Predicting Deliquescence Relative Humidities of Crystals and Crystal Mixtures
Abstract
1. Introduction
2. Theory
2.1. Solid–Liquid Equilibrium
2.2. Vapor-Liquid Equilibrium
2.3. Calculation of Deliquescence Relative Humidity
2.4. PC-SAFT
3. Results and Discussion
3.1. DRH Prediction of Single Components
3.2. DRH Prediction of Multicomponent Crystal Mixtures
3.2.1. DRH of a Binary Fructose/Glucose Crystal Mixture—Influence of the Crystal Ratio
3.2.2. DRH Values of Binary, Ternary and Quaternary Crystal Mixtures
3.3. Predicting DRH as a Function of Temperature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Notation
| thermodynamic activity | |
| Helmholtz energy/J | |
| difference of the heat capacity of the solid and the liquid component i at its melting point/kJ K−1 kg−1 | |
| melting enthalpy/kJ kg−1 | |
| Boltzmann constant/J K−1 | |
| binary interaction parameter | |
| number of segments of component i | |
| molar mass of component i/g mol−1 | |
| number experimental data points | |
| number of association sites | |
| pressure/bar | |
| gas constant/J mol−1 K−1 | |
| temperature/K | |
| dispersion energy parameter/K | |
| mass fraction of component i | |
| mole fraction of component i | |
| Greek symbols | |
| activity coefficient of component i | |
| association energy parameter/K | |
| association volume parameter | |
| density/g cm−3 | |
| stoichiometric coefficient of component i | |
| segment diameter/Å | |
| Subscripts | |
| calc | calculated |
| exp | experimental |
| component i, component j | |
| Superscripts | |
| L | liquid |
| trans | transition |
| S | solid |
| V | vapor |
| Abbreviations | |
| AA | ascorbic acid |
| ARD | average relative deviation |
| CA | citric acid anhydrate |
| DRH | deliquescence relative humidity |
| L | liquid phase |
| PC-SAFT | perturbed-chain statistical associating fluid theory |
| RH | relative humidity |
References
- Labuza, T.P.; Altunakar, B. Water Activity Prediction and Moisture Sorption Isotherms. In Water Activity in Foods: Fundamentals and Applications, 2nd ed.; Barbosa-Cánovas, G.V., Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 161–205. ISBN 9781118768310. [Google Scholar]
- Mauer, L.J.; Taylor, L.S. Water-solids interactions: Deliquescence. Annu. Rev. Food Sci. Technol. 2010, 1, 41–63. [Google Scholar] [CrossRef]
- Adams, J.R.; Merz, A.R. Hygroscopicity of Fertilizer Materials and Mixtures. Ind. Eng. Chem. 1929, 21, 305–307. [Google Scholar] [CrossRef]
- Peng, C.; Chan, C.K. The water cycles of water-soluble organic salts of atmospheric importance. Atmos. Environ. 2001, 35, 1183–1192. [Google Scholar] [CrossRef]
- Salameh, A.K.; Taylor, L.S. Deliquescence in binary mixtures. Pharm. Res. 2005, 22, 318–324. [Google Scholar] [CrossRef]
- Peng, C.; Chan, M.N.; Chan, C.K. The hygroscopic properties of dicarboxylic and multifunctional acids: Measurements and UNIFAC predictions. Environ. Sci. Technol. 2001, 35, 4495–4501. [Google Scholar] [CrossRef]
- Peng, C.; Chow, A.H.L.; Chan, C.K. Hygroscopic Study of Glucose, Citric Acid, and Sorbitol Using an Electrodynamic Balance: Comparison with UNIFAC Predictions. Aerosol Sci. Technol. 2001, 35, 753–758. [Google Scholar] [CrossRef]
- Hiatt, A.N.; Ferruzzi, M.G.; Taylor, L.S.; Mauer, L.J. Impact of deliquescence on the chemical stability of vitamins B1, B6, and C in powder blends. J. Agric. Food Chem. 2008, 56, 6471–6479. [Google Scholar] [CrossRef]
- Braga, D.; Giaffreda, S.L.; Grepioni, F.; Chierotti, M.R.; Gobetto, R.; Palladino, G.; Polito, M. Solvent effect in a “solvent free” reaction. CrystEngComm 2007, 9, 879–881. [Google Scholar] [CrossRef]
- Allan, M.; Mauer, L.J. Comparison of methods for determining the deliquescence points of single crystalline ingredients and blends. Food Chem. 2016, 195, 29–38. [Google Scholar] [CrossRef]
- Li, N.; Taylor, L.S.; Mauer, L.J. Heat transport model for the deliquescence kinetics of crystalline ingredients and mixtures. J. Food Eng. 2016, 169, 298–308. [Google Scholar] [CrossRef]
- Palmelund, H.; Rantanen, J.; Löbmann, K. Deliquescence Behavior of Deep Eutectic Solvents. Appl. Sci. 2021, 11, 1601. [Google Scholar] [CrossRef]
- Hawkes, S.J. Raoult’s Law Is a Deception. J. Chem. Educ. 1995, 72, 204. [Google Scholar] [CrossRef][Green Version]
- Salameh, A.K.; Mauer, L.J.; Taylor, L.S. Deliquescence Lowering in Food Ingredient Mixtures. J. Food Sci. 2006, 71, E10–E16. [Google Scholar] [CrossRef]
- Salameh, A.K.; Taylor, L.S. Role of deliquescence lowering in enhancing chemical reactivity in physical mixtures. J. Phys. Chem. B 2006, 110, 10190–10196. [Google Scholar] [CrossRef]
- Dupas-Langlet, M.; Benali, M.; Pezron, I.; Saleh, K.; Metlas-Komunjer, L. Deliquescence lowering in mixtures of NaCl and sucrose powders elucidated by modeling the water activity of corresponding solutions. J. Food Eng. 2013, 115, 391–397. [Google Scholar] [CrossRef]
- Ross, K.D. Estimation of water activity in intermediate moisture foods. Food Technol. 1975, 29, 26–34. [Google Scholar]
- Cazier, J.-B.; Gekas, V. Water activity and its prediction: A review. Int. J. Food Prop. 2001, 4, 35–43. [Google Scholar] [CrossRef]
- Prausnitz, J.M.; Lichtenthaler, R.N.; de Azevedo, E.G. Molecular Thermodynamics of Fluid-Phase Equilibria, 3rd ed.; Prentice Hall International Series in the Physical and Chemical Engineering: Upper Saddle River, NJ, USA, 1999; ISBN 0132440504. [Google Scholar]
- Veith, H.; Luebbert, C.; Sadowski, G. Correctly Measuring and Predicting Solubilities of Solvates, Hydrates, and Polymorphs. Cryst. Growth Des. 2020, 20, 723–735. [Google Scholar] [CrossRef]
- Gross, J.; Sadowski, G. Perturbed-Chain SAFT: An Equation of State Based on a Perturbation Theory for Chain Molecules. Ind. Eng. Chem. Res. 2001, 40, 1244–1260. [Google Scholar] [CrossRef]
- Gross, J.; Sadowski, G. Application of the Perturbed-Chain SAFT Equation of State to Associating Systems. Ind. Eng. Chem. Res. 2002, 41, 5510–5515. [Google Scholar] [CrossRef]
- Berthelot, D. Sur le mélange des gaz. Compt. Rendus 1898, 126, 1703–1706. [Google Scholar]
- Lorentz, H.A. Ueber die Anwendung des Satzes vom Virial in der kinetischen Theorie der Gase. Ann. Phys. 1881, 248, 127–136. [Google Scholar] [CrossRef]
- Wolbach, J.P.; Sandler, S.I. Using Molecular Orbital Calculations To Describe the Phase Behavior of Cross-associating Mixtures. Ind. Eng. Chem. Res. 1998, 37, 2917–2928. [Google Scholar] [CrossRef]
- Cameretti, L.F.; Sadowski, G. Modeling of aqueous amino acid and polypeptide solutions with PC-SAFT. Chem. Eng. Process. 2008, 47, 1018–1025. [Google Scholar] [CrossRef]
- Wysoczanska, K.; Sadowski, G.; Macedo, E.A.; Held, C. Toward Thermodynamic Predictions of Aqueous Vitamin Solubility: An Activity Coefficient-Based Approach. Ind. Eng. Chem. Res. 2019, 58, 7362–7369. [Google Scholar] [CrossRef]
- Crespo, E.A.; Silva, L.P.; Martins, M.A.R.; Bülow, M.; Ferreira, O.; Sadowski, G.; Held, C.; Pinho, S.P.; Coutinho, J.A.P. The Role of Polyfunctionality in the Formation of [Ch]Cl-Carboxylic Acid-Based Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2018, 57, 11195–11209. [Google Scholar] [CrossRef]
- Held, C.; Sadowski, G.; Carneiro, A.; Rodríguez, O.; Macedo, E.A. Modeling thermodynamic properties of aqueous single-solute and multi-solute sugar solutions with PC-SAFT. AIChE J. 2013, 59, 4794–4805. [Google Scholar] [CrossRef]
- Lange, L.; Lehmkemper, K.; Sadowski, G. Predicting the Aqueous Solubility of Pharmaceutical Cocrystals As a Function of pH and Temperature. Cryst. Growth Des. 2016, 16, 2726–2740. [Google Scholar] [CrossRef]
- Veith, H.; Wiechert, F.; Luebbert, C.; Sadowski, G. Combining crystalline and polymeric excipients in API solid dispersions—Opportunity or risk? Eur. J. Pharm. Biopharm. 2021, 158, 323–335. [Google Scholar] [CrossRef]
- Apelblat, A. Enthalpy of solution of oxalic, succinic, adipic, maleic, malic, tartaric, and citric acids, oxalic acid dihydrate, and citric acid monohydrate in water at 298.15 K. J. Chem. Thermodyn. 1986, 18, 351–357. [Google Scholar] [CrossRef]
- Allan, M.; Mauer, L.J. RH-temperature phase diagrams of hydrate forming deliquescent crystalline ingredients. Food Chem. 2017, 236, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Schröder, B.; Santos, L.M.; Marrucho, I.M.; Coutinho, J.A. Prediction of aqueous solubilities of solid carboxylic acids with COSMO-RS. Fluid Phase Equilib. 2010, 289, 140–147. [Google Scholar] [CrossRef]
- Lu, Q.; Zografi, G. Properties of citric acid at the glass transition. J. Pharm. Sci. 1997, 86, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Klímová, K.; Leitner, J. DSC study and phase diagrams calculation of binary systems of paracetamol. Thermochim. Acta 2012, 550, 59–64. [Google Scholar] [CrossRef]
- Raemy, A.; Schweizer, T.F. Thermal behaviour of carbohydrates studied by heat flow calorimetry. J. Therm. Anal. 1983, 28, 95–108. [Google Scholar] [CrossRef]
- Laube, F.; Klein, T.; Sadowski, G. Partition Coefficients of Pharmaceuticals as Functions of Temperature and pH. Ind. Eng. Chem. Res. 2015, 54, 3968–3975. [Google Scholar] [CrossRef]
- Abildskov, J.; Marrero, J. Organic Solutes Ranging from C2 to C41; Dechema: Frankfurt am Main, Germany, 2005; ISBN 38-97-46074-2. [Google Scholar]
- Good, D.J.; Rodríguez-Hornedo, N. Solubility Advantage of Pharmaceutical Cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Chang, S.S.; Bestul, A.B. Heat Capacity and Thermodynamic Properties of o -Terphenyl Crystal, Glass, and Liquid. J. Chem. Phys. 1972, 56, 503–516. [Google Scholar] [CrossRef]
- Young, F.E.; Jones, F.T.; Lewis, H.J. D-Fructose–Water Phase Diagram. J. Phys. Chem. B 1952, 56, 1093–1096. [Google Scholar] [CrossRef]
- Crestani, C.E.; Bernardo, A.; Costa, C.B.B.; Giulietti, M. Fructose Solubility in Mixed (Ethanol + Water) Solvent: Experimental Data and Comparison among Different Thermodynamic Models. J. Chem. Eng. Data 2013, 58, 3039–3045. [Google Scholar] [CrossRef]
- Lipasek, R.A.; Li, N.; Schmidt, S.J.; Taylor, L.S.; Mauer, L.J. Effect of temperature on the deliquescence properties of food ingredients and blends. J. Agric. Food Chem. 2013, 61, 9241–9250. [Google Scholar] [CrossRef] [PubMed]
- Jayasankar, A.; Good, D.J.; Rodríguez-Hornedo, N. Mechanisms by which moisture generates cocrystals. Mol. Pharm. 2007, 4, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, N.; Sadeghi, R. Osmotic properties of carbohydrate aqueous solutions. Fluid Phase Equilib. 2016, 417, 171–180. [Google Scholar] [CrossRef]

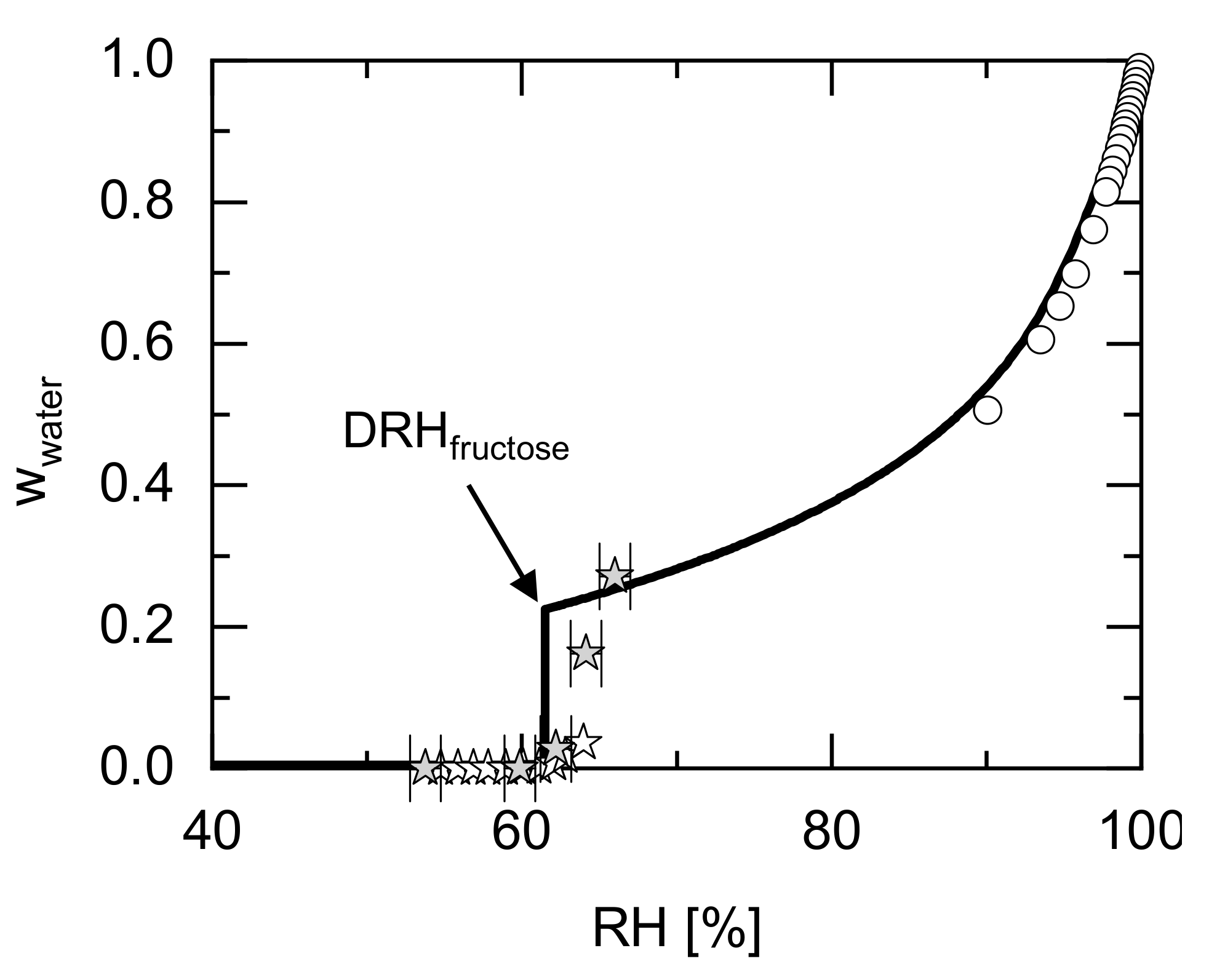

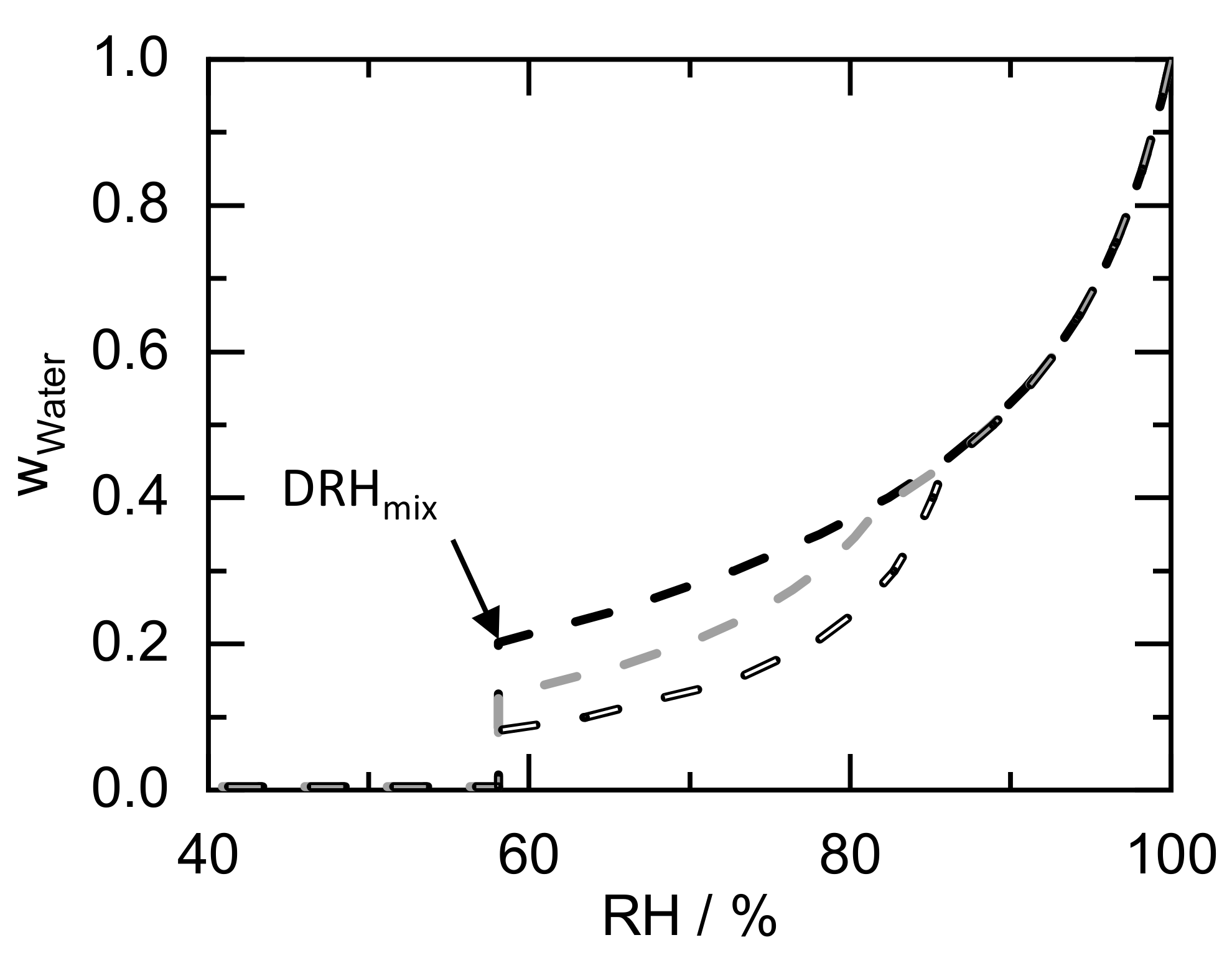
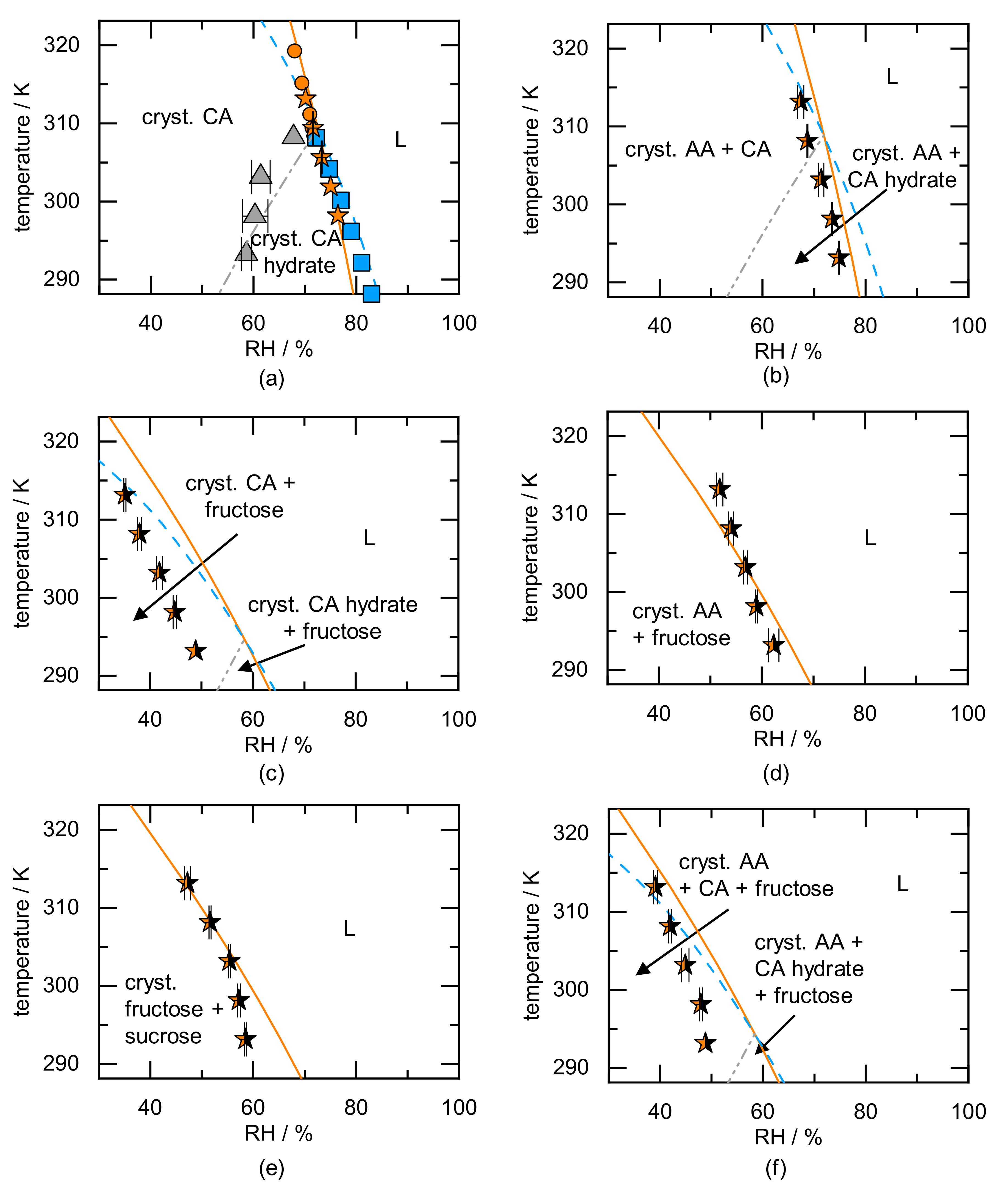
| Component | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|
| water | 18.02 | 0.06687 | A | 353.94 | 2425.67 | 0.0451 B | 1/1 | [26] |
| ascorbic acid | 176.13 | 0.06647 | 2.367 | 353.44 | 2600.68 | 0.039 | 4/1 | [27] |
| citric acid | 192.12 | 0.0445 | 2.723 | 227.18 | 2488 | 0.044 | 4/4 | [28] |
| fructose | 180.16 | 0.0410 | 2.849 | 237.19 | 5000 | 0.1 | 5/5 | [29] |
| glucose | 118.16 | 0.0368 | 2.986 | 244.53 | 5000 | 0.1 | 5/5 | [29] |
| nicotinamide | 122.12 | 0.0381 | 2.178 | 176.69 | 2195.3 | 0.02 | 2/2 | [30] |
| lactose | 342.3 | 0.0419 | 2.811 | 319.21 | 5000 | 0.1 | 8/8 | [29] |
| saccharin | 183.18 | 0.02393 | 4.125 | 383.54 | 853.03 | 0.02 | 1/1 | [31] |
| sucrose | 342.30 | 0.0435 | 2.827 | 297.39 | 5000 | 0.1 | 8/8 | [29] |
| Components | kij,T/K−1 | kij,b | Fitted to | Reference for Parameters |
|---|---|---|---|---|
| ascorbic acid/water | - | −0.018 | osmotic coefficients/density | [27] |
| citric acid/water | - | −0.07 | solubility | this work B |
| fructose/water | 1.40 × 10−4 | −0.097241 A | osmotic coefficients/density | [29] |
| glucose/water | 2.24 × 10−4 | −0.119186 A | osmotic coefficients/density | [29] |
| nicotinamide/water | 9.46 × 10−5 | −0.0294 | solubility | [30] |
| lactose/water | 1.84 × 10−4 | −0.08696 A | osmotic coefficients/density | [29] |
| saccharin/water | - | −0.01507 | solubility | [31] |
| sucrose/water | 2.56 × 10−4 | −0.113426 A | osmotic coefficients/density | [29] |
| Component | Ref. | |||
|---|---|---|---|---|
| ascorbic acid | 465.15 | 29.12 | 43 [27] | [34] |
| citric acid | 428.55 | 41.84 | 159.46 a [35] | [36] |
| fructose | 353.2 | 32.4 | - | [37] |
| glucose | 408.2 | 32.5 | - | [37] |
| nicotinamide | 401.15 | 28.0 | 78.12 [38] | [39] |
| lactose | 433.2 | 75.3 | - | [37] |
| saccharin | 502.9 | 32.1 | - | [40] |
| sucrose | 433.2 | 41.1 | - | [37] |
| DRH Prediction/% | DRH Measurement/% | Reference of Measurement | |||
|---|---|---|---|---|---|
| Component(s) | Raoult | PC-SAFT | DRH | ||
| Single components | |||||
| ascorbic acid (AA) | 96.9 | 97.4 | 97.5 ± 1 | 97 ± 0 | [44] |
| citric acid (CA) | 86.0 | 76.4 | 75.0 ± 1 | 78 * | [14] |
| citric acid hydrate | 87.4 | 79.4 | 78.0 ± 1 | 78 | [14] |
| fructose | 74.3 | 61.5 | 62.0 ± 1 | 61 | [14] |
| glucose | 91.1 | 89.4 | 91.0 ± 1 | 90 | [14] |
| nicotinamide (NA) | 89.6 | 93.6 | 94.5 ± 0.3 | [45] | |
| lactose | 95.0 | 94.2 | 95.0 ± 1 | 97 | [14] |
| saccharin | 100.0 | 100.0 | - | ||
| sucrose | 94.1 | 92.0 | 85.0 ± 1 | 85 | [14] |
| DRH Prediction/% | DRH Measurement/% | Reference of Measurement | |||
|---|---|---|---|---|---|
| Component (s) | Raoult | PC-SAFT | DRH | ||
| binary mixtures | |||||
| AA-CA | 82.8 | 75.7 | 74.4 ± 1 | 73.5 ± 0.1 * | [44] |
| AA-CA hydrate | 84.3 | 78.4 | 74 | 75 | [2] |
| AA-fructose | 71.2 | 61.3 | 58.9 ± 1 | 58.9 ± 0.2 | [44] |
| AA-sucrose | 91.0 | 87.7 | 85 | 83 | [2] |
| CA-fructose | 60.3 | 55.6 | 48.0 ± 1 | 47 | [14] |
| CA hydrate-fructose | 61.7 | 55.0 | 52 | 48 * | [2] |
| CA-glucose | 77.1 | 72.4 | 68.0 ± 1 | 67 * | [14] |
| CA hydrate-glucose | 78.5 | 74.3 | - | 68 | [2] |
| CA-sucrose | 80.1 | 75.3 | 64.0 ± 1 | 60 a | [14] |
| CA hydrate-sucrose | 81.6 | 77.8 | 65 | 56 * | [2] |
| fructose-glucose | 65.4 | 58.1 | 60 ± 1 | 54 | [14] |
| fructose-NA | 63.9 | 49.1 | 55.3 ± 0.3 | - | [45] |
| fructose-saccharin | 74.3 | 61.5 | 61.5 ± 0.3 | - | [45] |
| fructose-sucrose | 68.5 | 61.1 | 58 ± 1 | 56 | [14] |
| glucose-sucrose | 85.3 | 86.5 | 79 ± 1 | 78 | [14] |
| sucrose-NA | 83.7 | 76.1 | 80 ± 0.3 | - | [45] |
| ternary mixtures | |||||
| AA-CA-fructose | 57.2 | 55.4 | 47.9 ± 1 | 47.9 ± 0.3 | [44] |
| AA-CA hydrate-sucrose | 78.4 | 76.5 | 56 | 56 * | [2] |
| CA-fructose-glucose | 51.4 | 52.8 | 50 ± 1 | 43 a | [14] |
| CA hydrate-fructose-glucose | 52.8 | 51.9 | - | 47 * | [2] |
| CA-fructose-sucrose | 54.4 | 55.3 | 55 ± 1 | 44 a | [14] |
| CA-glucose-sucrose | 71.2 | 71.6 | 64 ± 1 | 59 a | [14] |
| fructose-glucose-sucrose | 59.6 | 57.7 | 58 ± 1 | 53 a | [14] |
| quaternary mixtures | |||||
| CA-fructose-glucose-sucrose | 45.5 | 52.5 | 54 ± 1 | 48 a | [14] |
| CA hydrate-fructose-glucose-sucrose | 47.0 | 1.9 | - | 44 * | [2] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veith, H.; Luebbert, C.; Sadowski, G. Predicting Deliquescence Relative Humidities of Crystals and Crystal Mixtures. Molecules 2021, 26, 3176. https://doi.org/10.3390/molecules26113176
Veith H, Luebbert C, Sadowski G. Predicting Deliquescence Relative Humidities of Crystals and Crystal Mixtures. Molecules. 2021; 26(11):3176. https://doi.org/10.3390/molecules26113176
Chicago/Turabian StyleVeith, Heiner, Christian Luebbert, and Gabriele Sadowski. 2021. "Predicting Deliquescence Relative Humidities of Crystals and Crystal Mixtures" Molecules 26, no. 11: 3176. https://doi.org/10.3390/molecules26113176
APA StyleVeith, H., Luebbert, C., & Sadowski, G. (2021). Predicting Deliquescence Relative Humidities of Crystals and Crystal Mixtures. Molecules, 26(11), 3176. https://doi.org/10.3390/molecules26113176






