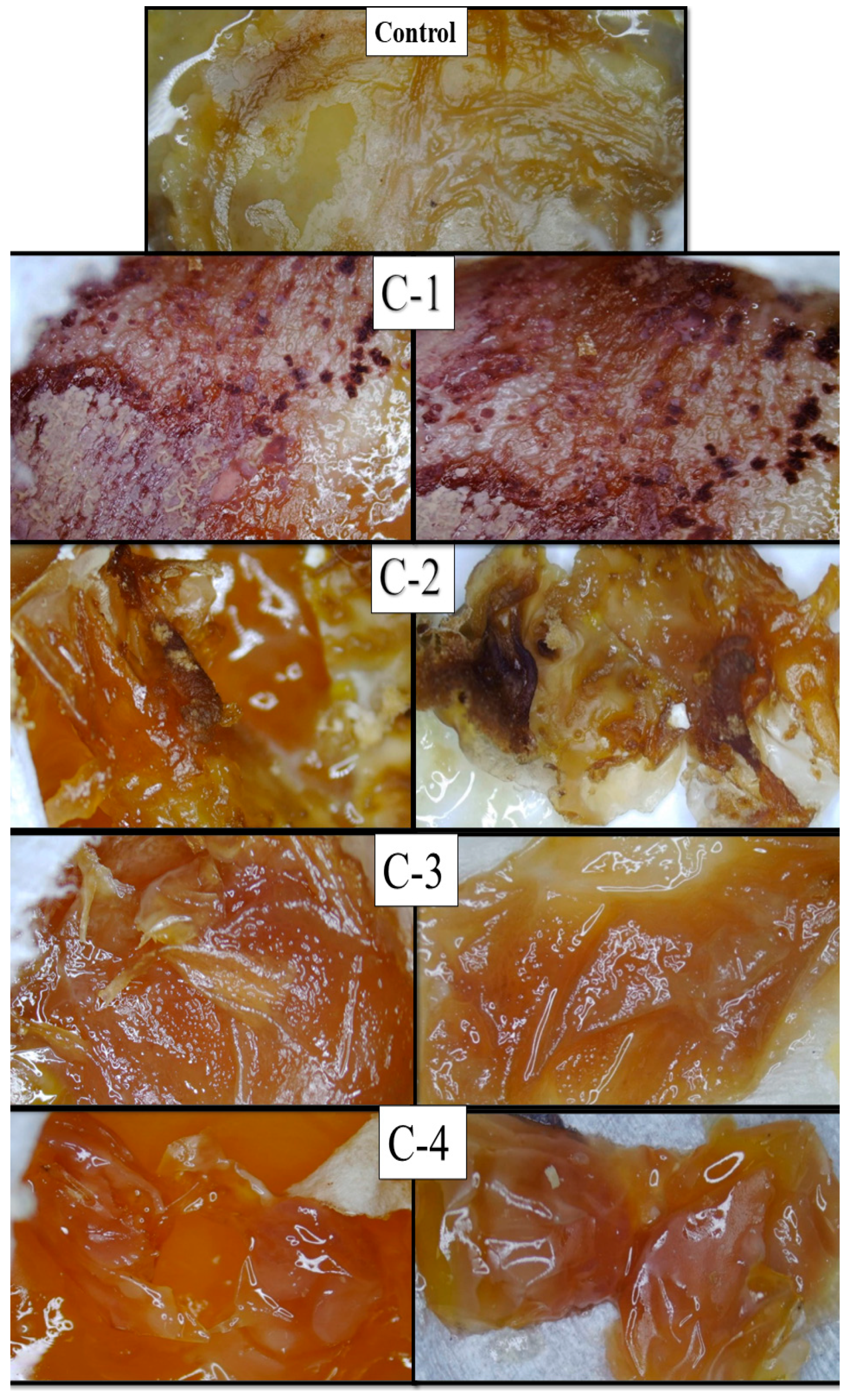
2.6. Chorioallantoic Membrane (CAM) Assay
3. Materials and Methods
3.1. Chemicals and Preparation Methods
3.1.1. Calotropis procera Extract Preparation
3.1.2. Preparation of Chitosan Solution
3.1.3. Synthesis of CS/Cp-LE Hydrogel
3.2. Characterization of Hydrogels
3.2.1. Fourier Transform Infrared Spectroscopy
3.2.2. Scanning Electron Microscope Imaging
3.2.3. Swelling Analysis
3.2.4. Thermogravimetric Analysis
3.2.5. CAM Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Health Organization. Hidden Cities: Unmasking and Overcoming Health Inequities in Urban Settings; World Health Organization: Geneva, Switzerland, 2020; pp. 1–126. ISBN 978 92 4 154803 8. [Google Scholar]
- Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Nie, J.; Wang, J. Carboxyl-modified poly (vinyl alcohol)-crosslinked chitosan hydrogel films for potential wound dressing. Carbohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Bedi, M.K.; Shenefelt, P.D. Herbal therapy in dermatology. Arch. Dermatol. 2002, 138, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Böttcher-Haberzeth, S.; Biedermann, T.; Reichmann, E. Tissue engineering of skin. Burns 2010, 36, 450–460. [Google Scholar] [CrossRef]
- Atala, A. Tissue engineering and regenerative medicine: Concepts for clinical application. Rejuvenation Res. 2004, 7, 15–31. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues—State of the art and future perspectives. J. Biomat. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef]
- Contreras-Ruiz, J.; Manzotti-Rodriguez, A.C. Multidisciplinary Management of Wound Healing in Diabetics. In Dermatology and Diabetes; Springer: Cham, Switzerland, 2018; pp. 199–223. [Google Scholar]
- Gupta, B.S.; Edwards, J.V. Textile materials and structures for topical management of wounds. In Advanced Textiles for Wound Care; Woodhead Publishing: Sawston, UK, 2019; pp. 55–104. [Google Scholar]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Boil. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef]
- de Lima, G.G.; de Lima, D.W.; de Oliveira, M.J.; Lugão, A.B.; Alcântara, M.T.; Devine, D.M.; de Sá, M.J. Synthesis and in vivo behavior of PVP/CMC/agar hydrogel membranes impregnated with silver nanoparticles for wound healing applications. ACS Appl. Bio Mater. 2018, 1, 1842–1852. [Google Scholar] [CrossRef]
- Thanh, N.T.; Hieu, M.H.; Phuong, N.T.M.; Thuan, T.D.B.; Thu, H.N.T.; Do Minh, T.; Dai, H.N.; Thi, H.N. Optimization and characterization of electrospun polycaprolactone coated with gelatin-silver nanoparticles for wound healing application. Mater. Sci. Eng. C 2018, 91, 318–329. [Google Scholar] [CrossRef]
- Bialik-Wąs, K.; Pluta, K.; Malina, D.; Barczewski, M.; Malarz, K.; Mrozek-Wilczkiewicz, A. Advanced SA/PVA-based hydrogel matrices with prolonged release of Aloe vera as promising wound dressings. Mater. Sci. Eng. C 2021, 120, 111667. [Google Scholar] [CrossRef]
- Jin, S.G.; Kim, K.S.; Kim, D.W.; Kim, D.S.; Seo, Y.G.; Go, T.G.; Youn, Y.S.; Kim, J.O.; Yong, C.S.; Choi, H.G. Development of a novel sodium fusidate-loaded triple polymer hydrogel wound dressing: Mechanical properties and effects on wound repair. Int. J. Pharm. 2016, 497, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Martínez-Ibarra, D.M.; Sánchez-Machado, D.I.; López-Cervantes, J.; Campas-Baypoli, O.N.; Sanches-Silva, A.; Madera-Santana, T.J. Hydrogel wound dressings based on chitosan and xyloglucan: Development and characterization. J. Appl. Polym. Sci. 2019, 136, 47342. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, Y.; Hao, J. Rapid-forming and self-healing agarose-based hydrogels for tissue adhesives and potential wound dressings. Biomacromolecules 2018, 19, 980–988. [Google Scholar] [CrossRef]
- Fu, L.H.; Qi, C.; Ma, M.G.; Wan, P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 1541–1562. [Google Scholar] [CrossRef]
- Avadi, M.R.; Sadeghi, A.M.M.; Mohammadpour, N.; Abedin, S.; Atyabi, F.; Dinarvand, R.; Rafiee-Tehrani, M. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomed. Nanotech. Biol. Med. 2010, 6, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Yar, M.; Gigliobianco, G.; Shahzadi, L.; Dew, L.; Siddiqi, S.A.; Khan, A.F.; Chaudhry, A.A.; Rehman, I.U.; MacNeil, S. Production of chitosan PVA PCL hydrogels to bind heparin and induce angiogenesis. Int. J. Polyme. Mater. Polym. Biomater. 2016, 65, 466–476. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Zhu, J.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Dongre, R.S. Introductory chapter: Multitask portfolio of chitin/chitosan: Biomatrix to quantum dot. Chitin-Chitosan Myriad Function. Sci. Technol. 2018, 1. [Google Scholar] [CrossRef]
- Gallo, M.; Naviglio, D.; Caruso, A.A.; Ferrara, L. Applications of chitosan as a functional food. In Novel Approaches of Nanotechnology in Food; Academic Press: Cambridge, MA, USA, 2016; pp. 425–464. [Google Scholar]
- d’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef] [PubMed]
- Meena, A.K.; Yadav, A.; Rao, M.M. Ayurvedic uses and pharmacological activities of Calotropis procera Linn. Asian J. Tradit. Med. 2011, 6, 45–53. [Google Scholar]
- Rasik, A.M.; Raghubir, R.; Gupta, A.; Shukla, A.; Dubey, M.P.; Srivastava, S.; Jain, H.K.; Kulshrestha, D.K. Healing potential of Calotropis procera on dermal wounds in Guinea pigs. J. Ethnopharmacol. 1999, 68, 261–266. [Google Scholar] [CrossRef]
- Buffoni, F.; Banchelli, G.; Cambi, S.; Ignesti, G.; Pirisino, R.; Raimondi, L.; Vannelli, G. Skin wound healing: Some biochemical parameters in Guinea-pig. J. Pharm. Pharmacol. 1993, 45, 784–790. [Google Scholar] [CrossRef]
- Chaiwut, P.; Rawdkuen, S.; Benjakul, S. Extraction of protease from Calotropis procera latex by polyethylene glycol–salts biphasic system. Process Biochem. 2010, 45, 1148–1155. [Google Scholar] [CrossRef]
- Mania, S.; Tylingo, R.; Augustin, E.; Gucwa, K.; Szwacki, J.; Staroszczyk, H. Investigation of an elutable N-propylphosphonic acid chitosan derivative composition with a chitosan matrix prepared from carbonic acid solution. Carbohydr. Polym. 2018, 179, 196–206. [Google Scholar] [CrossRef]
- Aleem, A.R.; Shahzadi, L.; Alvi, F.; Khan, A.F.; Chaudhry, A.A.; ur Rehman, I.; Yar, M. Thyroxin releasing chitosan/collagen based smart hydrogels to stimulate neovascularization. Mater. Design. 2017, 133, 416–425. [Google Scholar] [CrossRef]
- Poiyamozhi, A.; Sundaraganesan, N.; Karabacak, M.; Tanrıverdi, O.; Kurt, M. The spectroscopic (FTIR, FT-Raman, UV and NMR), first-order hyperpolarizability and HOMO–LUMO analysis of 4-amino-5-chloro-2-methoxybenzoic acid. J. Mol. Struct. 2012, 1024, 1–12. [Google Scholar] [CrossRef]
- Song, K.; Zhu, X.; Zhu, W.; Li, X. Preparation and characterization of cellulose nanocrystal extracted from Calotropis procera biomass. Bioresour. Bioprocess. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Jucá, T.L.; Ramos, M.V.; Moreno, F.B.M.B.; Viana de Matos, M.P.; Marinho-Filho, J.D.B.; Moreira, R.A.; Monteiro-Moreira, A.C.D.O. Insights on the phytochemical profile (cyclopeptides) and biological activities of Calotropis procera latex organic fractions. Sci. World J. 2013, 2013, 615454. [Google Scholar] [CrossRef]
- Yoganandam, K.; Ganeshan, P.; NagarajaGanesh, B.; Raja, K. Characterization studies on Calotropis procera fibers and their performance as reinforcements in epoxy matrix. J. Nat. Fibers. 2020, 17, 1706–1718. [Google Scholar] [CrossRef]
- Zarrinkhameh, M.; Zendehnam, A.; Hosseini, S.M. Fabrication of polyvinylchloride based nanocomposite thin film filled with zinc oxide nanoparticles: Morphological, thermal and optical characteristics. J. Indust. Engin. Chem. 2015, 30, 295–301. [Google Scholar] [CrossRef]
- EL-HAFIAN, E.A.; Elgannoudi, E.S.; Mainal, A.; Yahaya, A.H.B. Characterization of chitosan in acetic acid: Rheological and thermal studies. Turk. J. Chem. 2010, 34, 47–56. [Google Scholar]
- Valderruten, N.E.; Valverde, J.D.; Zuluaga, F.; Ruiz-Durántez, E. Synthesis and characterization of chitosan hydrogels cross-linked with dicarboxylic acids. React. Funct. Polym. 2014, 84, 21–28. [Google Scholar] [CrossRef]
- Aderounmua, A.O.; Omonisib, A.E.; Akingbasotec, J.A.; Makanjuolad, M.; Bejide, R.; Orafidiya, L.O.; Adelusolae, K.A. Wound-healing and potential anti-keloidal properties of the latex of Calotropis procera (Aiton) Asclepiadaceae in rabbits. Afr. J. Tradit. Complement. Altern. Med. 2003, 10, 574–579. [Google Scholar] [CrossRef]
- Verma, V.N. The chemical study of Calotropis. Int. Lett. Chem. Phys. Astron. 2014, 1, 74–90. [Google Scholar]
- Singhal, A.K.; Gupta, H.; Bhati, V.S. Wound healing activity of Argyreia nervosa leaves extract. Int. J. Appl. Basic Med. Res. 2011, 1, 36. [Google Scholar] [PubMed]
- Wu, W.S.; Wang, F.S.; Yang, K.D.; Huang, C.C.; Kuo, Y.R. Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J. Investing. Dermatol. 2006, 126, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Adamski, R.; Siuta, D. Mechanical, structural, and biological properties of chitosan/hydroxyapatite/silica composites for bone tissue engineering. Molecules 2021, 26, 1976. [Google Scholar] [CrossRef]

| Samples | CS (Wt %) | Cp-LE (Wt %) |
|---|---|---|
| Control | 100 | nil |
| C-1 | 60 | 40 |
| C-2 | 70 | 30 |
| C-3 | 80 | 20 |
| C-4 | 90 | 10 |
| Sample | Stages | T-onset (°C) | T-endset (°C) | W (%) |
|---|---|---|---|---|
| Control | I | 84.7 | 214.8 | 4 |
| II | 249.8 | 339.8 | 28 | |
| III | 353.6 | 621.4 | 14 | |
| C-1 | I | 99.7 | 202.3 | 1 |
| II | 224.8 | 259.8 | 3 | |
| III | 327.3 | 587.1 | 6 | |
| C-2 | I | 94.7 | 224.8 | 1 |
| II | 259.8 | 319.2 | 10 | |
| III | 349.8 | 584.6 | 4 | |
| C-3 | I | 79.7 | 214.8 | 1 |
| II | 244.9 | 324.8 | 15 | |
| III | 358.6 | 598.5 | 9 | |
| C-4 | I | 92.4 | 232.3 | 1 |
| II | 257.3 | 327.3 | 12 | |
| III | 353.6 | 606.2 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahid, M.; Lodhi, M.; Rehan, Z.A.; Tayyab, H.; Javed, T.; Shabbir, R.; Mukhtar, A.; EL Sabagh, A.; Adamski, R.; Sakran, M.I.; et al. Sustainable Development of Chitosan/Calotropis procera-Based Hydrogels to Stimulate Formation of Granulation Tissue and Angiogenesis in Wound Healing Applications. Molecules 2021, 26, 3284. https://doi.org/10.3390/molecules26113284
Zahid M, Lodhi M, Rehan ZA, Tayyab H, Javed T, Shabbir R, Mukhtar A, EL Sabagh A, Adamski R, Sakran MI, et al. Sustainable Development of Chitosan/Calotropis procera-Based Hydrogels to Stimulate Formation of Granulation Tissue and Angiogenesis in Wound Healing Applications. Molecules. 2021; 26(11):3284. https://doi.org/10.3390/molecules26113284
Chicago/Turabian StyleZahid, Muhammad, Maria Lodhi, Zulfiqar Ahmad Rehan, Hamna Tayyab, Talha Javed, Rubab Shabbir, Ahmed Mukhtar, Ayman EL Sabagh, Robert Adamski, Mohamed I. Sakran, and et al. 2021. "Sustainable Development of Chitosan/Calotropis procera-Based Hydrogels to Stimulate Formation of Granulation Tissue and Angiogenesis in Wound Healing Applications" Molecules 26, no. 11: 3284. https://doi.org/10.3390/molecules26113284
APA StyleZahid, M., Lodhi, M., Rehan, Z. A., Tayyab, H., Javed, T., Shabbir, R., Mukhtar, A., EL Sabagh, A., Adamski, R., Sakran, M. I., & Siuta, D. (2021). Sustainable Development of Chitosan/Calotropis procera-Based Hydrogels to Stimulate Formation of Granulation Tissue and Angiogenesis in Wound Healing Applications. Molecules, 26(11), 3284. https://doi.org/10.3390/molecules26113284







