Substituent Effects on EI-MS Fragmentation Patterns of 5-Allyloxy-1-aryl-tetrazoles and 4-Allyl-1-aryl-tetrazole-5-ones; Correlation with UV-Induced Fragmentation Channels
Abstract
1. Introduction
2. Results
2.1. 5-Allyloxy-1-aryl-tetrazoles
2.2. 4-Allyl-1-aryl-tetrazole-5-ones
3. Discussion
3.1. Electron Impact Mass Spectrometry
3.1.1. Fragmentation Patterns of 5-Allyloxy-1-aryl-tetrazoles
3.1.2. Fragmentation Patterns of 4-Allyl-1-aryltetrazole-5-ones
3.2. Photochemical Studies of Matrix-Isolated Tetrazoles
3.2.1. Tetrazolyl Ethers
3.2.2. Tetrazolones
3.3. Theoretical Studies
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bugalho, S.C.S.; Maçôas, E.M.S.; Cristiano, M.L.S.; Fausto, R. Low temperature matrix-isolation and solid state vibrational spectra of tetrazole. Phys. Chem. Chem. Phys. 2001, 3, 3541–3547. [Google Scholar] [CrossRef]
- Dhiman, N.; Kaur, K.; Jaitak, V. Tetrazoles as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Bioorg. Med. Chem. 2020, 28, 115599. [Google Scholar] [CrossRef]
- Ismael, A.; Abe, M.; Fausto, R.; Cristiano, M.L.S. Insights into the photochemistry of 5-aminotetrazole derivatives with applications in coordination chemistry. effect of the saccharyl moiety on the photostability. Pure Appl. Chem. 2020, 92, 49–62. [Google Scholar] [CrossRef]
- Smolobochkin, A.V.; Gazizov, A.S.; Burilov, A.R.; Pudovik, M.A.; Sinyashin, O.G. Ring opening reactions of nitrogen heterocycles. Russ. Chem. Rev. 2019, 88, 1104–1127. [Google Scholar] [CrossRef]
- Gao, C.; Chang, L.; Xu, Z.; Yan, X.F.; Ding, C.; Zhao, F.; Wu, X.; Feng, L.S. Recent advances of tetrazole derivatives as potential anti-tubercular and anti-malarial agents. Eur. J. Med. Chem. 2019, 163, 404–412. [Google Scholar] [CrossRef]
- Mittal, R.; Awasthi, S.K. Recent Advances in the Synthesis of 5-Substituted 1 H-Tetrazoles: A Complete Survey (2013–2018). Synthesis 2019, 51, 3765–3783. [Google Scholar] [CrossRef]
- Pagacz-Kostrzewa, M.; Sałdyka, M.; Gul, W.; Wierzejewska, M.; Khomenko, D.M.; Doroschuk, R.O. Infrared spectra and photochemistry of 2-(tetrazol-5-yl)benzoic acid isolated in nitrogen matrices. J. Photochem. Photobiol. A Chem. 2019, 371, 292–299. [Google Scholar] [CrossRef]
- Singh, H.; Chawla, A.S.; Kapoor, V.K.; Paul, D.; Malhotra, R.K. Medicinal chemistry of tetrazoles. Prog. Med. Chem. 1980, 17, 151–183. [Google Scholar]
- Malik, M.A.; Wani, M.Y.; Al-Thabaiti, S.A.; Shiekh, R.A. Tetrazoles as carboxylic acid isosteres: Chemistry and biology. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 15–37. [Google Scholar] [CrossRef]
- Burger, A. Isosterism and bioisosterism in drug design. Prog. Drug Res. 1991, 37, 287–371. [Google Scholar]
- Wittenberger, S.J. Recent developments in tetrazole chemistry. A review. Org. Prep. Proced. Int. 1994, 26, 499–531. [Google Scholar] [CrossRef]
- Gómez-Zavaglia, A.; Reva, I.D.; Frija, L.; Cristiano, M.L.; Fausto, R. Photochemistry of 1-phenyl-tetrazolone isolated in solid argon. J. Photochem. Photobiol. A Chem. 2006, 179, 243–255. [Google Scholar] [CrossRef]
- Araújo, N.C.; Brigas, A.F.; Cristiano, M.L.S.; Frija, L.M.T.; Guimarães, E.M.O.; Loureiro, R.M.S. Heteroaromatic benzyl ethers as intermediates for palladium-catalysed transfer hydrogenolysis of benzyl alcohols. J. Mol. Catal. A Chem. 2004, 215, 113–120. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Cristiano, M.L.S.; Guimarães, E.M.O.; Martins, N.C.; Loureiro, R.M.S.; Bickley, J.F. Palladium-catalysed reduction of heteroaromatic naphthyl ethers: Structural effects on reactivity. J. Mol. Catal. A Chem. 2005, 242, 241–250. [Google Scholar] [CrossRef]
- Cristiano, M.L.S.; Johnstone, R.A.W.; Price, P.J. Metal-assisted reactions. Part 25. Heterogeneous and homogeneous catalytic transfer hydrogenolysis of allyloxytetrazoles to yield alkenes or alkanes. J. Chem. Soc. Perkin Trans. 1996, 1, 1453–1459. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Ismael, A.; Cristiano, M.L.S. Photochemical transformations of tetrazole derivatives: Applications in organic synthesis. Molecules 2010, 15, 3757–3774. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Cristiano, M.L.S.; Gómez-Zavaglia, A.; Reva, I.; Fausto, R. Genesis of rare molecules using light-induced reactions of matrix-isolated tetrazoles. J. Photochem. Photobiol. C Photochem. Rev. 2014, 18, 71–90. [Google Scholar] [CrossRef]
- Ismael, A.; Cristiano, M.L.S.; Fausto, R.; Gómez-Zavaglia, A. Tautomer Selective Photochemistry in 1-(Tetrazol-5-yl)ethanol. J. Phys. Chem. A 2010, 114, 13076–13085. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zavaglia, A.; Reva, I.D.; Frija, L.; Cristiano, M.L.; Fausto, R. Molecular structure, vibrational spectra and photochemistry of 2-methyl-2H-tetrazol-5-amine in solid argon. J. Phys. Chem. A 2005, 109, 7967–7976. [Google Scholar] [CrossRef] [PubMed]
- Ismael, A.; Fausto, R.; Cristiano, M.L.S. Photochemistry of 1- and 2-Methyl-5-aminotetrazoles: Structural Effects on Reaction Pathways. J. Org. Chem. 2016, 81, 11656–11663. [Google Scholar] [CrossRef] [PubMed]
- Frija, L.M.T.; Reva, I.D.; Gómez-Zavaglia, A.; Cristiano, M.L.S.; Fausto, R. Photochemistry and vibrational spectra of matrix-isolated 5-ethoxy-1-phenyl-1H-tetrazole. J. Phys. Chem. A 2007, 111, 2879–2888. [Google Scholar] [CrossRef]
- Fraser, R.R.; Haque, K.E. Nuclear magnetic resonance and mass spectral properties of 5-aryltetrazoles. Can. J. Chem. 1968, 46, 2855–2859. [Google Scholar] [CrossRef]
- Fischer, G.W.; Herrmann, M.; Möder, M. Tetrazole compounds. 11 Electron impact mass spectrometry of 1-aryl-5-(l-acyl-2-dialkylaminovinyl)-1H-tetrazoles. J. Heterocycl. Chem. 1996, 33, 815–823. [Google Scholar] [CrossRef]
- Shurukhin, Y.V.; Dovgilevich, A.V.; Grandberg, I.I.; Baskunov, B.P. Mass spectral fragmentation of 1,5-disubstituted tetrazoles and rearrangement of the resulting nitrenes. Chem. Heterocycl. Compd. 1988, 24, 760–764. [Google Scholar] [CrossRef]
- Butler, R.N. Recent Advances in Tetrazole Chemistry. Adv. Heterocycl. Chem. 1977, 21, 323–435. [Google Scholar]
- Forkey, D.M.; Carpenter, W.R. Mass spectrometry of methyltetrazoles. Org. Mass Spectrom. 1969, 2, 433–445. [Google Scholar] [CrossRef]
- Maier, G.; Eckwert, J.; Bothur, A.; Reisenauer, H.P.; Schmidt, C. Photochemical Fragmentation of Unsubstituted Tetrazole, 1,2,3-Triazole, and 1,2,4-Triazole: First Matrix-Spectroscopic Identification of Nitrilimine HCNNH. Liebigs Ann. 1996, 1996, 1041–1053. [Google Scholar] [CrossRef]
- Gómez-Zavaglia, A.; Reva, I.D.; Frija, L.M.T.; Cristiano, M.L.S.; Fausto, R. Photochemistry of Tetrazole Derivatives in Cryogenic Rare Gas Matrices. In Photochemistry Research Progress; Sánchez, A., Gutierrez, S.J., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2008; pp. 295–324. [Google Scholar]
- Pagacz-Kostrzewa, M.; Mucha, M.; Welski, M.; Wierzejewski, M. Conformational properties and photochemistry of new allyl tetrazoles: Matrix isolation FTIR and computational approach. J. Photochem. Photobiol. A Chem. 2013, 251, 118–127. [Google Scholar] [CrossRef]
- Ismael, A.; Serpa, C.; Cristiano, M.L.S. Photochemistry of 1-allyl-4-aryltetrazolones in solution; structural effects on photoproduct selectivity. Photochem. Photobiol. Sci. 2013, 12, 272–283. [Google Scholar] [CrossRef]
- Araújo, N.C.P.; Barroca, P.M.M.; Bickley, J.F.; Brigas, A.F.; Cristiano, M.L.S.; Johnstone, R.A.W.; Loureiro, R.M.S.; Pena, P.C.A. Structural effects on sigmatropic shifts in heteroaromatic allyl ethers. J. Chem. Soc. Perkin Trans. 1 2002, 2, 1213–1219. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Reva, I.; Ismael, A.; Coelho, D.V.; Fausto, R.; Cristiano, M.L.S. Sigmatropic rearrangements in 5-allyloxytetrazoles. Org. Biomol. Chem. 2011, 9, 6040–6054. [Google Scholar] [CrossRef]
- Smith, R.M. Undrestanding Mass Spectra: A Basic Approach; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Abramovitch, R.A.; Kyba, E.P.; Scriven, E.F.V. Mass spectrometry of Aryl Azides. J. Org. Chem. 1971, 36, 3796–3803. [Google Scholar] [CrossRef]
- Bégué, D.; Dargelos, A.; Wentrup, C. Phenylnitrene Radical Cation Rearrangements. J. Phys. Chem. A 2018, 122, 8490–8496. [Google Scholar] [CrossRef]
- Bégué, D.; Dargelos, A.; Braybrook, C.; Wentrup, C. Phenylnitrene Radical Cation and Its Isomers from Tetrazoles, Nitrile Imines, Indazole, and Benzimidazole. J. Phys. Chem. A 2019, 123, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.; Orville-Thomas, W.J.; Gaufrès, R.; Müller, A. (Eds.) Matrix Isolation Spectroscopy; Springer: Dordrecht, The Netherlands, 1981. [Google Scholar]
- Gómez-Zavaglia, A.; Reva, I.D.; Frija, L.; Cristiano, M.L.S.; Fausto, R. Infrared spectrum and UV-induced photochemistry of matrix-isolated 5-methoxy-1-phenyl-1H-tetrazole. J. Photochem. Photobiol. A Chem. 2006, 180, 175–183. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Khmelinskii, I.V.; Serpa, C.; Reva, I.D.; Fausto, R.; Cristiano, M.L.S. Photochemistry of 5-allyloxy-tetrazoles: Steady-state and laser flash photolysis study. Org. Biomol. Chem. 2008, 6, 1046–1055. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Reva, I.D.; Gómez-Zavaglia, A.; Cristiano, M.L.S.; Fausto, R. UV-induced photochemistry of matrix-isolated 1-phenyl-4-allyl-tetrazolone. Photochem. Photobiol. Sci. 2007, 6, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Towards First Principle Calculation of Electron Impact Mass Spectra of Molecules. Angew. Chem. Int. Ed. 2013, 52, 6306–6312. [Google Scholar] [CrossRef]
- Borba, A.; Cabral, L.I.L.; Fausto, R.; Cristiano, M.L.S. Structure, vibrational spectroscopy, and photochemistry of 5-phenoxy-1-phenyltetrazole in argon and nitrogen cryomatrices. Can. J. Chem. 2015, 93, 1335–1344. [Google Scholar] [CrossRef]
- Cristiano, M.L.S.; Johnstone, R.A.W. A kinetic investigation of the thermal rearrangement of allyloxytetrazoles to N-allyltetrazolones. J. Chem. Soc. Perkin Trans. 2 1997, 2, 489–494. [Google Scholar]
- Cristiano, M.L.S.; Johnstone, R.A.W. Intramolecularity of the Thermal Rearrangement of Allyloxytetrazoles to N-Allyltetrazolones. J. Chem. Res. Part. S 1997, 5, 164–165. [Google Scholar] [CrossRef]
- Cristiano, M.L.S. Design and Synthesis of Photosensitive Materials for Lithographic Applications. Ph.D. Thesis, University of Liverpool, Liverpool, UK, 1994. [Google Scholar]





| Tetrazolyl Ether | R1 | R2 | Structure | [M]+· | Fragmentary Ions |
|---|---|---|---|---|---|
| 2a | H | H |  | 202 (1) | 77 (100), 41 (92), 119 (55), 91 (30), 133 (13), 105 (10), 173 (5). |
| 2b | H | CH3 | 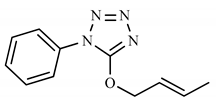 | 216 (4) | 119 (100), 55 (60), 77 (25), 91 (16), 162 (5). |
| 2c | H | C6H5 |  | 278 (<1) | 117 (100), 91 (14), 119 (13), 77 (8), 250 (4). |
| 2d | H | C7H12 | 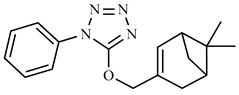 | 296 (<1) | 119 (100), 91 (80), 134 (56), 163 (43), 77 (40), 105 (23), 43 (10). |
| 2e | H | C6H4NO2 | 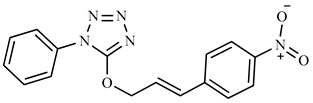 | 323 (2) | 119 (100), 116 (61), 91 (37), 43 (23), 77 (15), 162 (8), 295 (7). |
| 2f | NO2 | C6H5 | 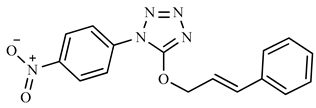 | 323 (5) | 117 (100), 118 (11), 91 (10), 295 (9), 77 (6). |
| 2g | F | C6H5 |  | 296 (4) | 117 (100), 137 (41), 91 (24), 109 (17), 118 (16), 77 (12), 104 (8), 105 (6), 268 (5). |
| 2h | NH2 | C6H5 |  | 293 (4) | 134 (100), 106 (51), 117 (34), 105 (25), 43 (15), 91 (11), 250 (6), 77(5). |
| Tetrazolone | R1 | R2 | Structure | [M]+· | Fragmentary Ions |
|---|---|---|---|---|---|
| 3b | H | CH3 | 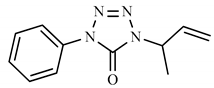 | 216 (11) | 119 (100), 91 (23), 55 (14), 77 (11). |
| 3c | H | C6H5 | 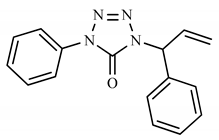 | 278 (5) | 117 (100), 91 (19), 119 (15), 77 (15), 250 (9). |
| 3f | NO2 | C6H5 |  | 323 (1) | 117 (100), 118 (22), 91 (11), 77 (8). |
| 3g | F | C6H5 |  | 296 (1) | 117 (100), 118 (10), 137 (8), 91 (6), 77 (6). |
| 3h | NH2 | C6H5 |  | 293 (11) | 134 (100), 117 (34), 106 (14), 91 (9), 43 (5). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secrieru, A.; Oumeddour, R.; Cristiano, M.L.S. Substituent Effects on EI-MS Fragmentation Patterns of 5-Allyloxy-1-aryl-tetrazoles and 4-Allyl-1-aryl-tetrazole-5-ones; Correlation with UV-Induced Fragmentation Channels. Molecules 2021, 26, 3282. https://doi.org/10.3390/molecules26113282
Secrieru A, Oumeddour R, Cristiano MLS. Substituent Effects on EI-MS Fragmentation Patterns of 5-Allyloxy-1-aryl-tetrazoles and 4-Allyl-1-aryl-tetrazole-5-ones; Correlation with UV-Induced Fragmentation Channels. Molecules. 2021; 26(11):3282. https://doi.org/10.3390/molecules26113282
Chicago/Turabian StyleSecrieru, Alina, Rabah Oumeddour, and Maria L. S. Cristiano. 2021. "Substituent Effects on EI-MS Fragmentation Patterns of 5-Allyloxy-1-aryl-tetrazoles and 4-Allyl-1-aryl-tetrazole-5-ones; Correlation with UV-Induced Fragmentation Channels" Molecules 26, no. 11: 3282. https://doi.org/10.3390/molecules26113282
APA StyleSecrieru, A., Oumeddour, R., & Cristiano, M. L. S. (2021). Substituent Effects on EI-MS Fragmentation Patterns of 5-Allyloxy-1-aryl-tetrazoles and 4-Allyl-1-aryl-tetrazole-5-ones; Correlation with UV-Induced Fragmentation Channels. Molecules, 26(11), 3282. https://doi.org/10.3390/molecules26113282







