Back to the Roots—An Overview of the Chemical Composition and Bioactivity of Selected Root-Essential Oils
Abstract
1. Introduction
2. Essential Oils from Selected Roots
2.1. Angelica archangelica
2.2. Armoracia rusticana
2.3. Carlina acaulis (and Related Species)
2.4. Chrysopogon zizanioides
2.5. Coleus forskohlii
2.6. Inula helenium
2.7. Sassafras albidum
2.8. Saussurea costus
2.9. Valeriana officinalis
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Figueiredo, A.C.; Barroso, J.; Pedro, L.; Scheffer, J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Kala, C.; Ali, S.S.; Ahmad, N.; Gilani, S.J.; Khan, N.A. Isothiocyanates: A review. Res. J. Pharmacogn. 2018, 5, 71–89. [Google Scholar] [CrossRef]
- Wnorowski, A.; Wnorowska, S.; Wojas, K.; Grenda, A.; Staniak, M.; Michalak, A.; Woźniak, S.; Matosiuk, D.; Biala, G.; Wójciak-Kosior, M.; et al. Toxicity of carlina oxide—A natural polyacetylene from the Carlina acaulis roots—In vitro and in vivo study. Toxins 2020, 12, 239. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.; Fierascu, I.; Dinu-Pirvu, C.; Fierascu, I.; Alina, P. The application of essential oils as a next-generation of pesticides: Recent developments and future perspectives. Z. Naturforsch. J. Biosci. 2019, 75, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Kavallieratos, N.G.; Boukouvala, M.C.; Ntalli, N.; Skourti, A.; Karagianni, E.S.; Nika, E.P.; Kontodimas, D.C.; Cappellacci, L.; Petrelli, R.; Cianfaglione, K.; et al. Effectiveness of eight essential oils against two key stored-product beetles, Prostephanus truncatus (Horn) and Trogoderma granarium everts. Food Chem. Toxicol. 2020, 139, 111255. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant. Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Doneanu, C.; Anitescu, G. Supercritical carbon dioxide extraction of Angelica archangelica L. root oil. J. Supercrit. Fluids 1998, 12, 59–67. [Google Scholar] [CrossRef]
- Wichtl, M. Teedrogen Und Phytopharmaka.; Wissenschaftliche Verlagsgesellschaft GmbH Stuttgart: Stuttgart, Germany, 2002; Volume 5. [Google Scholar]
- Pasqua, G.; Monacelli, B.; Silvestrini, A. Accumulation of essential oils in relation to root differentiation in Angelica archangelica L. Eur. J. Histochem. 2003, 47, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, M.; Goldberg, A.; Brinckmann, J. Herbal Medicine. Expanded Commission E Monographs; Integrative Medicine Communications: Boston, MA, USA, 2000. [Google Scholar]
- Nivinskiene, O.; Butkiene, R.; Mockutë, D. Changes in the chemical composition of essential oil of Angelica archangelica L. roots during storage. Chemija (Vilnius) 2003, 14, 52–56. [Google Scholar]
- Chauhan, R.S.; Nautiyal, M.C.; Cecotti, R.; Mella, M.; Tava, A. Variation in the essential oil composition of Angelica archangelica from three different altitudes in Western Himalaya, India. Ind. Crops Prod. 2016, 94, 401–404. [Google Scholar] [CrossRef]
- Paroul, N.; Rota, L.; Frizzo, C.; Santos, A.; Moyna, P.; Gower, A.; Serafini, L.; Cassel, E. Chemical composition of the volatiles of Angelica root obtained by hydrodistillation and supercritical CO2 extraction. J. Essent. Oil Res. 2002, 14, 282–285. [Google Scholar] [CrossRef]
- Nivinskienė, O.; Butkienė, R.; Mockutė, D. The chemical composition of the essential oil of Angelica archangelica L. roots growing wild in Lithuania. J. Essent. Oil. Res. 2005, 17, 373–377. [Google Scholar] [CrossRef]
- Letchamo, W.; Gosselin, A.; Hölzl, J. Growth and essential oil content of Angelica archangelica as influenced by light intensity and growing media. J. Essent. Oil Res. 1995, 7, 497–504. [Google Scholar] [CrossRef]
- Ojala, A.; Huopalahti, R.; Nykänen, A.; Kallio, H. Variation of Angelica archangelica subsp. archangelica (Apiaceae) in northern Fennoscandia: Variation in composition of essential oil. Ann. Bot. Fenn. 1986, 23, 325–332. [Google Scholar]
- IFRA-Standards. Available online: https://ifrafragrance.org/docs/default-source/ifra-code-of-practice-and-standards/49th-amendment/ifra-49th-amendment-(att-04)---index-of-ifra-standards.pdf?sfvrsn=6269aaf4_3 (accessed on 25 October 2020).
- Wedge, D.E.; Klun, J.A.; Tabanca, N.; Demirci, B.; Ozek, T.; Baser, K.H.C.; Liu, Z.; Zhang, S.; Cantrell, C.L.; Zhang, J. Bioactivity-guided fractionation and GC/MS fingerprinting of Angelica sinensis and Angelica archangelica root components for antifungal and mosquito deterrent activity. J. Agric. Food Chem. 2009, 57, 464–470. [Google Scholar] [CrossRef]
- Fraternale, D.; Flamini, G.; Ricci, D. Essential oil composition and antimicrobial activity of Angelica archangelica L.(Apiaceae) roots. J. Med. Food 2014, 17, 1043–1047. [Google Scholar] [CrossRef]
- Forsén, K. Aroma constituents of Angelica archangelica. Variation in the composition of the essential root oil of strains of var. norvegica and var. sativa. Rep. Kev Subart Res. Stat. 1979, 15, 1–7. [Google Scholar]
- Chalchat, J.-C.; Garry, R.-P. Essential oil of Angelica roots (Angelica archangelicaL.): Optimization of distillation, location in plant and chemical composition. J. Essent. Oil Res. 1997, 9, 311–319. [Google Scholar] [CrossRef]
- Acimovic, M.G.; Pavlovic, S.D.; Varga, A.O.; Filipovic, V.M.; Cvetkovic, M.T.; Stankovic, J.M.; Cabarkapa, I.S. Chemical composition and antibacterial activity of Angelica archangelica root essential oil. Nat. Prod. Commun. 2017, 12, 205–206. [Google Scholar] [PubMed]
- Fraternale, D.; Flamini, G.; Ricci, D. Essential oil composition of Angelica archangelica L. (Apiaceae) roots and its antifungal activity against plant pathogenic fungi. Plant Biosyst. 2016, 150, 558–563. [Google Scholar] [CrossRef]
- Shchipitsyna, O.S.; Efremov, A.A. Composition of ethereal oil isolated from various vegetative parts of angelica from the Siberian region. Russ. J. Bioorganic Chem. 2011, 37, 888–892. [Google Scholar] [CrossRef]
- Bernard, C.; Clair, G. Essential oils of three Angelica L. species growing in France. Part I. Root oils. J. Essent. Oil Res. 1997, 9, 289–294. [Google Scholar] [CrossRef]
- Kerrola, K.M.; Kallio, H.P. Extraction of volatile compounds of Angelica (Angelica archangelica L.) root by liquid carbon dioxide. J. Agric. Food Chem. 1994, 42, 2235–2245. [Google Scholar] [CrossRef]
- Nykänen, I.; Nykänen, L.; Alkio, M. Composition of Angelica root oils obtained by supercritical CO2 extraction and steam distillation. J. Essent. Oil Res. 1991, 3, 229–236. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Čomić, L.; Radulović, N.; Blagojević, P.; Denić, M.; Miltojević, A.; Rajković, J.; Mihajilov-Krstev, T. Antistaphylococcal activity of Inula helenium L. root essential oil: Eudesmane sesquiterpene lactones induce cell membrane damage. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1015–1025. [Google Scholar] [CrossRef]
- Prakash, B.; Singh, P.; Goni, R.; Raina, A.K.; Dubey, N.K. Efficacy of Angelica archangelica essential oil, phenyl ethyl alcohol and α- terpineol against isolated molds from walnut and their antiaflatoxigenic and antioxidant activity. J. Food Sci. Technol. 2015, 52, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Yu, C.-W.; Wu, S.-C.; Yih, K.-H. DPPH free-radical scavenging activity, total phenolic contents and chemical composition analysis of forty-two kinds of essential oils. J. Food Drug Anal. 2009, 17. [Google Scholar] [CrossRef]
- Pathak, S.; Wanjari, M.M.; Jain, S.K.; Tripathi, M. Evaluation of antiseizure activity of essential oil from roots of Angelica archangelica Linn. in mice. Indian J. Pharm. Sci. 2010, 72, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Bahr, T.A.; Rodriguez, D.; Beaumont, C.; Allred, K. The effects of various essential oils on epilepsy and acute seizure: A systematic review. Evid. Based Complement. Alternat. Med. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Fraternale, D.; Teodori, L.; Rudov, A.; Prattichizzo, F.; Olivieri, F.; Guidarelli, A.; Albertini, M.C. The in vitro activity of Angelica archangelica L. essential oil on inflammation. J. Med. Food 2018, 21, 1238–1243. [Google Scholar] [CrossRef]
- Nguyen, M.N. Study on Horseradish (Armoracia rusticana) Essential Oil and Comparison with the Related Species Debreceni Horseradish (Armoracia Macrocarpa); University of Debrecen: Debrecen, Hungary, 2016. [Google Scholar]
- Tomsone, L.; Kruma, Z.; Galoburda, R.; Talou, T. Composition of volatile compounds of horseradish roots (Armoracia rusticana L.) depending on the genotype. Rural Sustain. Res. 2013, 29, 1–10. [Google Scholar] [CrossRef]
- Shehata, A.; Mulwa, R.M.; Babadoost, M.; Uchanski, M.; Norton, M.A.; Skirvin, R.; Walters, S.A. Horseradish: Botany, horticulture, breeding. Hortic. Rev. 2009, 35, 221–261. [Google Scholar] [CrossRef]
- Uhl, S.R. Handbook of Spices, Seasonings, and Flavorings; Technomic Publishing Company Inc.: Lancaster, PA, USA, 2000. [Google Scholar]
- Agneta, R.; Möllers, C.; Rivelli, A.R. Horseradish (Armoracia rusticana), a neglected medical and condiment species with a relevant glucosinolate profile: A review. Genet. Resour. Crop. Evol. 2013, 60, 1923–1943. [Google Scholar] [CrossRef]
- Sampliner, D.; Miller, A. Ethnobotany of horseradish (Armoracia rusticana, Brassicaceae) and its wild relatives (Armoracia spp.): Reproductive biology and local uses in their native ranges. Econ. Bot. 2009, 63, 303–313. [Google Scholar] [CrossRef]
- Mazza, G. Volatiles in distillates of fresh, dehydrated and freeze dried horseradish. Can. Inst. Food Sci. Technol. J. 1984, 17, 18–23. [Google Scholar] [CrossRef]
- Cirimbei, M.R.; Dinică, R.; Gitin, L.; Vizireanu, C. Study on herbal actions of horseradish (Armoracia rusticana). J. Agroaliment. Proc. Technol. 2013, 19, 111–115. [Google Scholar]
- Petrović, S.; Drobac, M.; Ušjak, L.; Filipovic, V.; Milenković, M.; Niketic, M. Volatiles of roots of wild-growing and cultivated Armoracia macrocarpa and their antimicrobial activity, in comparison to horseradish, A. rusticana. Ind. Crops Prod. 2017, 109, 398–403. [Google Scholar] [CrossRef]
- Ren, J.J.; Zhang, D.; Hou, P.X.; Wu, H. Effects of horseradish oil (Armoracia rusticana) and eight isothiocyanates vapour treatment on the postharvest disease control and their efficacy as preservatives of mature green tomato. Plant. Dis. 2020, 104, 2688–2695. [Google Scholar] [CrossRef]
- Bentivenga, G.; D’Auria, M.; Mauriello, G.; Racioppi, R. SPME-GC-MS analysis of horseradish (Armoracia rusticana). Ital. J. Food Sci. 2004, 16, 487. [Google Scholar]
- Wu, H.; Zhang, G.-A.; Zeng, S.; Lin, K.-C. Extraction of allyl isothiocyanate from horseradish (Armoracia rusticana) and its fumigant insecticidal activity on four stored-product pests of paddy. Pest Manag. Sci. 2009, 65, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.Z.; Ding, R.X.; Ding, K.; Han, T.; Chen, X.N. Preparation of allyl isothiocyanate microencapsulation and its application in pork preservation. J. Food Process. Preserv. 2020, 44, e14709. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakamura, A.; Fujino, N.; Sawaguchi, Y.; Sato, M.; Kuda, T.; Kimura, B. Evaluation of the antibacterial activity of allyl isothiocyanate, clove oil, eugenol and carvacrol against spoilage lactic acid bacteria. LWT 2021, 145, 111263. [Google Scholar] [CrossRef]
- Nedorostova, L.; Kloucek, P.; Kokoska, L.; Stolcova, M.; Pulkrabek, J. Antimicrobial properties of selected essential oils in vapour phase against foodborne bacteria. Food Control. 2009, 20, 157–160. [Google Scholar] [CrossRef]
- Lin, C.M.; Kim, J.; Du, W.X.; Wei, C.I. Bactericidal activity of isothiocyanate against pathogens on fresh produce. J. Food Prot. 2000, 63, 25–30. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.-T.; Yin, J.-J. Effect of allyl isothiocyanate on antioxidants and fruit decay of blueberries. Food Chem. 2010, 120, 199–204. [Google Scholar] [CrossRef]
- Ogawa, T.; Isshiki, K. Antimicrobial activity of volatiles from edible herbs. Nippon. Shokubin Kagaku Kogaku Kaishi 1996, 43, 535–540. [Google Scholar] [CrossRef][Green Version]
- Chanjirakul, K.; Wang, S.; Wang, C.; Siriphanich, J. Natural volatile treatments increase free-radical scavenging capacity of strawberries and blackberries. J. Sci. Food Agric. 2007, 87, 1463–1472. [Google Scholar] [CrossRef]
- Colussi, R.; Ferreira da Silva, W.M.; Biduski, B.; Mello El Halal, S.L.; da Rosa Zavareze, E.; Guerra Dias, A.R. Postharvest quality and antioxidant activity extension of strawberry fruit using allyl isothiocyanate encapsulated by electrospun zein ultrafine fibers. LWT Food Sci. Technol. 2021, 143, 111087. [Google Scholar] [CrossRef]
- Kloucek, P.; Smid, J.; Flesar, J.; Havlik, J.; Titera, D.; Rada, V.; Drabek, O.; Kokoska, L. In Vitro inhibitory activity of essential oil vapors against Ascosphaera apis. Nat. Prod. Commun. 2012, 7, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An overview of their antimicrobial activity against human infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [PubMed]
- Nedorostova, L.; Kloucek, P.; Urbanova, K.; Kokoska, L.; Smid, J.; Urban, J.; Valterova, I.; Stolcova, M. Antibacterial effect of essential oil vapours against different strains of Staphylococcus aureus, including MRSA. Flavour Fragr. J. 2011, 26, 403–407. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Phan-a-god, S.; Shin, I.-S. Antibacterial activities of isothiocyanates extracted from horseradish (Armoracia rusticana) root against antibiotic-resistant bacteria. Food Sci. Biotechnol. 2015, 24, 1029–1034. [Google Scholar] [CrossRef]
- Bertoti, R.; Vasas, G.; Gonda, S.; Nguyen, N.M.; Szoke, E.; Jakab, A.; Pocsi, I.; Emri, T. Glutathione protects Candida albicans against horseradish volatile oil. J. Basic Microbiol. 2016, 56, 1071–1079. [Google Scholar] [CrossRef]
- Choi, K.-D.; Kim, H.-Y.; Shin, I. Antifungal activity of isothiocyanates extracted from horseradish (Armoracia rusticana) root against pathogenic dermal fungi. Food Sci. Biotechnol. 2017, 26, 847–852. [Google Scholar] [CrossRef]
- Kim, S.-I.; Roh, J.-Y.; Kim, D.-H.; Lee, H.-S.; Ahn, Y.-J. Insecticidal activities of aromatic plant extracts and essential oils against Sitophilus oryzae and Callosobruchus chinensis. J. Stored Prod. Res. 2003, 39, 293–303. [Google Scholar] [CrossRef]
- Tsao, R.; Peterson, C.; Coats, J. Glucosinolate breakdown products as insect fumigants and their effect on carbon dioxide emission of insects. BMC Ecol. 2002, 2, 5. [Google Scholar] [CrossRef]
- Park, I.K.; Choi, K.S.; Kim, D.H.; Choi, I.H.; Kim, L.S.; Bak, W.C.; Choi, J.W.; Shin, S.C. Fumigant activity of plant essential oils and components from horseradish (Armoracia rusticana), anise (Pimpinella anisum) and garlic (Allium sativum) oils against Lycoriella ingenua (Diptera: Sciaridae). Pest Manag. Sci. 2006, 62, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Akinkurolere, R.O.; Zhang, H. Fumigant activity of plant essential oil from Armoracia rusticana (L.) on Plodia interpunctella (Lepidoptera: Pyralidae) and Sitophilus zeamais (Coleoptera: Curculionidae). Afr. J. Biotechnol. 2011, 10, 1200–1205. [Google Scholar]
- Xie, L.D.; He, Y.P.; Chen, S.S.; Xu, L.; He, J. A study of fumigation toxicity of horseradish essential oil against two stored grain insects. In Proceedings of the 10th International Working Conference on Stored Product Protection, 27 June–2 July 2010; Estoril, Portugal; pp. 464–468. [Google Scholar]
- Yun, Y.-K.; Kim, H.-K.; Kim, J.-R.; Hwang, K.; Ahn, Y.-J. Contact and fumigant toxicity of Armoracia rusticana essential oil, allyl isothiocyanate and related compounds to Dermatophagoides farinae. Pest Manag. Sci. 2012, 68, 788–794. [Google Scholar] [CrossRef]
- Yeger, H.; Mokhtari, R.B. Perspective on dietary isothiocyanates in the prevention, development and treatment of cancer. J. Cancer Metastasis Treat. 2020, 2020, 26. [Google Scholar] [CrossRef]
- Majewska, A.; Bałasińska, B.; Dąbrowska, B. Antioxidant properties of leaf and root extract and oil from different types of horseradish (Armoracia rusticana Gaertn.). Folia Hortic. 2004, 16, 15–22. [Google Scholar]
- Hudlikar, R.; Wang, L.; Wu, R.; Li, S.; Peter, R.; Shannar, A.; Chou, P.J.; Liu, X.; Liu, Z.; Kuo, H.-C.; et al. Epigenetics/epigenomics and prevention of early stages of cancer by isothiocyanates. Cancer Prev. Res. 2021, 14, 151–164. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Preetha, P.; Haque, S.; Akhter, N.; Khan, S.; Ahmed, S.; Hussain, A. Dietary isothiocyanates inhibit cancer progression by modulation of epigenome. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021; pp. 30281–30289. [Google Scholar] [CrossRef]
- Osipitan, O.O.; Shi, Y.; Di Pasqua, A.J. Phenethyl isothiocyanate-containing carbomer gel for use against squamous cell carcinoma. Pharmaceutics 2021, 13, 106. [Google Scholar] [CrossRef]
- Zhang, Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res. 2010, 54, 127–135. [Google Scholar] [CrossRef]
- Thangarasu, R.; Pachaiappan, P.; Subbaiyan, T. Anti-estrogenic and anti-cell proliferative effect of allyl isothiocyanate in chemoprevention of chemically induced mammary carcinogenesis in rats. Pathol. Oncol. Res. 2020, 26, 913–925. [Google Scholar] [CrossRef]
- Chiang, J.H.; Tsai, F.J.; Hsu, Y.M.; Yin, M.C.; Chiu, H.Y.; Yang, J.S. Sensitivity of allyl isothiocyanate to induce apoptosis via ER stress and the mitochondrial pathway upon ROS production in colorectal adenocarcinoma cells. Oncol. Rep. 2020, 44, 1415–1424. [Google Scholar] [CrossRef]
- Chang, P.-Y.; Tsai, F.-J.; Bau, D.-T.; Hsu, Y.-M.; Yang, J.-S.; Tu, M.-G.; Chiang, S.-L. Potential effects of allyl isothiocyanate on inhibiting cellular proliferation and inducing apoptotic pathway in human cisplatin-resistant oral cancer cells. J. Formos. Med. Assoc. 2021, 120, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H. Flora Europaea: Plantaginaceae to Compositae (and Rubiaceae); Cambridge University Press: Cambridge, UK, 1964; Volume 4. [Google Scholar]
- Benelli, G.; Pavela, R.; Petrelli, R.; Nzekoue, F.K.; Cappellacci, L.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Sut, S.; Dall’Acqua, S.; et al. Carlina oxide from Carlina acaulis root essential oil acts as a potent mosquito larvicide. Ind. Crops Prod. 2019, 137, 356–366. [Google Scholar] [CrossRef]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Załuski, D.; Verpoorte, R. Historical and traditional medical applications of Carlina acaulis L. - A critical ethnopharmacological review. J. Ethnopharmacol. 2019, 239, 111842. [Google Scholar] [CrossRef]
- Strzemski, M.; Dresler, S.; Sowa, I.; Czubacka, A.; Agacka-Mołdoch, M.; Płachno, B.J.; Granica, S.; Feldo, M.; Wójciak-Kosior, M. The impact of different cultivation systems on the content of selected secondary metabolites and antioxidant activity of Carlina acaulis plant material. Molecules 2019, 25, 146. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M. Food medicine and minor nourishment in the folk traditions of Central Italy (Marche, Abruzzo and Latium). Fitoterapia 2003, 74, 515–544. [Google Scholar] [CrossRef]
- GazzettaUfficiale. 2018. Available online: https://www.gazzettaufficiale.it/eli/gu/2018/09/26/224/sg/pdf (accessed on 25 October 2020).
- Cousyn, G.; Dalfrà, S.; Scarpa, B.; Geelen, J. Project BELFRIT-Harmonizing the use of plants in food supplements in the European Union: Belguim, France and Italy-A first step. Eur. Food Feed L. Rev. 2013, 8, 187. [Google Scholar]
- Đorđević, S.; Lakušić, B.; Petrović, S.; Niketić, M. Morpho-anatomical characteristics of Carlina acaulis subsp. caulescens and C. acanthifolia subsp. utzka (Asteraceae). Arh. Farm. 2004, 54, 773–783. [Google Scholar]
- Stojanović-Radić, Z.; Čomić, L.; Radulović, N.; Blagojević, P.; Mihajilov-Krstev, T.; Rajković, J. Commercial Carlinae radix herbal drug: Botanical identity, chemical composition and antimicrobial properties. Pharm. Biolog. 2012, 50, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, S.; Petrovic, S.; Ristic, M.; Djokovic, D. Composition of Carlina acanthifolia root essential oil. Chem. Nat. Compd. 2005, 41, 410–412. [Google Scholar] [CrossRef]
- Schilcher, H.; Hagels, H. Carlinae radix: Verfälschung, verwechslung oder ersatzdroge. Dtsch. Apoth. Ztg. 1990, 130, 2186–2190. [Google Scholar]
- Chalchat, J.C.; Djordjevic, S.; Gorunovic, M. Composition of the essential oil from the root of Carlina acaulis L. Asteraceae. J. Essent. Oil Res. 1996, 8, 577–578. [Google Scholar] [CrossRef]
- Vitkova, A.; Evstatieva, L. Plants rich in inulin from family Asteraceae. God. Sofiskiya Univ. Kliment Okhridski Kn. 2002, 2, 107–111. [Google Scholar]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Agacka-Mołdoch, M.; Drączkowski, P.; Matosiuk, D.; Kurach, Ł.; Kocjan, R.; Dresler, S. Application of raman spectroscopy for direct analysis of Carlina acanthifolia subsp. utzka root essential oil. Talanta 2017, 174, 633–637. [Google Scholar] [CrossRef]
- Dordević, S.; Petrović, S.; Dobrić, S.; Milenković, M.; Vucićević, D.; Zizić, S.; Kukić, J. Antimicrobial, anti-inflammatory, anti-ulcer and antioxidant activities of Carlina acanthifolia root essential oil. J. Ethnopharmacol. 2007, 109, 458–463. [Google Scholar] [CrossRef]
- Benelli, G.; Rizzo, R.; Zeni, V.; Govigli, A.; Samková, A.; Sinacori, M.; Verde, G.; Pavela, R.; Cappellacci, L.; Petrelli, R.; et al. Carlina acaulis and Trachyspermum ammi essential oils formulated in protein baits are highly toxic and reduce aggressiveness in the medfly, Ceratitis capitata. Ind. Crops Prod. 2021, 161, 113191. [Google Scholar] [CrossRef]
- Dresler, S.; Hawrylak-Nowak, B.; Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Hanaka, A.; Gołoś, I.; Skalska-Kamińska, A.; Cieślak, M.; Kováčik, J. Metabolic changes induced by silver ions in Carlina acaulis. Plants 2019, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.; Hamoud, R.; Sporer, F.; Tahrani, A.; Wink, M. Carlina oxide- A natural polyacetylene from Carlina acaulis (Asteraceae) with potent antitrypanosomal and antimicrobial properties. Planta Med. 2011, 77, 1905–1911. [Google Scholar] [CrossRef]
- Mami, I.; Belabbes, R.; Dib, M.; Boufeldja, T.; Costa, J.; Muselli, A. Biological activities of Carlina oxide isolated from the roots of Carthamus caeruleus. J. Nat. Prod. 2020, 10, 145–152. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Petrelli, R.; Cappellacci, L.; Buccioni, M.; Palmieri, A.; Canale, A.; Benelli, G. Outstanding insecticidal activity and sublethal effects of Carlina acaulis root essential oil on the housefly, Musca domestica, with insights on its toxicity on human cells. Food Chem. Toxicol. 2020, 136, 111037. [Google Scholar] [CrossRef]
- Benelli, G.; Pavoni, L.; Zeni, V.; Ricciardi, R.; Cosci, F.; Cacopardo, G.; Gendusa, S.; Spinozzi, E.; Petrelli, R.; Cappellacci, L.; et al. Developing a highly stable Carlina acaulis essential oil nanoemulsion for managing Lobesia botrana. Nanomaterials 2020, 10, 1867. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Cappellacci, L.; Petrelli, R.; Spinozzi, E.; Aguzzi, C.; Zeppa, L.; Ubaldi, M.; et al. Encapsulation of Carlina acaulis essential oil and carlina oxide to develop long-lasting mosquito larvicides: Microemulsions versus nanoemulsions. J. Pest Sci. 2021. [Google Scholar] [CrossRef]
- Bhushan, B.; Sharma, S.K.; Singh, T.; Singh, L.; Arya, H. Vetiveria Zizanioides (Linn.) Nash: A pharmacological overview. Int. Res. J. Pharm. 2013, 4, 18–20. [Google Scholar] [CrossRef]
- Chomchalow, N.; Chapman, K. Other uses and utilizations of vetiver. AU J. Technol. 2003, 7, 81–91. [Google Scholar]
- Mallavarapu, G.R.; Syamasundar, K.V.; Ramesh, S.; Rao, B.R.R. Constituents of south Indian vetiver oils. Nat. Prod. Commun. 2012, 7, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Massardo, D.R.; Senatore, F.; Alifano, P.; Giudice, L.; Pontieri, P. Vetiver oil production correlates with early root growth. Biochem. Syst. Ecol. 2006, 34, 376–382. [Google Scholar] [CrossRef]
- Pandey, J.; Verma, R.; Singh, S. Suitability of aromatic plants for phytoremediation of heavy metal contaminated areas: A review. Int. J. Phytoremediat. 2019, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.C.; Nasrulhaq Boyce, A.; Bin Abas, M.R.; Mahmood, N.; Han, F. Evaluation of Vetiver grass uptake efficiency in single and mixed heavy metal contaminated soil. Environ. Process. 2020, 7, 207–226. [Google Scholar] [CrossRef]
- Ramírez-Rueda, R.; Marinho, J.; Salvador, M. Bioguided identification of antimicrobial compounds from Chrysopogon zizaniodes (L.) roberty root essential oil. Future Microbiol. 2019, 14, 1179–1189. [Google Scholar] [CrossRef]
- Chintani, Y.S.; Butarbutar, E.S.; Nugroho, A.P.; Sembiring, T. Uptake and release of chromium and nickel by Vetiver grass (Chrysopogon zizanioides (L.) Roberty). SN Appl. Sci. 2021, 3, 285. [Google Scholar] [CrossRef]
- Balasankar, D.; Vanilarasu, K.; Preetha, P.; Rajeswari, S.; Umadevi, M.; Bhowmik, D. Traditional and medicinal uses of vetiver. J. Med. Plants Stud. 2013, 1, 191–200. [Google Scholar]
- Rajasekhar, C.H.; Kokila, B.N.; Rakesh; Rajesh, B. Potentiating effect of Vetiveria zizanioides root extract and essential oil on phenobarbital induced sedation-hypnosis in swiss albino mice. J. Exp. Pharmacol. 2014, 4, 89–93. [Google Scholar]
- Suyono, H.; Jong, F.X.; Wijaya, S. Lavender, cedarwood, vetiver balm work as anti-stress treatment by reducing plasma cortisol level. Rec. Nat. Prod. 2020, 8, 10–12. [Google Scholar]
- Thubthimthed, S.; Thisayakorn, K.; Rerk-am, U.; Tangstirapakdee, S.; Suntorntanasat, T. Vetiver oil and its sedative effect. In Proceedings of the 3rd International Vetiver Conference, Guangzhou, China, 6–9 October 2003; pp. 492–494. [Google Scholar]
- Han, X.; Parker, T.L. Biological activity of vetiver (Vetiveria zizanioides) essential oil in human dermal fibroblasts. Cogent Med. 2017, 4, 1298176. [Google Scholar] [CrossRef]
- Sinha, S.; Jothiramajayam, M.; Ghosh, M.; Mukherjee, A. Evaluation of toxicity of essential oils palmarosa, citronella, lemongrass and vetiver in human lymphocytes. Food Chem. Toxicol. 2014, 68, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Weyerstahl, P.; Marschall, H.; Splittgerber, U.; Wolf, D.; Surburg, H. Constituents of Haitian vetiver oil. Flavour Fragr. J. 2000, 15, 395–412. [Google Scholar] [CrossRef]
- Ouyang, J.; Bae, H.; Jordi, S.; Dao, Q.M.; Dossenbach, S.; Dehn, S.; Lingnau, J.B.; Kanta De, C.; Kraft, P.; List, B. The smelling principle of vetiver oil, unveiled by chemical synthesis. Angew. Chem. Int. Ed. Engl. 2021, 60, 5666–5672. [Google Scholar] [CrossRef]
- Council of Europe. N. s. o. f., 1st Ed. ed; Council of Europe: Strasbourg, France, 2000; Volume I. [Google Scholar]
- Champagnat, P.; Figueredo, G.; Chalchat, J.-C.; Carnat, A.-P.; Bessière, J.-M. A study on the composition of commercial Vetiveria zizanioides oils from different geographical origins. J. Essent. Oil Res. 2006, 18, 416–422. [Google Scholar] [CrossRef]
- Belhassen, E.; Filippi, J.-J.; Brévard, H.; Joulain, D.; Baldovini, N. Volatile constituents of vetiver: A review. Flavour. Fragr. J. 2015, 30, 26–82. [Google Scholar] [CrossRef]
- Adams, R.P.; Pandey, R.N.; Dafforn, M.R.; James, S.A. Vetiver DNA-fingerprinted cultivars: Effects of environment on growth, oil yields and composition. J. Essent. Oil Res. 2003, 15, 363–371. [Google Scholar] [CrossRef]
- Ganguly, R.N.; Trivedi, G.K. Chemosystemization of Indian vetiver oil through biogenetic missing links.; Aspect Publishing: London, UK, 1990; pp. 119–126. [Google Scholar]
- Soidrou, S.H.; Farah, A.; Satrani, B.; Ghanmi, M.; Jennan, S.; Hassane, S.O.S.; Lachkar, M.; El Abed, S.; Ibnsouda Koraichi, S.; Bousta, D. Fungicidal activity of four essential oils from Piper capense, Piper borbonense and Vetiveria zizanoides growing in Comoros against fungi decay wood. J. Essent. Oil Res. 2013, 25, 216–223. [Google Scholar] [CrossRef]
- Cheaha, D.; Issuriya, A.; Manor, R.; Kwangjai, J.; Rujiralai, T.; Kumarnsit, E. Modification of sleep-waking and electroencephalogram induced by vetiver essential oil inhalation. J. Intercult. Ethnopharmacol. 2016, 5, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Atif, M.; Ilavenil, S.; Devanesan, S.; AlSalhi, M.S.; Choi, K.C.; Vijayaraghavan, P.; Alfuraydi, A.A.; Alanazi, N.F. Essential oils of two medicinal plants and protective properties of jack fruits against the spoilage bacteria and fungi. Ind. Crops Prod. 2020, 147, 112239. [Google Scholar] [CrossRef]
- Chou, S.-T.; Lai, C.-P.; Lin, C.-C.; Shih, Y. Study of the chemical composition, antioxidant activity and anti-inflammatory activity of essential oil from Vetiveria zizanioides. Food Chem. 2012, 134, 262–268. [Google Scholar] [CrossRef]
- Del Giudice, L.; Massardo, D.R.; Pontieri, P.; Bertea, C.M.; Mombello, D.; Carata, E.; Tredici, S.M.; Talà, A.; Mucciarelli, M.; Groudeva, V.I.; et al. The microbial community of Vetiver root and its involvement into essential oil biogenesis. Environ. Microbiol. 2008, 10, 2824–2841. [Google Scholar] [CrossRef] [PubMed]
- Thara Saraswathi, K.J.; Jayalakshmi, N.R.; Vyshali, P.; Kameshwari, M.N.S. Comparitive study on essential oil in natural and in vitro regenerated plants of Vetiveria zizanioides (Linn.) Nash. Am. Eurasian J. Agric. Environ. Sci. 2011, 10, 458–463. [Google Scholar]
- Adams, R.P.; Habte, M.; Park, S.; Dafforn, M.R. Preliminary comparison of vetiver root essential oils from cleansed (bacteria- and fungus-free) versus non-cleansed (normal) vetiver plants. Biochem. System. Ecol. 2004, 32, 1137–1144. [Google Scholar] [CrossRef]
- Rotkittikhun, P.; Kruatrachue, M.; Pokethitiyook, P.; Baker, A.J. Tolerance and accumulation of lead in Vetiveria zizanioides and its effect on oil production. J. Environ. Biol. 2010, 31, 329–334. [Google Scholar]
- Sulastri, Y.; Tampubolon, K. Aromatic plants: Phytoremediation of cadmium heavy metal and the relationship to essential oil production. Int. J. Adv. Sci. Technol. 2019, 8, 1064–1069. [Google Scholar]
- Gautam, M.; Agrawal, M. Application potential of Chrysopogon zizanioides (L.) Roberty for the remediation of red mud-treated soil: An analysis via determining alterations in essential oil content and composition. Int. J. Phytoremediat. 2021, 1–9. [Google Scholar] [CrossRef]
- Pripdeevech, P.; Wongpornchai, S.; Promsiri, A. Highly volatile constituents of Vetiveria zizanioides roots grown under different cultivation conditions. Molecules 2006, 11, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, X.; Kim, H. Antioxidant, Anticarcinogenic and Termiticidal Activities of Vetiver Oil. In Proceedings of the 3rd International Vetiver Conference, Guangzhou, China, 6–9 October 2003. [Google Scholar]
- Champagnat, P.; Sidibé, L.; Forestier, C.; Carnat, A.; Chalchat, J.-C.; Lamaison, J.-L. Antimicrobial activity of essential oils from Vetiveria nigritana and Vetiveria zizanioides roots. J. Essent. Oil Bear. Plants 2007, 10, 519–524. [Google Scholar] [CrossRef]
- Burger, P.; Landreau, A.; Watson, M.; Janci, L.; Cassisa, V.; Kempf, M.; Azoulay, S.; Fernandez, X. Vetiver essential oil in cosmetics: What is new? Medicines 2017, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dwivedi, G.R.; Darokar, M.P.; Srivastava, S.K. Antimycobacterial activity of fractions and isolated compounds from Vetiveria zizanioides. Med. Chem. Res. 2011, 21, 1283–1289. [Google Scholar] [CrossRef]
- Efe, D. The evaluation of the antibacterial activity of Vetiveria zizanioides (L.) Nash grown in Giresun, Turkey. Alinteri J. Agr. Sci. 2019, 34, 21–24. [Google Scholar] [CrossRef]
- Nidhi, D.; Raghav, C.S.; Gupta, R.L.; Chhonkar, S.S. Chemical composition and antifungal activity of vetiver oil of North and South India against Rhizoctonia solani. Pest Res. J. 2010, 22, 63–67. [Google Scholar]
- Nararak, J.; Sathantriphop, S.; Chauhan, K.; Tantakom, S.; Eiden, A.L.; Chareonviriyaphap, T. Avoidance behavior to essential oils by Anopheles minimus, a malaria vector in Thailand. J. Am. Mosq. Control. Assoc. 2016, 32, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Khater, H.F.; Ali, A.M.; Abouelella, G.A.; Marawan, M.A.; Govindarajan, M.; Murugan, K.; Abbas, R.Z.; Vaz, N.P.; Benelli, G. Toxicity and growth inhibition potential of vetiver, cinnamon, and lavender essential oils and their blends against larvae of the sheep blowfly, Lucilia sericata. Int. J. Dermatol. 2018, 57, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Bulgarini, G.; Bortolini, S.; Maistrello, L. Repellent activity of essential oils on adults of Halyomorpha halys (Heteroptera: Pentatomidae) in different physiological-behavioural phases. J. Appl. Entomol. 2021, 1–12. [Google Scholar] [CrossRef]
- Bhardwaj, U.; Chahal, K.; Dhillon, N.K.; Kaur, R. Nematicidal activity of vetiver essential oil and its fractions against Meloidogyne incognita. Indian J. Nematol. 2017, 47, 43–50. [Google Scholar]
- Jain, S.; Nowicki, S.; Eisner, T.; Meinwald, J. Insect Repellents from Vetiver Oil: I. Zizanal and Epizizanal; Tetrahedron Letters: Amsterdam, The Netherlands, 1982; pp. 4639–4642. [Google Scholar]
- Campos, R.N.; Nascimento Lima, C.B.; Passos Oliveira, A.; Albano Araújo, A.P.; Fitzgerald Blank, A.; Barreto Alves, P.; Nascimento Lima, R.; Albano Araújo, V.; Santana, A.S.; Bacci, L. Acaricidal properties of vetiver essential oil from Chrysopogon zizanioides (Poaceae) against the tick species Amblyomma cajennense and Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Vet. Parasitol. 2015, 212, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Saiyudthong, S.; Pongmayteegul, S.; Marsden, C.A.; Phansuwan-Pujito, P. Anxiety-like behaviour and c-fos expression in rats that inhaled vetiver essential oil. Nat. Prod. Res. 2015, 29, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Itano, I.S.S.; Yuka, A.; Tanabe, T.; Sorai, Y.; Nakagawa, A.; Takebayashi, N. The preliminary study about effects of essential oils by near infrared spectroscopy; The relations between fragrance and stress with blood volume in the frontal lobe of brain. Aroma 2006, Res 28, 54–59. [Google Scholar]
- Matsubara, E.; Shimizu, K.; Fukagawa, M.; Ishizi, Y.; Kakoi, C.; Hatayama, T.; Nagano, J.; Okamoto, T.; Ohnuki, K.; Kondo, R. Volatiles emitted from the roots of Vetiveria zizanioides suppress the decline in attention during a visual display terminal task. Biomed. Res. 2012, 33, 299–308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, P.; Tripathi, L. Anticonvulsant potential of essential oil of Vetiveria zizanioides Linn. Nash. Asian J. Chem. 2013, 25, 5111–5112. [Google Scholar] [CrossRef]
- Kumar, R.; Ruckmani, A.; Saradha, S.; Arunkumar, R.; Prabhu, R.; Madhavi, E.; Devi, T. Evaluation of antiepileptic activity of Vetiveria zizanioides oil in mice. Int. J. Pharm. 2014, 25, 248–251. [Google Scholar]
- Sivakumar, L.; Chellappan, D.R.; Sriramavaratharajan, V.; Murugan, R. Root essential oil of Chrysopogon zizanioides relaxes rat isolated thoracic aorta—An ex vivo approach. Z. Naturforsch. C J. Biosci. 2021, 76, 161–168. [Google Scholar] [CrossRef]
- Kim, H.; Chen, F.; Wang, X.; Chung, H.Y.; Jin, Z. Evaluation of antioxidant activity of vetiver (Vetiveria zizanioides L.) oil and identification of its antioxidant constituents. J. Agric. Food Chem. 2005, 53, 7691–7695. [Google Scholar] [CrossRef]
- Sinha, S.; Jothiramajayam, M.; Ghosh, M.; Jana, A.; Chatterji, U.; Mukherjee, A. Vetiver oil (Java) attenuates cisplatin-induced oxidative stress, nephrotoxicity and myelosuppression in Swiss albino mice. Food Chem. Toxicol. 2015, 81, 120–128. [Google Scholar] [CrossRef]
- Peng, H.-Y.; Lai, C.-C.; Lin, C.-C.; Chou, S.-T. Effect of Vetiveria zizanioides essential oil on melanogenesis in melanoma cells: Downregulation of tyrosinase expression and suppression of oxidative stress. Sci. World J. 2014, 2014, 213013. [Google Scholar] [CrossRef]
- Raja, S.; Rani, J.; Mohapatra, S.; Devaraj, A. Effect of Vetiveria zizanioides on experimentally induced dyslipidemia. Int. J. Pharma Bio Sci. 2017, 8, 351–359. [Google Scholar] [CrossRef]
- Shivaprasad, H.N.; Pandit, S.; Bhanumathy, M.; Manohar, D.; Jain, V.; Thandu, S.A.; Su, X. Ethnopharmacological and phytomedical knowledge of Coleus forskohlii: An approach towards its safety and therapeutic value. Orient. Pharm. Exp. Med. 2014, 14, 301–312. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Chaubey, M.; Khatoon, S.; Rawat, A.; Mehrotra, S. Pharmacognostic evaluation of Coleus forskohlii. Pharm. Biol. 2002, 40, 129–134. [Google Scholar] [CrossRef]
- Paul, M.; Radha, A.; Kumar, D.S. On the high value medicinal plant Coleus forskohlii Briq. Hygeia 2013, 5, 69–78. [Google Scholar]
- Kavitha, C.; Rajamani, K.; Vadivel, E. Coleus forskohlii: A comprehensive review on morphology, phytochemistry and pharmacological aspects. J. Med. Plant. Res. 2010, 4, 278–285. [Google Scholar]
- Chowdhury, A.R.; Sharma, M. GC-MS investigations on the essential oil from Coleus forskohlii (Willd.) Briq. Indian Perfum. 1998, 42, 15–16. [Google Scholar]
- Lukhoba, C.W.; Simmonds, M.S.; Paton, A.J. Plectranthus: A review of ethnobotanical uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [CrossRef]
- Salehi, B.; Staniak, M.; Czopek, K.; Stępień, A.; Dua, K.; Wadhwa, R.; Kumar Chellappan, D.; Sytar, O.; Brestic, M.; Ganesh Bhat, N.; et al. The therapeutic potential of the labdane diterpenoid forskolin. Appl. Sci. 2019, 9, 4089. [Google Scholar] [CrossRef]
- Alasbahi, R.H.; Melzig, M.F. Plectranthus barbatus: A review of phytochemistry, ethnobotanical uses and pharmacology—Part 1. Planta Med. 2010, 76, 653–661. [Google Scholar] [CrossRef]
- Misra, L.N.; Tyagi, B.R.; Ahmad, A.; Bahl, J.R. Variability in the chemical composition of the essential oil of Coleus forskohlii genotypes. J. Essent. Oil Res. 1994, 6, 243–247. [Google Scholar] [CrossRef]
- Majeed, M.; Prakash, S. Composition and Methods Containing an Antimicrobial Essential Oil Extended from Coleus forskohlii. U.S. Patent 6,607,712 B2, 19 August 2003. [Google Scholar]
- Gelmini, F.; Squillace, P.; Testa, C.; Sparacino, A.; Angioletti, S.; Beretta, G. GC MS characterisation and biological activity of essential oils from different vegetative organs of Plectranthus barbatus and Plectranthus caninus cultivated in north Italy. Nat. Prod. Res. 2015, 29, 993–998. [Google Scholar] [CrossRef]
- Kerntopf, M.R.; de Albuquerque, R.L.; Machado, M.I.L.; Matos, F.J.A.; Craveiro, A.A. Essential oils from leaves, stems and roots of Plectranthus barbatus Andr.(Labiatae) grown in Brazil. J. Essent. Oil Res. 2002, 14, 101–102. [Google Scholar] [CrossRef]
- Rana, P.S.; Saklani, P.; Chandel, C. Influence of altitude on secondary metabolites and antioxidant activity of Coleus forskohlii root extracts. Res. J. Medicinal. Plant. 2020, 14, 43–52. [Google Scholar] [CrossRef]
- Tamboli, E.T.; Chester, K.; Ahmad, S. Quality control aspects of herbs and botanicals in developing countries: Coleus forskohlii Briq a case study. J. Pharm. Bioallied Sci. 2015, 7, 254. [Google Scholar] [CrossRef]
- Takshak, S.; Agrawal, S.B. The role of supplemental ultraviolet-B radiation in altering the metabolite profile, essential oil content and composition, and free radical scavenging activities of Coleus forskohlii, an indigenous medicinal plant. Environ. Sci. Pollut. Res. 2016, 23, 7324–7337. [Google Scholar] [CrossRef]
- Chatterjee, B.; Vittal, R.R. Quorum sensing modulatory and biofilm inhibitory activity of Plectranthus barbatus essential oil: A novel intervention strategy. Arch. Microbiol. 2021, 203, 1767–1778. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Dimitrijević, M.; Genčić, M.; Pejčić, M.; Radulović, N. Anticandidal activity of Inula helenium root essential oil: Synergistic potential, anti-virulence efficacy and mechanism of action. Ind. Crops Prod. 2020, 149, 112373. [Google Scholar] [CrossRef]
- Konishi, T.; Kondo, S.; Uchiyama, N. Larvicidal activities of sesquiterpenes from Inula helenium (Compositae) against Aedes albopictus (Diptera: Culicidae) and Paratanytarsus grimmii (Diptera: Chironomidae). Appl. Entomol. Zool. 2008, 43, 77–81. [Google Scholar] [CrossRef][Green Version]
- Buza, V.; Matei, M.-C.; Ștefănuț, L.C. Inula helenium: A literature review on ethnomedical uses, bioactive compounds and pharmacological activities. Lucr. Ştiinţ. Ser. Med. Vet. 2020, 63, 53–59. [Google Scholar]
- Sowndhararajan, K.; Cho, H.; Yu, B.; Song, J.; Kim, S. Effect of inhalation of essential oil from Inula helenium L. root on electroencephalographic (EEG) activity of the human brain. Eur. J. Integr. Med. 2016, 8, 453–457. [Google Scholar] [CrossRef]
- Bourrel, C.; Vilarem, G.; Perineau, F. Chemical analysis, bacteriostatic and fungistatic properties of the essential oil of elecampane (Inula helenium L.). J. Essent. Oil Res. 1993, 5, 411–417. [Google Scholar] [CrossRef]
- Paulsen, E. Contact sensitization from Compositae-containing herbal remedies and cosmetics. Contact Derm. 2002, 47, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Deriu, A.; Zanetti, S.; Sechi, L.A.; Marongiu, B.; Piras, A.; Porcedda, S.; Tuveri, E. Antimicrobial activity of Inula helenium L. essential oil against gram positive, gram negative bacteria and Candida ssp. Int. J. Antimicrob. Agents. 2008, 31, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ochoa, L.; Vilarem, G.; Mouloungui, Z.; Medina-Gonzalez, Y. Comparing the effect of utilization of ethyl heptanoate as co-solvent during extraction of essential oil of Inula helenium in a hydrodistillation process. Chem. Nat. Compd. 2012, 47, 995–997. [Google Scholar] [CrossRef]
- Rasul, A.; Khan, M.; Ali, M.; Li, J.; Li, X. Targeting apoptosis pathways in cancer with alantolactone and isoalantolactone. Sci. World J. 2013, 5, 248532. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xie, Y.; Hu, H. Antitumor activity and mechanism of costunolide and dehydrocostus lactone: Two natural sesquiterpene lactones from the Asteraceae family. Biomed. Pharmacother. 2020, 125, 109955. [Google Scholar] [CrossRef]
- Lepoittevin, J.-P.; Berl, V.; Giménez-Arnau, E. α-Methylene-γ-butyrolactones: Versatile skin bioactive natural products. Chem. Rec. 2009, 9, 258–270. [Google Scholar] [CrossRef]
- Qiu, J.; Luo, M.; Wang, J.; Dong, J.; Li, H.; Leng, B.; Zhang, Q.; Dai, X.; Zhang, Y.; Niu, X.; et al. Isoalantolactone protects against Staphylococcus aureus pneumonia. FEMS Microbiol. Lett. 2011, 324, 147–155. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, Y.; Wen, Z.; Ci, X.; Xia, L.; Wang, Y.; Deng, X.; Wang, J. Isoalantolactone enhances the antimicrobial activity of penicillin G against Staphylococcus aureus by inactivating β-lactamase during protein translation. Pathogens 2020, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Gierlikowska, B.; Gierlikowski, W.; Demkow, U. Alantolactone enhances the phagocytic properties of human macrophages and modulates their proinflammatory functions. Front. Pharmacol. 2020, 11, 1339. [Google Scholar] [CrossRef]
- Lu, N.; Lv, Q.; Sun, X.; Zhou, Y.; Guo, Y.; Qiu, J.; Zhang, P.; Wang, J. Isoalantolactone restores the sensitivity of gram-negative Enterobacteriaceae carrying MCR-1 to carbapenems. J. Cell. Mol. Med. 2020, 24, 2475–2483. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, L.; Ma, Z.; Sui, Y.; Xie, J.N.; Ma, T.; Yang, L. Antifungal effects of alantolactone on Candida albicans: An in vitro study. bioRxiv 2021. [Google Scholar] [CrossRef]
- Budán, F.; Nan, M.; Kocsis, B.; Laczko-Zöld, E. Antimicrobial activity and potential secondary signal transduction mechanisms of elecampane (Inula helenium L.) root extract. Plant. Cell Biotechnol. Mol. Biol. 2021, 22, 86–92. [Google Scholar]
- Liu, C.; Mishra, A.; He, B.; Tan, R. Antimicrobial activities of isoalantolactone, a major sesquiterpene lactone of Inula racemosa. Chin. Sci. Bull. 2001, 46, 498–501. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Abate, L.; Fronczek, F.R.; Franzblau, S.G.; Quijano, L.; Fischer, N.H. Antimycobacterial eudesmanolides from Inula helenium and Rudbeckia subtomentosa. Planta Med. 1999, 65, 351–355. [Google Scholar] [CrossRef]
- Rezeng, C.; Yuan, D.; Long, J.; Suonan, D.; Yang, F.; Li, W.; Tong, L.; Jiumei, P. Alantolactone exhibited anti-herpes simplex virus 1 (HSV-1) action in vitro. Biosci. Trends 2016, 9, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, C.L.; Pridgeon, J.W.; Fronczek, F.R.; Becnel, J.J. Structure-activity relationship studies on derivatives of eudesmanolides from Inula helenium as toxicants against Aedes aegypti larvae and adults. Chem. Biodivers. 2010, 7, 1681–1697. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lan, Y.L.; Xing, J.S.; Lan, X.Q.; Wang, L.T.; Zhang, B. Alantolactone plays neuroprotective roles in traumatic brain injury in rats via anti-inflammatory, anti-oxidative and anti-apoptosis pathways. Am. J. Transl. Res. 2018, 10, 368–380. [Google Scholar] [PubMed]
- Ding, Y.-H.; Song, Y.-D.; Wu, Y.-X.; He, H.-Q.; Yu, T.-H.; Hu, Y.-D.; Zhang, D.-P.; Jiang, H.-C.; Yu, K.-K.; Li, X.-Z.; et al. Isoalantolactone suppresses LPS-induced inflammation by inhibiting TRAF6 ubiquitination and alleviates acute lung injury. Acta Pharmacol. Sin. 2019, 40, 64–74. [Google Scholar] [CrossRef]
- Chun, J.; Choi, R.J.; Khan, S.; Lee, D.S.; Kim, Y.C.; Nam, Y.J.; Lee, D.U.; Kim, Y.S. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-κB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int. Immunopharmacol. 2012, 14, 375–383. [Google Scholar] [CrossRef]
- Chen, H.-L.; Lin, S.C.; Li, S.; Tang, K.-T.; Lin, C.-C. Alantolactone alleviates collagen-induced arthritis and inhibits Th17 cell differentiation through modulation of STAT3 signalling. Pharmaceutical. Biol. 2021, 59, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Cakir, M.; Karayigit, M.O.; Eken, A.; Donmez-Altuntas, H. The effect of alantolactone on the development of multiple sclerosis. Proceedings 2019, 40, 16. [Google Scholar] [CrossRef]
- Seo, J.Y.; Lim, S.S.; Kim, J.; Lee, K.W.; Kim, J.-S. Alantolactone and isoalantolactone prevent amyloid β25–35-induced toxicity in mouse cortical neurons and scopolamine-induced cognitive impairment in mice. Phytother. Res. 2017, 31, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Gierlikowska, B.; Gierlikowski, W.; Bekier, K.; Skalicka-Woźniak, K.; Czerwińska, M.E.; Kiss, A.K. Inula helenium and Grindelia squarrosa as a source of compounds with anti-inflammatory activity in human neutrophils and cultured human respiratory epithelium. J. Ethnopharmacol. 2020, 249, 1–11. [Google Scholar] [CrossRef]
- Dang, X.; He, B.; Ning, Q.; Liu, Y.; Guo, J.; Niu, G.; Chen, M. Alantolactone suppresses inflammation, apoptosis and oxidative stress in cigarette smoke-induced human bronchial epithelial cells through activation of Nrf2/HO-1 and inhibition of the NF-κB pathways. Respir. Res. 2020, 21, 95. [Google Scholar] [CrossRef]
- Lu, J.; Kuang, Z.; Chen, T.; Ye, C.; Hou, W.; Tang, L.; Chen, Y.; He, R. Isoalantolactone inhibits RANKL-induced osteoclast formation via multiple signaling pathways. Int. Immunopharmacol. 2020, 84, 106550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, L.; Xiong, J.; Xie, L.; Ying, S.; Jia, Y.; Yao, Y.; Song, X.; Zeng, Z.; Yuan, J. Isoalantolactone inhibits pancreatic cancer proliferation by regulation of PI3K and Wnt signal pathway. PLoS ONE 2021, 16, e0247752. [Google Scholar] [CrossRef]
- Cao, P.; Xia, Y.; He, W.; Zhang, T.; Hong, L.; Zheng, P.; Shen, X.; Liang, G.; Cui, R.; Zou, P. Enhancement of oxaliplatin-induced colon cancer cell apoptosis by alantolactone, a natural product inducer of ROS. Int. J. Biol. Sci. 2019, 15, 1676–1684. [Google Scholar] [CrossRef]
- Bashir, A.Y.; Zaky, M.; Khan, B.; Hussain, A.; Abdelbaset, G.; Rehman, A.; Din, Z.; Shah, S.R.; Alshwmi, M. Alantolactone enhances cisplatin anticancer activity in A549 cells through inhibition of STAT3 activation and mitochondrial dependent pathway. Biocell 2020, 44, 502–509. [Google Scholar]
- Liu, J.; Liu, M.; Wang, S.; He, Y.; Huo, Y.; Yang, Z.; Cao, X. Alantolactone induces apoptosis and suppresses migration in MCF-7 human breast cancer cells via the p38 MAPK, NF-κB and Nrf2 signaling pathways. Int. J. Mol. Med. 2018, 42, 1847–1856. [Google Scholar] [CrossRef]
- He, W.; Cao, P.; Xia, Y.; Hong, L.; Zhang, T.; Shen, X.; Zheng, P.; Shen, H.; Zhao, Y.; Zou, P. Potent inhibition of gastric cancer cells by a natural compound via inhibiting TrxR1 activity and activating ROS-mediated p38 MAPK pathway. Free Radic. Res. 2019, 53, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yang, L.; Kang, Y.; Chen, M.; Lin, S.; Xiang, Y.; Li, C.; Dai, X.; Huang, X.; Liang, G.; et al. Alantolactone sensitizes human pancreatic cancer cells to EGFR inhibitors through the inhibition of STAT3 signaling: Alantolactone mediates chemosensitization to EGFR inhibitors. Mol. Carcinogen. 2018, 58, 565–576. [Google Scholar] [CrossRef]
- Shi, C.; Lan, W.; Wang, Z.; Yang, D.; Wei, J.; Liu, Z.; Teng, Y.; Gu, M.; Yuan, T.; Cao, F.; et al. Alantolactone inhibits cell autophagy and promotes apoptosis via AP2M1 in acute lymphoblastic leukemia. Cancer Cell Int. 2020, 20, 442. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Yi, F.; Rasul, A.; Li, T.; Wang, N.; Gao, H.; Gao, R.; Ma, T. Alantolactone induces apoptosis in glioblastoma cells via GSH depletion, ROS generation, and mitochondrial dysfunction. IUBMB Life 2012, 64, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zou, S.; Ren, T.; Zhao, L.-J.; Yu, L.-F.; Li, X.-Y.; Yan, X.; Zhang, L.-J. Alantolactone suppresses the metastatic phenotype and induces the apoptosis of glioblastoma cells by targeting LIMK kinase activity and activating the cofilin/G-actin signaling cascade. Int. J. Mol. Med. 2021, 47, 68. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Q.; Chen, H.; Shi, L.; He, M.; Liu, H.; Li, T.; Lü, M.; Deng, M.; Luo, G. Inhibition of growth of esophageal cancer by alantolactone via wnt/βcatenin signaling. Anticancer Agents Med. Chem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Peng, Y.; Wang, M.; Li, X. Intestinal absorption of isoalantolactone and alantolactone, two sesquiterpene lactones from radix inulae, using Caco-2 Cells. Eur. J. Drug. Metab. Pharmacokinet. 2019, 44, 295–303. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, S.B.; Chun, J.; Song, K.H.; Kim, Y.S.; Chung, S.J.; Cho, H.J.; Yoon, I.S.; Kim, D.D. High body clearance and low oral bioavailability of alantolactone, isolated from Inula helenium, in rats: Extensive hepatic metabolism and low stability in gastrointestinal fluids. Biopharm. Drug Dispos. 2016, 37, 156–167. [Google Scholar] [CrossRef]
- Zwaving, J.H.; Bos, R. Composition of the essential oil from the root of Sassafras albidum (Nutt.) Nees. J. Essent. Oil Res. 1996, 8, 193–195. [Google Scholar] [CrossRef]
- French, L.G. The sassafras tree and designer drugs: From herbal tea to ecstasy. J. Chem. Educ. 1995, 72, 484. [Google Scholar] [CrossRef]
- Francis, J.K. Sassafras albidum (Nutt.) Nees sassafras. In Wildland Shrubs of the United States and Its Territories: Thamnic Descriptions; Agriculture, U.S.D., Ed.; Southern Research Station: Asheville, NC, USA, 2004; Volume 1, p. 681. [Google Scholar] [CrossRef]
- Gant, R.E.; Clebsch, E.E.C. The allelopathic influences of Sassafras albidum in old-field succession in Tennessee. Ecology 1975, 56, 604–615. [Google Scholar] [CrossRef]
- Magnaghi, R.M. Sassafras and its role in early America, 1562–1662. Terr. Incogn. 1997, 29, 10–21. [Google Scholar] [CrossRef]
- Kemprai, P.; Protim Mahanta, B.; Sut, D.; Barman, R.; Banik, D.; Lal, M.; Proteem Saikia, S.; Haldar, S. Review on safrole: Identity shift of the ‘candy shop’ aroma to a carcinogen and deforester. Flavour. Fragr. J. 2020, 35, 5–23. [Google Scholar] [CrossRef]
- Kamdem, D.P.; Gage, D.A. Chemical composition of essential oil from the root bark of Sassafras albidum. Planta Med. 1995, 61, 574–575. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information (2020). PubChem Compound Summary for CID 5144, S.R.O. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Safrole (accessed on 20 November 2020).
- Sethi, M.L.; Rao, G.S.; Chowdhury, B.K.; Morton, J.F.; Kapadia, G.J. Identification of volatile constituents of Sassafras albidum root oil. Phytochemistry 1976, 15, 1773–1775. [Google Scholar] [CrossRef]
- Simić, A.; Soković, M.D.; Ristić, M.; Grujić-Jovanović, S.; Vukojević, J.; Marin, P.D. The chemical composition of some Lauraceae essential oils and their antifungal activities. Phytother. Res. 2004, 18, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Huhn, C.; Pütz, M.; Dahlenburg, R.; Pyell, U. Sassafras Oils as Precursors for the Production of Synthetic Drugs: Profiling via MEKC-UVD. Beiträge zum XIV, GTFCh-Symposium, Chemistry. Mosbach, Germany, 14–16 April 2005; pp. 198–207. [Google Scholar]
- Schäffer, M.; Gröger, T.; Pütz, M.; Zimmermann, R. Forensic profiling of sassafras oils based on comprehensive two-dimensional gas chromatography. Forensic Sci. Int. 2013, 229, 108–115. [Google Scholar] [CrossRef]
- Hagan, E.C.; Jenner, P.M.; Jones, W.I.; Fitzhugh, O.G.; Long, E.L.; Brouwer, J.G.; Webb, W.K. Toxic properties of compounds related to safrole. Toxicol. Appl. Pharmacol. 1965, 7, 18–24. [Google Scholar] [CrossRef]
- Ioannides, C.; Delaforge, M.; Parke, D.V. Safrole: Its metabolism, carcinogenicity and interactions with cytochrome P450. Food Cosmet. Toxicol. 1981, 19, 657–666. [Google Scholar] [CrossRef]
- Wislocki, P.G.; Miller, E.C.; Miller, J.A.; McCoy, E.C.; Rosenkranz, H.S. Carcinogenic and mutagenic activities of safrole, 1′-hydroxysafrole, and some known or possible metabolites. Cancer Res. 1977, 37, 1883–1891. [Google Scholar]
- Lima, L. Safrole and the versatility of a natural biophore. Rev. Virtual Química 2015, 7, 495–538. [Google Scholar] [CrossRef]
- Miller, E.C.; Swanson, A.B.; Phillips, D.H.; Fletcher, T.L.; Liem, A.; Miller, J.A. Structure-activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 1983, 43, 1124–1134. [Google Scholar] [PubMed]
- Ueng, Y.-F.; Hsieh, C.-H.; Don, M.-J.; Chi, C.-W.; Ho, L.-K. Identification of the main human cytochrome P450 enzymes involved in safrole 1‘-hydroxylation. Chem. Res. Toxicol. 2004, 17, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, J.D.H.; Diem, M.; Wesseling, S.; Vervoort, J.; Oostenbrink, C.; Rietjens, I. Molecular dynamics and in vitro quantification of safrole DNA adducts reveal DNA adduct persistence due to limited DNA distortion resulting in inefficient repair. Chem. Res. Toxicol. 2020, 33, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- U.F.D.A. CFR-Code of Federal Regulation Title 21. In Safrole. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=189.180 (accessed on 11 November 2020).
- Yu, F.S.; Yang, J.S.; Yu, C.S.; Lu, C.C.; Chiang, J.H.; Lin, C.W.; Chung, J.G. Safrole induces apoptosis in human oral cancer HSC-3 cells. J. Dent. Res. 2011, 90, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Valerio, L.G. Investigating DNA adduct formation by flavor chemicals and tobacco byproducts in electronic nicotine delivery system (ENDS) using in silico approaches. Toxicol. Appl. Pharmacol. 2020, 398, 115026. [Google Scholar] [CrossRef] [PubMed]
- U.F.D.A. CFR-Code Title 21; Safrole-Free Extract of Sassafras. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.580 (accessed on 11 November 2020).
- Wang, C.-K.; Hwang, L.S. Phenolic compounds of betel quid chewing juice. Food Sci. 1993, 20, 458–471. [Google Scholar]
- Huang, J.-K.; Huang, C.-J.; Chen, W.-C.; Liu, S.-I.; Hsu, S.-S.; Chang, H.-T.; Tseng, L.-L.; Chou, C.-T.; Chang, C.-H.; Jan, C.-R. Independent [Ca2+]i increases and cell proliferation induced by the carcinogen safrole in human oral cancer cells. Naunyn-Schmiedeberg Arch. Pharmacol. 2005, 372, 88–94. [Google Scholar] [CrossRef]
- Jeng, J.H.; Chang, M.C.; Hahn, L.J. Role of areca nut in betel quid-associated chemical carcinogenesis: Current awareness and future perspectives. Oral Oncol. 2001, 37, 477–492. [Google Scholar] [CrossRef]
- Ling, L.J.; Hung, S.L.; Tseng, S.C.; Chen, Y.T.; Chi, L.Y.; Wu, K.M.; Lai, Y.L. Association between betel quid chewing, periodontal status and periodontal pathogens. Oral Microbiol. Immun. 2001, 16, 364–369. [Google Scholar] [CrossRef]
- Hung, S.-L.; Chen, Y.-L.; Chen, Y.-T. Effects of safrole on the defensive functions of human neutrophils. J. Periodont. Res. 2003, 38, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-Y.; Lin, J.-C.; Chang, C.-W.; Ho, W.-H.; Chen, Y.-T.; Peng, J.-L.; Hung, S.-L. Inhibitory effects of safrole on phagocytosis, intracellular reactive oxygen species, and the activity of myeloperoxidase released by human polymorphonuclear leukocytes. J. Periodontol. 2009, 80, 1330–1337. [Google Scholar] [CrossRef]
- Ni, Y.-L.; Shen, H.-T.; Lee, M.-W.; Yeh, K.-L.; Chiang, C.-Y.; Kuan, Y.-H. Safrole-induced expression of proinflammatory responses is associated with phosphorylation of mitogen-activated protein kinase family and the nuclear factor-κB/inhibitor of κB pathway in macrophages. Ci Ji Yi Xue Za Zhi 2020, 32, 344–350. [Google Scholar] [CrossRef]
- Chien, K.-J.; Yang, M.-L.; Tsai, P.-K.; Su, C.-H.; Chen, C.-H.; Horng, C.-T.; Yeh, C.-H.; Chen, W.-Y.; Lin, M.-L.; Chen, C.-J.; et al. Safrole induced cytotoxicity, DNA damage, and apoptosis in macrophages via reactive oxygen species generation and akt phosphorylation. Environ. Toxicol. Pharmacol. 2018, 64, 94–100. [Google Scholar] [CrossRef]
- Lin, H.-C.; Cheng, H.-H.; Huang, C.-J.; Chen, W.-C.; Chen, I.S.; Liu, S.-I.; Hsu, S.-S.; Chang, H.-T.; Huang, J.-K.; Chen, J.-S.; et al. Safrole-induced cellular Ca2+ increases and death in human osteosarcoma cells. Pharm. Res. 2006, 54, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.S.; Huang, A.C.; Yang, J.S.; Yu, C.C.; Lin, C.C.; Chung, H.K.; Huang, Y.P.; Chueh, F.S.; Chung, J.G. Safrole induces G0/G1 phase arrest via inhibition of cyclin E and provokes apoptosis through endoplasmic reticulum stress and mitochondrion-dependent pathways in human leukemia HL-60 cells. Anticancer Res. 2012, 32, 1671–1679. [Google Scholar]
- Song, X.; Yin, Z.; Ye, K.; Wei, Q.; Jia, R.; Zhou, L.; Du, Y.; Xu, J.; Liang, X.; He, C.; et al. Anti-hepatoma effect of safrole from Cinnamomum longepaniculatum leaf essential oil in vitro. Int. J. Clin. Exp. Patho 2014, 7, 2265–2272. [Google Scholar]
- Yu, F.-S.; Yang, J.-S.; Yu, C.-S.; Chiang, J.-H.; Lu, C.-C.; Chung, H.-K.; Yu, C.-C.; Wu, C.-C.; Ho, H.-C.; Chung, J.-G. Safrole suppresses murine myelomonocytic leukemia WEHI-3 cells in vivo, and stimulates macrophage phagocytosis and natural killer cell cytotoxicity in leukemic mice. Environ. Toxicol. 2013, 28, 601–608. [Google Scholar] [CrossRef]
- Barreiro, E.J.; Fraga, C.A.M. A utilização do safrol, principal componente químico do óleo de sassafráz, na síntese de substâncias bioativas na cascata do ácido araquidônico: Antiinflamatórios, analgésicos e anti-trombóticos. Química Nova 1999, 22, 744–759. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Qian, C.; Chen, X. Synthetic and mechanistic investigation of piperonyl butoxide from dihydrosafrole. Res. Chem. Intermed. 2012, 38, 147–160. [Google Scholar] [CrossRef]
- Vilela, R.F.; Costa, N.A.S.; Souza, H.D.S.; Cruz, L.E.G.; Assis, K.M.S.d.; Lima, E.O.; Cordeiro, L.V.; Lira, B.F.; Athayde-Filho, P.F.d.; Rocha, G.B.; et al. Synthesis, in silico study, theoretical stereochemistry elucidation and antifungal activity of new imides derived from safrole. J. Braz. Chem. Soc. 2020, 31, 2091–2103. [Google Scholar] [CrossRef]
- Xu, Z.; Chang, J.; Zhang, P.; Guan, X.; Chen, Y.; Fan, H. Collagen modified with epoxidized safrole for improving antibacterial activity. RSC Adv. 2017, 7, 50300–50306. [Google Scholar] [CrossRef]
- Almeida, R.S.; Freitas, P.R.; Araujo, A.C.J.; Menezes, I.R.A.; Santos, E.L.; Tintino, S.R.; Moura, T.F.; Filho, J.R.; Ferreira, V.A.; Silva, A.C.A.; et al. GC-MS profile and enhancement of antibiotic activity by the essential oil of Ocotea odorifera and safrole: Inhibition of Staphylococcus aureus efflux pumps. Antibiotics 2020, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Vizcaíno Páez, S.; Pineda Mejía, R.; Garcia, C.; Gil, J.; Durango, D. Metabolism and antifungal activity of safrole, dillapiole, and derivatives against Botryodiplodia theobromae and Colletotrichum acutatum. Bol. Latinoam. Caribe Plantas Med. Aromat. 2016, 15, 1–17. [Google Scholar]
- Yi, C.-G.; Choi, B.-R.; Park, H.-M.; Park, C.-G.; Ahn, Y.-J. Fumigant toxicity of plant essential oils to Thrips palmi (Thysanoptera: Thripidae) and Orius strigicollis (Heteroptera: Anthocoridae). J. Econ. Entomol. 2006, 99, 1733–1738. [Google Scholar] [CrossRef]
- Wong, C.; Crystal, K.; Coats, J. Three molecules found in rosemary or nutmeg essential oils repel ticks (Dermacentor variabilis) more effectively than DEET in a no-human assay. Pest Manag. Sci. 2021, 77, 1348–1354. [Google Scholar] [CrossRef]
- Drug Enforcement Administration (DEA). Safrole and Sassafras Oil Are Used in the Illicit Manufacture of MDMA. Springfield, V.D.E.A.D. Available online: https://www.deadiversion.usdoj.gov/chem_prog/advisories/safrole.htm (accessed on 11 November 2020).
- The Plant List (2013). Version 1.1. Available online: http://www.theplantlist.org/ (accessed on 8 November 2020).
- Nadda, R.K.; Ali, A.; Goyal, R.C.; Khosla, P.K.; Goyal, R. Aucklandia costus (syn. Saussurea costus): Ethnopharmacology of an endangered medicinal plant of the Himalayan region. J. Ethnopharmacol. 2020, 263, 113199. [Google Scholar] [CrossRef]
- Negi, J.; Bisht, V.K.; Bhandari, A.K.; Bhatt, V.; Sati, M.; Mohanty, J.; Sundriyal, R. Antidiarrheal activity of methanol extract and major essential oil contents of Saussurea lappa Clarke. Afr. J. Pharm. Pharmacol. 2013, 7, 474–477. [Google Scholar] [CrossRef]
- Kuniyal, C.; Rawat, Y.; Oinam, S.; Kuniyal, J.C.; Vishvakarma, S.C.R. Kuth (Saussurea lappa) cultivation in the cold desert environment of the Lahaul valley, northwestern Himalaya, India: Arising threats and need to revive socio-economic values. Biodivers. Conserv. 2005, 14, 1035–1045. [Google Scholar] [CrossRef]
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Saussurea costus: Botanical, chemical and pharmacological review of an ayurvedic medicinal plant. J. Ethnopharmacol. 2007, 110, 379–390. [Google Scholar] [CrossRef]
- Chandavarkar, R. The Wealth of India: A dictionary of Indian raw materials and industrial products (industrial product“ part I). Ind. Med. Gaz. 1949, 84, 476–477. [Google Scholar]
- Shah, N.C. Herbal folk medicines in Northern India. J. Ethnopharmacol. 1982, 6, 293–301. [Google Scholar] [CrossRef]
- Madhuri, K.; Elango, K.; Ponnusankar, S. Saussurea lappa (kuth root): Review of its traditional uses, phytochemistry and pharmacology. Orient. Pharm. Exp. Med. 2011, 12, 1–9. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Fu, X.; Liu, C.; Xu, Y.; Ji, W.; Fan, J.; Chen, L.; Fang, L.; Huang, Y.; et al. Volatile oil from Saussurea lappa exerts antitumor efficacy by inhibiting epithelial growth factor receptor tyrosine kinase-mediated signaling pathway in hepatocellular carcinoma. Oncotarget 2016, 7, 79761–79773. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, S.; Pancholi, S.; Mohan, S.; Sultan, M.; Tripathi, P.; Alshahrani, S.; Sidiqui, M.; Farasani, A.; Taha, M. Antinociceptive activity of the essential oil formulation from the roots of Saussaura lappa Clarks. Curr. Top. Nutraceutical. Res. 2020, 19, 127–132. [Google Scholar] [CrossRef]
- Zahara, K.; Tabassum, S.; Sabir, S.; Arshad, M.; Qureshi, R.; Amjad, M.S.; Chaudhari, S.K. A review of therapeutic potential of Saussurea lappa - An endangered plant from Himalaya. Asian Pac. J. Trop. Med. 2014, 7, 60–69. [Google Scholar] [CrossRef]
- Semwal, R.; Joshi, K.; Pandian, A.; Badoni, P.; Semwal, D. Biological applications and secondary metabolites of Saussurea costus (Falc.) Lipsch. J. Convent. Knowl. Holist. Health 2020, 4, 201. [Google Scholar]
- Bhattacharya, S.K. Handbook of Medicinal Plants; Pointer Publisher’s: Jaipur, India, 2001. [Google Scholar]
- Placzek, M.; Frömel, W.; Eberlein, B.; Gilbertz, K.P.; Przybilla, B. Evaluation of phototoxic properties of fragrances. Acta Derm. Venereol. 2007, 87, 312–316. [Google Scholar] [CrossRef]
- Benedetto, C.; D’Auria, M.; Mecca, M.; Prasad, P.; Singh, P.; Singh, S.; Sinisgalli, C.; Milella, L. Chemical and biological evaluation of essential oil from Saussurea costus (Falc.) Lipsch. from Garhwal Himalaya collected at different harvesting periods. Nat. Prod. Res. 2019, 33, 2355–2358. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Taha, M.M.E.; Alhazmi, H.A.; Ahsan, W.; Rehman, Z.U.; Bratty, M.; Makeen, H. Phytochemical profiling of costus (Saussurea lappa Clarke) root essential oil, and its antimicrobial and toxicological effects. Trop. J. Pharm. Res. 2019, 18, 2155–2160. [Google Scholar] [CrossRef]
- Marotti, M.; Piccaglia, R.; Giovanelli, E.; Deans, S.G.; Eaglesham, E. Effects of planting time and mineral fertilization on peppermint (mentha x piperita l.) essential oil composition and its biological activity. Flavour Fragr. J. 1994, 9, 125–129. [Google Scholar] [CrossRef]
- Chang, K.-M.; Kim, G.-H. Comparison of volatile aroma components from Saussurea lappa CB Clarke root oils. J. Food Sci. Nutr. 2008, 13, 128–133. [Google Scholar] [CrossRef]
- Liu, Z.L.; He, Q.; Chu, S.; Wang, C.F.; Du, S.; Deng, Z. Essential oil composition and larvicidal activity of Saussurea lappa roots against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2011, 110, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Lammari, N.; Demautis, T.; Louaer, O.; Meniai, A.H.; Casabianca, H.; Bensouici, C.; Devouassoux, G.; Fessi, H.; Bentaher, A.; Elaissari, A. Nanocapsules containing Saussurea lappa essential oil: Formulation, characterization, antidiabetic, anti-cholinesterase and anti-inflammatory potentials. Int. J. Pharm. 2021, 593, 120138. [Google Scholar] [CrossRef]
- Gwari, G.; Bhandari, U.; Andola, H.; Lohani, H.; Chauhan, N. Volatile constituents of Saussurea costus roots cultivated in Uttarakhand Himalayas, India. Pharmacogn. Res. 2013, 5, 179–182. [Google Scholar] [CrossRef]
- Kim, D.Y.; Choi, B.Y. Costunolide a bioactive sesquiterpene lactone with diverse therapeutic potential. Int. J. Mol. Sci. 2019, 20, 2926. [Google Scholar] [CrossRef]
- Woo, J.H.; Ahn, J.H.; Jang, D.S.; Choi, J.H. Effect of dehydrocostus lactone isolated from the roots of Aucklandia lappa on the apoptosis of endometriotic cells and the alternative activation of endometriosis-associated macrophages. Am. J. Chinese Med. 2019, 47, 1289–1305. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, W.-X.; He, Z.-Q.; Wu, B.-S.; Shen, Z.-F.; Shang, H.-T.; Chen, T.; Wang, Q.; Chen, Y.; Han, S.-T. The possible anti-inflammatory effect of dehydrocostus lactone on DSS-induced colitis in mice. Evid. Based Complement. Altern. Med. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.C.; Feng, N.; Li, W.W.; Tu, P.F.; Chen, J.P.; Han, J.Y.; Zeng, K.W. Costunolide plays an anti-neuroinflammation role in lipopolysaccharide-induced BV2 microglial activation by targeting cyclin-dependent kinase 2. Molecules 2020, 25, 2840. [Google Scholar] [CrossRef]
- Heydarirad, G.; Cramer, H.; Choopani, R. Topical Costus sp. preparation as palliative care for chemotherapy-induced peripheral neuropathy of patients: A randomized placebo-controlled pilot trial. J. Altern. Complement. Med. 2020, 26, 809–814. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Fan, J.; Lin, X.; Liu, C.; Xu, Y.; Ji, W.; Yan, C.; Su, C. Costunolide and dehydrocostuslactone combination treatment inhibit breast cancer by inducing cell cycle arrest and apoptosis through c-Myc/p53 and AKT/14-3-3 pathway. Sci. Rep. 2017, 7, 41254. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.-X.; Wang, Y.; Gu, X.; Xue, Y.; Wu, Q.; Yan, C. Metabolic transformation of breast cancer in a MCF-7 xenograft mouse model and inhibitory effect of volatile oil from Saussurea lappa Decne treatment. Metabolomics 2014, 11, 636–656. [Google Scholar] [CrossRef]
- Peng, Z.X.; Wang, Y.; Gu, X.; Wen, Y.Y.; Yan, C. A platform for fast screening potential anti-breast cancer compounds in traditional Chinese medicines. Biomed. Chromatogr. 2013, 27, 1759–1766. [Google Scholar] [CrossRef]
- Huang, H.; Park, S.; Zhang, H.; Park, S.; Kwon, W.; Kim, E.; Zhang, X.; Jang, S.; Yoon, D.; Choi, S.-K.; et al. Targeting AKT with costunolide suppresses the growth of colorectal cancer cells and induces apoptosis in vitro and in vivo. J. Exp. Clin. Cancer Res. 2021, 40, 114. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, Y.-C.; Lim, J.S. Costunolide, a sesquiterpene lactone, suppresses skin cancer via induction of apoptosis and blockage of cell proliferation. Int. J. Mol. Sci. 2021, 22, 2075. [Google Scholar] [CrossRef] [PubMed]
- Chopra, C.L.; Bhatia, M.C.; Chopra, I.C. In vitro antibacterial activity of oils from Indian medicinal plants I. J. Am. Pharm. Assoc. 1960, 49, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wang, J.; Luo, H.; Du, X.; Li, H.; Luo, M.; Dong, J.; Chen, Z.; Deng, X. The effects of subinhibitory concentrations of costus oil on virulence factor production in Staphylococcus aureus. J. Appl. Microbiol. 2010, 110, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, C.L.; Nunez, I.S.; Castaneda-Acosta, J.; Foroozesh, M.; Fronczek, F.R.; Fischer, N.H.; Franzblau, S.G. Antimycobacterial activities of dehydrocostus lactone and its oxidation products. J. Nat. Prod. 1998, 61, 1181–1186. [Google Scholar] [CrossRef]
- Luna-Herrera, J.; Costa, M.; González, H.; Castilho, P. Synergistic antimycobacterial activities of sesquiterpene lactones from Laurus spp. J. Antimicrob. Chemother. 2007, 59, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Oltra, J.E.; Alvarez, M.; Raslan, D.S.; Saude, D.A.; Akssira, M. New sources and antifungal activity of sesquiterpene lactones. Fitoterapia 2000, 71, 60–64. [Google Scholar] [CrossRef]
- Chen, H.-C.; Chou, C.-K.; Lee, S.-D.; Wang, J.-C.; Yeh, S.-F. Active compounds from Saussurea lappa Clarks that suppress hepatitis B virus surface antigen gene expression in human hepatoma cells. Antiviral Res. 1995, 27, 99–109. [Google Scholar] [CrossRef]
- Manzoor, F.; Samreen, K.; Parveen, Z. Larvicidal activity of essential oils against Aedes aegypti and Culex quinquefasciatus larvae (Diptera: Culicidae). J. Anim. Plant Sci. 2013, 23, 420–424. [Google Scholar]
- Huntose, Y.; Pandey, K.; Dwivedi, M. Response of herbal drug (kustha) on psychological and neurobehavior changes during labour. In Proceedings of the South-East Asian Seminar on Herbs and Herbal Medicines, Patna, India, 16–19 January 1999; pp. 63–68. [Google Scholar]
- Dosoky, N.S.; Setzer, W.N. Maternal reproductive toxicity of some essential oils and their constituents. Int. J. Mol. Sci. 2021, 22, 2380. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, H.; Ueda, R.; Matsumoto, K.; Kawanishi, K.; Kato, A. Effect of dehydrocostus lactone and costunolide from Saussurea root on the central nervous system in mice. Phytomedicine 1996, 3, 147–153. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zhang, X.; Ren, G.; Wang, L.; Li, J.; Wang, M.; Ren, T.; Zhao, Y.; Yang, M.; et al. The combination of Aquilaria sinensis (Lour.) Gilg and Aucklandia costus Falc. volatile oils exerts antidepressant effects in a CUMS-induced rat model by regulating the HPA Axis and levels of neurotransmitters. Front. Pharmacol. 2021, 11, 614413. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, H.; Ueda, R.; Matsumoto, K.; Kawanishi, K.; Kato, K. Effects of sesquiterpenoids from “Oriental incenses” on acetic acid-induced writhing and D2 and 5-HT2A receptors in rat brain. Phytomedicine 2000, 7, 417–422. [Google Scholar] [CrossRef]
- Sundaresan, N.; Narayanan, K.; Ilango, K. Valeriana officinalis: A review of its traditional uses, phytochemistry and pharmacology. Asian J. Pharm. CLin. Res. 2018, 11, 36. [Google Scholar] [CrossRef][Green Version]
- Heuberger, H.L.U.; Schmatz, R.; Tegtmeier, M. Baldrian (Valeriana officinalis L.). Handbuch des Arznei- und Gewürzpflanzenanbaus. Band 4 Arznei- und Gewürzpflanzen A-K; Hoppe B, Ed.; Arznei und Gewürzpflanzen A-K: Verein für Arznei- und Gewürzpflanzen SALUPLANTA e.V.: Bernburg, Germany, 2012; Volume 4. [Google Scholar]
- Bos, R.; Woerdenbag, H.J.; Hendriks, H.; Scheffer, J.J.C. Composition of the essential oils from underground parts of Valeriana officinalis L. s.l. and several closely related taxa. Flavour Fragr. J. 1997, 12, 359–370. [Google Scholar] [CrossRef]
- Cornara, L.; Ambu, G.; Trombetta, D.; Denaro, M.; Alloisio, S.; Frigerio, J.; Labra, M.; Ghimire, G.; Valussi, M.; Smeriglio, A. Comparative and functional screening of three species traditionally used as antidepressants: Valeriana officinalis L. Valeriana jatamansi Jones ex Roxb. and Nardostachys jatamansi (D.Don) DC. Plants 2020, 9, 994. [Google Scholar] [CrossRef] [PubMed]
- Khin, M.; Thidar, S.; Than, N. Some bioactivities screening and extraction of essential oil from Valeriana officinalis L. (Kantbalu). Myanmar Korea Conf. Res. J. Myanmar 2020, 3, 1675–1679. [Google Scholar]
- Houghton, P.J.E. Valerian: The Genus Valeriana, 1st ed.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1997. [Google Scholar]
- G.-V.P.V.G. European Pharmacopoeia. Baldrianwurzel Valerianae radix s.l., 9th ed.; Deutscher Apotheker Verlag: Stuttgard, Germany, 2017. [Google Scholar]
- Krystal, A.; Ressler, I. The use of valerian in neuropsychiatry. CNS Spectrums 2001, 6, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Shinjyo, N.; Waddell, G.; Green, J. Valerian root in treating sleep problems and associated disorders—A systematic review and meta-analysis. J. Evid. Based Integr. Med. 2020, 25, 1–31. [Google Scholar] [CrossRef]
- Houghton, P.J. The scientific basis for the reputed activity of Valerian. J. Pharm. Pharmacol. 1999, 51, 505–512. [Google Scholar] [CrossRef]
- EMA. European Union Herbal Monograph on Valeriana officinalis, L. Radix; Committee on Herbal Medicinal Products (HMPC): London, UK, 2016. [Google Scholar]
- Letchamo, W.; Ward, W.; Heard, B.; Heard, D. Essential oil of Valeriana officinalis L. cultivars and their antimicrobial activity as influenced by harvesting time under commercial organic cultivation. J. Agric. Food Chem. 2004, 52, 3915–3919. [Google Scholar] [CrossRef]
- Morteza, E.; Akbari, G.; Mohammad, S.; Sanavi, M.; Foghi, B.; Abdoli, M.; Farahani, H. Planting density influence on variation of the essential oil content and compositions in valerian (Valeriana officinalis L.) under different sowing dates. Afr. J. Microbiol. Res. 2009, 3, 897–902. [Google Scholar]
- Li, S.-H.; Shi, G.-Q. Studies on volatile constituents in valeriana oil and its application in cigarettes flavoring. J. Zhengzhou Univ. Light Ind. 2009, 14, 27–29. [Google Scholar]
- Wang, J.; Zhao, J.; Liu, H.; Zhou, L.; Liu, Z.L.; Wang, J.; Han, J.; Yu, Z.; Yang, F. Chemical analysis and biological activity of the essential oils of two valerianaceous species from China: Nardostachys chinensis and Valeriana officinalis. Molecules 2010, 15, 6411–6422. [Google Scholar] [CrossRef]
- Pop, M.; Sand, C.; Bobit, D.; Antofie, M.-M.; Barbu, H.; Pavel, P.-B.; Muntean, L.; Mircea, S. Studies concerning the production of volatile oil, rhizomes and roots, to different genotypes of Valeriana officinalis L. An. Univ. Oradea, Fasc. Biol. 2010, 17, 332–335. [Google Scholar]
- Raal, A.; Arak, E.; Orav, A.; Kailas, T.; Müürisepp, M. Variation in the composition of the essential oil of commercial Valeriana officinalis L. roots from different countries. J. Essent. Oil Res. 2008, 20, 524–529. [Google Scholar] [CrossRef]
- Feng, Y.-X.; Wang, Y.; Chen, Z.-Y.; Guo, S.-S.; You, C.-X.; Du, S.-S. Efficacy of bornyl acetate and camphene from Valeriana officinalis essential oil against two storage insects. Environ. Sci. Pollut. Res. 2019, 26, 16157–16165. [Google Scholar] [CrossRef]
- Choi, S.-A.; Lee, H.-S. Acaricidal and antibacterial toxicities of Valeriana officinalis oils obtained by steam distillation extraction. J. Appl. Biol. Chem. 2019, 62, 19–23. [Google Scholar] [CrossRef]
- Pavlovic, M.; Kovacevic, N.; Tzakou, O.; Couladis, M. The essential oil of Valeriana officinalis L. s.l. growing wild in Western Serbia. J. Essent. Oil Res. 2004, 16, 397–399. [Google Scholar] [CrossRef]
- Morteza, E.; Joorabloo, A. Evaluation of medicinal plant Valerian (Valeriana officinalis L.) essential oil composition cultivated at Gamsar zone in Iran. J. Pharm. Sci. Innov. 2012, 1, 87–88. [Google Scholar]
- Seidler-Lozykowska, K.; Mielcarek, S.; Baraniak, M. Content of essential oil and valerenic acids in valerian (Valeriana offcinalis L.) roots at the selected developmental phases. J. Essent. Oil Res. 2009, 21, 413–416. [Google Scholar] [CrossRef]
- Bos, R.; Woerdenbag, H.J.; Putten, F.M.V.; Hendriks, H.; Scheffer, J. Seasonal variation of the essential oil, valerenic acid and derivatives, and velopotriates in Valeriana officinalis roots and rhizomes, and the selection of plants suitable for phytomedicines. Planta Med. 1998, 64, 143–147. [Google Scholar] [CrossRef]
- Nakurte, C.I.; Mežaka, I.; Taškova, I.; Primavera, A.; Vecvanags, A.; Kronberga, A. Seasonal changes in chemical composition of Valeriana officinalis L. roots in natural conditions and organic production system in Latvia. Plant Biol. Crop Res. Chem. Interm. 2021, 4, 1031. [Google Scholar]
- Penzkofer, M.; Ziegler, E.; Heuberger, H. Contents of essential oil, valerenic acids and extractives in different parts of the rootstock of medicinal valerian (Valeriana officinalis L. s.l.). J. Appl. Res. Med. Aromat. Plants 2014, 1, 98–106. [Google Scholar] [CrossRef]
- Penzkofer, M.; Baron, A.; Naumann, A.; Krähmer, A.; Schulz, H.; Heuberger, H. Characterization of essential oil distribution in the root cross-section of Valeriana officinalis L. s.l. by using histological imaging techniques. Plant Methods 2018, 14, 41. [Google Scholar] [CrossRef]
- Morteza, E.; Akbari, G.; Ali, S.; Sanavi, M.; Farahani, H. Determination of the vegetative and reproductive characteristics of valerian (Valeriana officinalis L.) under sowing dates and planting densities at Iran. J. Med. Plant. Res. 2010, 4, 857–861. [Google Scholar]
- Tabatabaei, S.J. Effects of cultivation systems on the growth, and essential oil content and composition of Valerian. J. Herbs Spices Med. Plants 2008, 14, 54–67. [Google Scholar] [CrossRef]
- Javan Gholiloo, M.; Yarnia, M.; Ghorttapeh, A.H.; Farahvash, F.; Daneshian, A.M. Evaluating effects of drought stress and bio-fertilizer on quantitative and qualitative traits of valerian (Valeriana officinalis l.). J. Plant Nutr. 2019, 42, 1417–1429. [Google Scholar] [CrossRef]
- Mustafavi, S.H.; Shekari, F.; Maleki, H.H. Influence of exogenous polyamines on antioxidant defence and essential oil production in valerian (Valeriana officinalis L.) plants under drought stress. Acta Agric. Slov. 2016, 107, 81–91. [Google Scholar] [CrossRef]
- Lopes, D.; Strobl, H.; Kolodziejczyk, P. Influence of drying and distilling procedures on the chemical composition of Valerian oil (Valeriana officinalis L.). J. Essent. Oil Bear. Plants 2013, 8, 134–139. [Google Scholar] [CrossRef]
- Safaralie, A.; Fatemi, S.; Sefidkon, F. Essential oil composition of Valeriana officinalis L. roots cultivated in Iran. Comparative analysis between supercritical CO2 extraction and hydrodistillation. J. Chromatogr. A 2008, 1180, 159–164. [Google Scholar] [CrossRef]
- Komori, T.; Matsumoto, T.; Motomura, E.; Shiroyama, T. The sleep-enhancing effect of valerian inhalation and sleep-shortening effect of lemon inhalation. Chem. Senses 2006, 31, 731–737. [Google Scholar] [CrossRef]
- Hosoi, J.; Tanida, M.; Tsuchiya, T. Mitigation of stress-induced suppression of contact hypersensitivity by odorant inhalation. Br. J. Dermatol. 2001, 145, 716–719. [Google Scholar] [CrossRef]
- Rafiee, M.; Kiani, Z.; Moezi, S.A.; Rad, G.H.M. The effects of lavender, valerian, and oxazepam on anxiety among hospitalized patients with coronary artery disease. Mod. Care J. 2018, 15, e68390. [Google Scholar] [CrossRef]
- Mirghafourvand, M. The comparison of the impact of lavender and Valerian aromatherapy on reduction of the active phase among nulliparous women: A double blind randomized controlled trial. Int. J. Med. Res. Health Sci. 2016, 5, 532–538. [Google Scholar]
- Komori, T. Effects of lemon and valerian inhalation on autonomic nerve activity in depressed and healthy subjects. Int. J. Essent. Oil Ther. 2009, 3, 3–8. [Google Scholar] [CrossRef]
- Hendriks, H.; Bos, R.; Allersma, D.P.; Malingré, T.M.; Koster, A.S. Pharmacological screening of valerenal and some other components of essential oil of Valeriana officinalis. Planta Med. 1981, 42, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-X.; Wang, Y.; Geng, Z.-F.; Zhang, D.; Almaz, B.; Du, S.-S. Contact toxicity and repellent efficacy of Valerianaceae spp. to three stored-product insects and synergistic interactions between two major compounds camphene and bornyl acetate. Ecotoxicol. Environ. Saf. 2019, 190, 110106. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Hu, J.F.; Zhou, L.; Liu, Z.L. Evaluation of fumigant toxicity of essential oils of Chinese medicinal herbs against Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). J. Entomol. Zool. 2014, 2, 164–169. [Google Scholar]
- Bin, Z. Study on antimicrobial and antioxidant activities of essential oils from Valeriana officinalis L. and Valeriana jatamansi Jones. Nat. Prod. Res. Dev. 2013, 25, 1037–1040. [Google Scholar]
- Miao, T.; Zheng, H.; Wang, X.; Lan, Y.; Zhang, Y.; Song, Y.; Li, L. Optimization of extraction process of Valeriana officinalis L. root essential oil and study on its anti-free radical activity. IOP Conf. Ser. Earth Environ. Sci. 2020, 526, 012067. [Google Scholar] [CrossRef]
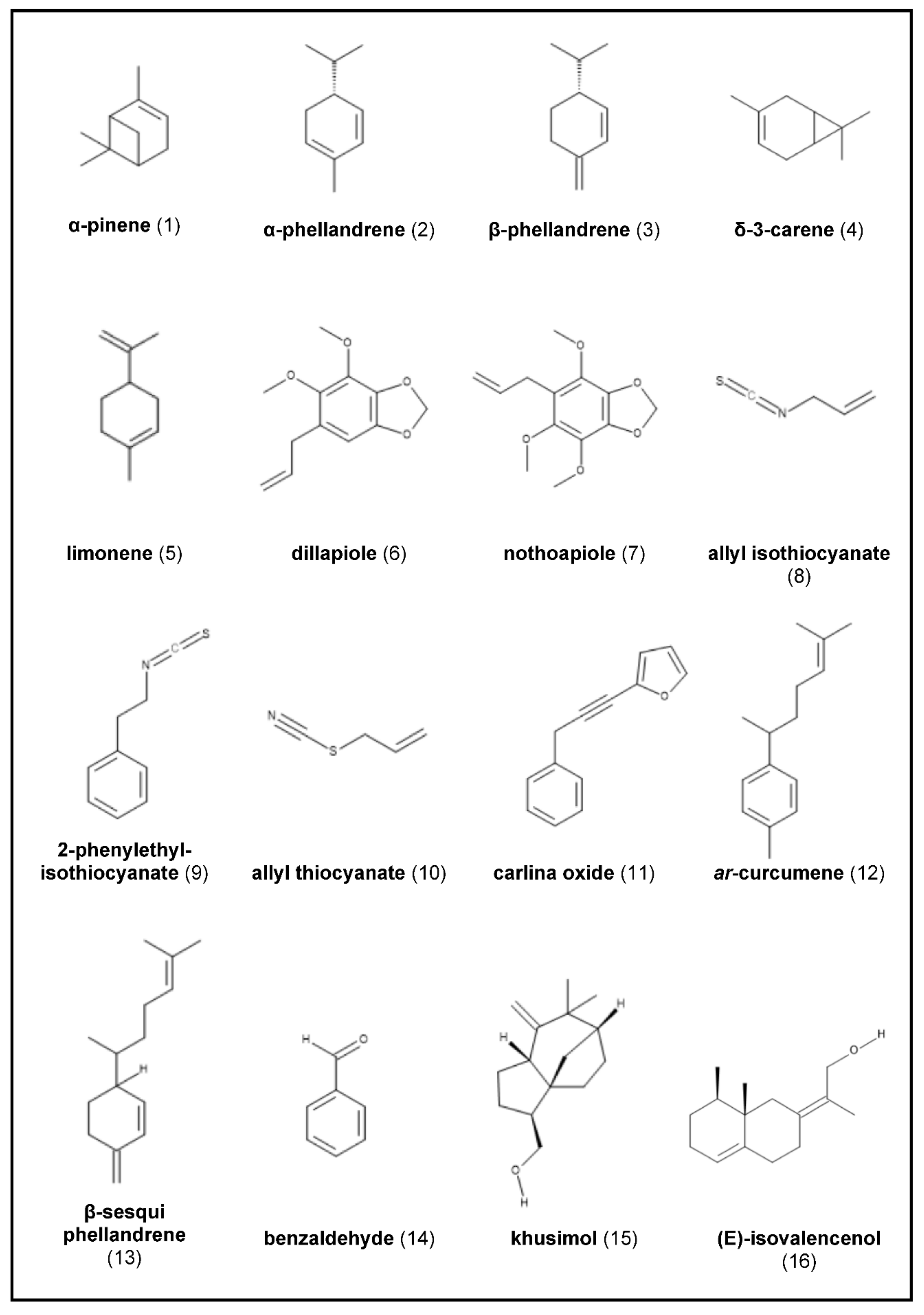
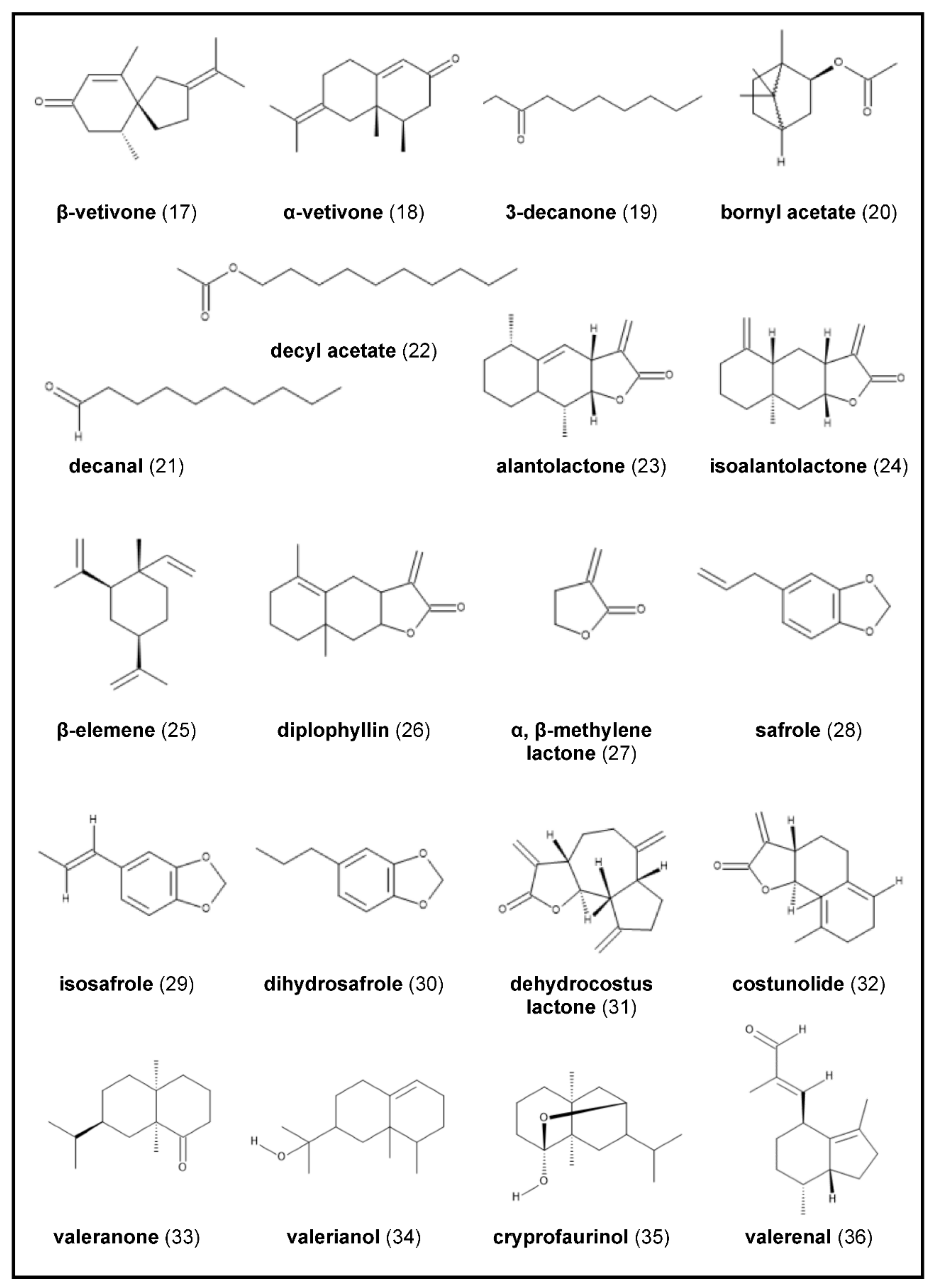
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunz, K.; Stappen, I. Back to the Roots—An Overview of the Chemical Composition and Bioactivity of Selected Root-Essential Oils. Molecules 2021, 26, 3155. https://doi.org/10.3390/molecules26113155
Lunz K, Stappen I. Back to the Roots—An Overview of the Chemical Composition and Bioactivity of Selected Root-Essential Oils. Molecules. 2021; 26(11):3155. https://doi.org/10.3390/molecules26113155
Chicago/Turabian StyleLunz, Karin, and Iris Stappen. 2021. "Back to the Roots—An Overview of the Chemical Composition and Bioactivity of Selected Root-Essential Oils" Molecules 26, no. 11: 3155. https://doi.org/10.3390/molecules26113155
APA StyleLunz, K., & Stappen, I. (2021). Back to the Roots—An Overview of the Chemical Composition and Bioactivity of Selected Root-Essential Oils. Molecules, 26(11), 3155. https://doi.org/10.3390/molecules26113155





