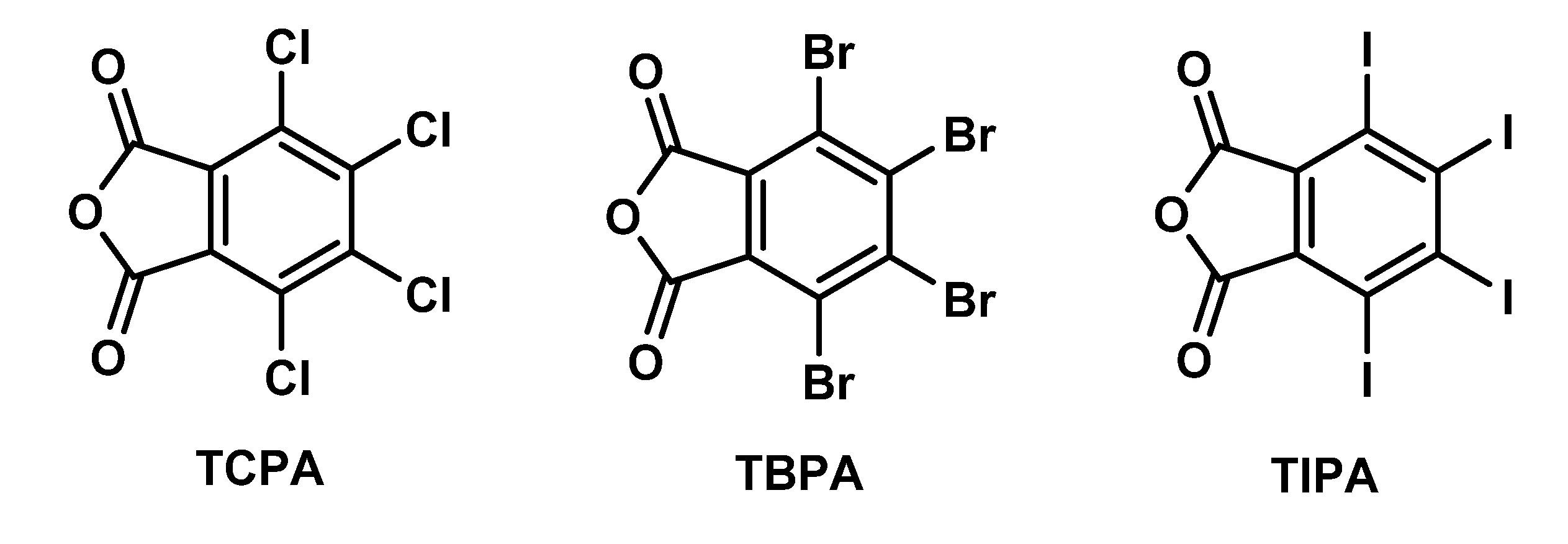
Comparative Structural Study of Three Tetrahalophthalic Anhydrides: Recognition of X···O(anhydride) Halogen Bond and πh···O(anhydride) Interaction
Abstract
1. Introduction
2. Results and Discussion
2.1. General Consideration
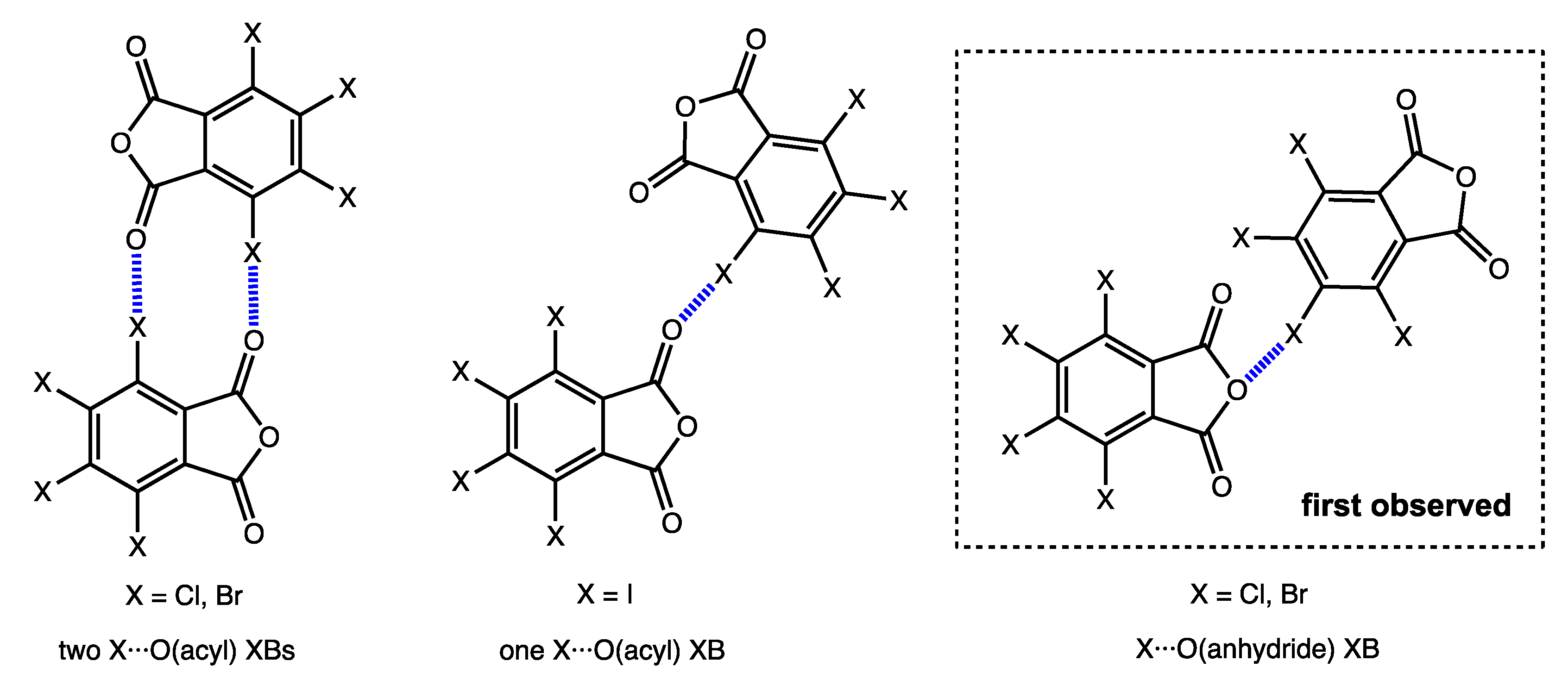
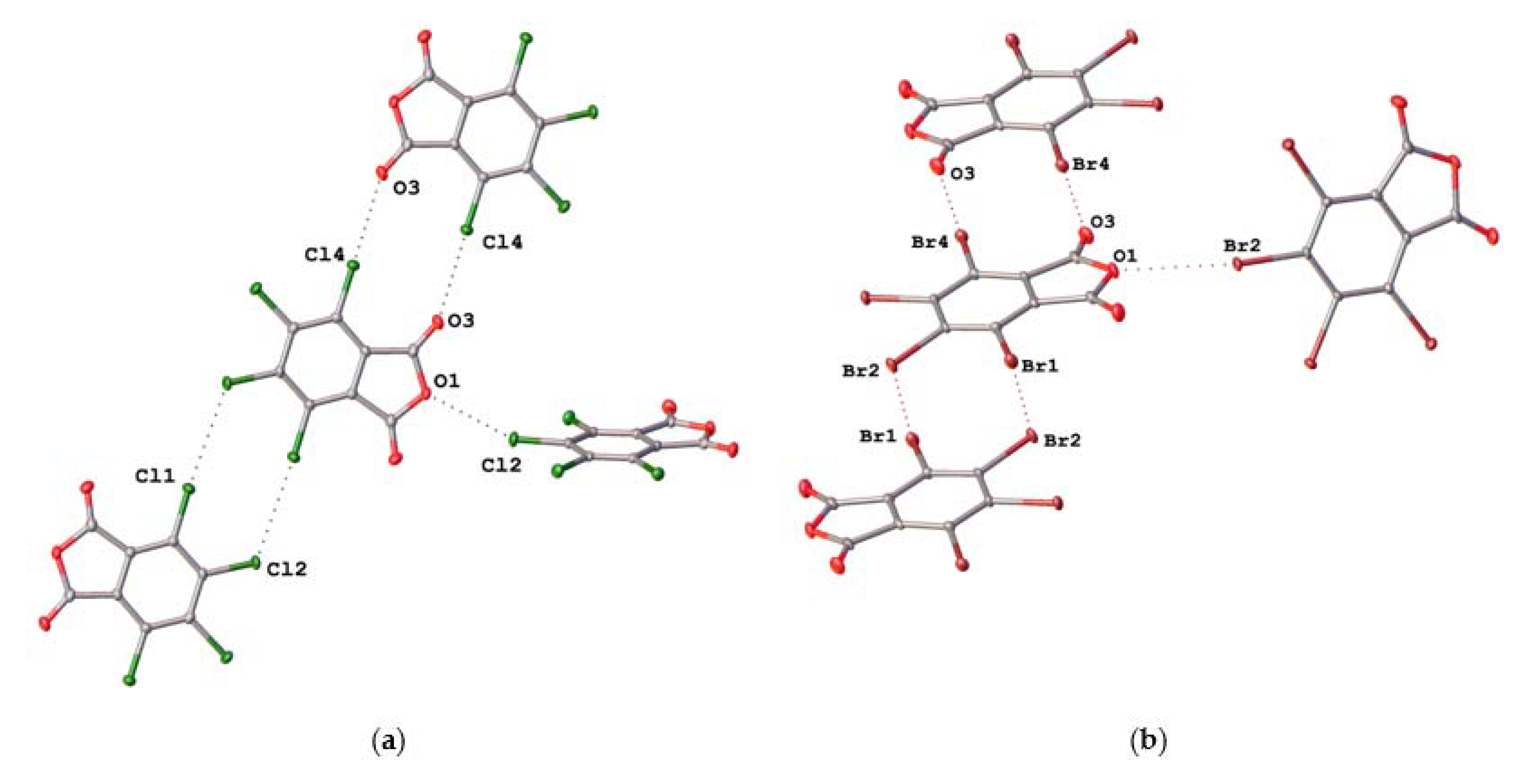
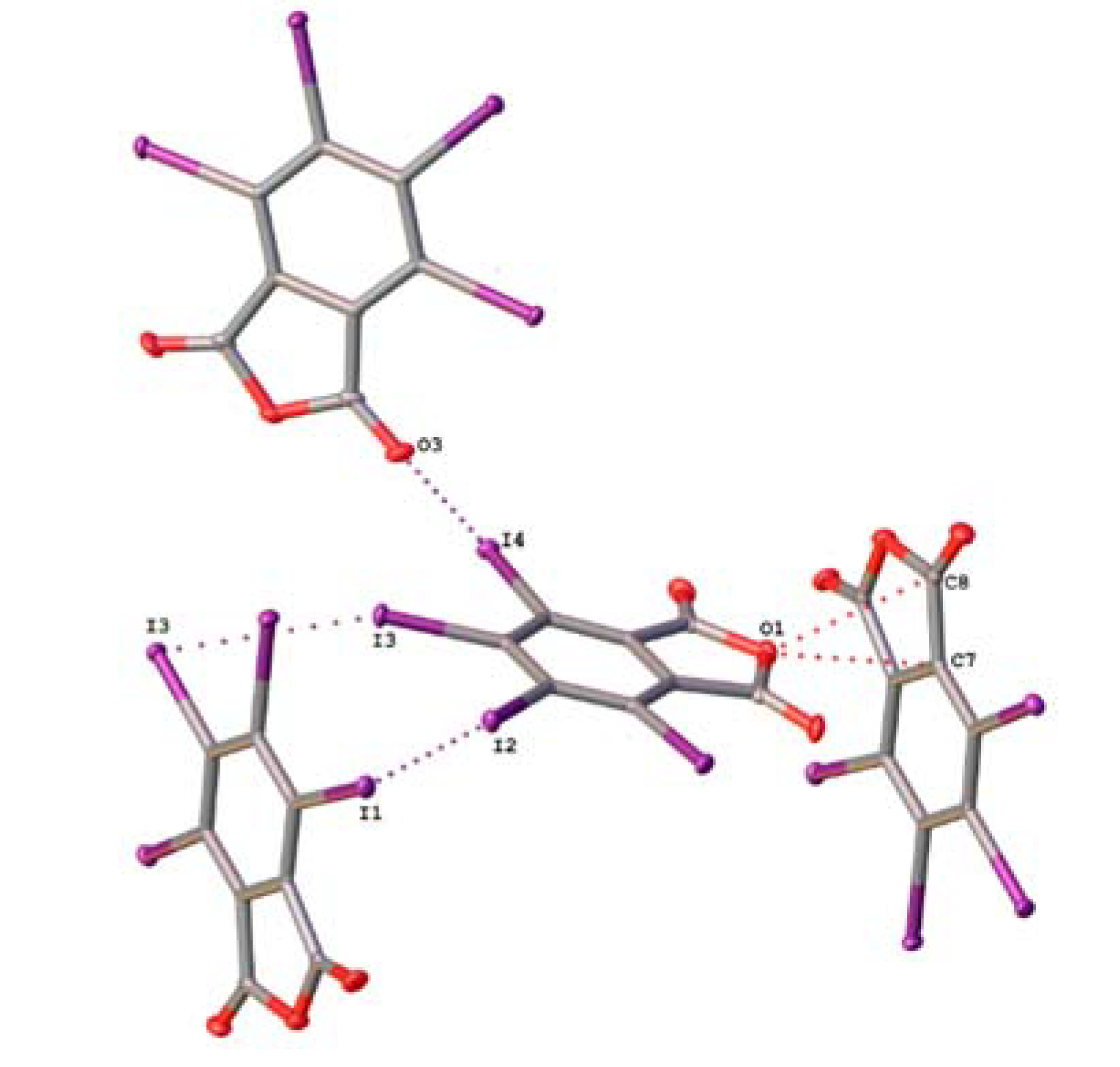
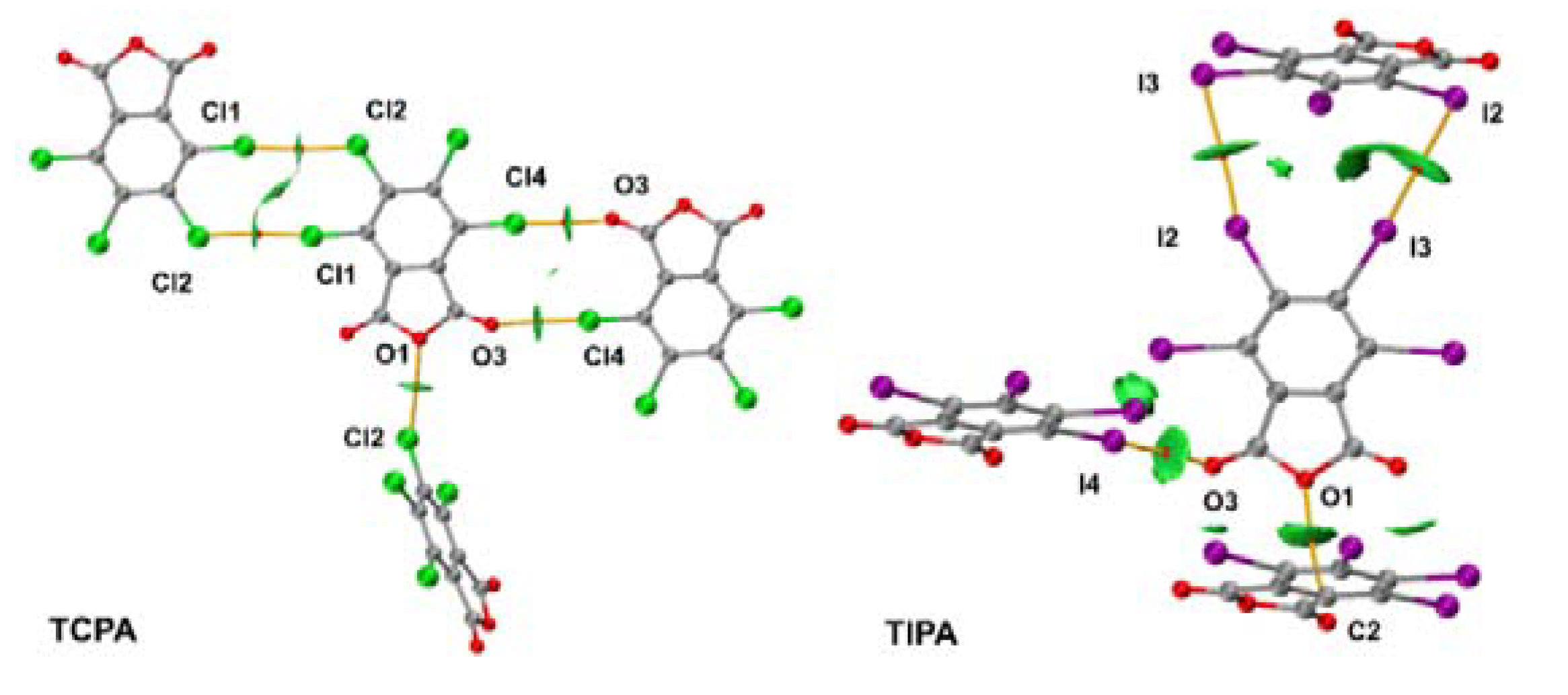
2.2. Noncovalent Interactions with Anhydride Oxygen
2.3. Other Halogen Bonds in TXPA Crystals
2.4. Lp···πh Interactions with Halogen and Oxygen Atom
2.5. Theoretical Calculations
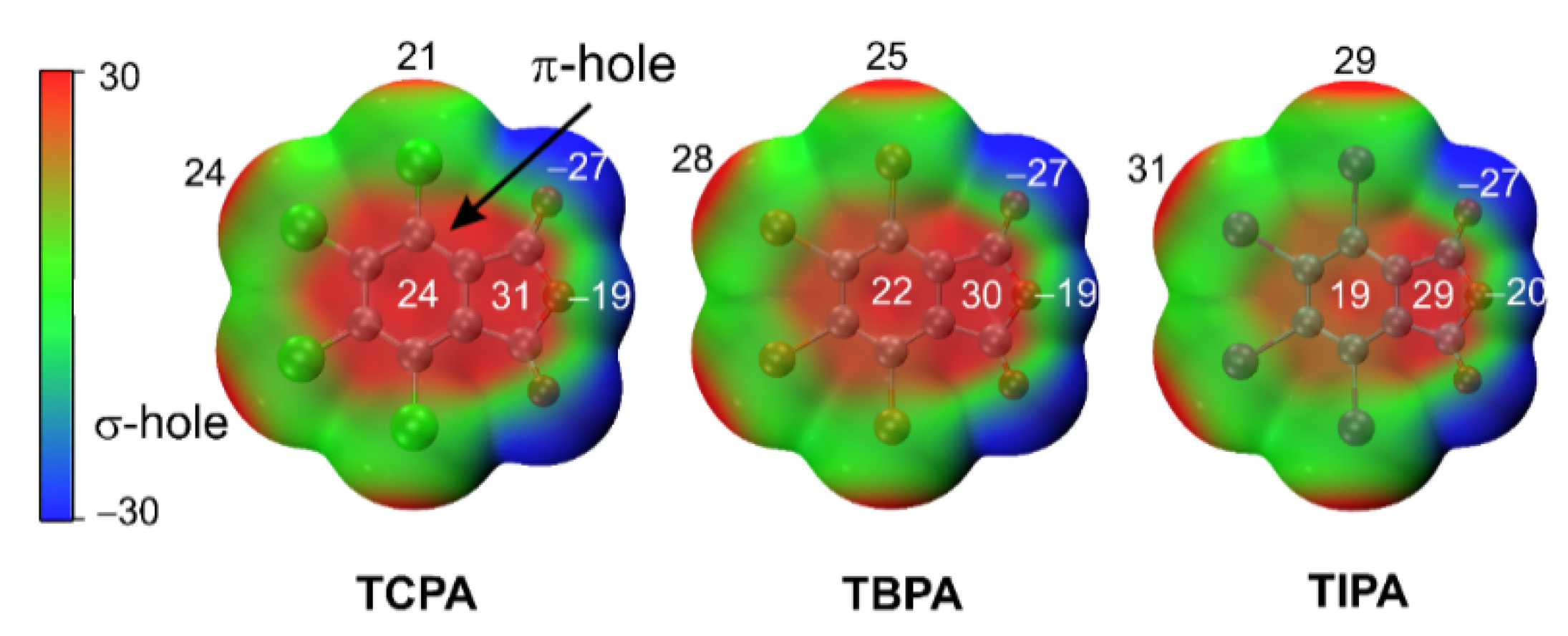
2.5.1. Molecular Electrostatic Potentials
2.5.2. The Quantum Theory of Atoms in Molecules and the Independent Gradient Model
2.5.3. Natural Bond Orbital Analysis
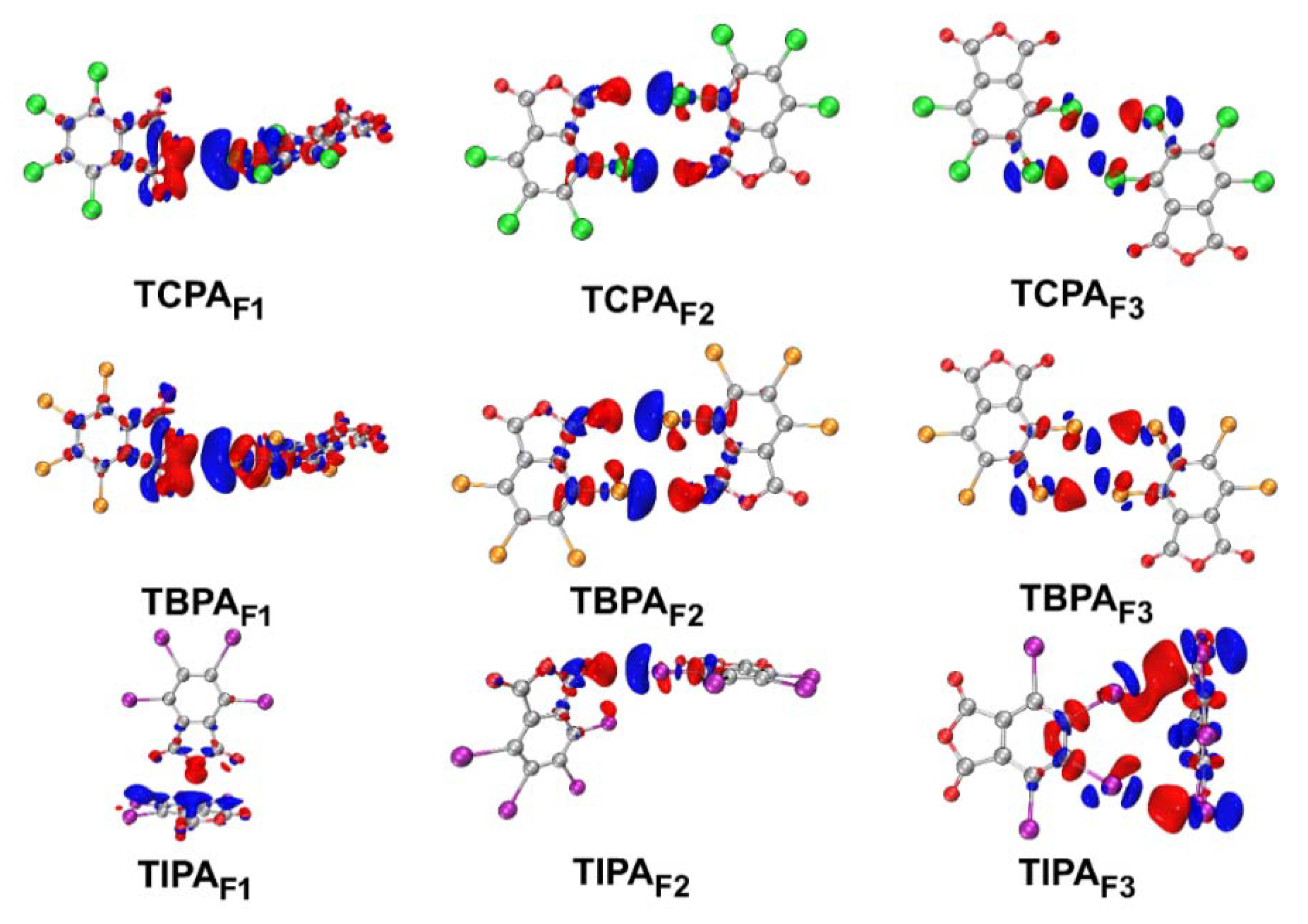
2.5.4. Electron Density Difference Maps
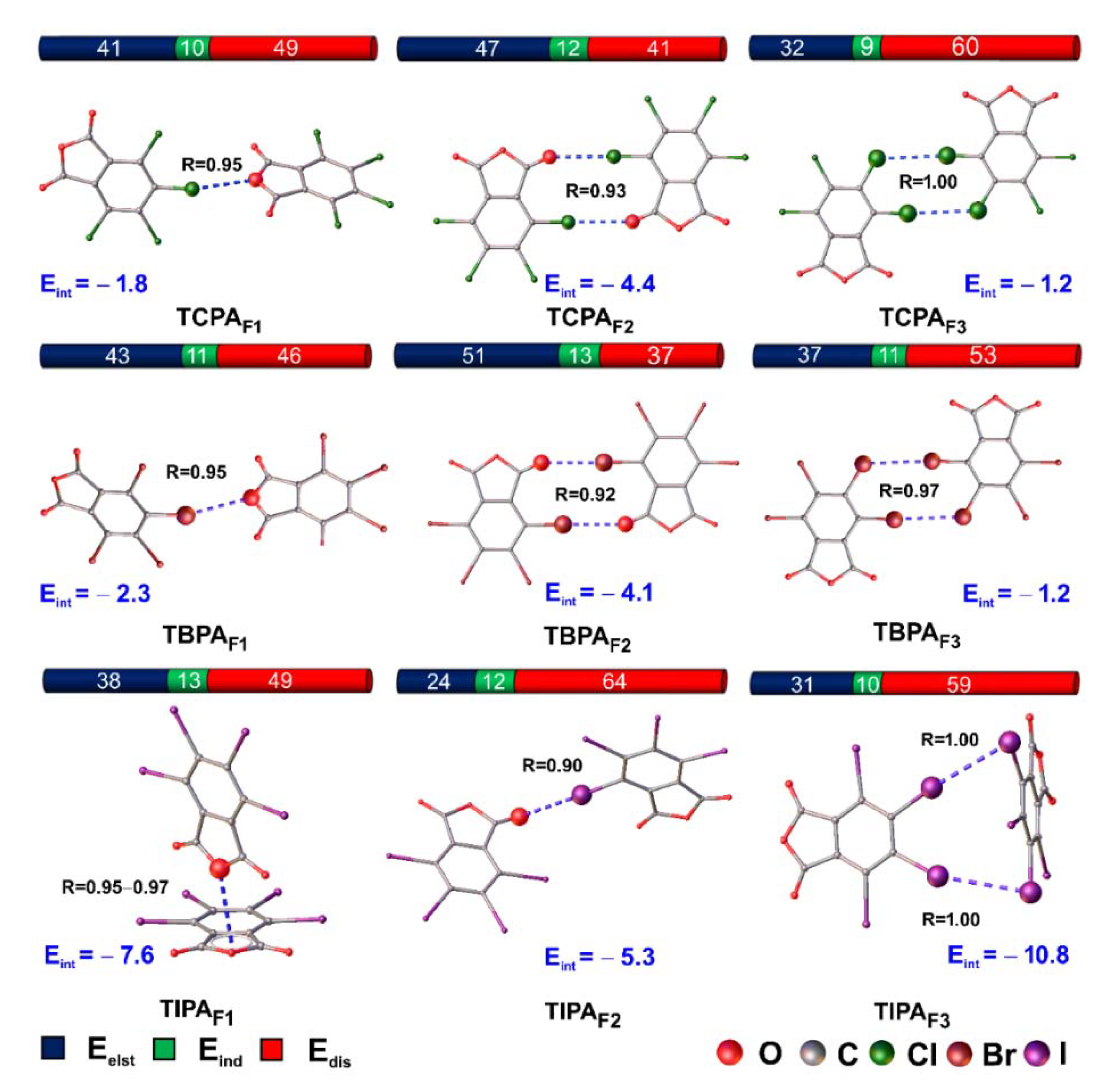
2.5.5. Interaction Energies and SAPT-Based Decomposition
2.5.6. Fragment Optimization in Vacuum
3. Material and Methods
3.1. Crystallography
3.2. Computational Study
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shakya, S.; Khan, I.M. Charge Transfer Complexes: Emerging and Promising Colorimetric Real-Time Chemosensors for Hazardous Materials. J. Hazard. Mater. 2021, 403, 123537. [Google Scholar] [CrossRef] [PubMed]
- Tayi, A.S.; Shveyd, A.K.; Sue, A.C.-H.; Szarko, J.M.; Rolczynski, B.S.; Cao, D.; Kennedy, T.J.; Sarjeant, A.A.; Stern, C.L.; Paxton, W.F.; et al. Room-Temperature Ferroelectricity in Supramolecular Networks of Charge-Transfer Complexes. Nature 2012, 488, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Kobayashi, K.; Kumai, R.; Minami, N.; Kagawa, F.; Tokura, Y. Quantum Ferroelectricity in Charge-Transfer Complex Crystals. Nat. Commun. 2015, 6, 7469. [Google Scholar] [CrossRef] [PubMed]
- Isakov, D.; Vasilev, S.; Gomes, E.d.M.; Almeida, B.; Shur, V.Y.; Kholkin, A.L. Probing Ferroelectric Behaviour in Charge-Transfer Organic Meta-Nitroaniline. Appl. Phys. Lett. 2016, 109, 162903. [Google Scholar] [CrossRef]
- Miller, J.S.; Epstein, A.J.; Reiff, W.M. Ferromagnetic Molecular Charge-Transfer Complexes. Chem. Rev. 1988, 88, 201–220. [Google Scholar] [CrossRef]
- Lei, Y.-L.; Jin, Y.; Zhou, D.-Y.; Gu, W.; Shi, X.-B.; Liao, L.-S.; Lee, S.-T. White-Light Emitting Microtubes of Mixed Organic Charge-Transfer Complexes. Adv. Mater. 2012, 24, 5345–5351. [Google Scholar] [CrossRef]
- Shen, D.; Chen, W.-C.; Lo, M.-F.; Lee, C.-S. Charge-Transfer Complexes and Their Applications in Optoelectronic Devices. Mater. Today Energy 2021, 20, 100644. [Google Scholar] [CrossRef]
- Alves, H.; Molinari, A.S.; Xie, H.; Morpurgo, A.F. Metallic Conduction at Organic Charge-Transfer Interfaces. Nat. Mater. 2008, 7, 574–580. [Google Scholar] [CrossRef]
- Jeszka, J.K.; Ulański, J.; Kryszewski, M. Conductive Polymer: Reticulate Doping with Charge-Transfer Complex. Nature 1981, 289, 390–391. [Google Scholar] [CrossRef]
- Hussain, Z.; Zou, W.; Murdoch, B.J.; Nafady, A.; Field, M.R.; Ramanathan, R.; Bansal, V. Metal–Organic Charge Transfer Complexes of Pb(TCNQ) 2 and Pb(TCNQF 4) 2 as New Catalysts for Electron Transfer Reactions. Adv. Mater. Interfaces 2020, 7, 2001111. [Google Scholar] [CrossRef]
- Srivastava, R.D.; Gupta, P.D. Charge transfer interaction between tetrabromophthalic anhydride and aromatic hydrocarbons. Spectrochim. Acta Part A Mol. Spectrosc. 1968, 24, 373–376. [Google Scholar] [CrossRef]
- Wilkerson, A.K.; Chodak, J.B.; Strouse, C.E. X-ray structure determination of the 1:1 charge transfer complex of naphthalene and tetrachlorophthalic anhydride at -153.deg. J. Am. Chem. Soc. 1975, 97, 3000–3004. [Google Scholar] [CrossRef]
- Harada, J.; Ohtani, M.; Takahashi, Y.; Inabe, T. Molecular Motion, Dielectric Response, and Phase Transition of Charge-Transfer Crystals: Acquired Dynamic and Dielectric Properties of Polar Molecules in Crystals. J. Am. Chem. Soc. 2015, 137, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Rudman, R. Tetrachlorophthalic anhydride: A study of the carbon–chlorine bond. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1971, 27, 262–269. [Google Scholar] [CrossRef]
- Sake Gowda, D.S.; Rudman, R. Refinement of tetrachlorophthalic anhydride and tetrabromophthalic anhydride. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1982, 38, 2842–2845. [Google Scholar] [CrossRef]
- Ito, K.; Moriya, K.; Kashino, S.; Haisa, M. Topochemical Studies. VII. The Crystal and Molecular Structures of Tetrachlorophthalic Acid Hemihydrate and Tetrabromophthalic Anhydride. Bull. Chem. Soc. Jpn. 1975, 48, 3078–3084. [Google Scholar] [CrossRef]
- Uchida, T.; Nakano, H.; Kozawa, K. Tetrachlorophthalic anhydride (TCPA), a refinement. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1982, 38, 2963–2965. [Google Scholar] [CrossRef]
- Sake Gowda, D.S.; Rudman, R. Polymorphism in the tetrahalophthalic anhydrides. 2. The crystal and molecular structures of ordered tetraiodophthalic anhydride. J. Phys. Chem. 1982, 86, 4356–4360. [Google Scholar] [CrossRef]
- Baykov, S.V.; Filimonov, S.I.; Rozhkov, A.V.; Novikov, A.S.; Ananyev, I.V.; Ivanov, D.M.; Kukushkin, V.Y. Reverse Sandwich Structures from Interplay between Lone Pair−π-Hole Atom-Directed C···dz2[M] and Halogen Bond Interactions. Cryst. Growth Des. 2020, 20, 995–1008. [Google Scholar] [CrossRef]
- Ivanov, D.M.; Baykov, S.V.; Novikov, A.S.; Romanenko, G.; Bokach, N.A.; Evarestov, R.A.; Kukushkin, V.Y. Noncovalent Sulfoxide–Nitrile Coupling Involving Four-Center Heteroleptic Dipole–Dipole Interactions between the Sulfinyl and Nitrile Groups. Cryst. Growth Des. 2020, 20, 3417–3428. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Eliseeva, A.A.; Baykov, S.V.; Galmés, B.; Frontera, A.; Kukushkin, V.Y. One-Pot Route to X-perfluoroarenes (X = Br, I) Based on FeIII-Assisted C–F Functionalization and Utilization of These Arenes as Building Blocks for Crystal Engineering Involving Halogen Bonding. Cryst. Growth Des. 2020, 20, 5908–5921. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Novikov, A.S.; Ivanov, D.M.; Bolotin, D.S.; Bokach, N.A.; Kukushkin, V.Y. Structure-Directing Weak Interactions with 1,4-Diiodotetrafluorobenzene Convert One-Dimensional Arrays of [MII(acac)2] Species into Three-Dimensional Networks. Cryst. Growth Des. 2018, 18, 3626–3636. [Google Scholar] [CrossRef]
- Baykov, S.V.; Presnukhina, S.I.; Novikov, A.S.; Shetnev, A.A.; Boyarskiy, V.P.; Kukushkin, V.Y. 2,5-Dibromothiophenes: Halogen Bond Involving Packing Patterns and Their Relevance to Solid-State Polymerization. Cryst. Growth Des. 2021, 21, 2526–2540. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Clark, T.; Riley, K.E.; Politzer, P. σ-Holes, π-holes and electrostatically-driven interactions. J. Mol. Model. 2012, 18, 541–548. [Google Scholar] [CrossRef]
- Galmés, B.; Franconetti, A.; Frontera, A. Nitropyridine-1-Oxides as Excellent π-Hole Donors: Interplay between σ-Hole (Halogen, Hydrogen, Triel, and Coordination Bonds) and π-Hole Interactions. Int. J. Mol. Sci. 2019, 20, 3440. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Kumar, P.S.V.; Raghavendra, V.; Subramanian, V. Bader’s Theory of Atoms in Molecules (AIM) and its Applications to Chemical Bonding. J. Chem. Sci. 2016, 128, 1527–1536. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1994; ISBN 978-0-19-855865-1. [Google Scholar]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, P.; Varadwaj, A.; Marques, H. Halogen Bonding: A Halogen-Centered Noncovalent Interaction Yet to Be Understood. Inorganics 2019, 7, 40. [Google Scholar] [CrossRef]
- Zhu, S.; Asim Khan, M.; Wang, F.; Bano, Z.; Xia, M. Rapid removal of toxic metals Cu2+ and Pb2+ by amino trimethylene phosphonic acid intercalated layered double hydroxide: A combined experimental and DFT study. Chem. Eng. J. 2020, 392, 123711. [Google Scholar] [CrossRef]
- Huang, W.; Lin, R.; Zhao, X.; Li, Q.; Huang, Y.; Ye, G. How does a weak interaction change from a reactive complex to a saddle point in a reaction? Comput. Theor. Chem. 2020, 1173, 112640. [Google Scholar] [CrossRef]
- Lefebvre, C.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Piquemal, J.-P.; Hénon, E. The Independent Gradient Model: A New Approach for Probing Strong and Weak Interactions in Molecules from Wave Function Calculations. ChemPhysChem 2018, 19, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Babinský, M.; Bouzková, K.; Pipíška, M.; Novosadová, L.; Marek, R. Interpretation of Crystal Effects on NMR Chemical Shift Tensors: Electron and Shielding Deformation Densities. J. Phys. Chem. A 2013, 117, 497–503. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zheng, B.; Zhou, F.; Jiao, Y.; Liu, Y.; Ding, X.; Lu, T. A theoretical investigation on Cu/Ag/Au bonding in XH 2 P···MY(X = H, CH3, F, CN, NO2; M = Cu, Ag, Au; Y = F, Cl, Br, I) complexes. J. Chem. Phys. 2018, 148, 194106. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Parrish, R.M.; Sherrill, C.D.; Turney, J.M.; Schaefer, H.F. Large-scale symmetry-adapted perturbation theory computations via density fitting and Laplace transformation techniques: Investigating the fundamental forces of DNA-intercalator interactions. J. Chem. Phys. 2011, 135, 174107. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Jaeger, H.M.; Carrell, E.J.; Tschumper, G.S.; Sherrill, C.D. Accurate Interaction Energies for Problematic Dispersion-Bound Complexes: Homogeneous Dimers of NCCN, P2, and PCCP. J. Chem. Theory Comput. 2011, 7, 2842–2851. [Google Scholar] [CrossRef]
- Hayes, I.C.; Stone, A.J. An intermolecular perturbation theory for the region of moderate overlap. Mol. Phys. 1984, 53, 83–105. [Google Scholar] [CrossRef]
- Jeziorski, B.; Moszynski, R.; Szalewicz, K. Perturbation Theory Approach to Intermolecular Potential Energy Surfaces of van der Waals Complexes. Chem. Rev. 1994, 94, 1887–1930. [Google Scholar] [CrossRef]
- Patkowski, K. Recent developments in symmetry-adapted perturbation theory. WIREs Comput. Mol. Sci. 2020, 10. [Google Scholar] [CrossRef]
- Katlenok, E.A.; Rozhkov, A.V.; Levin, O.V.; Haukka, M.; Kuznetsov, M.L.; Kukushkin, V.Y. Halogen Bonding Involving Palladium(II) as an XB Acceptor. Cryst. Growth Des. 2020, 21, 1159–1177. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Beer, P.D. Sigma-Hole Interactions in Anion Recognition. Chem 2018, 4, 731–783. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. σ-holes and π-holes: Similarities and differences. J. Comput. Chem. 2018, 39, 464–471. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Interaction and Polarization Energy Relationships in σ-Hole and π-Hole Bonding. Crystals 2020, 10, 76. [Google Scholar] [CrossRef]
- Liu, N.; Li, Q.; McDowell, S.A.C. Reliable Comparison of Pnicogen, Chalcogen, and Halogen Bonds in Complexes of 6-OXF2-Fulvene (X = As, Sb, Se, Te, Be, I) With Three Electron Donors. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Klein, J.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Piquemal, J.-P.; Hénon, E. New Way for Probing Bond Strength. J. Phys. Chem. A 2020, 124, 1850–1860. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Y.; Wang, Z.; Lu, T.; Yang, Q.; Liu, Y.; Zheng, B. A new type of halogen bond involving multivalent astatine: An ab initio study. Phys. Chem. Chem. Phys. 2019, 21, 15310–15318. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, T.; Ding, L.; Wang, G.; Wang, Z.; Zheng, B.; Liu, Y.; Ding, X.L. Cooperativity effects between regium-bonding and pnicogen-bonding interactions in ternary MF···PH3O···MF (M = Cu, Ag, Au): An ab initio study. Mol. Phys. 2020, 118, e1784478. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mahdavinia, G.; Javaheri, M.; Sobhi, H.R. A theoretical study of substitution effects on halogen–π interactions. Mol. Phys. 2014, 112, 1160–1166. [Google Scholar] [CrossRef]
- Riley, K.E.; Hobza, P. Investigations into the Nature of Halogen Bonding Including Symmetry Adapted Perturbation Theory Analyses. J. Chem. Theory Comput. 2008, 4, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Riley, K.E.; Hobza, P. The relative roles of electrostatics and dispersion in the stabilization of halogen bonds. Phys. Chem. Chem. Phys. 2013, 15, 17742. [Google Scholar] [CrossRef]
- Riley, K.E.; Murray, J.S.; Fanfrlík, J.; Řezáč, J.; Solá, R.J.; Concha, M.C.; Ramos, F.M.; Politzer, P. Halogen bond tunability II: The varying roles of electrostatic and dispersion contributions to attraction in halogen bonds. J. Mol. Model. 2013, 19, 4651–4659. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- CrysAlis Pro; Yarnton: Oxfordshire, UK, 2013.
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef]
- Pantazis, D.A.; Neese, F. All-Electron Scalar Relativistic Basis Sets for the Actinides. J. Chem. Theory Comput. 2011, 7, 677–684. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm. J. Mol. Graph. Model. 2012, 38, 314–323. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Chen, Q. Intermolecular interaction characteristics of the all-carboatomic ring, cyclo[18]carbon: Focusing on molecular adsorption and stacking. Carbon 2021, 171, 514–523. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Carroll, M.T.; Cheeseman, J.R.; Chang, C. Properties of atoms in molecules: Atomic volumes. J. Am. Chem. Soc. 1987, 109, 7968–7979. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Turney, J.M.; Simmonett, A.C.; Parrish, R.M.; Hohenstein, E.G.; Evangelista, F.A.; Fermann, J.T.; Mintz, B.J.; Burns, L.A.; Wilke, J.J.; Abrams, M.L.; et al. Psi4: An open-source ab initio electronic structure program. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 556–565. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Karafiloglou, P.; Landis, C.R.; Weinhold, F. NBO7; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2018. [Google Scholar]
| X-ray Structure | Contributions of Various Intermolecular Contacts to the Molecular Hirshfeld Surface 1 |
|---|---|
| TCPA | Cl–O 30.5%, Cl–Cl 25.2%, C–Cl 22.2%, C–O 16.8%, O–O 5.0% |
| TBPA | Br–O 30.9%, Br–Br 25.8%, C–Br 22.7%, C–O 16.1%, O–O 4.4% |
| TIPA | I–I 36.5%, I–O 24.4%, C–I 13.1%, C–O 12.5%, O–O 7.4%, C–C 6.1% |
| Compound | Contact | d(X···Y), Å | R 1 | ∠(C–X···Y), ° |
|---|---|---|---|---|
| TCPA | C4–Cl2···O1 | 3.0972(14) | 0.95 | 155.23(7) |
| C6–Cl4···O3 | 3.0373(16) | 0.93 | 173.08(7) | |
| C3–Cl1···Cl2 | 3.4978(7) | 1.00 | 174.58(7) | |
| TBPA | C4–Br2···O1 | 3.1873(19) | 0.95 | 155.48(9) |
| C6–Br4···O3 | 3.093(2) | 0.92 | 175.23(9) | |
| C3–Br1···Br2 | 3.5816(4) | 0.97 | 173.89(8) | |
| TIPA | C6–I4···O3 | 3.141(3) | 0.90 | 177.59(9) |
| C4–I2···I1 | 3.7497(6) | 0.95 | 174.00(8) | |
| C5–I3···I3 | 3.7760(5) | 0.95 | 157.67(9) | |
| C4–I2···C3 | 3.651(3) | 0.99 | 141.25(10) |
| Structure | X | d(X···O), Å | ∠(C–X···O), ° | R 1 |
|---|---|---|---|---|
| VILFIF | Br | 3.468(5) | 166.3(2) | 1.03 |
| WEXKEP | Br | 3.333(6) | 170.6(4) | 0.99 |
| LIZCOM | Cl | 3.2970(9) | 170.68(4) | 1.01 |
| Compound | Contact | d(X···Y), Å | R 1 |
|---|---|---|---|
| TCPA | Cl4···C2 | 3.399(2) | 0.99 |
| O2···C1 | 2.939(3) | 0.91 | |
| O2···C2 | 2.951(2) | 0.92 | |
| O3···C8 | 3.209(2) | 1.00 | |
| TBPA | Br4···C2 | 3.584(3) | 1.00 |
| O2···C1 | 3.076(4) | 0.96 | |
| O2···C2 | 3.043(3) | 0.95 | |
| TIPA | O1···C7 | 3.057(4) | 0.95 |
| O1···C8 | 3.127(4) | 0.97 |
| Cluster | Contact | ρb | ∇2ρb | Vb | Gb | Sign(λ2)ρb | IGMH-IBSI |
|---|---|---|---|---|---|---|---|
| [TCPA]5 | C4–Cl2···O1 | 0.0076 | 0.0347 | −0.0056 | 0.0066 | −0.0076 | 0.008 |
| C6–Cl4···O3 | 0.0076 | 0.0377 | –0.0050 | 0.0072 | –0.0076 | 0.009 | |
| C3–Cl1···Cl2 | 0.0055 | 0.0226 | −0.0028 | 0.0042 | −0.0055 | 0.006 | |
| [TBPA]5 | C4–Br2···O1 | 0.0077 | 0.0313 | −0.0046 | 0.0062 | −0.0077 | 0.008 |
| C6–Br4···O3 | 0.0082 | 0.0362 | −0.0053 | 0.0072 | −0.0082 | 0.009 | |
| C3–Br1···Br2 | 0.0073 | 0.0237 | −0.0039 | 0.0237 | −0.0073 | 0.008 | |
| [TIPA]6 | C6–I4···O3 | 0.0098 | 0.0399 | −0.0061 | 0.0080 | −0.0098 | 0.011 |
| C4–I2···I1 | 0.0101 | 0.0244 | −0.0046 | 0.0053 | −0.0101 | 0.011 | |
| C5–I3···I3 | 0.0101 | 0.0233 | −0.0045 | 0.0051 | −0.0101 | 0.011 | |
| C4–I2···C3 | 0.0078 | 0.0318 | −0.0045 | 0.0062 | −0.0078 | 0.007 |
| Cluster | Contact | Transition | E(2) | ∆occ σ*(X–C) |
|---|---|---|---|---|
| [TCPA]5 | C4–Cl2···O1 | LP(O) → σ*(Cl–C) | 0.5 | 3 |
| C6–Cl4···O3 | LP(O) → σ*(Cl–C) | 0.9 | 2 | |
| C3–Cl1···Cl2 | LP(Cl) → σ*(Cl–C) | 0.7 | 4 | |
| [TBPA]5 | C4–Br2···O1 | LP(O) → σ*(Br–C) | 0.8 | 5 |
| C6–Br4···O3 | LP(O) → σ*(Br–C) | 1.4 | 4 | |
| C3–Br1···Br2 | LP(Br) → σ*(Br–C) | 1.8 | 9 | |
| [TIPA]6 | C6–I4···O3 | LP(O) → σ*(I–C) | 2.0 | 6 |
| C4–I2···I1 | LP(I) → σ*(I–C) | 3.8 | 25 | |
| C5–I3···I3 | LP(I) → σ*(I–C) | 3.5 | 25 | |
| C4–I2···C3 | LP(O) → π*(O/C–C)/σ*(C–C) | 0.9 | – |
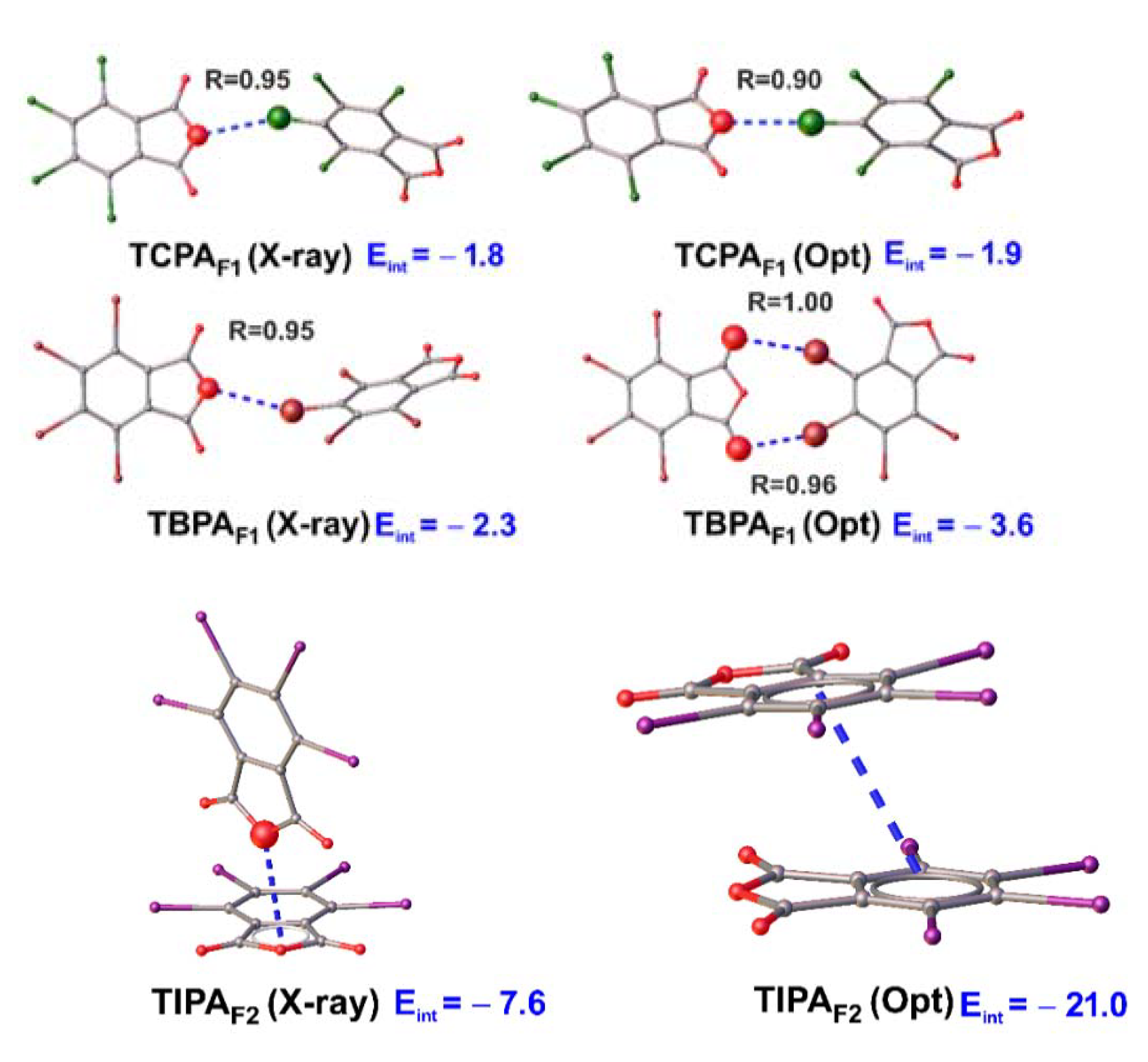
| Fragments | Important Contact | Eelst | Eind | Eexch | Edis | Eint(SAPT) 1 |
|---|---|---|---|---|---|---|
| TCPAF1 | C4–Cl2···O1 | −1.6 | −0.4 | 2.0 | −1.9 | −1.8 |
| TCPAF1opt | C4–Cl2···O1 | −2.1 | −0.51 | 3.0 | −2.2 | −1.9 |
| TCPAF2 | C6–Cl4···O3 | −3.9 | −1.0 | 3.9 | −3.4 | −4.4 2 |
| TCPAF3 | C3–Cl1···Cl2 | −1.8 | −0.5 | 4.6 | −3.4 | −1.2 |
| TBPAF1 | C4–Br2···O1 | −2.0 | −0.5 | 2.5 | −2.3 | −2.3 |
| TBPAF1opt | C4–Br2···O1 C4–Br2···O1 | −5.0 | −1.0 | 6.9 | −4.5 | −3.6 2 |
| TBPAF2 | C6–Br4···O3 | −6.4 | −1.6 | 6.6 | −4.6 | −6.0 |
| TBPAF2 | C6–Br4···O3 | −3.5 | −0.7 | 2.2 | −2.0 | −4.1 2 |
| TBPAF3 | C3–Br1···Br2 | −3.4 | −1.0 | 8.1 | −4.9 | −1.2 |
| TIPAF1 | C6–I4···O3 | −4.6 | −1.5 | 6.7 | −5.9 | −5.3 |
| TIPAF1opt | π···π | −10.3 | −3.4 | 27.9 | −35.1 | −21.0 |
| TIPAF2 | C4–I2···I1 C5–I3···I3 | −6.5 | −3.3 | 16.4 | −17.4 | −10.8 2 |
| TIPAF3 | I2··· π | −4.5 | −1.4 | 6.8 | −8.5 | −7.6 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baykov, S.V.; Semenov, A.V.; Katlenok, E.A.; Shetnev, A.A.; Bokach, N.A. Comparative Structural Study of Three Tetrahalophthalic Anhydrides: Recognition of X···O(anhydride) Halogen Bond and πh···O(anhydride) Interaction. Molecules 2021, 26, 3119. https://doi.org/10.3390/molecules26113119
Baykov SV, Semenov AV, Katlenok EA, Shetnev AA, Bokach NA. Comparative Structural Study of Three Tetrahalophthalic Anhydrides: Recognition of X···O(anhydride) Halogen Bond and πh···O(anhydride) Interaction. Molecules. 2021; 26(11):3119. https://doi.org/10.3390/molecules26113119
Chicago/Turabian StyleBaykov, Sergey V., Artem V. Semenov, Eugene A. Katlenok, Anton A. Shetnev, and Nadezhda A. Bokach. 2021. "Comparative Structural Study of Three Tetrahalophthalic Anhydrides: Recognition of X···O(anhydride) Halogen Bond and πh···O(anhydride) Interaction" Molecules 26, no. 11: 3119. https://doi.org/10.3390/molecules26113119
APA StyleBaykov, S. V., Semenov, A. V., Katlenok, E. A., Shetnev, A. A., & Bokach, N. A. (2021). Comparative Structural Study of Three Tetrahalophthalic Anhydrides: Recognition of X···O(anhydride) Halogen Bond and πh···O(anhydride) Interaction. Molecules, 26(11), 3119. https://doi.org/10.3390/molecules26113119







