Isomers of Terpyridine as Ligands in Coordination Polymers and Networks Containing Zinc(II) and Cadmium(II)
Abstract
1. Introduction
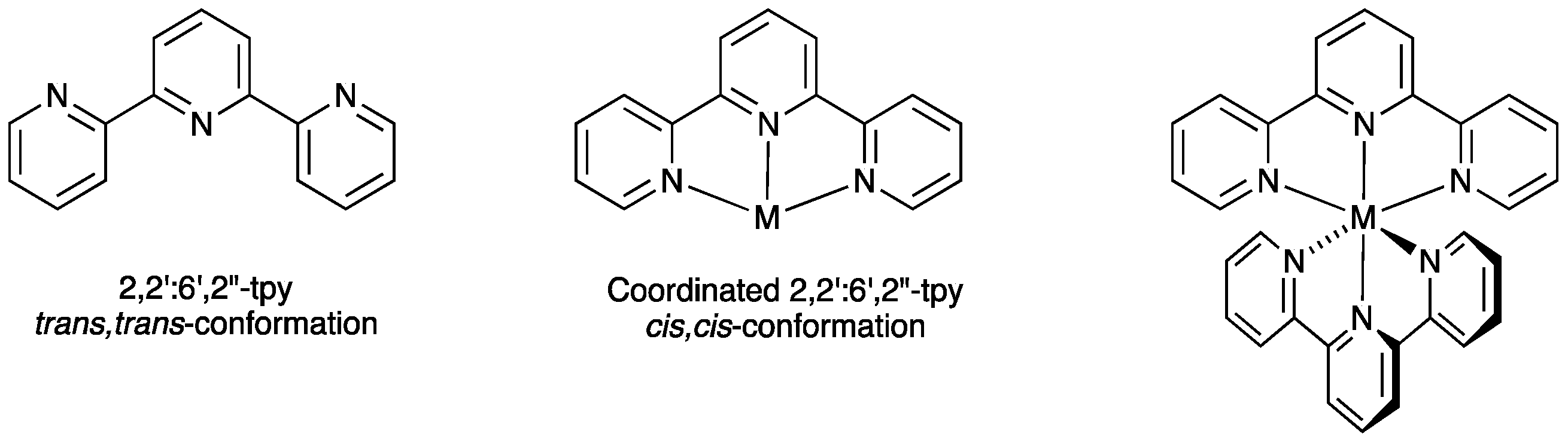
2. 2,2′:6′,2″-Terpyridine
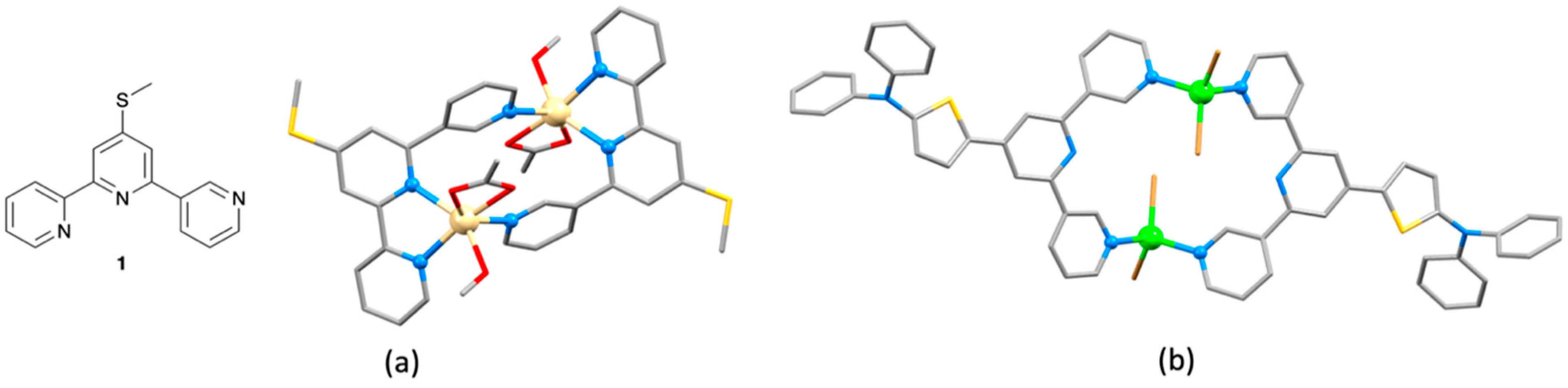
2.1. Assemblies with {M(4′-pyridinyl-2,2′:6′,2″-tpy)Xn} (M = Zn, Cd) Building Blocks
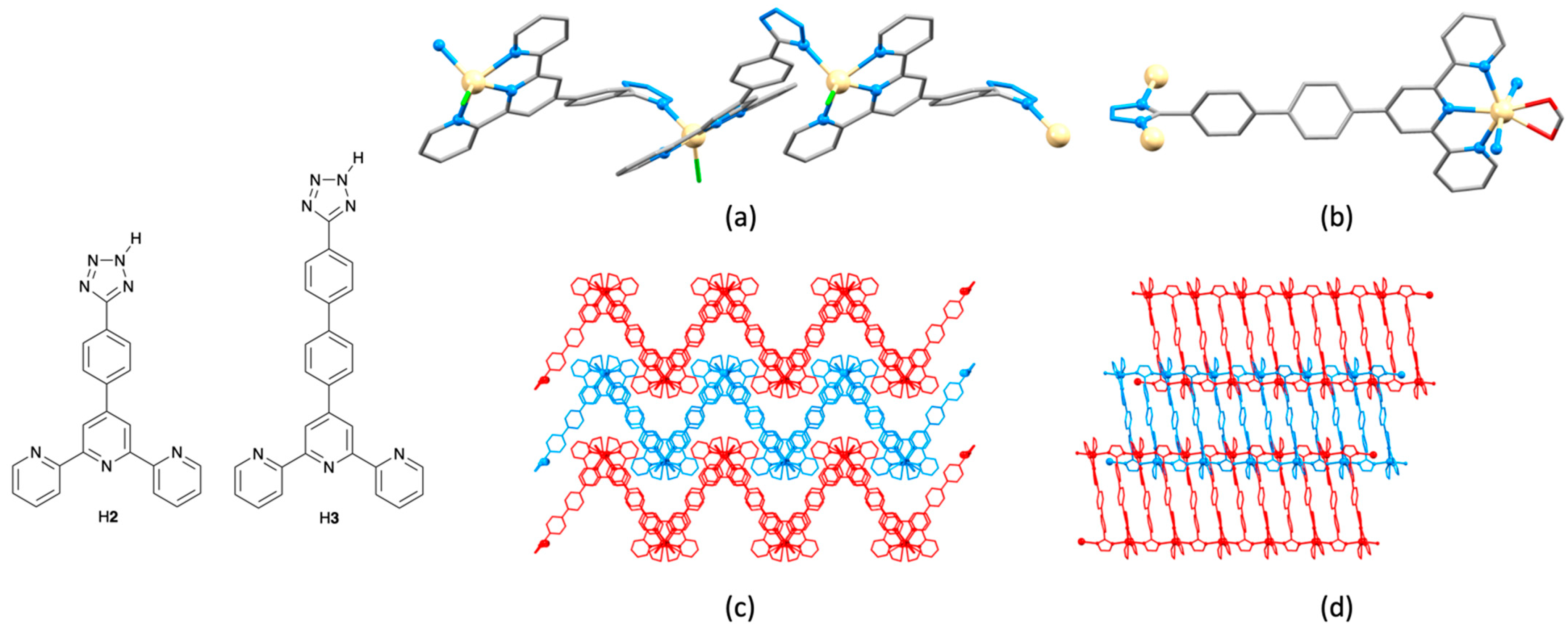
2.2. Assemblies with {Cd(4′-(4-(5-tetrazolato)phenyl)-2,2′:6′,2″-tpy)Xn} Building Blocks
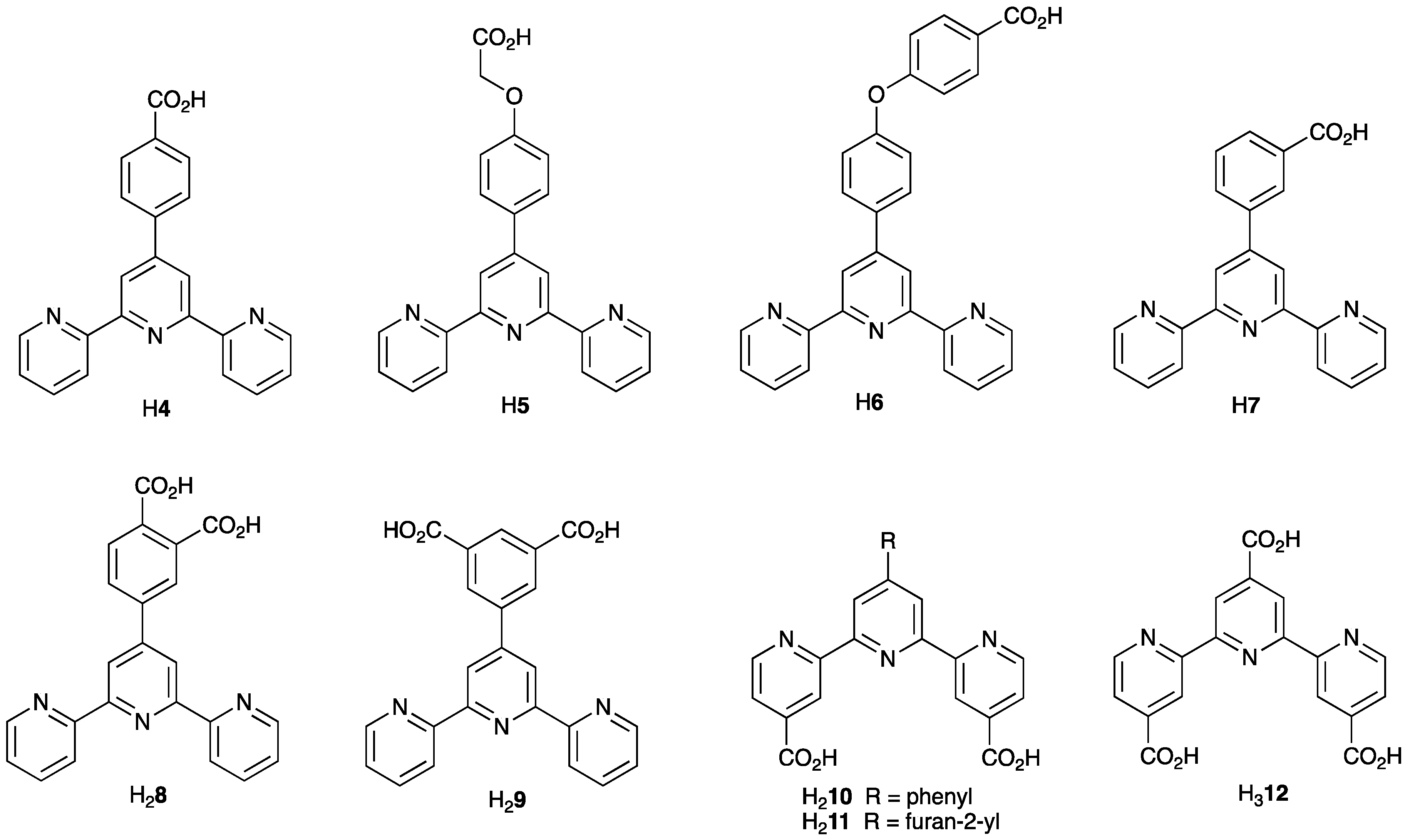
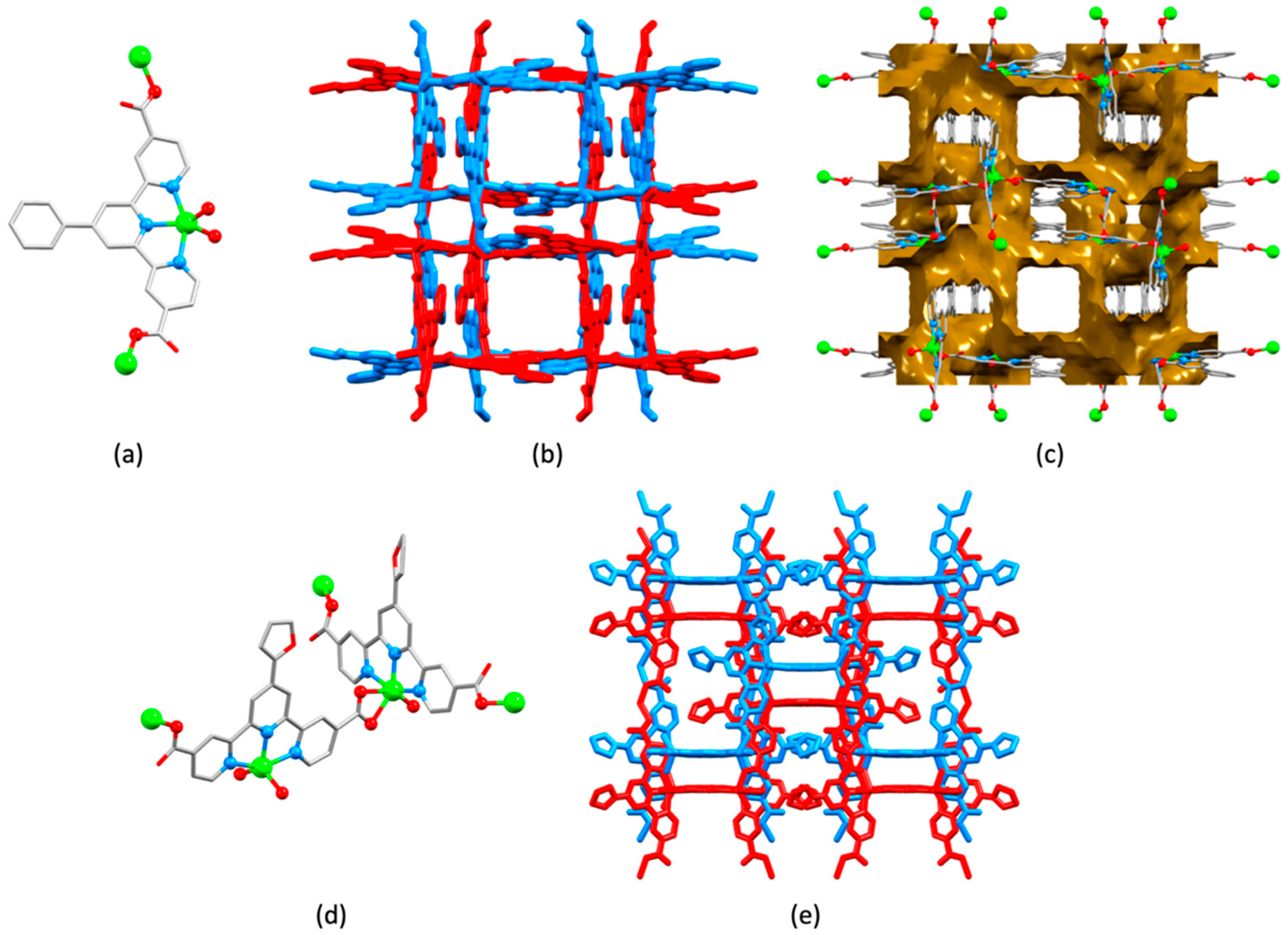
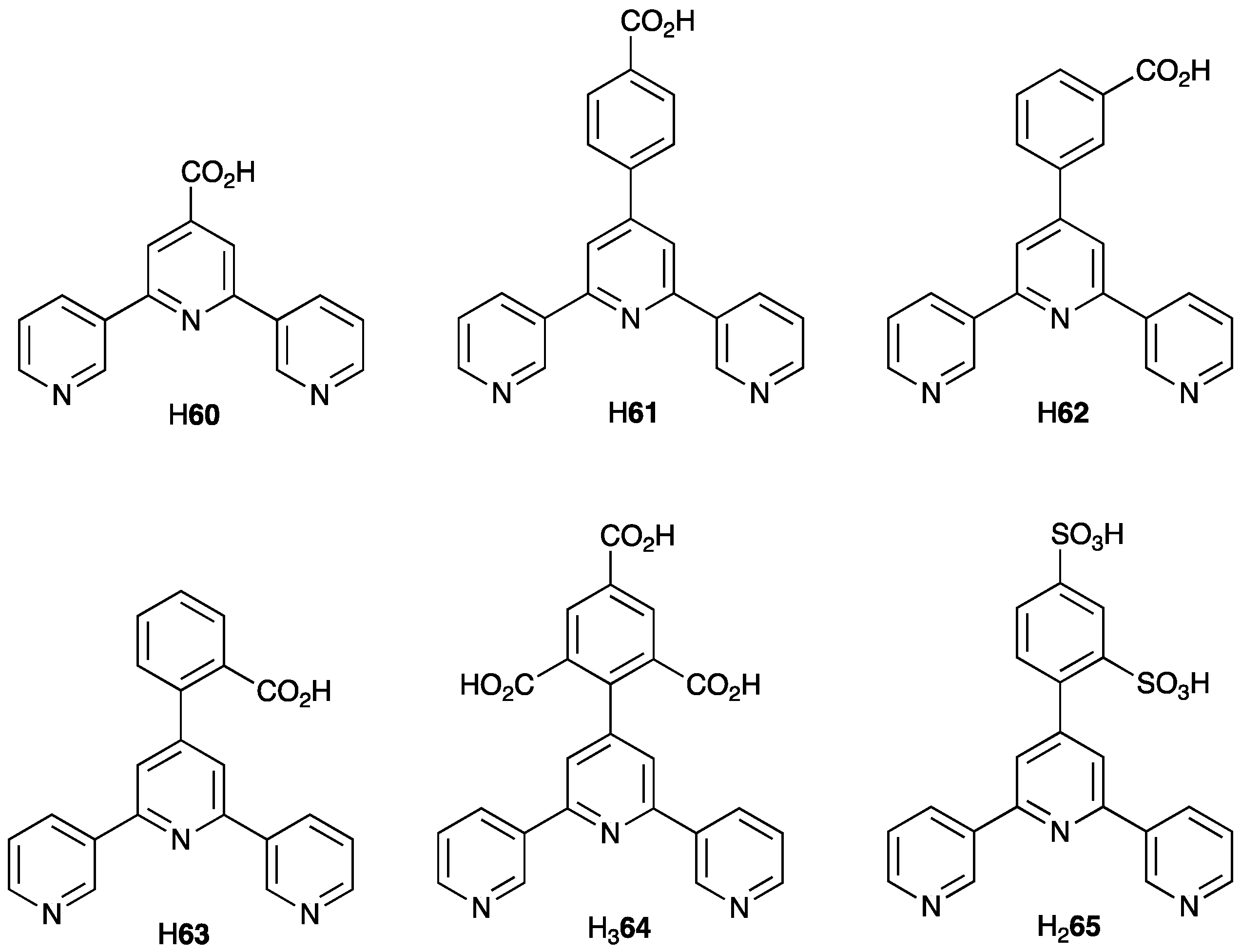
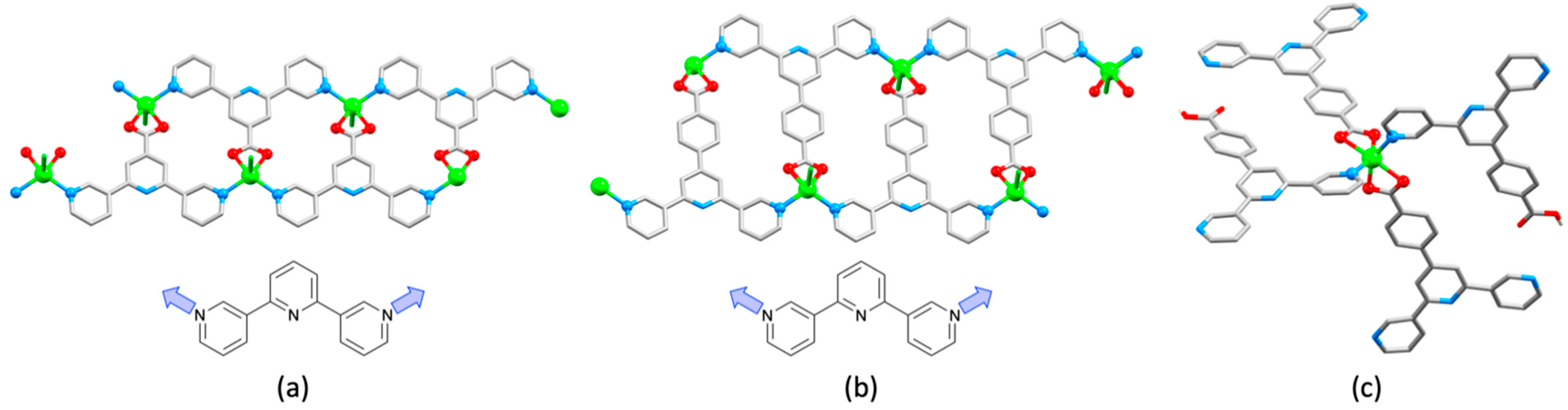
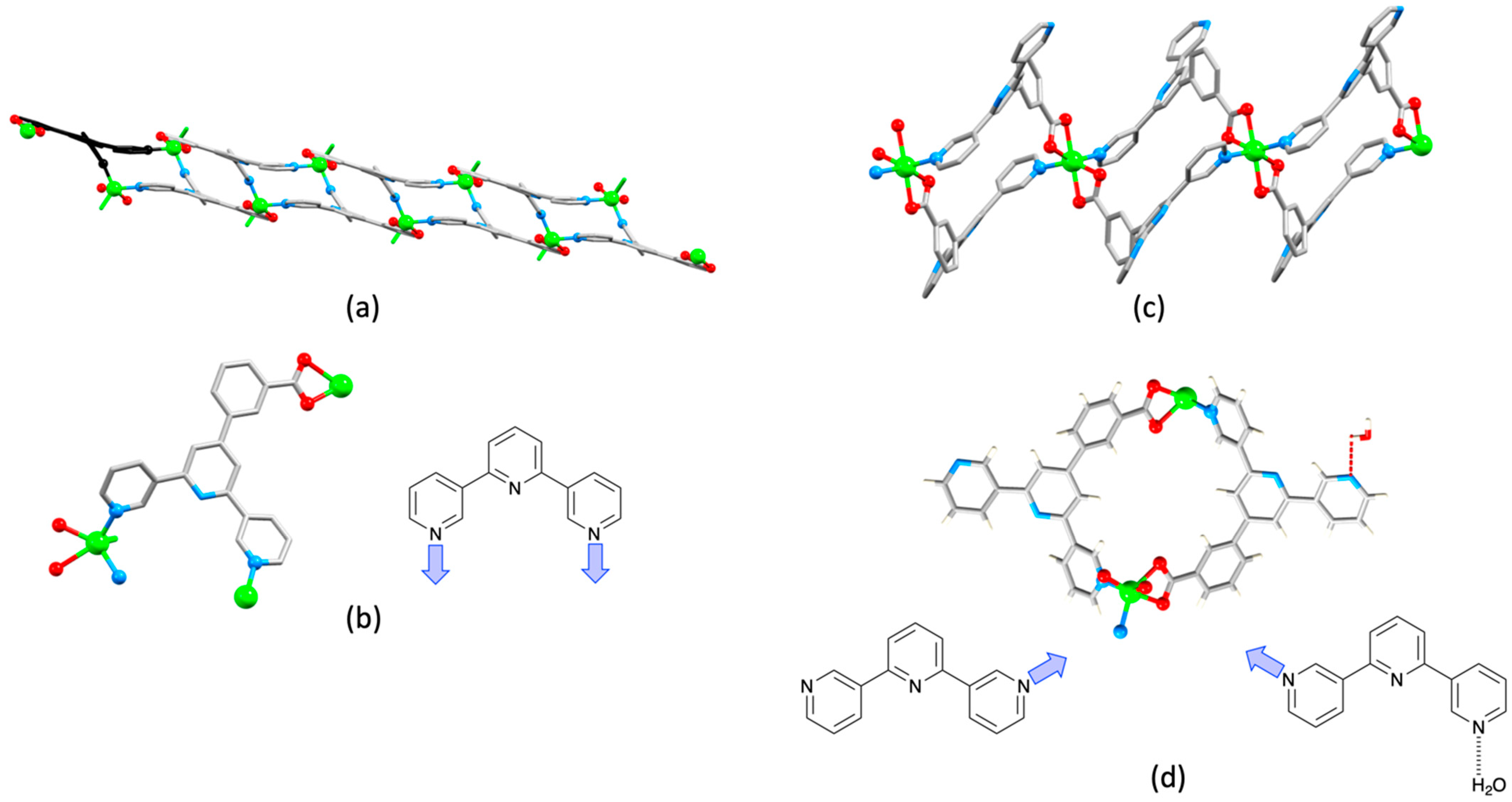
2.3. Assemblies Containing Zn(II) and 2,2′:6′,2″-tpy Ligands Functionalized with Carboxylate Donors
2.4. An Assembly Containing Zn(II) and the Conjugate Base of 4′-Hydroxy-2,2′:6′,2″-terpyridine
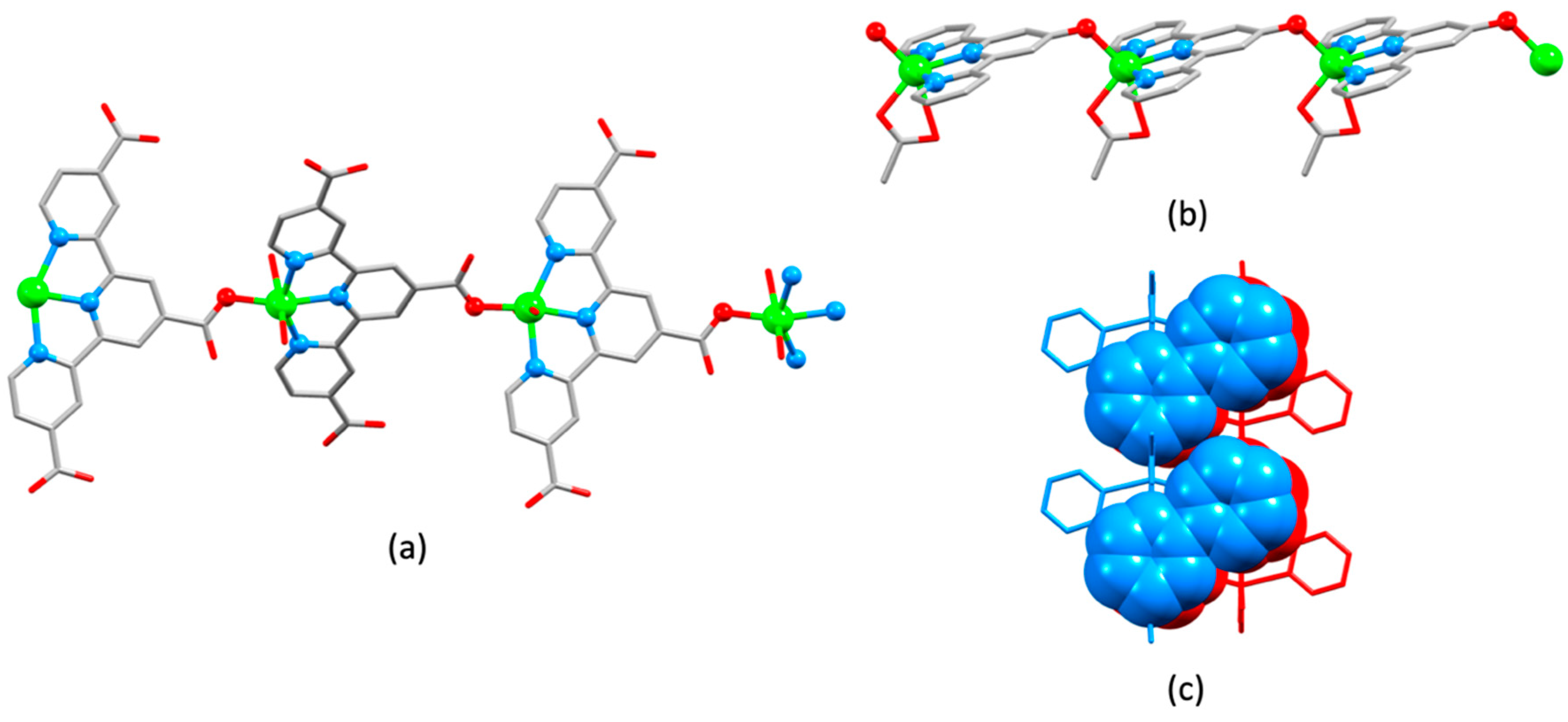
2.5. Assemblies Containing Cd(II) and 2,2′:6′,2″-tpy Ligands Functionalized with Carboxylate or Sulfonate Donors
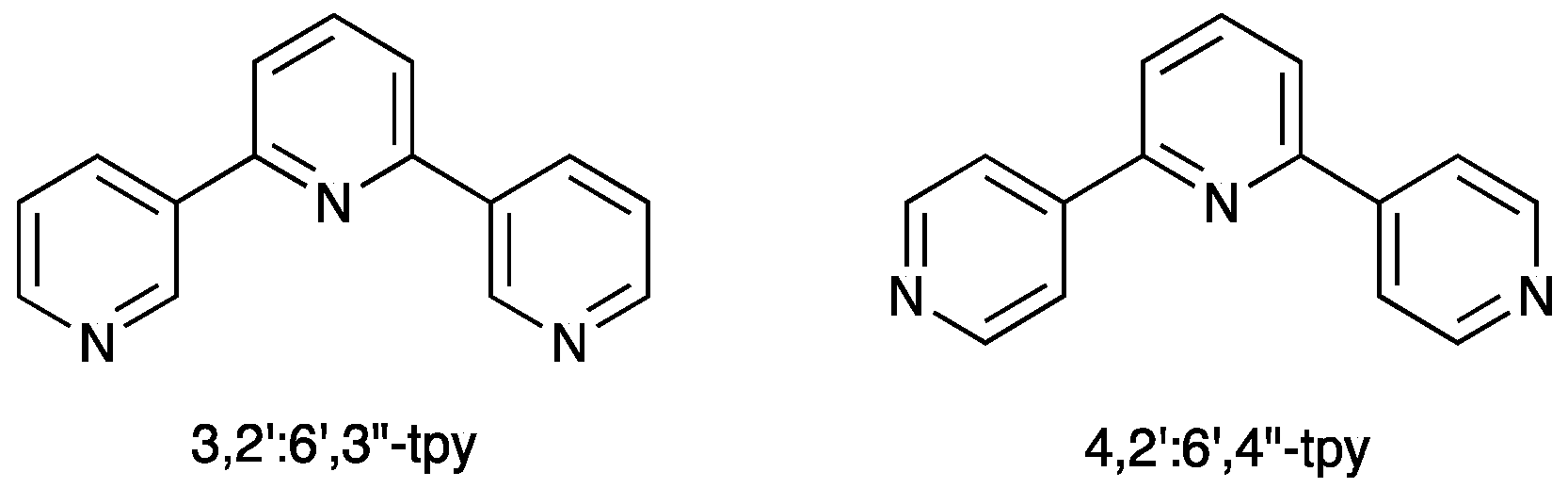
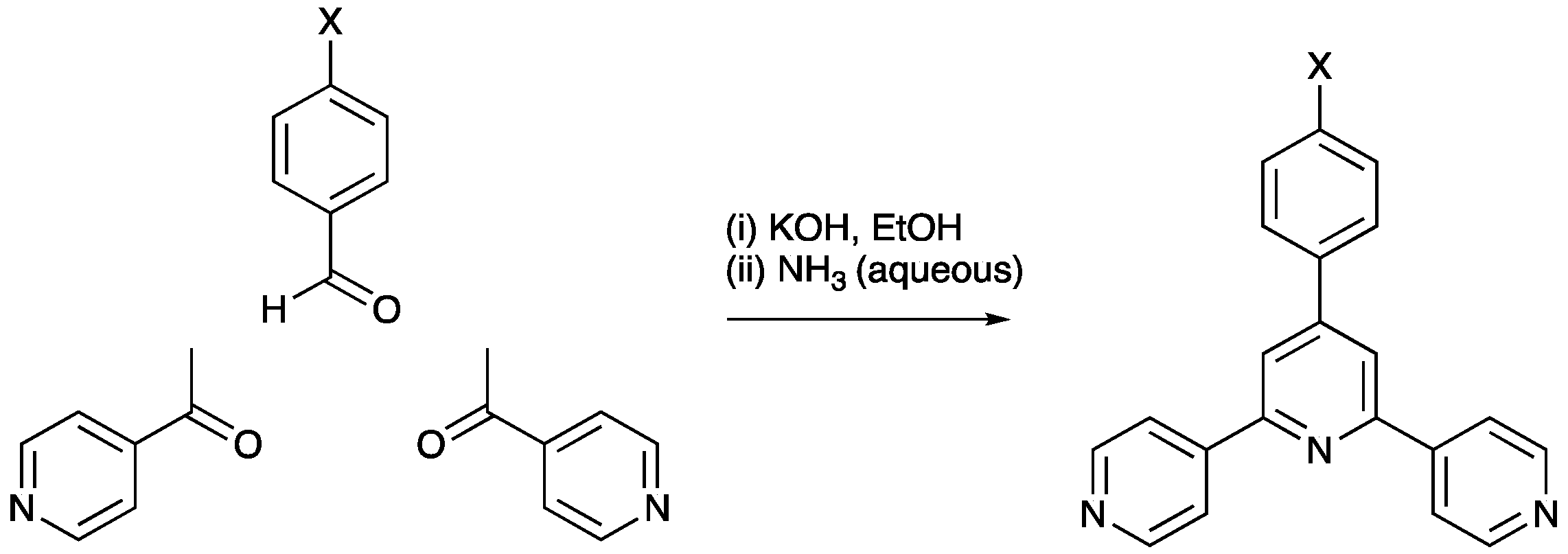
3. 4,2′:6′,4″-Terpyridine
3.1. A 1D-Coordination Polymer with an Unfunctionalized 4,2′:6′,4″-tpy
3.2. Assemblies Containing Zinc(II) and 4′-Functionalized 4,2′:6′,4″-tpy Ligands with Coordinatively Innocent Substituents
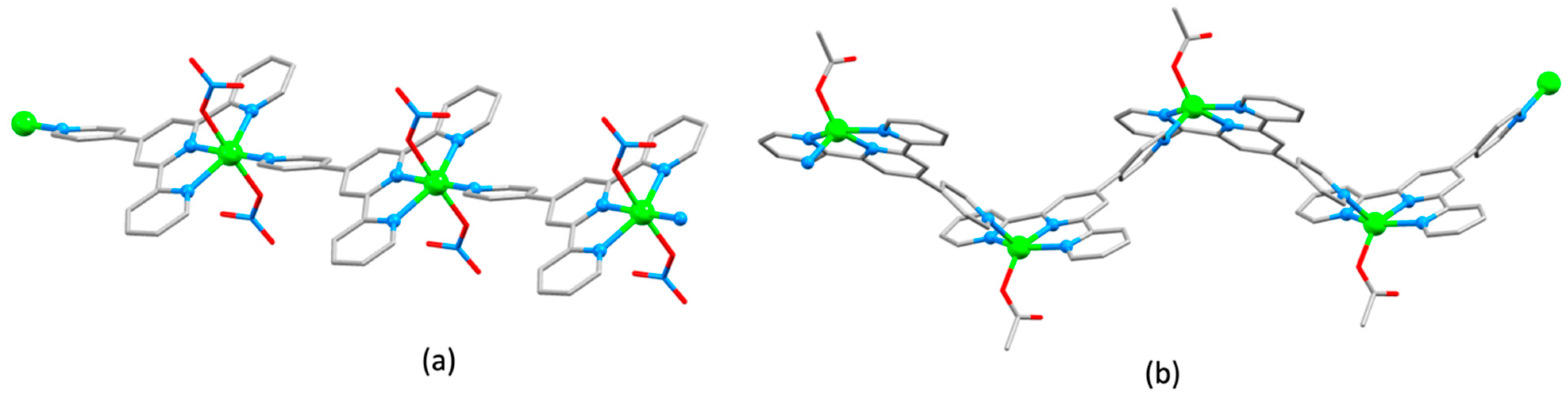
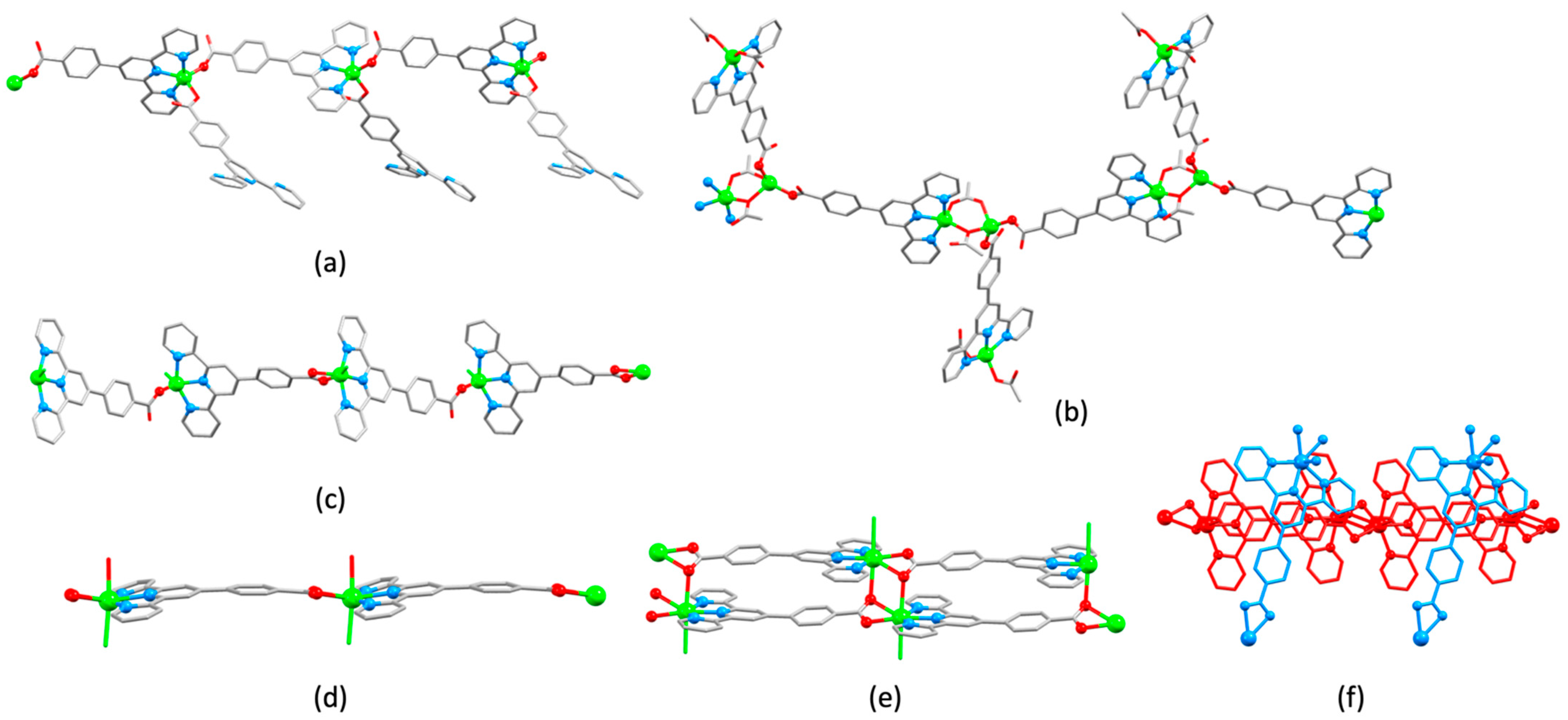
3.3. Assemblies Containing 4′-Functionalized 4,2′:6′,4″-tpy Ligands Connected by {Zn2μ-OAc)4} Paddle-Wheel Units
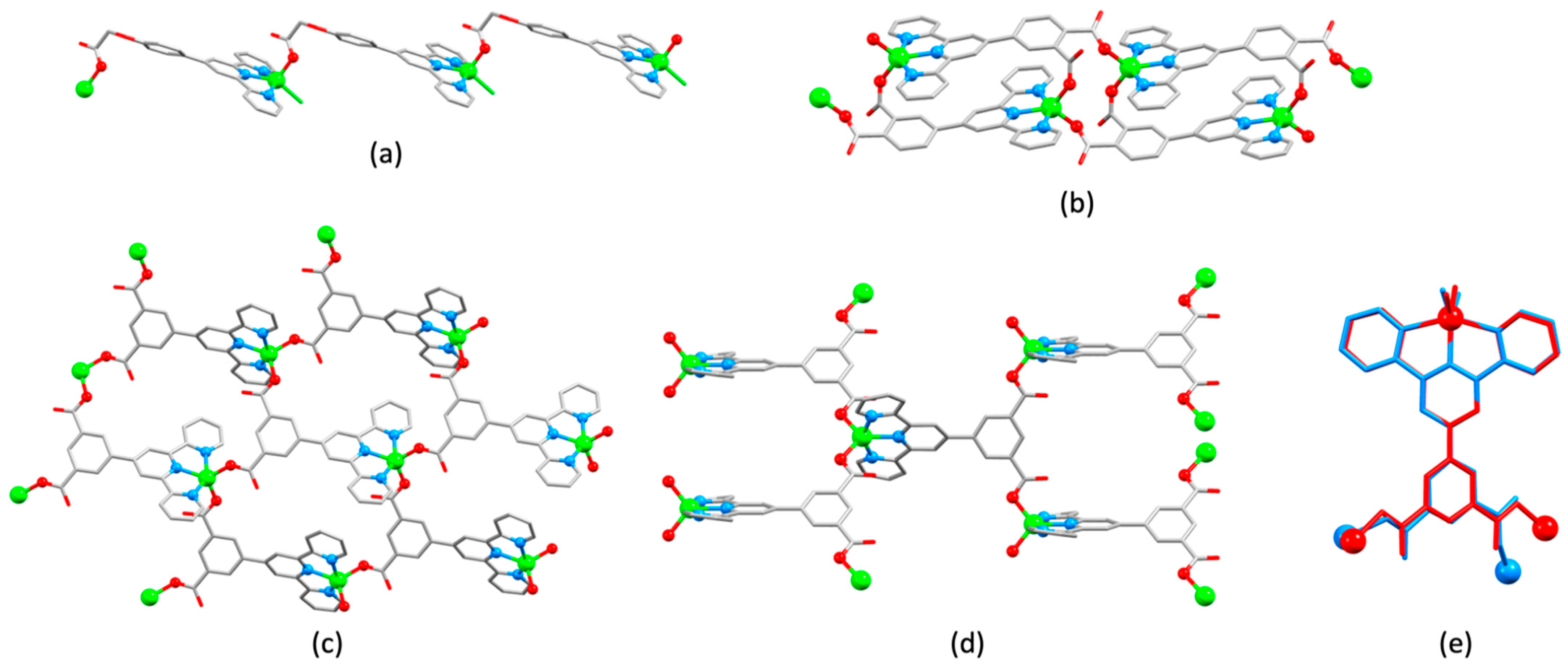
3.4. Assemblies Containing Zinc(II) and 4,2′:6′,4″-tpy Ligands Functionalized in the 4′-Position with Carboxylates
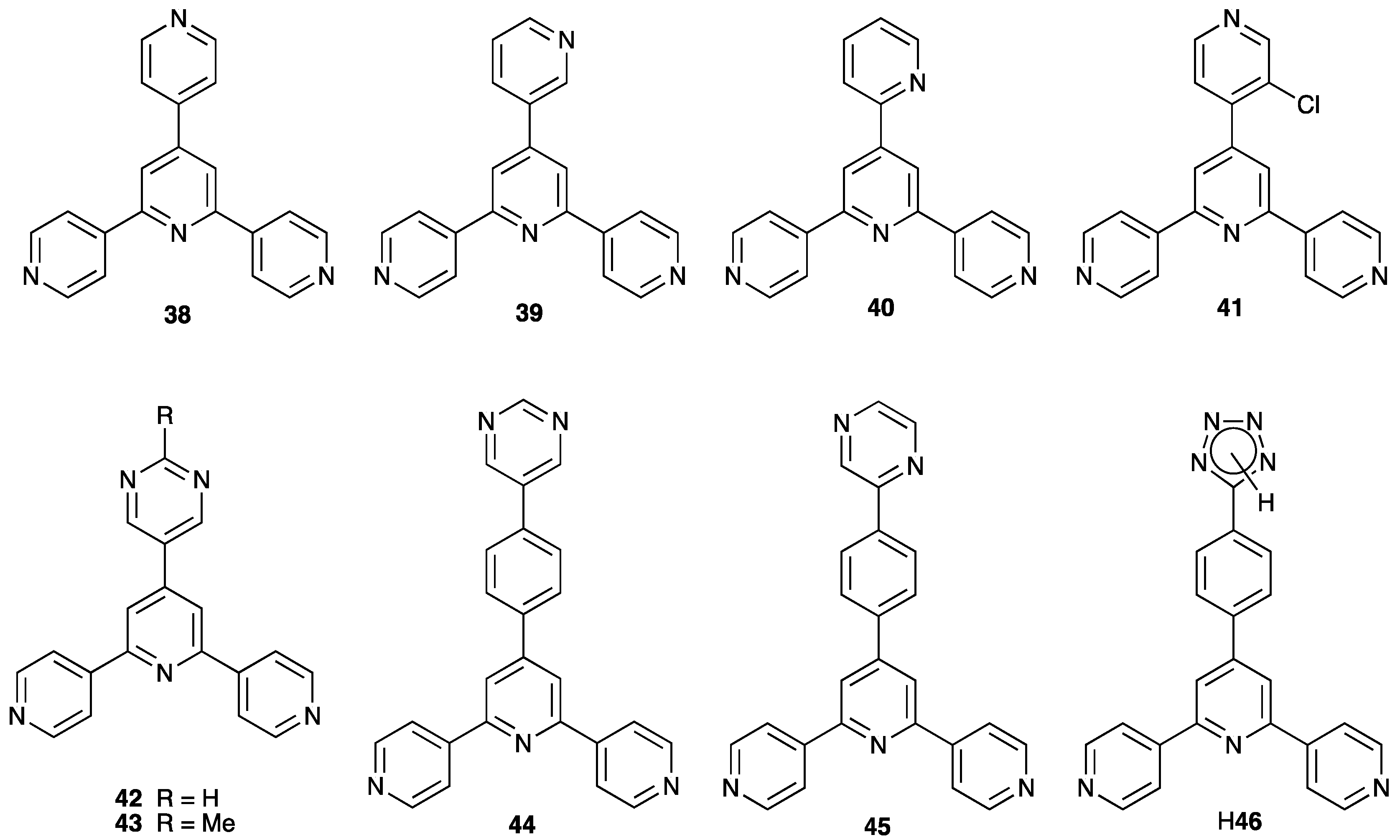
3.5. Assemblies Containing Zinc(II) and 4,2′:6′,4″-tpy Ligands Functionalized in the 4′-Position with a Pyridinyl Group or Other Nitrogen Heterocycles
3.6. An Assembly Containing Zinc(II) and a 4,2′:6′,4″-tpy Ligand Functionalized in the 4′-Position with an Oxygen Heterocycle
3.7. Assemblies Containing Cadmium(II) and 4′-Functionalized 4,2′:6′,4″-tpy Ligands with Coordinatively Innocent Substituents
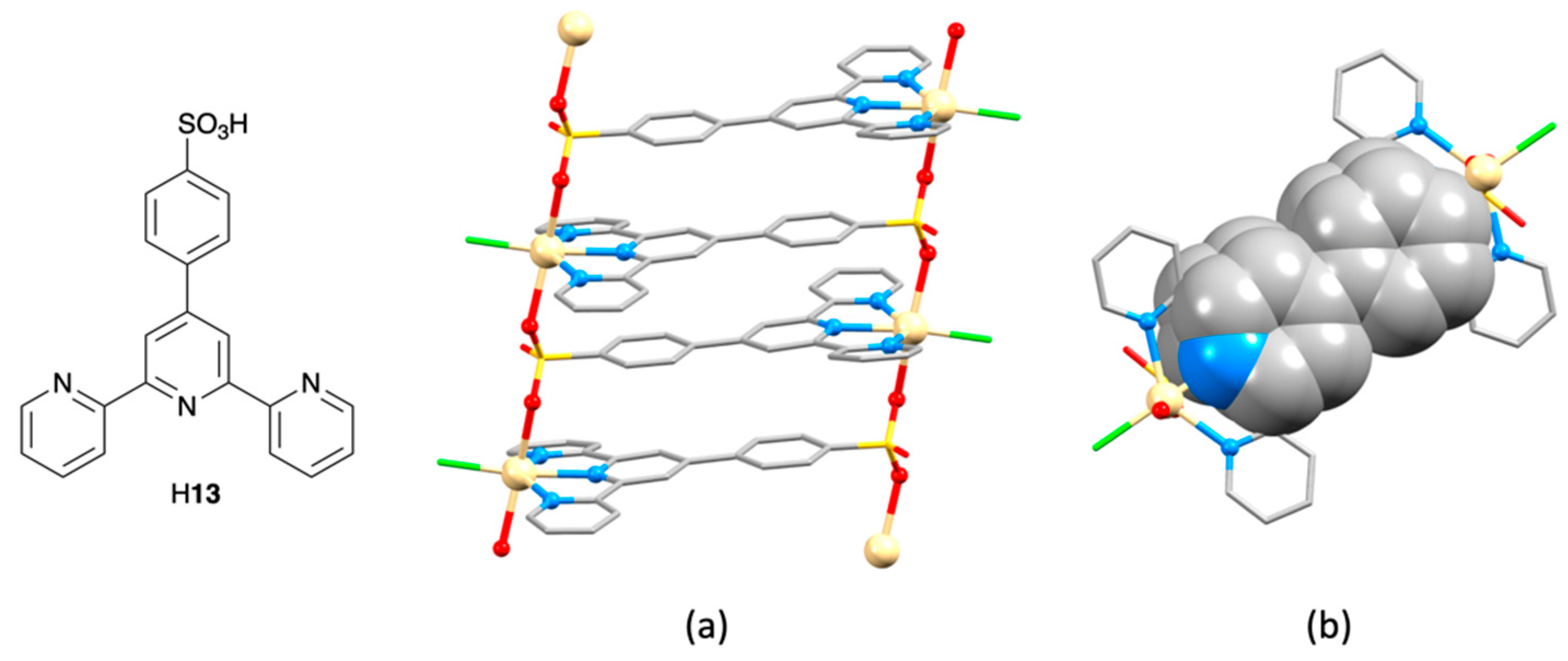
3.8. Assemblies Containing Cadmium(II) and 4,2′:6′,4″-tpy Ligands Functionalized in the 4′-Position with Carboxylate or Sulfonate Donors
3.9. Assemblies Containing Cadmium(II) and 4,2′:6′,4″-tpy Ligands Functionalized in the 4′-Position with a Pyridinyl Group
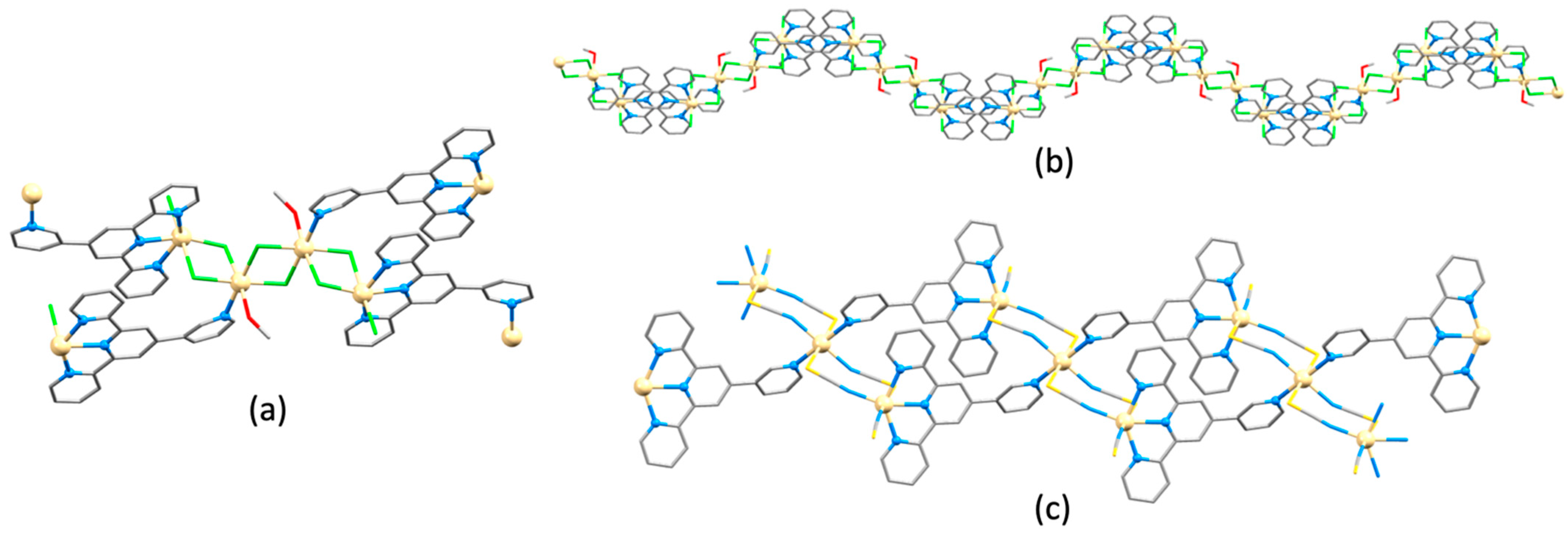
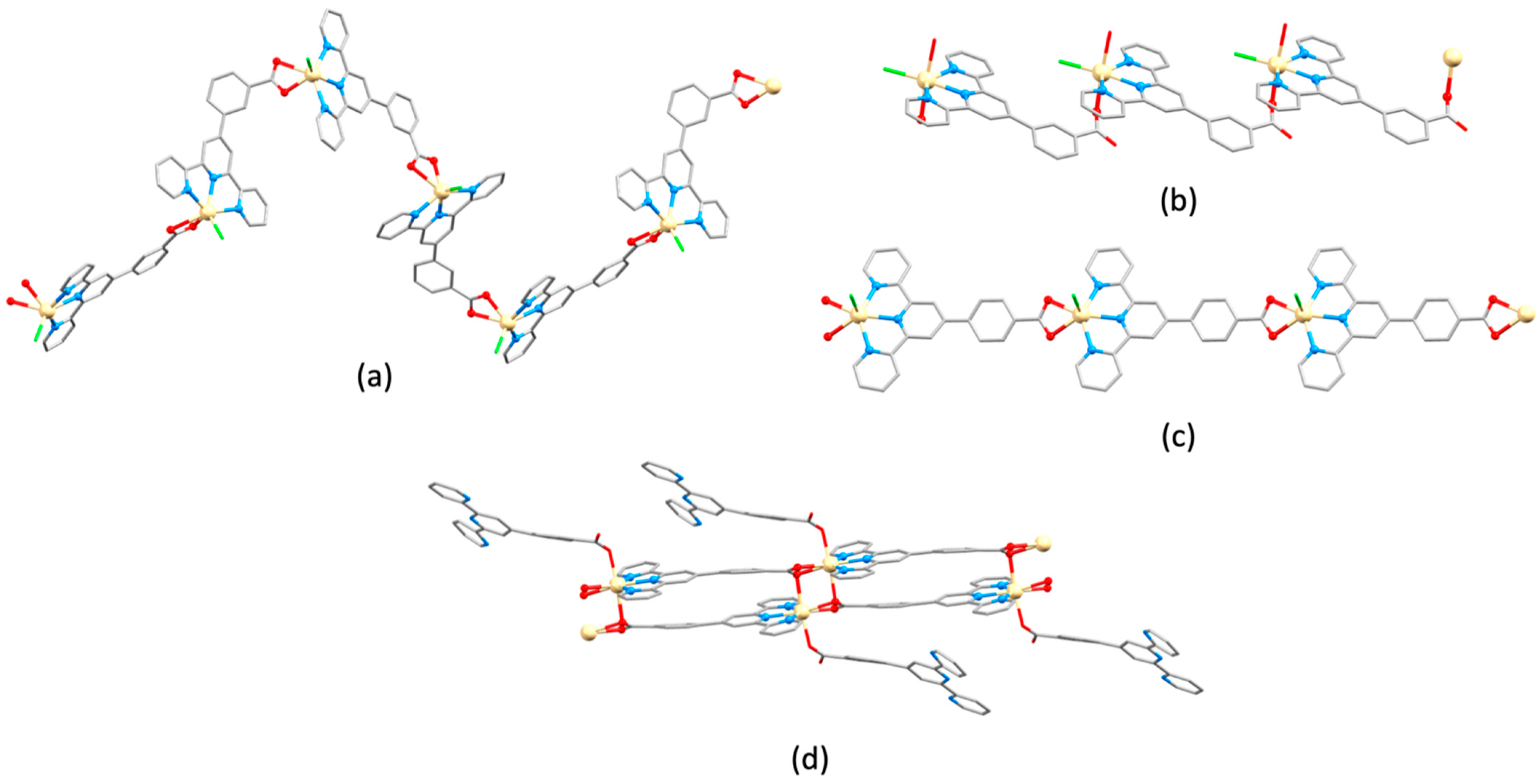
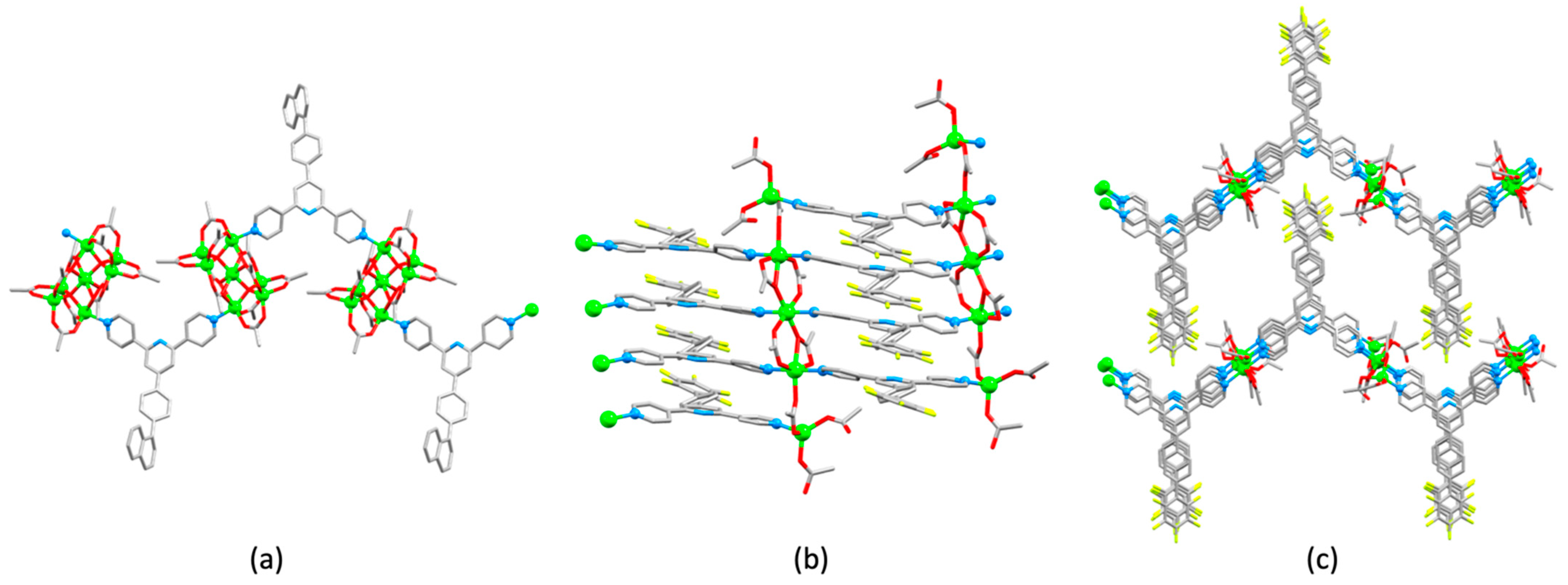
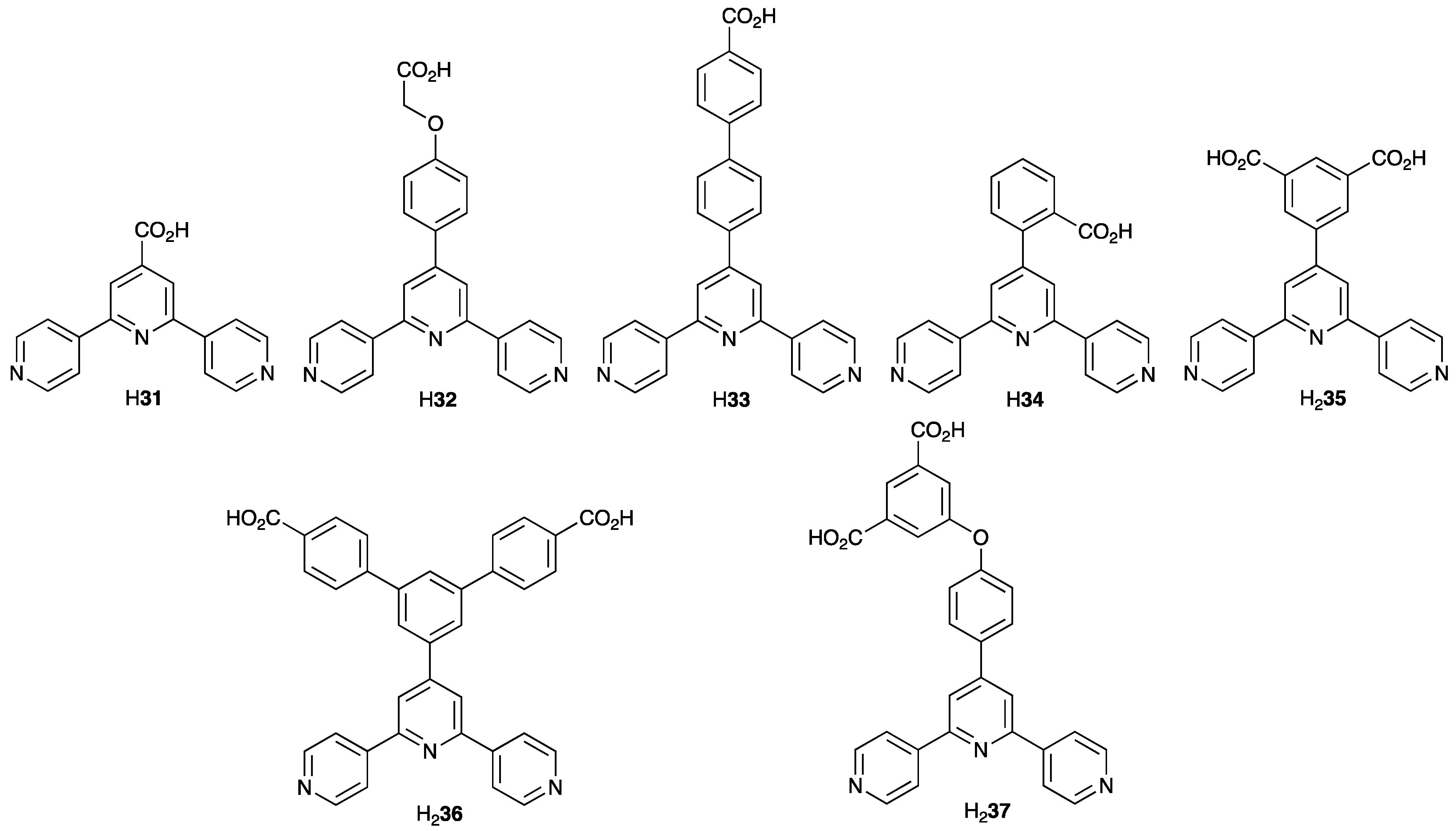
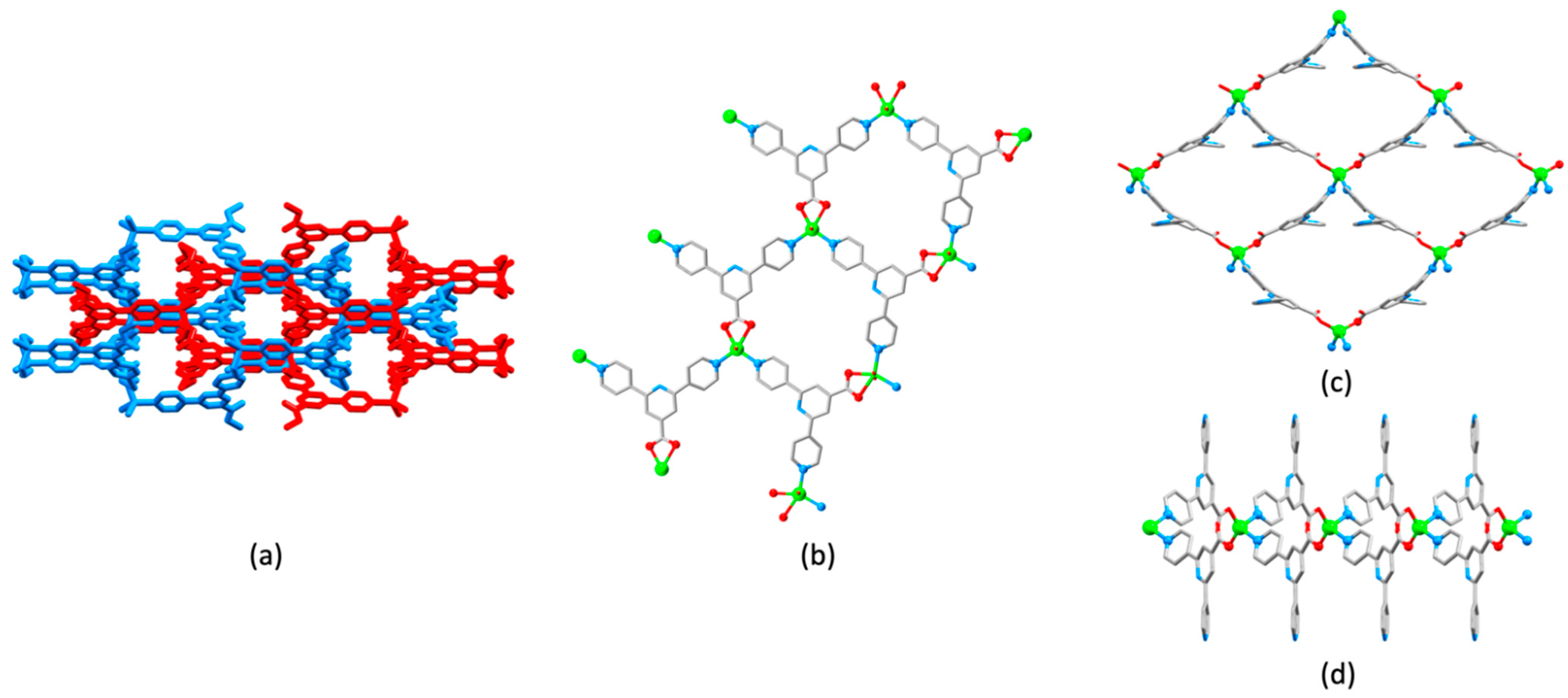
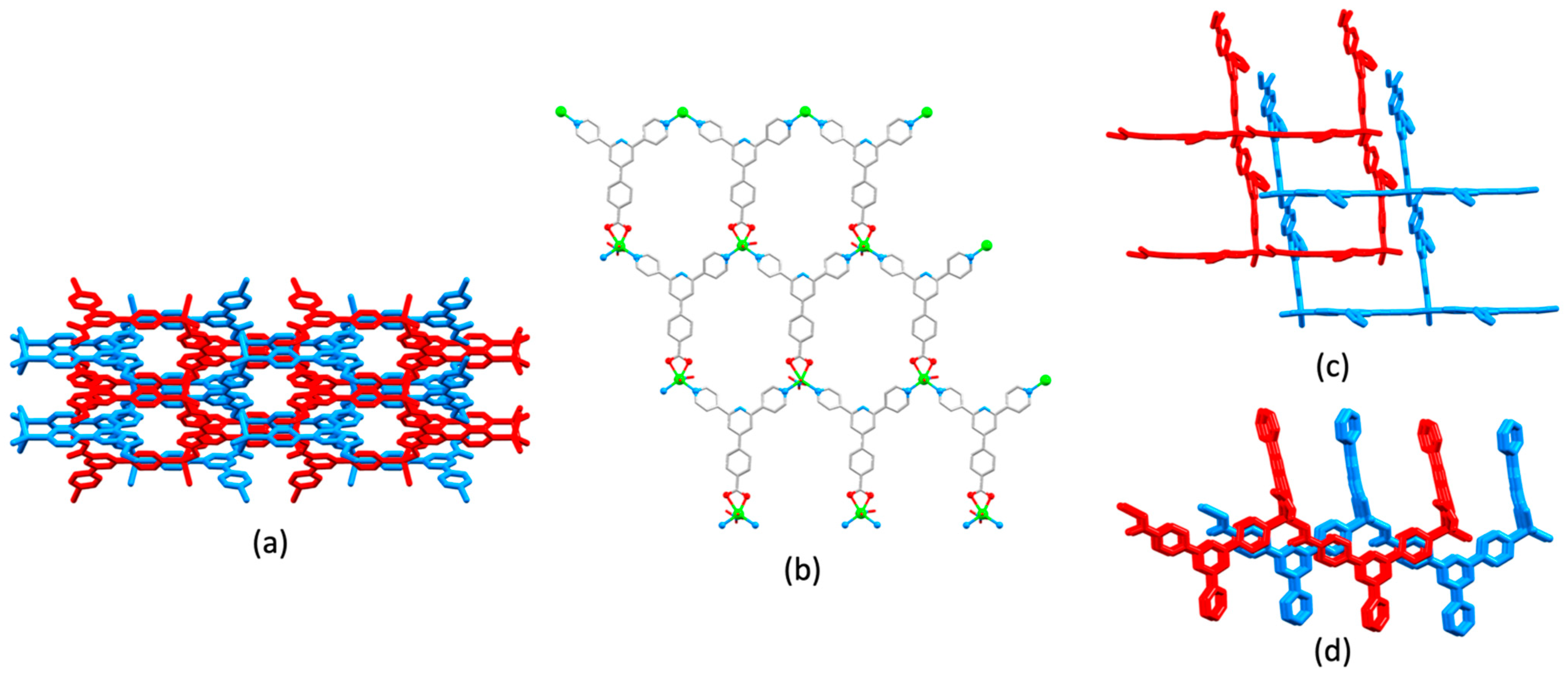
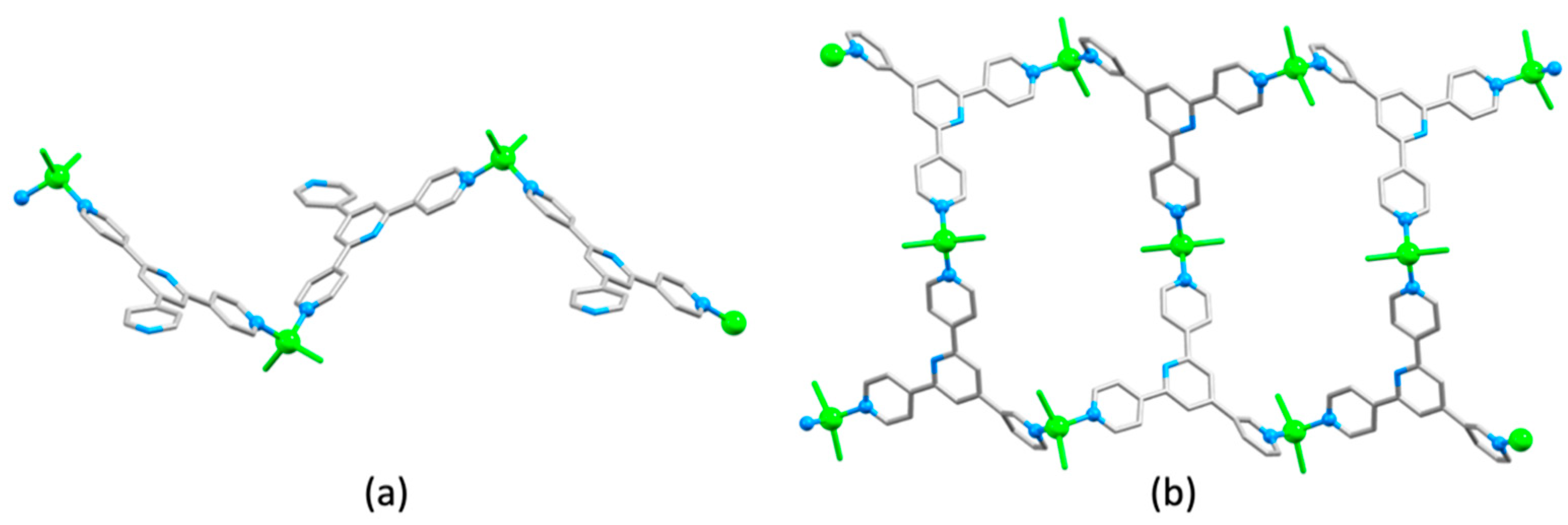
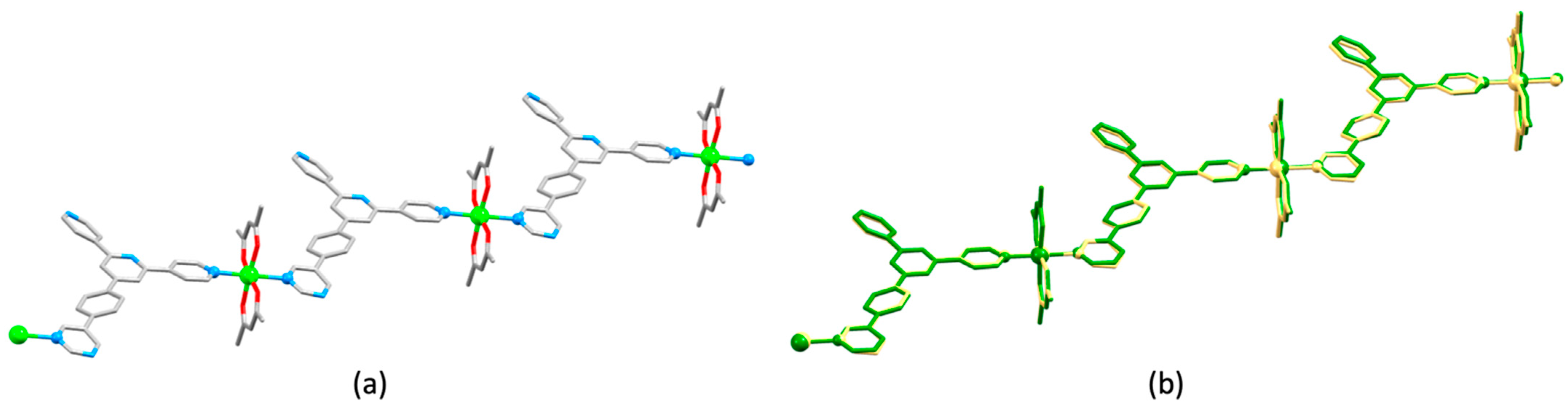
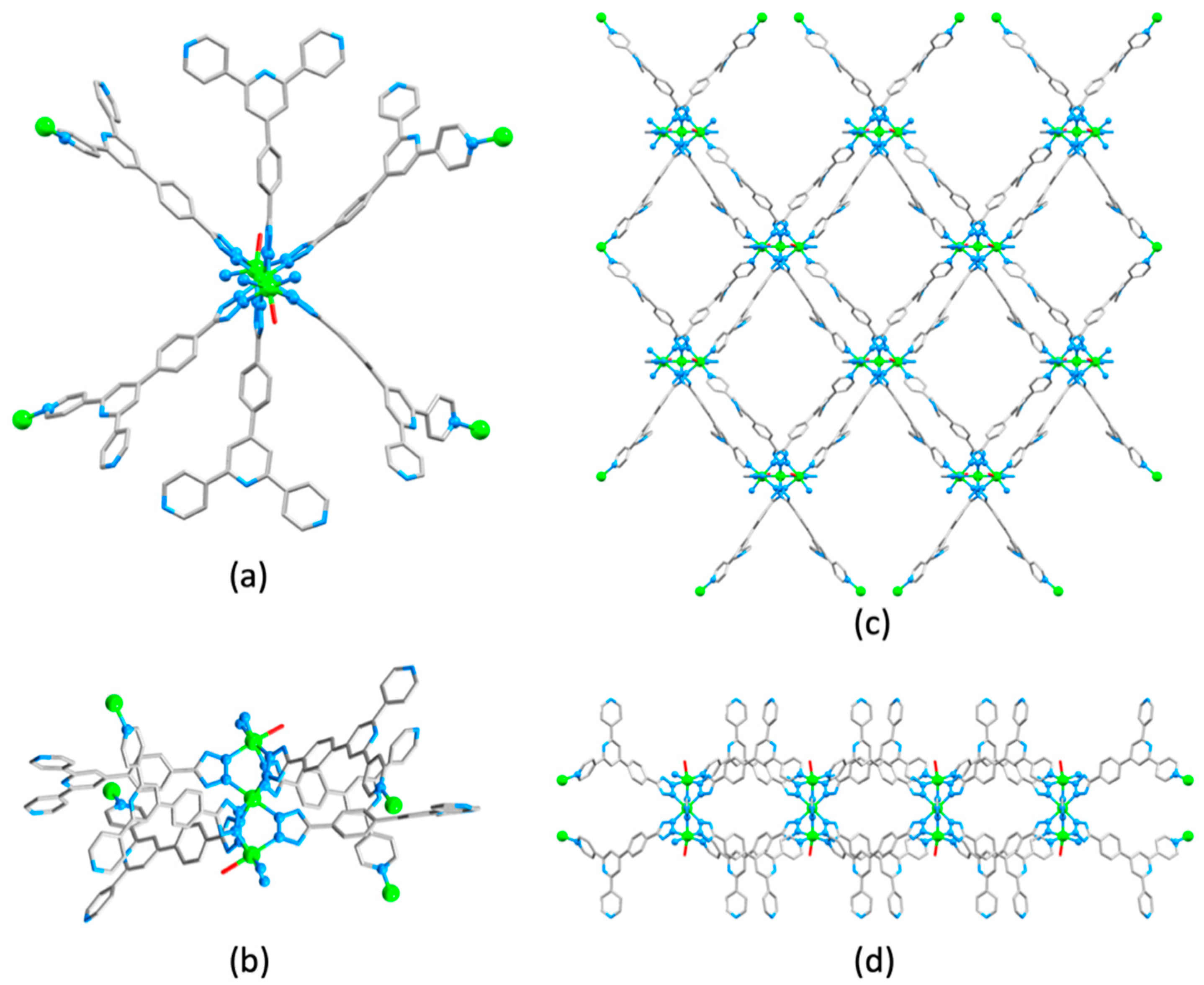
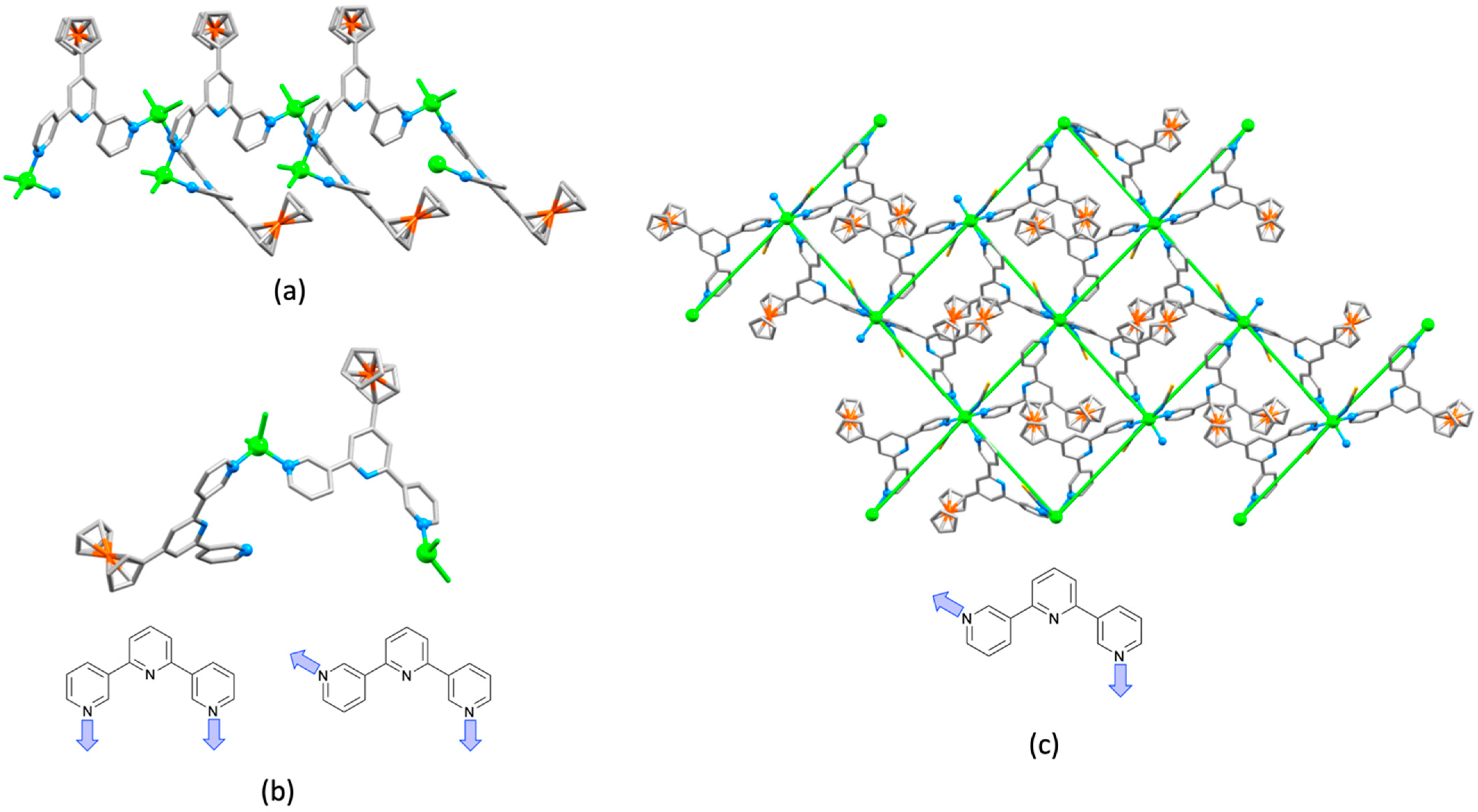
3.10. Ligands Containing Two 4,2′:6′,4″-tpy Metal-Binding Domains: 4-Connecting Nodes
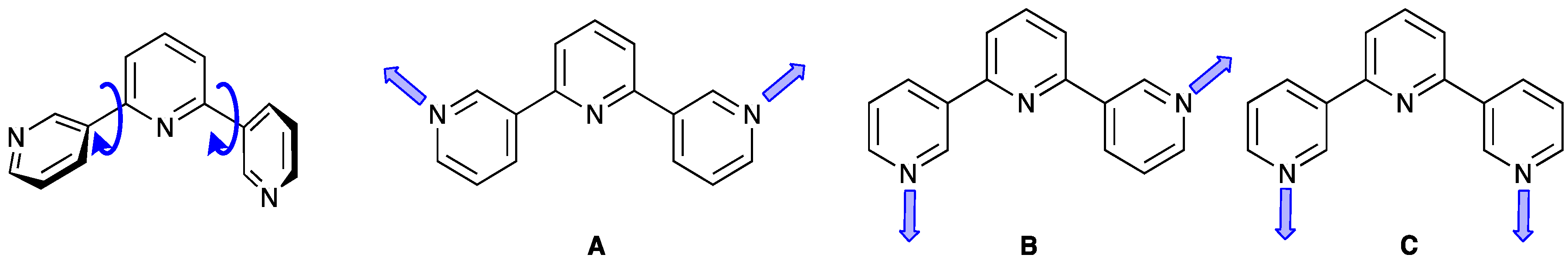
4. 3,2′:6′,3″-Terpyridine
4.1. Assemblies Containing Zinc(II) and 4′-Functionalized 3,2′:6′,3″-tpy Ligands with Coordinatively Innocent Substituents
4.2. Assemblies Containing Zinc(II) and 3,2′:6′,3″-tpy Ligands Functionalized with Carboxylate or Sulfonate Donors
4.3. Assemblies Containing Cadmium(II) and 3,2′:6′,3″-tpy Ligands
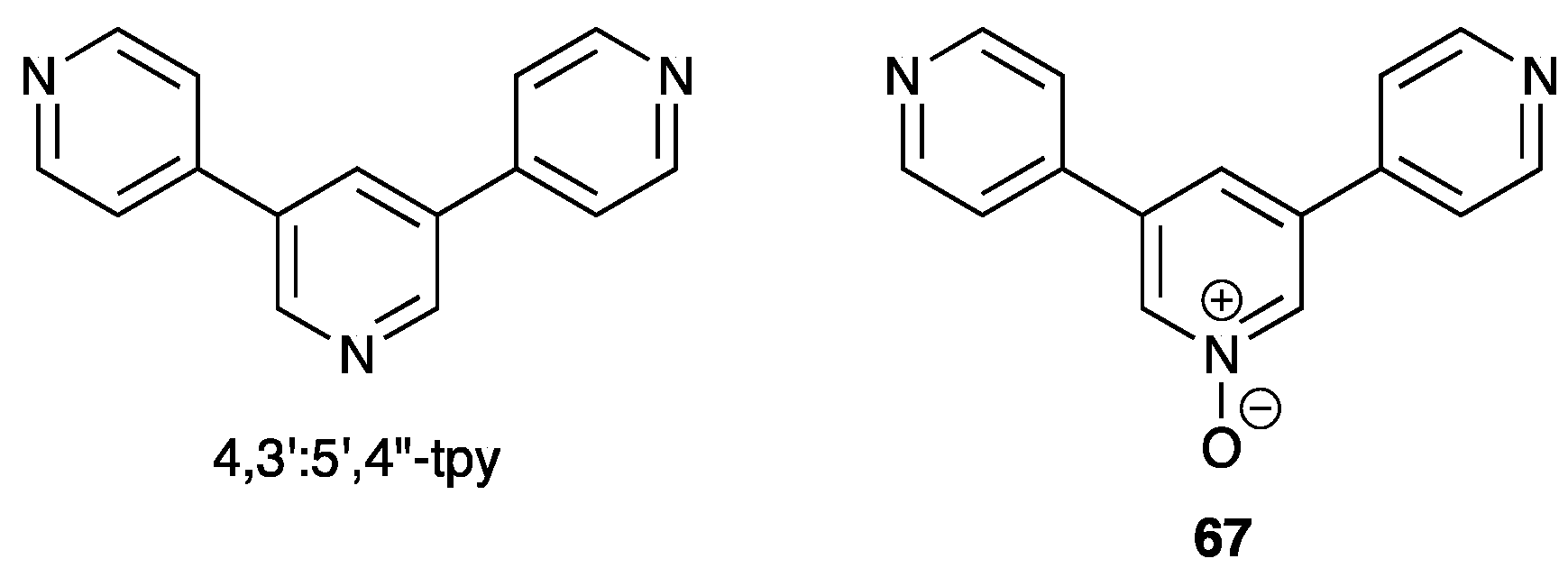
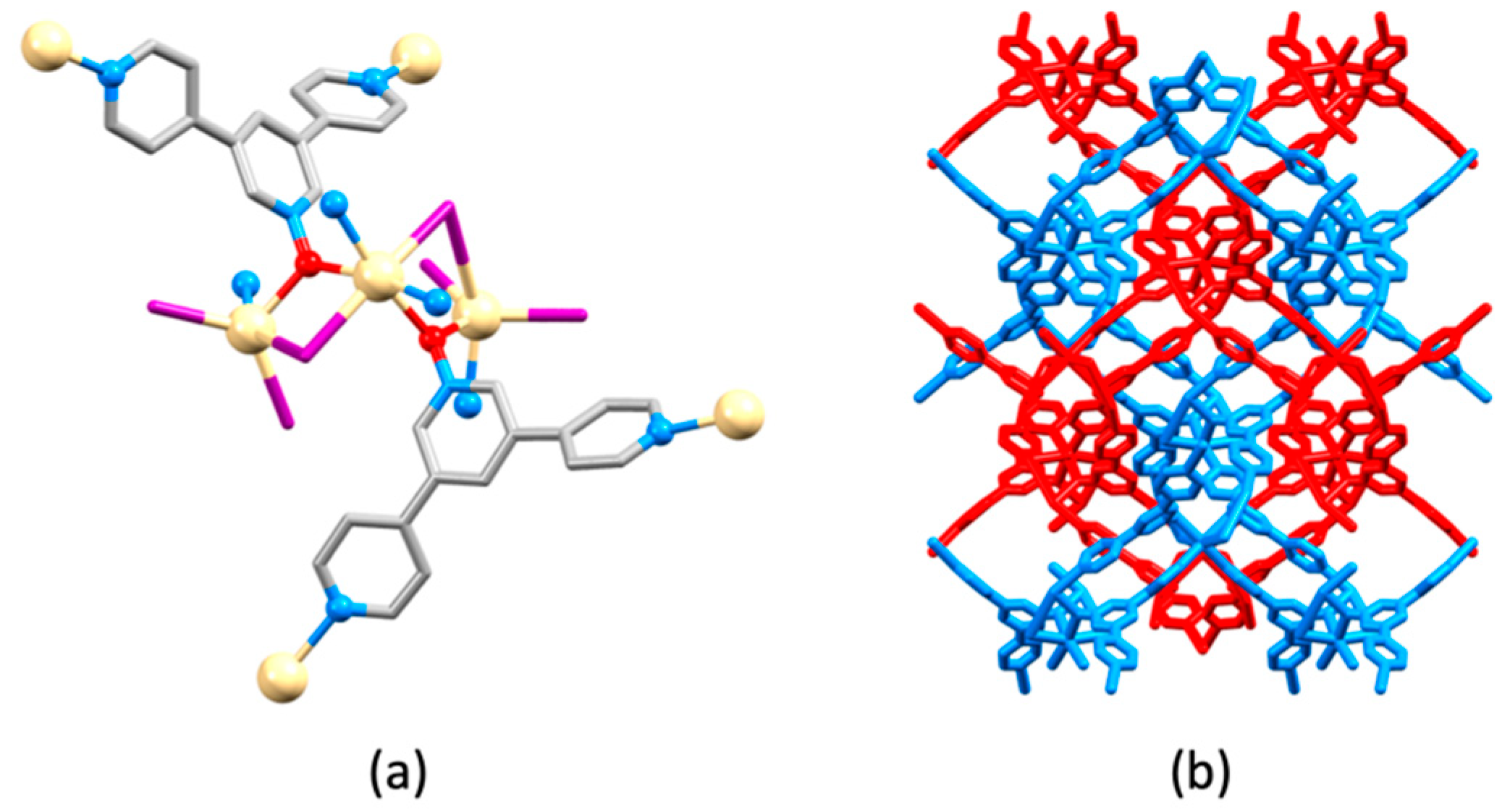
5. 4,3′:5′,4″-Terpyridine
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Constable, E.C. The Coordination Chemistry of 2,2′:6′,2″-Terpyridine and Higher Oligopyridines. Adv. Inorg. Chem. 1986, 30, 69–121. [Google Scholar] [CrossRef]
- Constable, E.C. 2,2′:6′,2″-Terpyridines: From chemical obscurity to common supramolecular motifs. Chem. Soc. Rev. 2007, 36, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH Verlag & Co.: Weinheim, Germany, 2006. [Google Scholar]
- Wei, C.; He, Y.; Shi, X.; Song, Z. Terpyridine-metal complexes: Applications in catalysis and supramolecular chemistry. Coord. Chem. Rev. 2019, 385, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Housecroft, C.E. More Hydra than Janus-non-classical coordination modes in complexes of oligopyridine ligands. Coord. Chem. Rev. 2017, 350, 84–104. [Google Scholar] [CrossRef]
- Kröhnke, F. The Specific Synthesis of Pyridines and Oligopyridines. Synthesis 1976, 1–24. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G.S. A facile route to sterically hindered and non-hindered 4′-aryl-2,2′:6′,2′′- terpyridines. Synlett 2005, 1251–1254. [Google Scholar] [CrossRef]
- Rocco, D.; Housecroft, C.E.; Constable, E.C. Synthesis of Terpyridines: Simple Reactions–What Could Possibly Go Wrong? Molecules 2019, 24, 1799. [Google Scholar] [CrossRef]
- Hannon, M.J.; Painting, C.L.; Errington, W. Self-assembly of supramolecular boxes. Chem. Commun. 1997, 307–308. [Google Scholar] [CrossRef]
- Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Switching the conformation of 3,2′:6′,3′′-tpy domains in 4′-(4-n-alkyloxyphenyl)-3,2′:6′,3′′-terpyridines. Molecules 2020, 25, 3162. [Google Scholar] [CrossRef]
- Wang, T.-T.; Zhang, J.-L.; Hua, H.-M.; Cheng, Y.; Xue, L.-L.; Wanga, X.; Wang, B.-Z. Syntheses, structures and luminescent properties of Zn/Cd coordination polymers based on 4′-(2-carboxyphenyl)-3,2′:6′,3″-terpyridine. Polyhedron 2018, 151, 43–50. [Google Scholar] [CrossRef]
- Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Directing 2D-coordination networks: Combined effects of a conformationally flexible 3,2′:6′,3″-terpyridine and chain length variation in 4′-(4-n-alkyloxyphenyl) substituents. Molecules 2020, 25, 1663. [Google Scholar] [CrossRef] [PubMed]
- Rocco, D.; Novak, S.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Manipulating the conformation of 3,2′:6′,3′′-terpyridine in [Cu2(μ-OAc)4(3,2′:6′,3′′-tpy)]n 1D-polymers. Chemistry 2021, 3, 15. [Google Scholar] [CrossRef]
- Zhao, M.; Tan, J.; Su, J.; Zhang, J.; Zhang, S.; Wu, J.; Tian, J. Syntheses, crystal structures and third-order nonlinear optical properties of two series of Zn(II) complexes using the thiophene-based terpyridine ligands. Dyes Pigm. 2016, 130, 216–225. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Cryst. 2002, B58, 389–397. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Guo, L.; Li, D.-L. Synthesis, crystal structure and photoluminescence of a one-dimensional zinc(II) coordination polymer with substituted terpyridine. Chin. J. Struct. Chem. 2010, 29, 1098–1102. [Google Scholar]
- Momeni, B.Z.; Heydari, S. Design of novel copper(II) and zinc(II) coordination polymers based on the 4′-functionalized terpyridines. Polyhedron 2015, 97, 94–102. [Google Scholar] [CrossRef]
- Gou, L.; Wu, Q.-R.; Hu, H.-M.; Qin, T.; Xue, G.-L.; Yang, M.-L.; Tang, Z.-X. An investigation of the positional isomeric effect of terpyridine derivatives: Self-assembly of novel cadmium coordination architectures driven by N-donor covalence and π…π non-covalent interactions. Polyhedron 2008, 27, 1517–1526. [Google Scholar] [CrossRef]
- Gou, L.; Zhang, B.; Hu, H.-M.; Chen, X.-L.; Wang, B.-C.; Wu, Q.-R.; Qin, T.; Tang, Z.-X. Syntheses and characterization of two novel cadmium(II) coordination polymers derived from pyridyl substituted terpyridine and diphenate mixed ligands. J. Mol. Struct. 2008, 889, 244–250. [Google Scholar] [CrossRef]
- Wang, Z. CSD Communication2019 (refcode QIVRAR). [CrossRef]
- Wang, Z. CSD Communication2019 (refcode QIVQUK). [CrossRef]
- Wang, Z. CSD Communication2019 (refcode QIVREV). [CrossRef]
- Wang, Z.; Zhu, C.-Y.; Wei, Z.-W.; Fan, Y.-N.; Pan, M. CSD Communication2020 (refcode PUCBUN). [CrossRef]
- Wang, Z.; Zhu, C.-Y.; Wei, Z.-W.; Fan, Y.-N.; Pan, M. Breathing-Ignited Long Persistent Luminescence in a Resilient Metal−Organic Framework. Chem. Mater. 2020, 32, 841–848. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.-H.; Liu, Z.-F.; Chen, X.-X.; Xiao, Y.; Zheng, F.-K.; Guo, G.-C. An Azole-Based Metal−Organic Framework toward Direct White-Light Emissions by the Synergism of Ligand-Centered Charge Transfer and Interligand π−π Interactions. Cryst. Growth Des. 2016, 16, 3969–3975. [Google Scholar] [CrossRef]
- Yang, J.; Hu, R.-X.; Zhang, M.-B. Construction of monomers and chains assembled by 3d/4f metals and 4′-(4-carboxyphenyl)-2,2′:6′,2″-terpyridine. J. Solid State Chem. 2012, 196, 398–403. [Google Scholar] [CrossRef]
- Li, H.-Z.; Wang, F. A zinc(II) coordination polymer based on carboxyphenyl-terpyridine ligand with novel hydrogen-bond topology. Inorg. Chim. Acta 2020, 502, 119351. [Google Scholar] [CrossRef]
- Gai, Y.-L.; Jiang, F.-L.; Chen, L.; Bu, Y.; Wu, M.-Y.; Zhou, K.; Pan, J.; Hong, M.-C. A series of novel zinc(II) entangled coordination polymers based on carboxyphenyl-terpyridine ligands. Dalton Trans. 2013, 42, 9954–9965. [Google Scholar] [CrossRef]
- Yuan, H. CSD Communication2020 (refcode NUCBAR). [CrossRef]
- Xie, J.; Wu, Q.-R.; Hu, H.-M.; Cheng, Y.; Yang, M.-L.; Dong, F.-X.; Xue, G.-L. Synthesis, crystal structure and luminescence of zinc(II) coordination polymers based on a flexible bifunctional terpyridyl carboxylic ligand. Polyhedron 2014, 83, 92–101. [Google Scholar] [CrossRef]
- Liang, X.; Jia, Y.; Zhan, Z.; Hu, M. A highly selective multifunctional Zn-coordaintion polymer sensor for detection of Cr(III), Cr(VI) ion, and TNP molecule. Appl. Organometal. Chem. 2019, 33, e4988. [Google Scholar] [CrossRef]
- Bai, N.N.; Hou, L.; Gao, R.-C.; Liang, J.-Y.; Yang, F.; Wang, Y.-Y. Five 1D to 3D Z(II)/Mn(II)-CPs based on dicarboxyphenyl-terpyridine ligand: Stepwise adsorptivity and magnetic properties. CrystEngComm 2017, 19, 4789–4796. [Google Scholar] [CrossRef]
- Young, D.C.; Yang, H.; Telfer, S.G.; Kruger, P.E. An Isoreticular Series of Zinc(II) Metal−Organic Frameworks Derived from Terpyridylcarboxylate Ligands. Inorg. Chem. 2017, 56, 12224–12231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, W.; Wu, X.-Y.; Zhang, L.; Lu, C.-Z. Synthesis, Photoluminescence, and Gas Adsorption Properties of a New Furan-Functionalized MOF and Direct Carbonization for Synthesis of Porous Carbon. Cryst. Growth Des. 2016, 16, 475–482. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, Z.; Lin, H.; Wang, R.; Zhang, L.; Sun, D. Synthesis, structure, and properties of a 3D porous Zn(II) MOF constructed from a terpyridine-based ligand. RSC Adv. 2016, 6, 16575–16580. [Google Scholar] [CrossRef]
- Zhang, S.R.; Wang, W.; Xu, G.-J.; Yao, C.; Xu, Y.H.; Su, Z.-M. A fluorescent sensor for selective, sensitive, and recyclable detection of mercury(II) in aqueous solution based on a zinc(II) coordination polymer. Inorg. Chem. Comm. 2018, 89, 73–77. [Google Scholar] [CrossRef]
- Bai, C.; Xu, B.; Hu, H.-M.; Yang, M.-L.; Xue, G. Cadmium(II) coordination polymers constructed from a bis-functionalized ligand 4′-(3-carboxyphenyl)-2,2′:6′,2″-terpyridine: Synthesis, structure and luminescence. Polyhedron 2017, 124, 1–11. [Google Scholar] [CrossRef]
- Tseng, T.-W.; Yang, M.-L.; Luo, T.-T. A key route to designing huge eight-fold interpenetrated coordination networks with ths-type topology: Synthesis, structures, and topological characteristics. J. Solid State Chem. 2015, 221, 345–350. [Google Scholar] [CrossRef]
- Wu, Q.-R.; Wang, J.-J.; Hu, H.-M.; Wang, B.-C.; Wu, X.-L.; Fu, F.; Li, D.-S.; Yang, M.-L.; Xue, G.-L. Two novel cadmium(II) coordination polymers based on bis-functionalized ligand 4′-(4-carboxyphenyl)-2,2′:6′,2″-terpyridine. Inorg. Chem.Comm. 2010, 13, 715–719. [Google Scholar] [CrossRef]
- Du, Y.; Liu, J.; Shao, C.; Yang, L. Synthesis and characterization of luminescent metal-organic frameworks for the selective recognition of Cu2+ cation and Tryptophan. J. Alloys Compd. 2019, 781, 904–912. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Q.; Sa, R.; Wu, K. A white-light-emitting Ln MOF with color properties improved via Eu3+ doping: An alternative approach to a rational design for solid-state lighting. Chem. Commun. 2014, 50, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Barquin, M.; Cancela, J.; González Garmendia, M.J.; Quintanilla, J.; Amador, U. Coordination compounds of 4,2′-6′,4″-terpyridine, [MCl2(4,2′-6′,4″-terpyridine)], M = Mn(II), Co(II), Ni(II), Cu(II) or Zn(II). Crystal structure of catena-poly[(dichlorozinc)-μ-(4,2′-6′,4″-terpyridine)]. Polyhedron 1998, 17, 2373–2378. [Google Scholar] [CrossRef]
- Housecroft, C.E. 4,2′:6′,4′′-Terpyridines: Diverging and diverse building blocks in coordination polymers and metallomacrocycles. Dalton Trans. 2014, 43, 6594–6604. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Li, D. A new ligand 4′-phenyl-4,2′:6′,4″-terpyridine and its 1D helical zinc(II) coordination polymer: Syntheses, structures and photoluminescent properties. Inorg. Chem. Comm. 2005, 8, 190–193. [Google Scholar] [CrossRef]
- Li, X.-Z.; Li, M.; Li, Z.; Hou, J.-Z.; Huang, X.-C.; Li, D. Concomitant and Controllable Chiral/Racemic Polymorphs: From Achirality to Isotactic, Syndiotactic, and Heterotactic Chirality. Angew. Chem. Int. Ed. 2008, 47, 6371–6374. [Google Scholar] [CrossRef]
- Yang, P.; Wang, M.-S.; Shen, J.-J.; Li, M.-X.; Wang, Z.-X.; Shao, M.; He, X. Seven novel coordination polymers constructed by rigid 4-(4-carboxyphenyl)-terpyridine ligands: Synthesis, structural diversity, luminescence and magnetic properties. Dalton Trans. 2014, 43, 1460–1470. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, X.; Hu, H.-M.; Shen, S.-S.; An, R.; Xue, G.-L. Syntheses, structures and luminescent properties of two new zinc coordination polymers based on 4′-(4-aminephenyl)-4,2′:6′,4″-terpyridine. Inorg. Chem. Comm. 2014, 48, 26–29. [Google Scholar] [CrossRef]
- Su, J.; Zhang, J.; Tian, X.; Zhao, M.; Song, T.; Yu, J.; Cui, Y.; Qian, G.; Zhong, H.; Luo, L.; et al. A series of multifunctional coordination polymers based on terpyridine and zinc halide: Second-harmonic generation and two-photon absorption properties and intracellular imaging. J. Mater. Chem. B 2017, 5, 5458–5463. [Google Scholar] [CrossRef]
- Cave, G.W.V.; Raston, C.L. Helical Polymeric Network of [ZnCl2(4′-aryl-4,2′:6′,4″-terpyridine)]n. J. Supramol. Chem. 2002, 2, 317–319. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Prescimone, A.; Vujovic, S.; Zampese, J.A. Environmental control in the assembly of metallomacrocycles and one-dimensional polymers with 4,2′:6′:4′′-terpyridine linkers and zinc(II) nodes. CrystEngComm 2014, 16, 8691–8699. [Google Scholar] [CrossRef]
- Beves, J.E.; Constable, E.C.; Housecroft, C.E.; Kepert, C.J.; Neuburger, M.; Price, D.J.; Schaffner, S. The conjugate acid of bis{4′-(4-pyridyl)-2,2′:6′,2″-terpyridine}iron(II) as a self-complementary hydrogen-bonded building block. CrystEngComm 2007, 9, 1073–1077. [Google Scholar] [CrossRef]
- Constable, E.C.; Zhang, G.; Housecroft, C.E.; Neuburger, M.; Zampese, J.A. Assembling and dissembling zinc-containing coordination polymers of 4′-phenyl-4,2′:6′,4″-terpyridine. CrystEngComm 2010, 12, 2146–2152. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Kopecky, P.; Neuburger, M.; Zampese, J.A.; Zhang, G. Coordination polymers with divergent 4′-tert-butyl-4,2′:6′,4″-terpyridine linkers: From aryl-aryl to ball-and-socket packing. CrystEngComm 2012, 14, 446–452. [Google Scholar] [CrossRef]
- Xiao, L.; Zhu, L.; Zeng, Q.; Liu, Q.; Zhang, J.; Li, S.; Zhou, H.; Zhang, S.; Wu, J.; Tian, Y. Novel metal-organic hybrid materials constructed by ferrocenyl terpyridine derivatives and ZnIIX2 (X = Cl–, Br–, I–, SCN– and CH3COO–). J. Organomet. Chem. 2015, 789–790, 22–28. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Coordination behaviour of 1-(4,2′:6′,4′′-terpyridin-4′-yl)ferrocene and 1-(3,2′:6′,3′′-terpyridin-4′-yl)ferrocene: Predictable and unpredictable assembly algorithms. Aust. J. Chem. 2017, 70, 468–477. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Schönle, J.; Vujovic, S.; Zampese, J.A. Coordination polymers with 4′-(4-(anthracen-9-yl)phenyl)- and 4′-(4-(naphthalen-1-yl)phenyl)-4,2′:6′,4″-terpyridines: Mono-, di and heptazinc(II) nodes. Polyhedron 2013, 62, 260–267. [Google Scholar] [CrossRef]
- Constable, E.C.; Zhang, G.; Housecroft, C.E.; Zampese, J.A. Zinc(II) coordination polymers, metallohexacycles and metallocapsules–Do we understand self-assembly in metallosupramolecular chemistry: Algorithms or serendipity? CrystEngComm 2011, 13, 6864–6870. [Google Scholar] [CrossRef]
- Constable, E.C.; Zhang, G.; Coronado, E.; Housecroft, C.E.; Neuburger, M. Not just size and shape: Spherically symmetrical d5 and d10 metal ions give different coordination nets with 4,2′:6′,4″-terpyridines. CrystEngComm 2010, 12, 2139–2145. [Google Scholar] [CrossRef]
- Zhang, G.; Jia, Y.-X.; Chen, W.; Lo, W.-F.; Brathwaite, N.; Golen, J.A.; Rheingold, A.L. Diverse zinc(II) coordination assemblies built on divergent 4,2′:6′,4″-terpyridine derivatives: Syntheses, structures and catalytic properties. RSC Adv. 2015, 5, 15870–15879. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Schönle, J.; Vujovic, S.; Zampese, J.A. Molecular recognition between 4′-(4-biphenylyl)-4, 2′: 6′, 4″-terpyridine domains in the assembly of d9 and d10 metal ion-containing one-dimensional coordination polymers. Polyhedron 2013, 60, 120–129. [Google Scholar] [CrossRef]
- Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Zampese, J.A.; Crochet, A. Greasy tails switch 1D-coordination [Zn2(OAc)4(4′-(4-ROC6H4)-4,2′:6′,4′′-tpy)]n polymers to discrete [Zn2(OAc)4(4′-(4-ROC6H4)-4,2′:6′,4′′-tpy)2] complexes. CrystEngComm 2014, 16, 9915–9929. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Vujovic, S.; Zampese, J.A.; Crochet, A.; Batten, S.R. Do perfluoroarene..arene and C–H…F interactions make a difference to 4,2′:6′,4″-terpyridine-based coordination polymers? CrystEngComm 2013, 15, 10068–10078. [Google Scholar] [CrossRef]
- Köberl, M.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. From molecules to materials: Molecular paddle-wheel synthons of macromolecules, cage compounds and metal–organic frameworks. Dalton Trans. 2011, 40, 6834–6859. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yuan, C.-M.; Hu, H.-M.; Wang, T.-T.; Zhou, C.-S. Structural diversity of a series of terpyridyl carboxylate coordination polymers: Luminescent sensor and magnetic properties. J. Solid State Chem. 2018, 258, 588–601. [Google Scholar] [CrossRef]
- Yuan, F.; Zhu, Q.-E.; Hu, H.-M.; Xie, J.; Xu, B.; Yuan, C.-M.; Yang, M.L.; Dong, F.-X.; Xue, G.-L. Three novel coordination polymers based on bifunctionalized ligand 4′-carboxy-4,2′:6′,4′′-terpyridine. Inorg. Chim. Acta 2013, 397, 117–123. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Gao, G.-M.; Liu, J.; Wang, H.-Y. Two metal–organic frameworks based on carboxyphenyl–terpyridine ligands: Synthesis, structure and highly luminescent sensing of nitrobenzene. Polyhedron 2018, 152, 11–16. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, T.-T.; Hu, H.-M.; Li, C.-T.; Zhou, C.-S.; Wang, X.; Xue, G. Two luminescent d10 metal coordination polymers assembled from a semirigid terpyridyl carboxylate ligand with high selective detecting of Cu2+, Cr2O72– and acetone. J. Solid State Chem. 2017, 251, 79–89. [Google Scholar] [CrossRef]
- Yuan, F.; An, R.; Hu, H.-M.; Shen, S.-S.; Wang, X.; Yang, M.-L.; Xue, G. Syntheses, structures and luminescent properties of two new two-fold interpenetrating 2D coordination polymers based on 4′-(4-carboxyphenyl)-4,2′:6′,4″-terpyridine. Inorg. Chem. Comm. 2015, 56, 1–4. [Google Scholar] [CrossRef]
- Zhang, H.-N.; Yuan, F.; Hu, H.-M.; Shen, S.-S.; Xue, G.-L. A 3D Zn(II) coordination polymer with a new semi-rigid tripodal ligand tecton showing 4-connected three-fold interpenetrating diamond network and helical character. Inorg. Chem. Comm. 2013, 34, 51–54. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, J.; Gao, X.; Ji, G.; Wang, D.; Liu, Z. A multi-chemosensor based on Zn-MOF: Ratio-dependent color transition detection of Hg(II) and highly sensitive sensor of Cr(VI). Sens. Actuators B 2018, 269, 164–172. [Google Scholar] [CrossRef]
- Xu, B.; Xie, J.; Hu, H.-M.; Yang, X.-L.; Dong, F.-X.; Yang, M.-L.; Xue, G.-L. Synthesis, Crystal Structure, and Luminescence of Zn/Cd Coordination Polymers with a New Fuctionalized Terpyridyl Carboxylate Ligand. Cryst. Growth Des. 2014, 14, 1629–1641. [Google Scholar] [CrossRef]
- Sheng, L.; Li, Q.; Wang, H.; Feng, L. Two novel Mn(II) and Zn(II) complexes: Crystal structures and anti-prostatic cancer activity. Main Group Chem. 2018, 17, 229–234. [Google Scholar] [CrossRef]
- Shi, Y.; Song, T.-Q.; Cao, C.-S.; Zhao, B. CSD Communication2019 (refcode HOSMEK). [CrossRef]
- Shi, Y. CSD Communication2018 (refcode QIDQUS). [CrossRef]
- Liu, B.; Hou, L.; Wu, W.-P.; Dou, A.-N.; Wang, Y.-Y. Highly selective luminescence sensing for Cu2+ ions and selective CO2 capture in a doubly interpenetrated MOF with Lewis basic pyridyl sites. Dalton Trans. 2015, 44, 4423–4427. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-S.; Wu, Y.-P.; Zhao, J.; Zhang, J.; Lu, J.Y. Metal-organic frameworks based upon non-zeotype 4-connected topology. Coord. Chem. Rev. 2014, 261, 1–27. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Luo, Z.; Wang, J.; Li, Y.; Han, Y.; Liu, J. Fluorescence detection of Mn2+, Cr2O72– and nitroexplosives and photocatalytic degradation of methyl violet and rhodamine B based on two stable metal–organic frameworks. RSC Adv. 2017, 7, 10415–10423. [Google Scholar] [CrossRef]
- Wang, B.-C.; Wu, Q.-R.; Hu, H.-M.; Chen, X.-L.; Yang, Z.-H.; Shangguan, Y.-Q.; Yang, M.-L.; Xue, G.-L. Four novel Zn(II)/Cd(II) metal–organic frameworks constructed from 4′-(4-pyridyl)-4,2′:6′,4′′-terpyridine: Hydrothermal synthesis, crystal structures, and luminescent properties. CrystEngComm 2010, 12, 485–492. [Google Scholar] [CrossRef]
- Heine, J.; auf der Günne, J.S.; Dehnen, S. Formation of a Strandlike Polycatenane of Icosahedral Cages for Reversible One-Dimensional Encapsulation of Guests. J. Am. Chem. Soc. 2011, 133, 10018–10021. [Google Scholar] [CrossRef]
- Yang, X.-L.; Shangguan, Y.-Q.; Hun, H.-M.; Xu, B.; Wang, B.-C.; Xie, J.; Yuan, F.; Yang, M.-L.; Dong, F.-X.; Xue, G.-L. Synthesis, crystal structures and luminescent properties of zinc(II) metal–organic frameworks constructed from terpyridyl derivative ligand. J. Solid State Chem. 2014, 216, 13–22. [Google Scholar] [CrossRef]
- Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Zampese, J.A. 4′-(Pyrimidin-5-yl)- and 4′-(2-methylpyrimidin-5-yl)- 4,2′:6′,4″-terpyridines: Selective coordination to zinc(II) through the 4,2′:6′,4″-terpyridine domain. Polyhedron 2014, 81, 98–104. [Google Scholar] [CrossRef]
- Granifo, J.; Gavino, R.; Freire, E.; Baggio, R. A novel hybrid terpyridine–pyrimidine ligand and the supramolecular structures of two of its complexes with Zn(II) and acetylacetonato: The underlying role of non-covalent π…π contacts and C–H…X (O, N, π) hydrogen bonds. J. Mol. Struct. 2014, 1063, 102–108. [Google Scholar] [CrossRef]
- Granifo, J.; Gavino, R.; Suárez, S.; Baggio, R. Structural characterization of a hybrid terpyridine–pyrazine ligand and its one-dimensional ZnII coordination polymer: A computational approach to conventional and nonconventional intermolecular interactions. Acta Crystallogr. 2019, 75C, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H. CSD Communication2020 (refcode NUBZUI). [CrossRef]
- Constable, E.C.; Zhang, G.; Housecroft, C.E.; Neuburger, M.; Zampese, J.A. Adding the second dimension with cadmium: Two-dimensional sheets assembled from cadmium(II) and 4′-phenyl-4,2′:6′,4″-terpyridine and locked by π-stacked interactions. CrystEngComm 2009, 11, 2279–2281. [Google Scholar] [CrossRef]
- Singh, U.P.; Narang, S.; Pachfule, P.; Banerjee, R. Variation of CO2 adsorption in isostructural Cd(II)/Co(II) based MOFs by anion modulation. CrystEngComm 2014, 16, 5012–5020. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Manipulating connecting nodes through remote alkoxy chain variation in coordination networks with 4′-alkoxy-4,2′:6′,4″-terpyridine linkers. CrystEngComm 2015, 17, 6483–6492. [Google Scholar] [CrossRef]
- Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Prescimone, A. Assembling coordination ladders with 4′-(4-methoxyphenyl)-4,2′:6′,4″-terpyridine as rails and rungs. Inorg. Chem. Commun. 2014, 49, 41–43. [Google Scholar] [CrossRef]
- Constable, E.C.; Zhang, G.; Housecroft, C.E.; Neuburger, M.; Zampese, J.A. Sheet, ladder or chain? Small substituents in 4′-phenyl-4,2′:6′,4″-terpyridines control dimensionality in cadmium(II) coordination polymers. CrystEngComm 2010, 12, 3733–3739. [Google Scholar] [CrossRef]
- Yuan, F.; Xie, J.; Hu, H.-M.; Yuan, C.-M.; Xu, B.; Yang, M.-L.; Dong, F.-X.; Xue, G.-L. Effect of pH/metal ion on the structure of metal–organic frameworks based on novel bifunctionalized ligand 4′-carboxy-4,2′:6′,4″-terpyridine. CrystEngComm 2013, 15, 1460–1467. [Google Scholar] [CrossRef]
- Wen, L.; Ke, X.; Qiu, L.; Zou, Y.; Zhou, L.; Zhao, J.; Li, D. Assembly of Two Porous Cadmium(II) Frameworks: Selective Adsorption and Luminescent Property. Cryst. Growth Des. 2012, 12, 4083–4089. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, J.; Liu, M.; Ji, G.; Liu, Z. Fabrication of a Luminescence-Silent System Based on a Post- Synthetic Modification Cd-MOFs: A Highly Selective and Sensitive Turn-on Luminescent Probe for Ascorbic Acid Detection. Inorg. Chem. 2019, 58, 6167–6174. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, J.-D.; Chen, Y.-T.; Zheng, S.-R.; Fan, J.; Zhang, W.-G. Syntheses, structures, and properties of nine d10 or p-block coordination polymers based on a ligand containing both terpyridyl and sulfo groups. CrystEngComm 2015, 17, 5538–5550. [Google Scholar] [CrossRef]
- Shen, S.-S.; Bai, C.; Hu, H.-M.; Yuan, F.; Wang, X.; Xue, G. Syntheses, Structures, and Luminescent Properties of Two Cadmium(II) Coordination Compounds based on a Sulfonate Functionalized Terpyridine Ligand. Z. Anorg. Allg. Chem. 2015, 641, 1772–1776. [Google Scholar] [CrossRef]
- Zhang, S.-F.; Li, J. Effect of Anionic Ancillary Ligands on Terpyridine Based Coordination Polymers: Synthesis, Crystal Structure and Fluorescence Property. Chin. J. Struct. Chem. 2016, 35, 811–817. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Vujovic, S.; Zampese, J.A. 2D→2D Parallel interpenetration of (4,4) sheets constructed from a ditopic bis(4,2′:6′,4′′-terpyridine). CrystEngComm 2014, 16, 3494–3497. [Google Scholar] [CrossRef]
- Vujovic, S.; Constable, E.C.; Housecroft, C.E.; Morris, C.D.; Neuburger, M.; Prescimone, A. Engineering 2D→2D parallel interpenetration using long alkoxy-chain substituents. Polyhedron 2015, 92, 77–83. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. A double-stranded 1D-coordination polymer assembled using the tetravergent ligand 1,1′-bis(4,2′:6′,4″-terpyridin-4′-yl)ferrocene. Inorg. Chem. Commun. 2016, 70, 118–120. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Neuburger, M.; Constable, E.C.; Housecroft, C.E. What a difference a tail makes: 2D → 2D parallel interpenetration of sheets to interpenetrated nbo networks using ditopic-4,2′:6′,4″-terpyridine ligands. CrystEngComm 2017, 19, 2894–2902. [Google Scholar] [CrossRef]
- Xiao, L.; Tian, Y. CSD Communication2015 (refcode VUKMOF). [CrossRef]
- Tian, Y.; Xiao, L. CSD Communication2015 (refcode CACXEM). [CrossRef]
- Xiao, L.; Tian, Y. CSD Communication2015 (refcode OGOYIU). [CrossRef]
- Li, N.; Guo, H.-L.; Hu, H.-M.; Song, J.; Xu, B.; Yang, M.-L.; Dong, F.-X.; Xue, G.-L. Hydrothermalsyntheses, crystal structures and luminescence properties of zinc(II) and cadmium(II) coordination polymers based on bifunctional 3,2′:6′,3″-terpyridine-4′-carboxylic acid. J. Solid State Chem. 2013, 198, 416–423. [Google Scholar] [CrossRef]
- Li, N.; Zhu, Q.-E.; Hu, H.-M.; Guo, H.-L.; Xie, J.; Yang, F.; Dong, F.-X.; Yang, M.-L.; Xue, G.-L. Hydrothermal syntheses, crystal structures and luminescence properties of zinc(II) coordination polymers constructed by bifunctional 4′-(4-carboxyphenyl)-3,2′:6′,3″-terpyridine. Polyhedron 2013, 49, 207–215. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, B.; Luo, F.; Tang, G.; Zhang, C. Two 4′-(4-carboxyphenyl)-3,2′:6′,3″-terpyridine-based luminescent Zn(II) coordination polymers for detection of 2,4,6-trinitrophenol. Polyhedron 2019, 169, 51–57. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, M.-L.; Hu, H.-M.; Xu, B.; Wang, X.; Xue, G. Syntheses, structures and luminescence for zinc coordination polymers based on a multifunctional 4′-(3-carboxyphenyl)-3,2′:6′,3″-terpyridine ligand. J. Solid State Chem. 2016, 239, 121–130. [Google Scholar] [CrossRef]
- Wang, J.; Lu, L.; Ding, Q.; Zhang, S.-L.; Wang, J.; Singh, A.; Kumar, A.; Ma, A.J. Multi-responsive luminescent 2D Zn (II)-based coordination polymer for detection of trinitrophenol and Fe3+. J. Coord. Chem. 2020, 73, 307–316. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.-J.; He, J.-E.; Chen, Y.-Y.; Zheng, S.-R.; Fan, J.; Zhang, W.-G. Construction of New Coordination Polymers from 4′-(2,4-disulfophenyl)-3,2′:6′,3″-terpyridine: Polymorphism, pH-dependent syntheses, structures, and properties. J. Solid State Chem. 2016, 233, 444–454. [Google Scholar] [CrossRef]
- Cheng, J.-Y.; Ding, F.-W.; Wang, P.; Zhao, C.-W.; Dong, Y.-B. Synthesis, Structure, and Ligand-Centered Catalytic Properties of MII Coordination Polymers (M = ZnII, CdII, HgII)with Open Pyridyl N-Oxide Sites. ChemPlusChem 2016, 81, 743–751. [Google Scholar] [CrossRef]
- Xu, B.; Luo, F.; Tang, G.; Zhang, J. A 4′-(4-carboxyphenyl)-3,2′:6′,3″-terpyridine-based luminescent cadmium(II) coordination polymer for the detection of 2,4,6-trinitrophenol. Acta Crystallogr. 2019, C75, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Xu, H.; Song, X.-W.; Meng, Y.; Chen, J.-J. Three d10 coordination polymers based on rigid ligands with flexible functional groups: Syntheses, structures and luminescence. Inorg. Chem. Commun. 2017, 84, 229–233. [Google Scholar] [CrossRef]
- Thébault, F.; Barnett, S.A.; Blake, A.J.; Wilson, C.; Champness, N.R.; Schröder, M. Control of Copper(I) Iodide Architectures by Ligand Design: Angular versus Linear Bridging Ligands. Inorg. Chem. 2006, 45, 6179–6187. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Biradha, K.; Fujita, M.; Sakamoto, S.; Yamaguchi, K. A Chiral M6L4 Cage Complex Assembled from a D2h-Symmetric Ligand: Self-Assembly, Structure, and Chirality Observation. Bull. Chem. Soc. Jpn. 2002, 75, 559–565. [Google Scholar] [CrossRef]
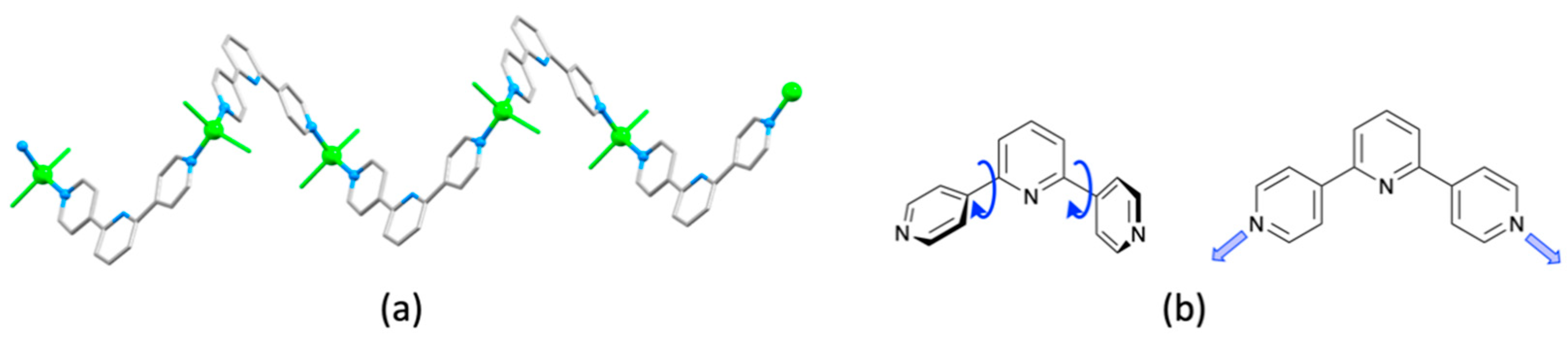
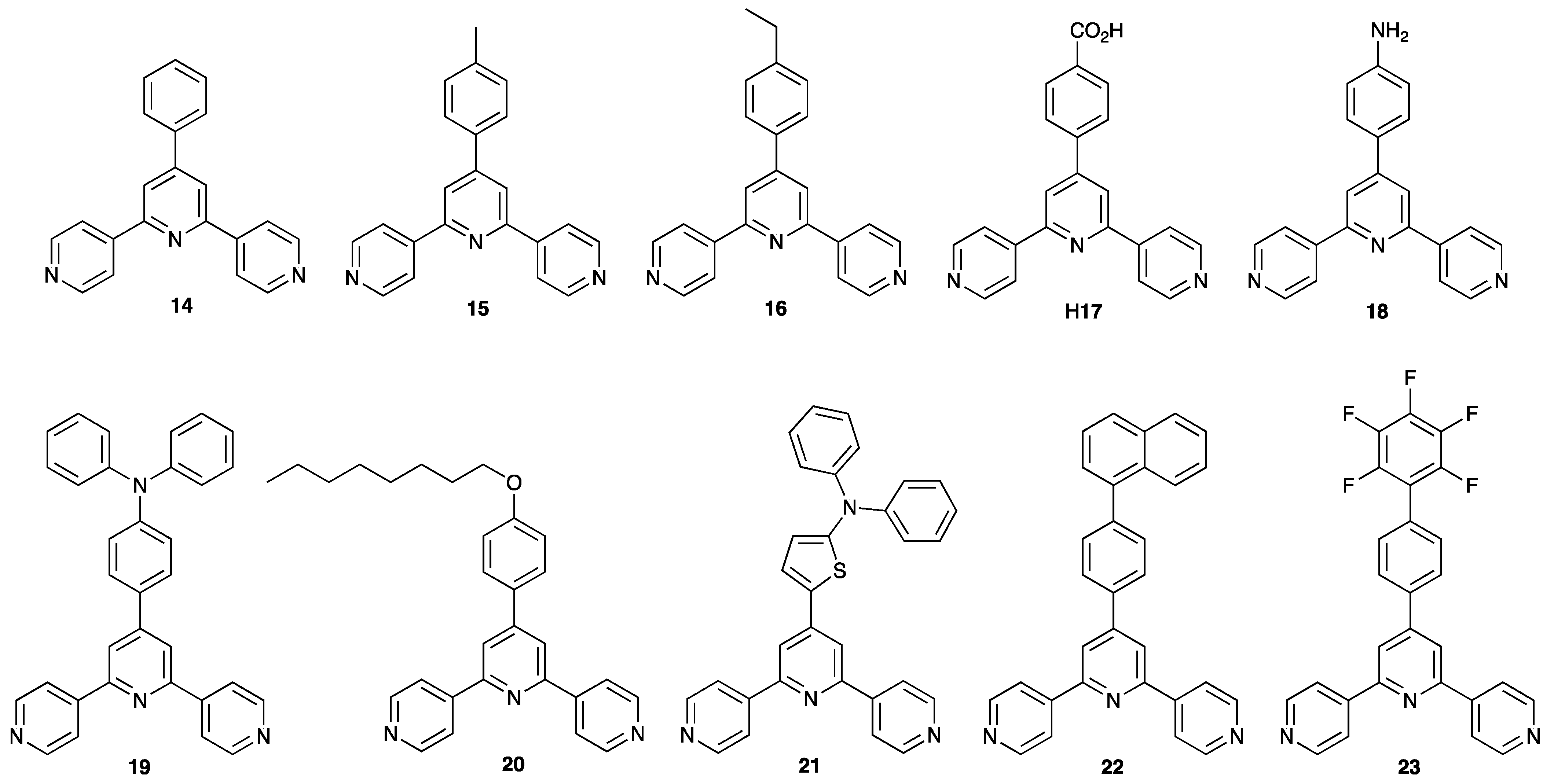
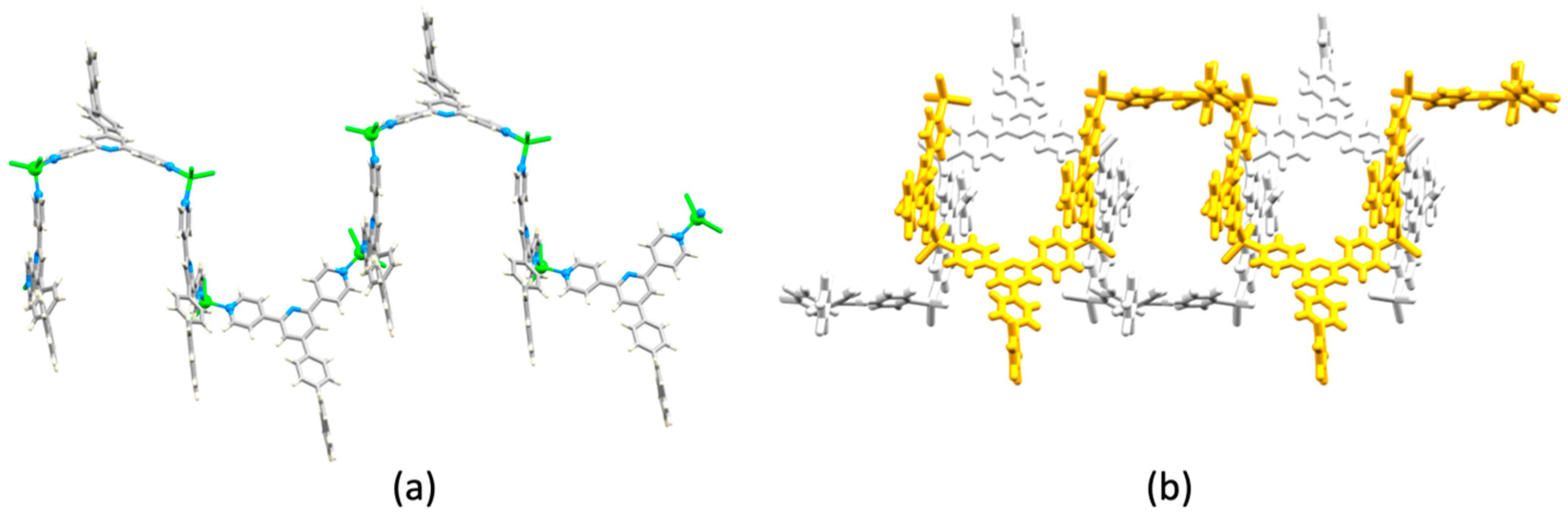
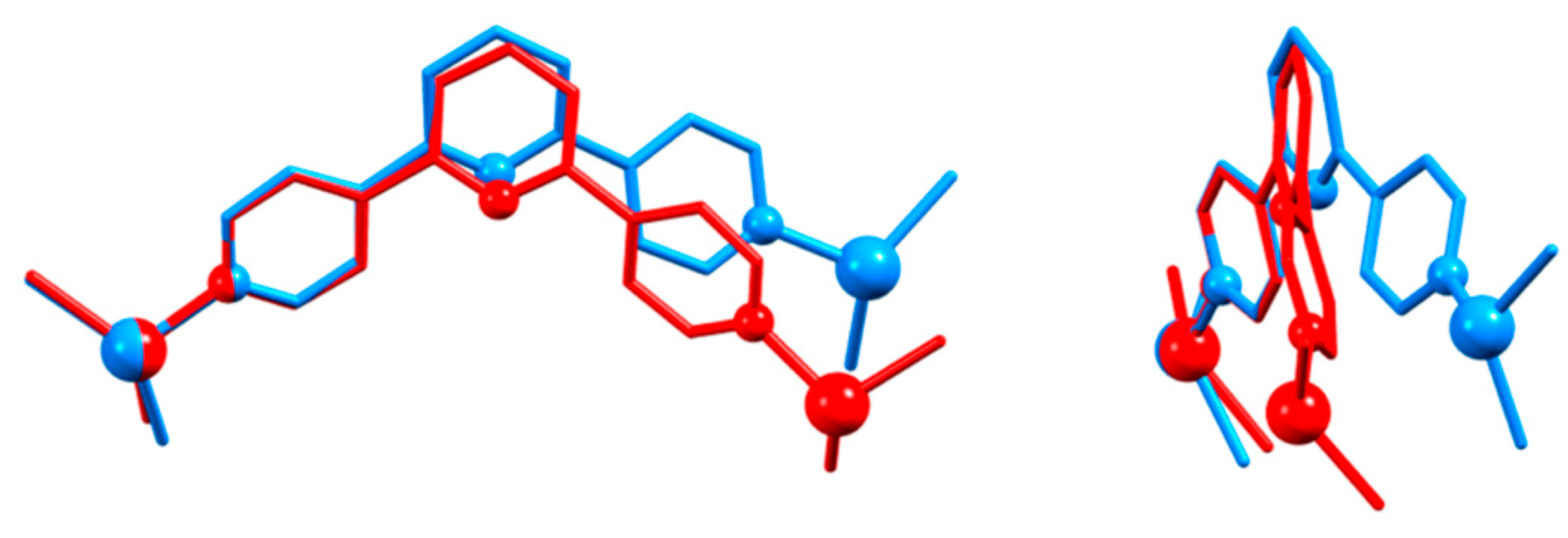
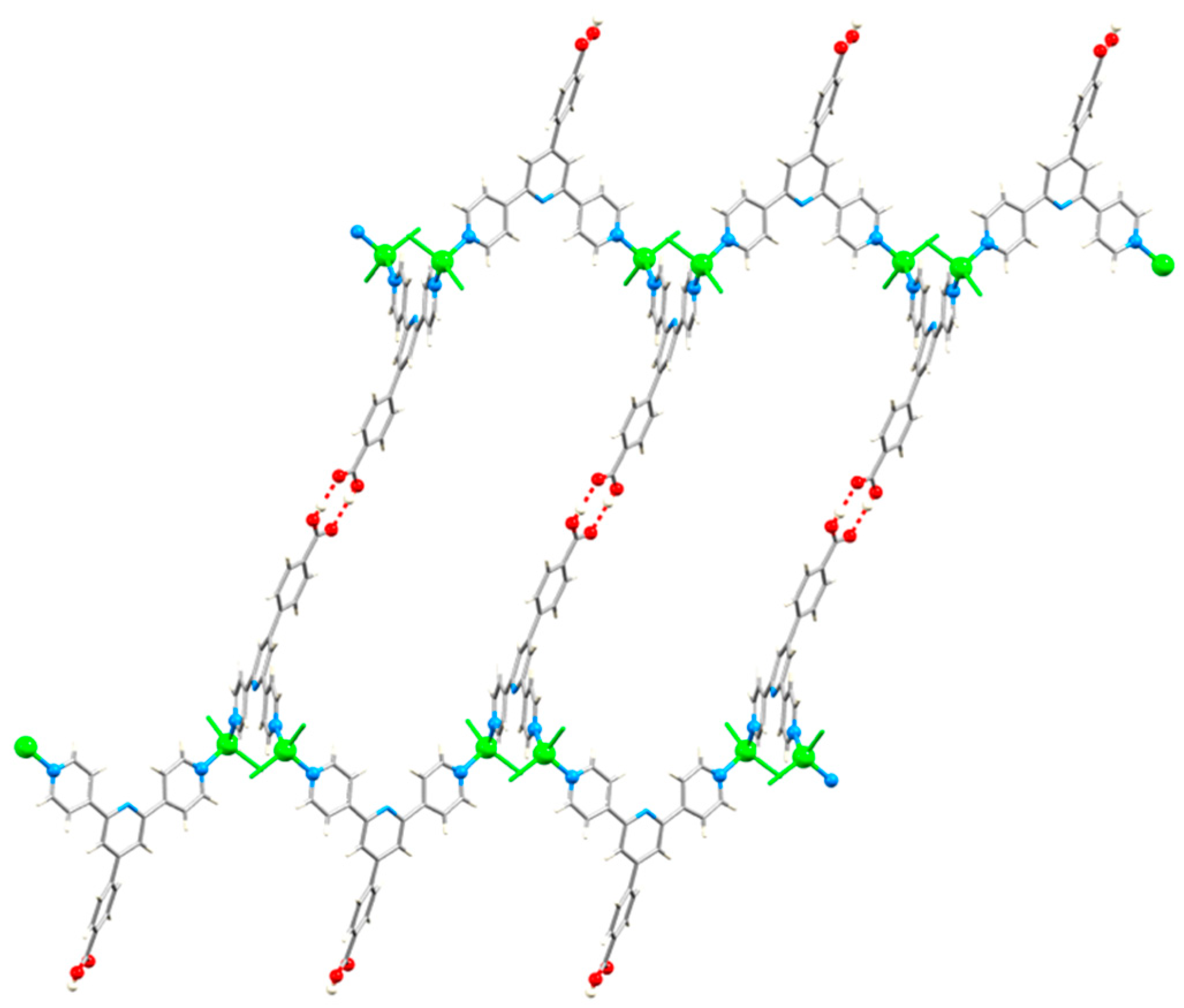
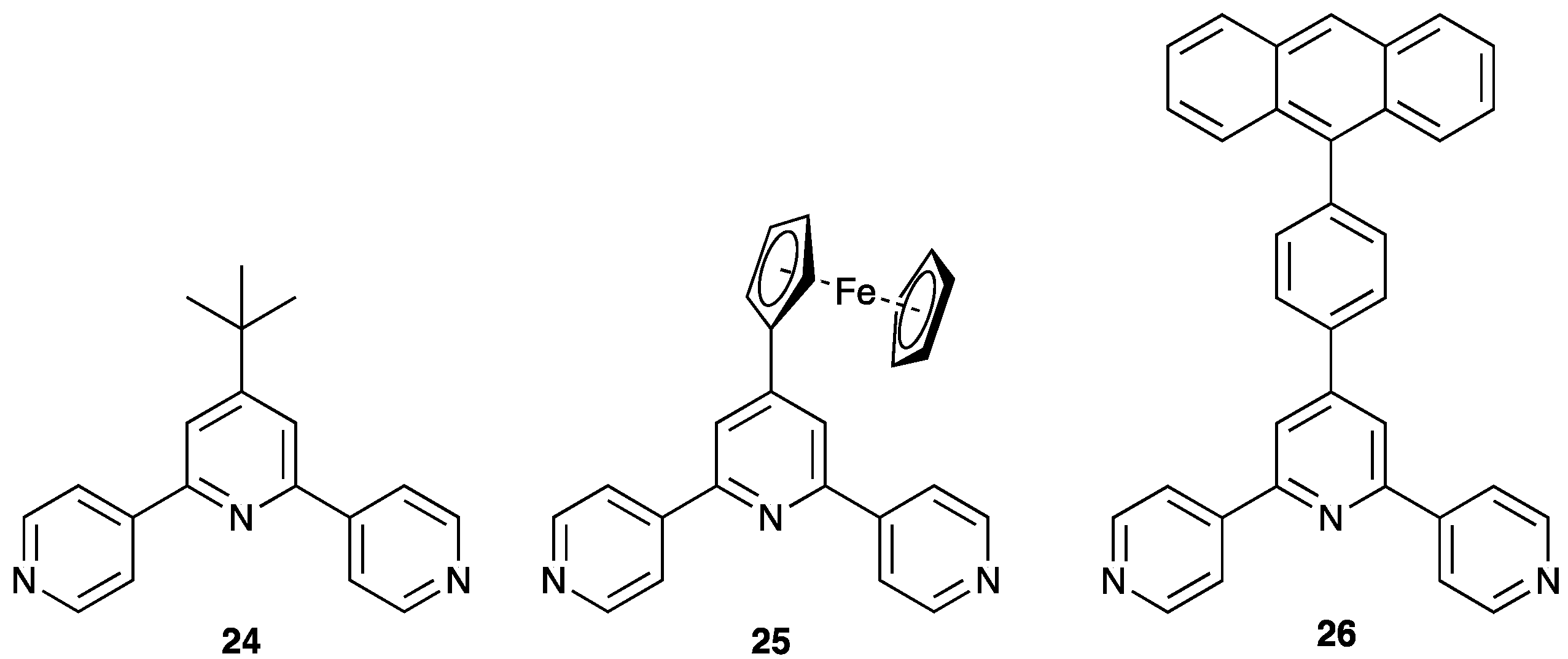
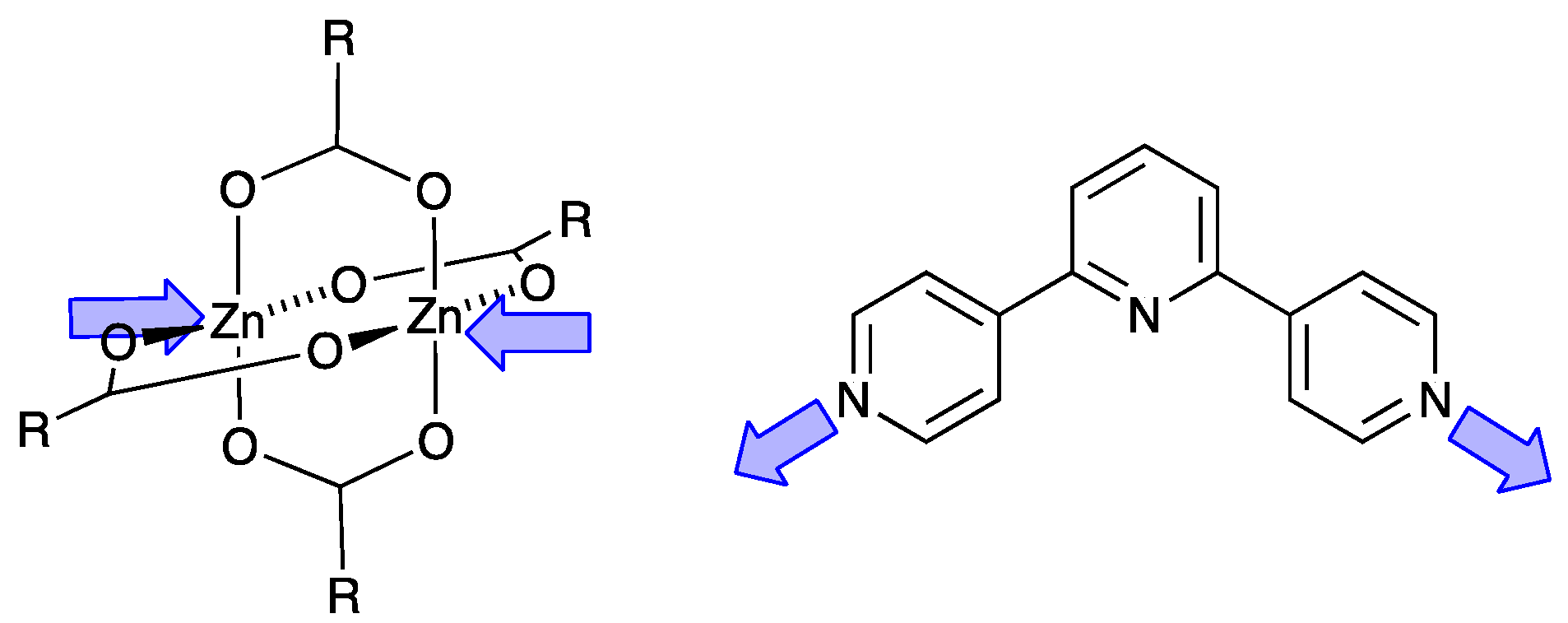
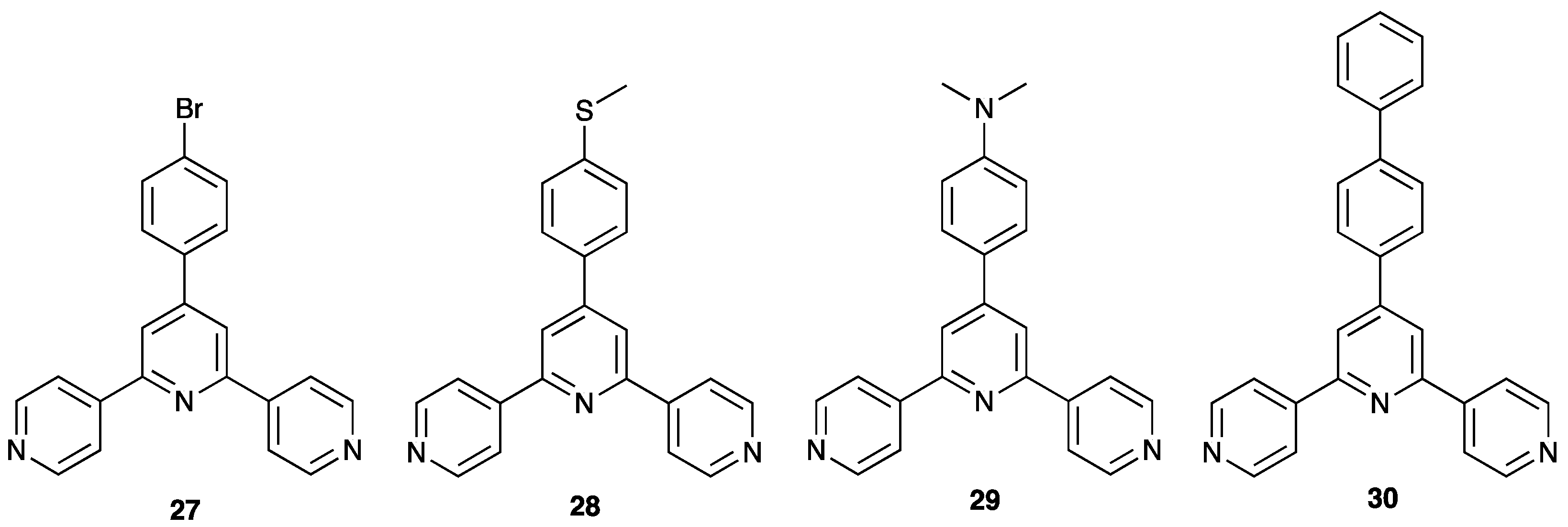
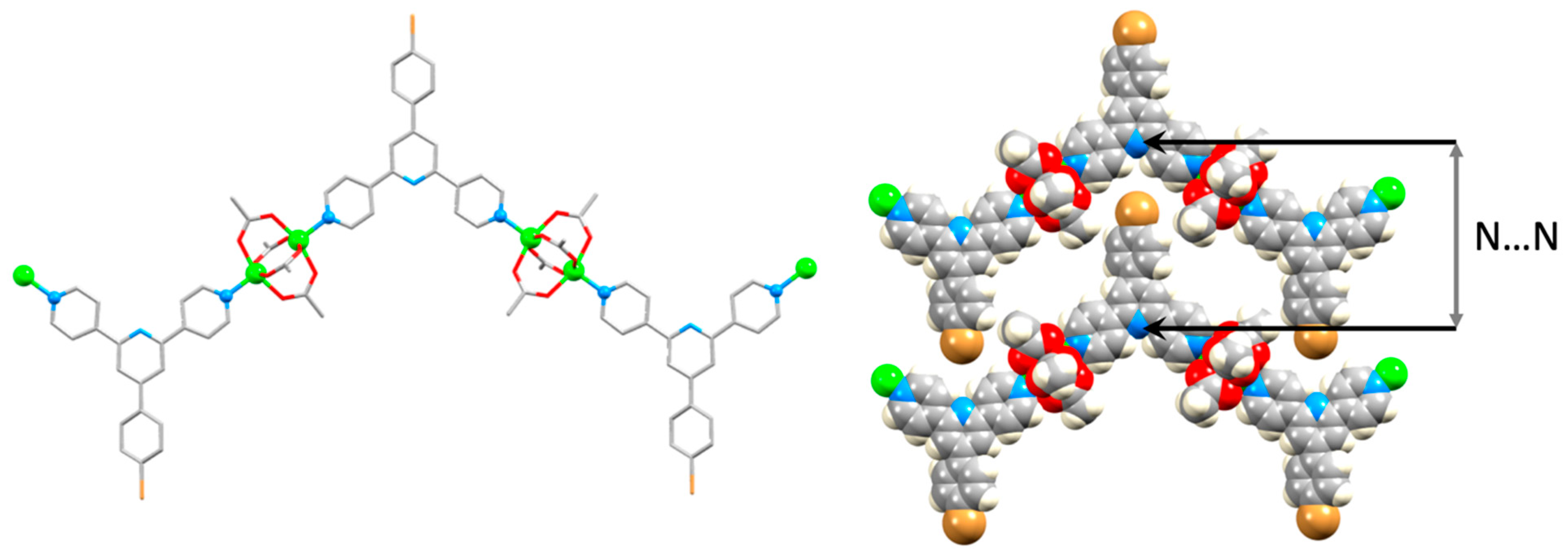
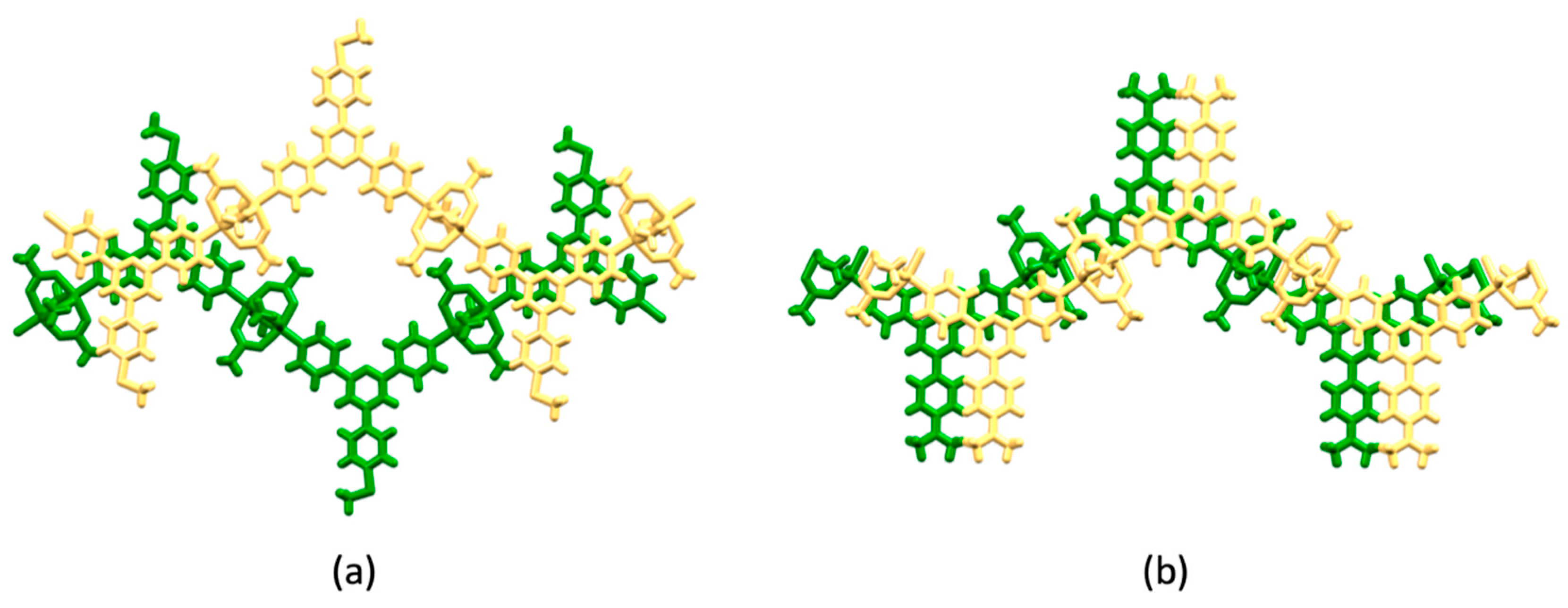
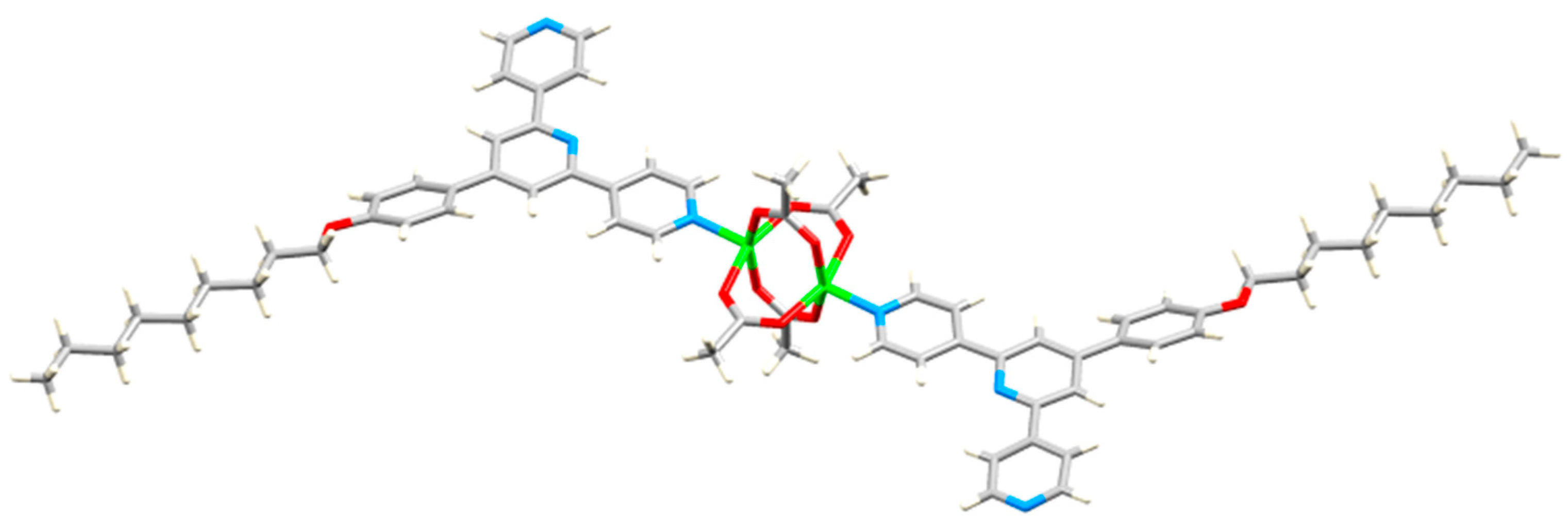
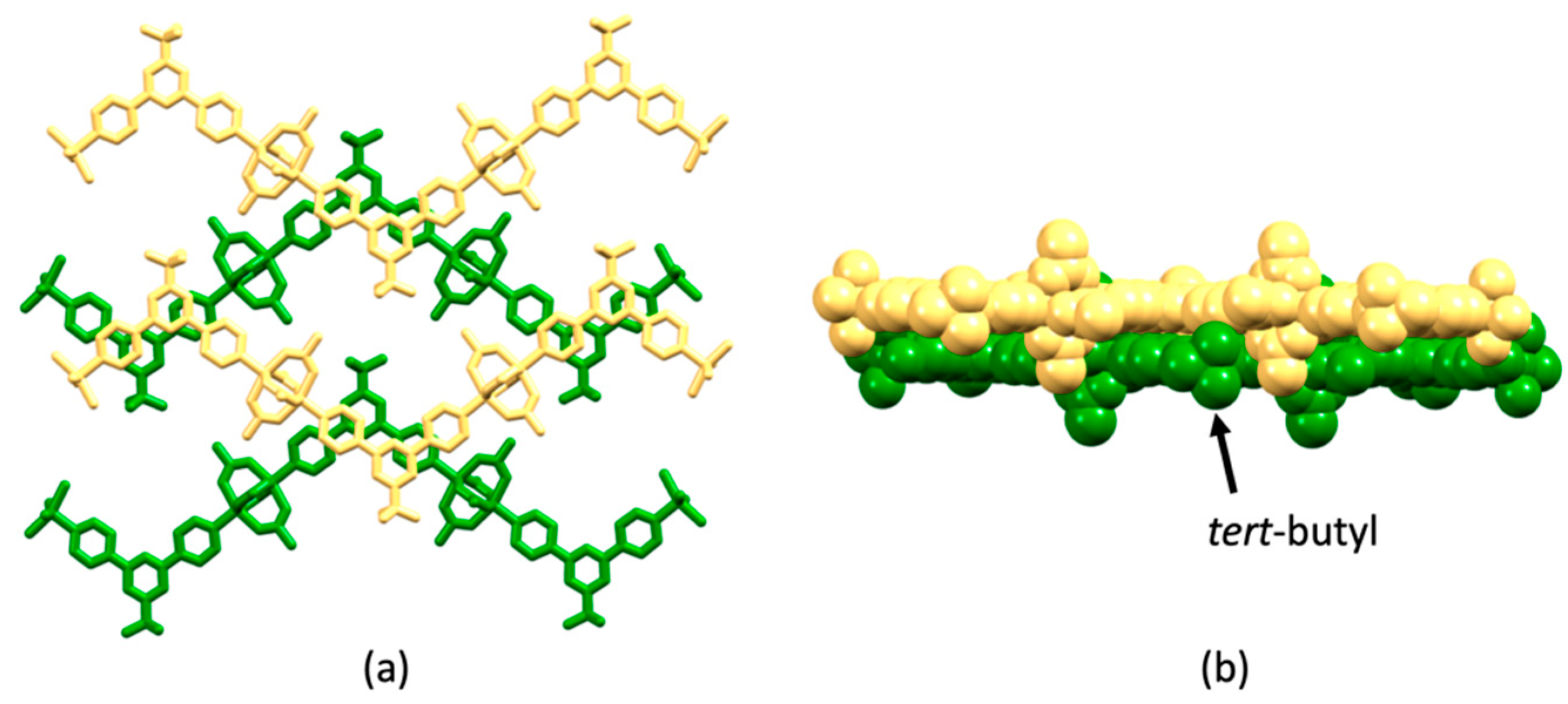
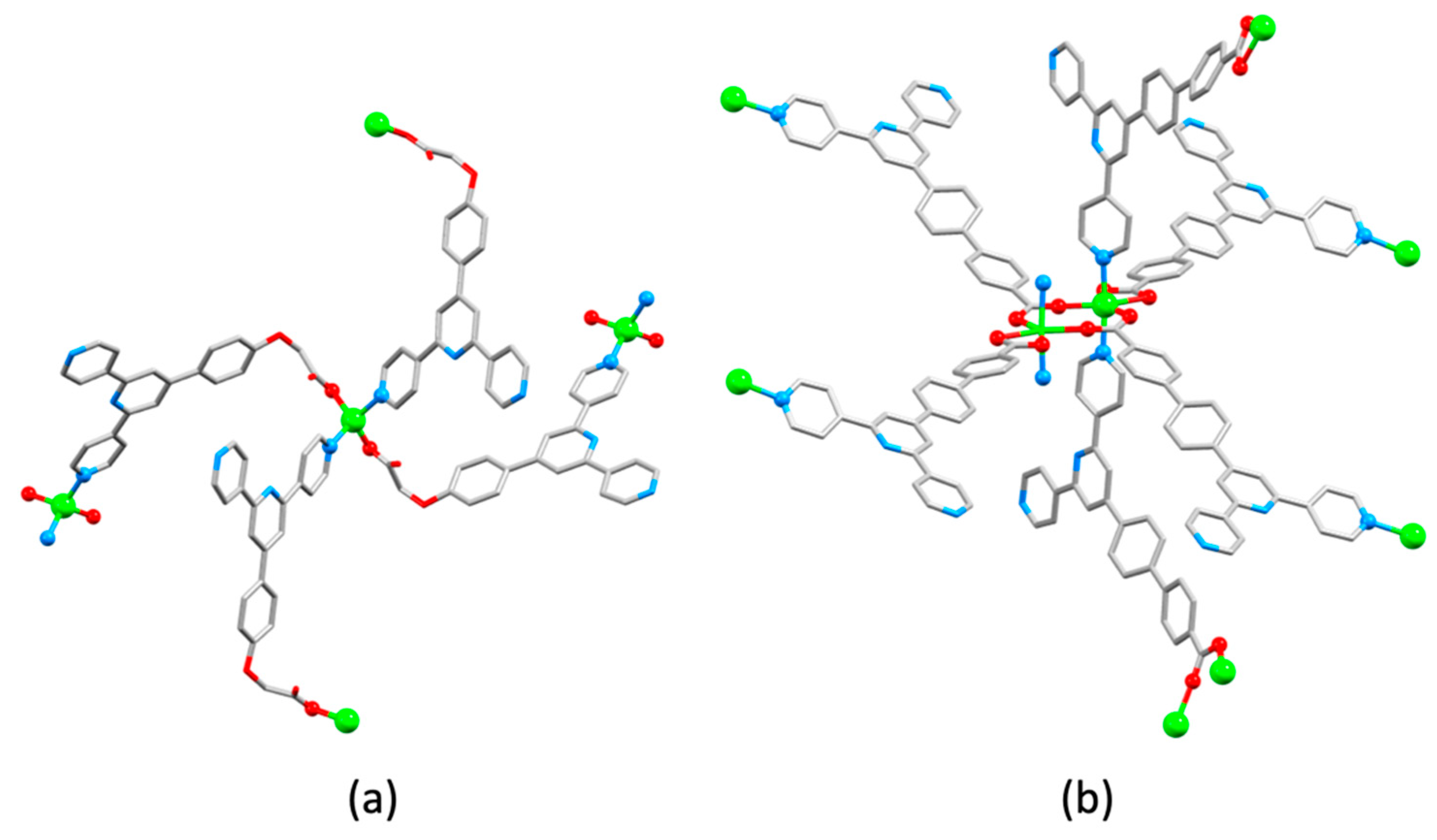
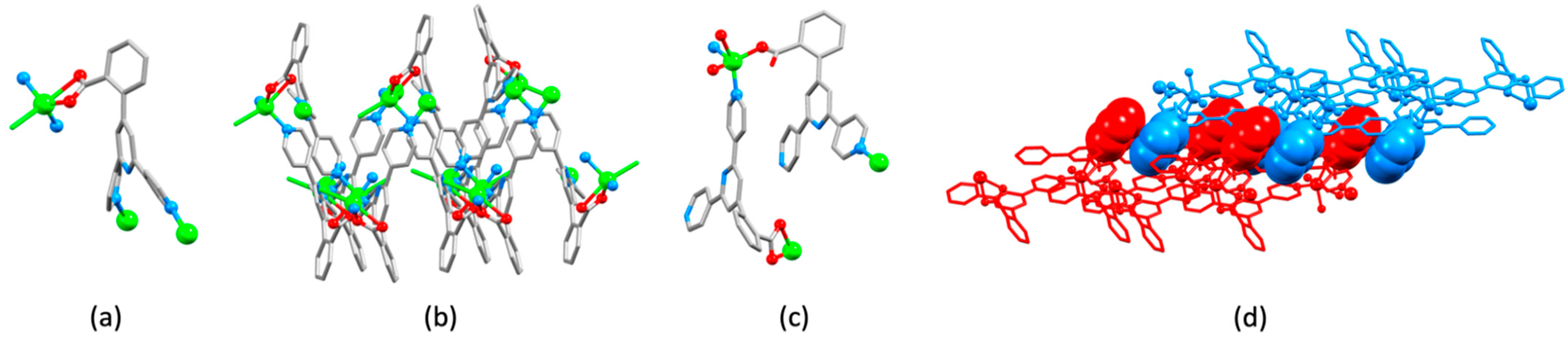
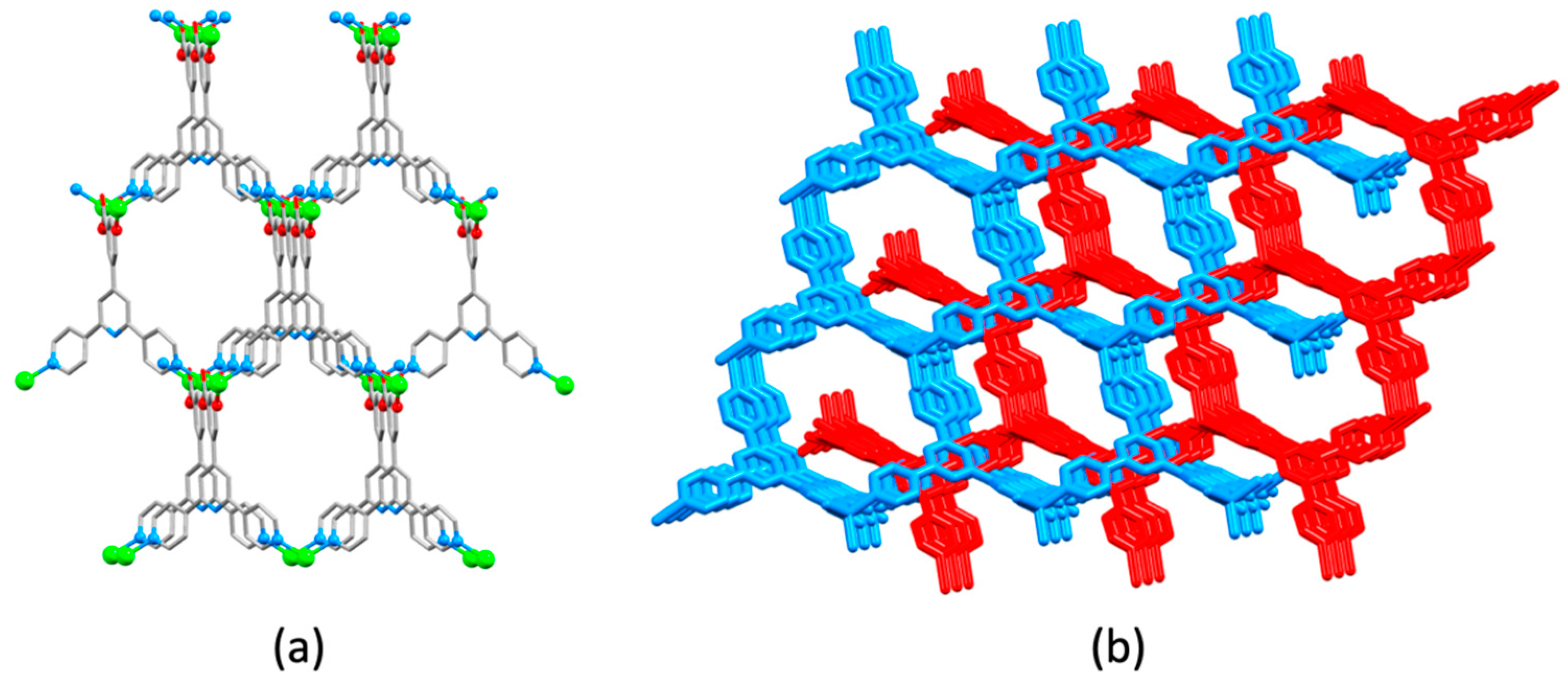
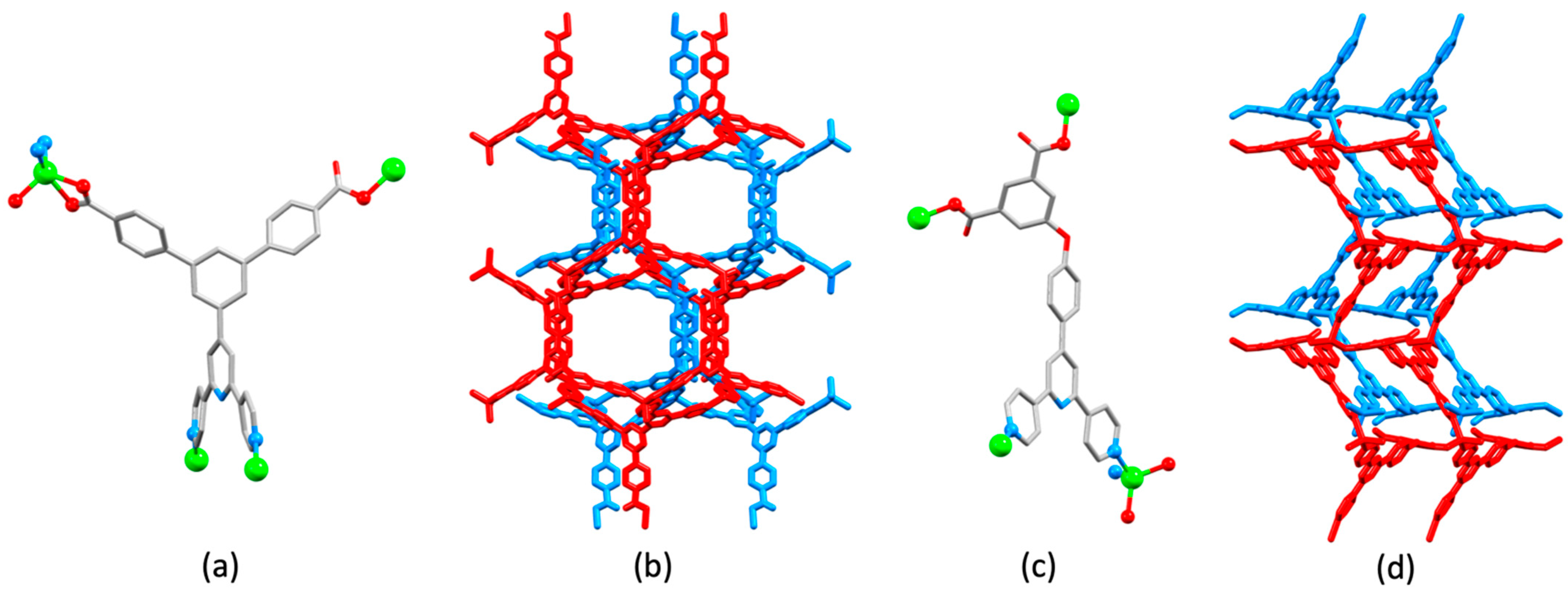
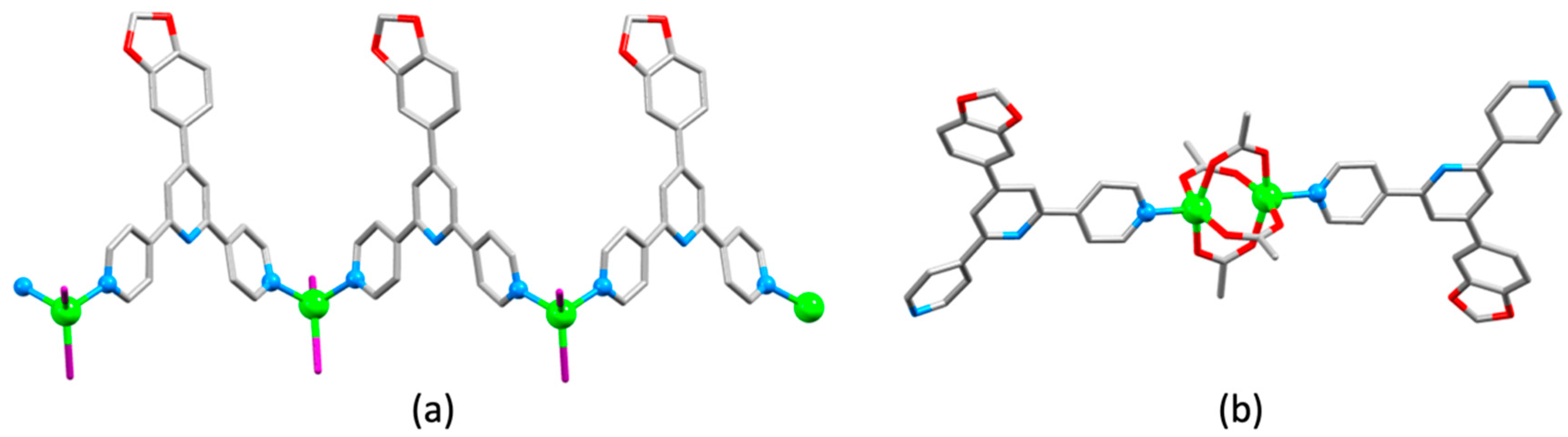
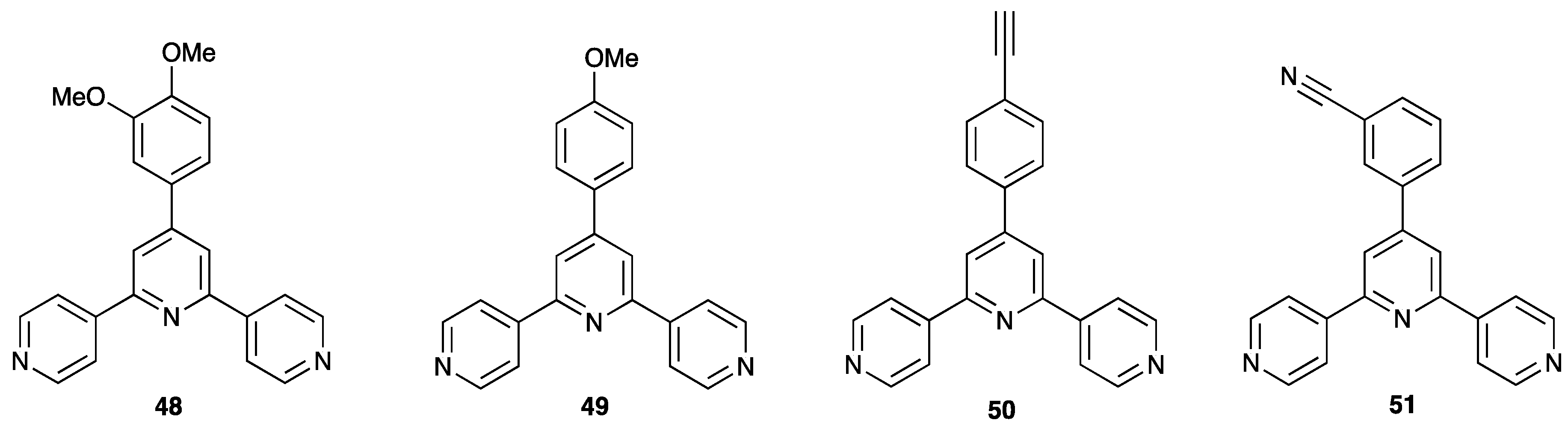
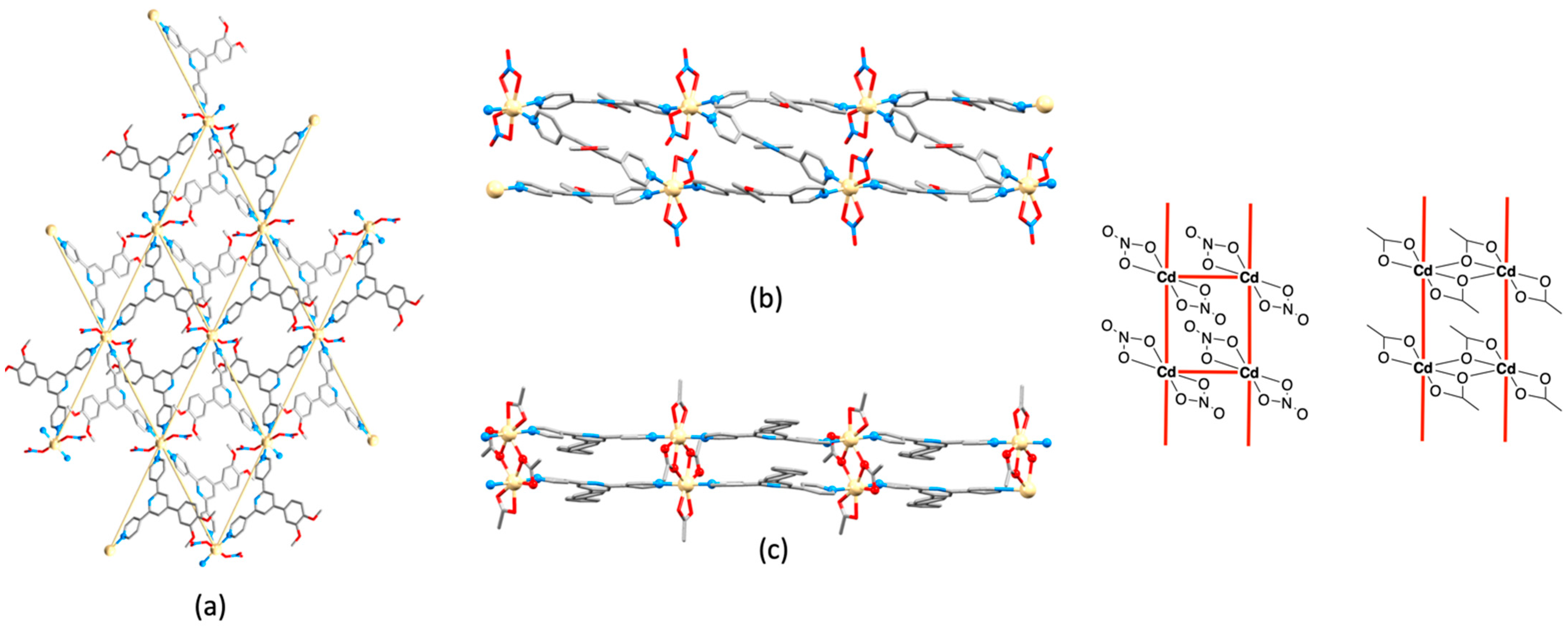
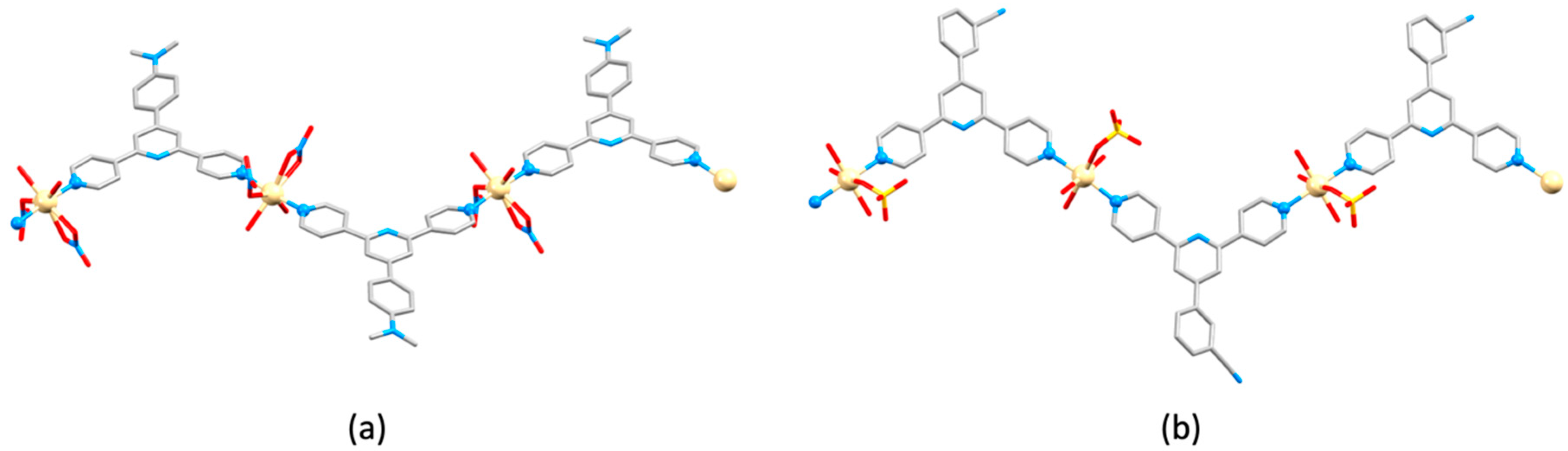
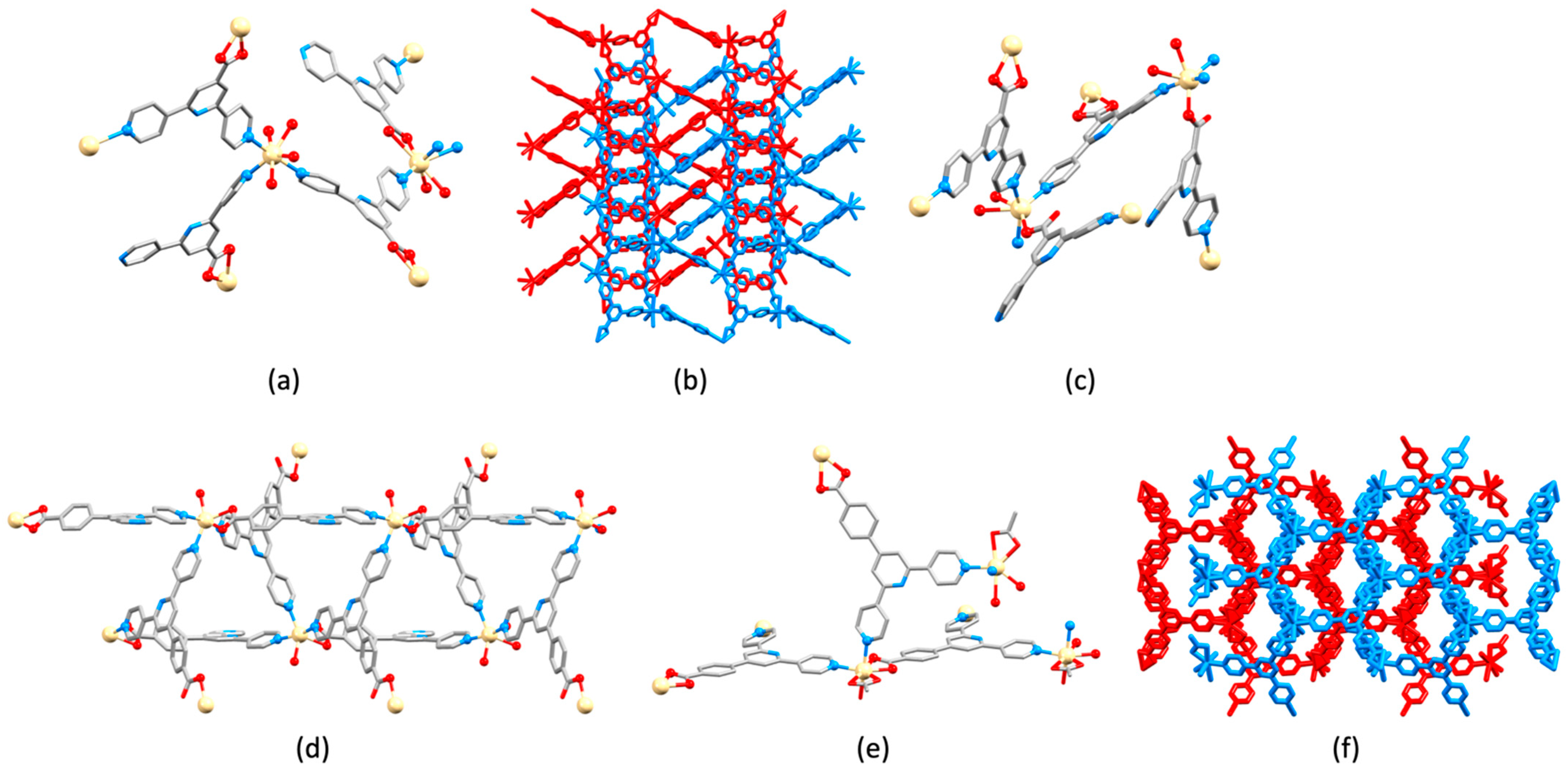
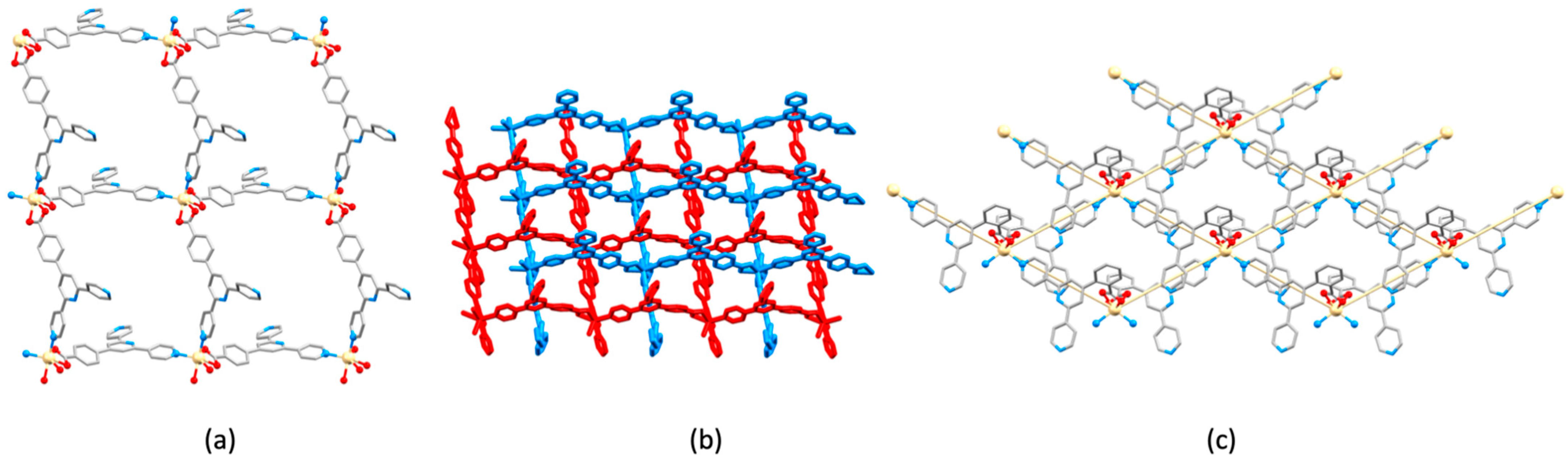
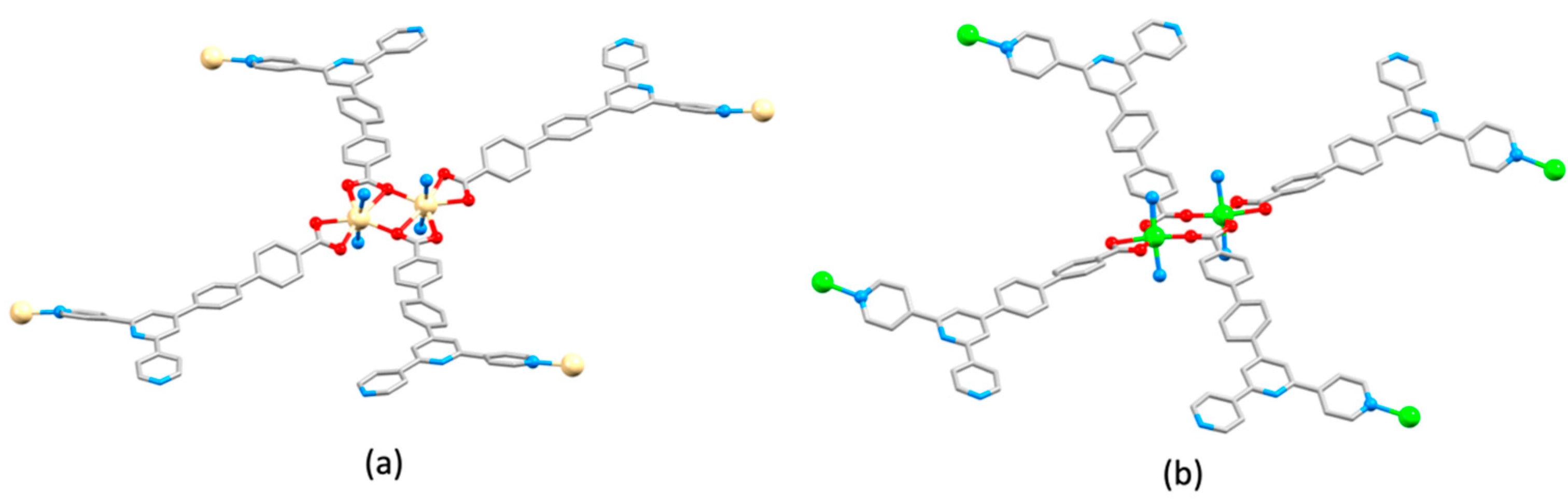
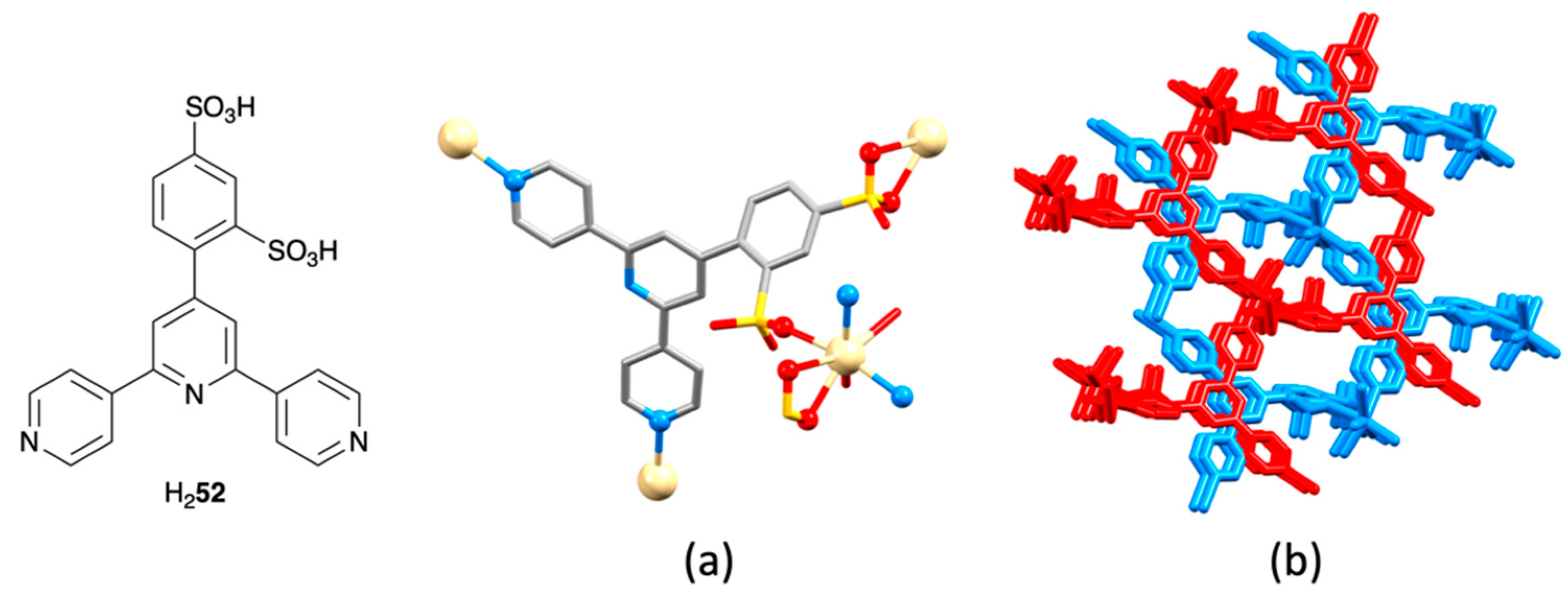
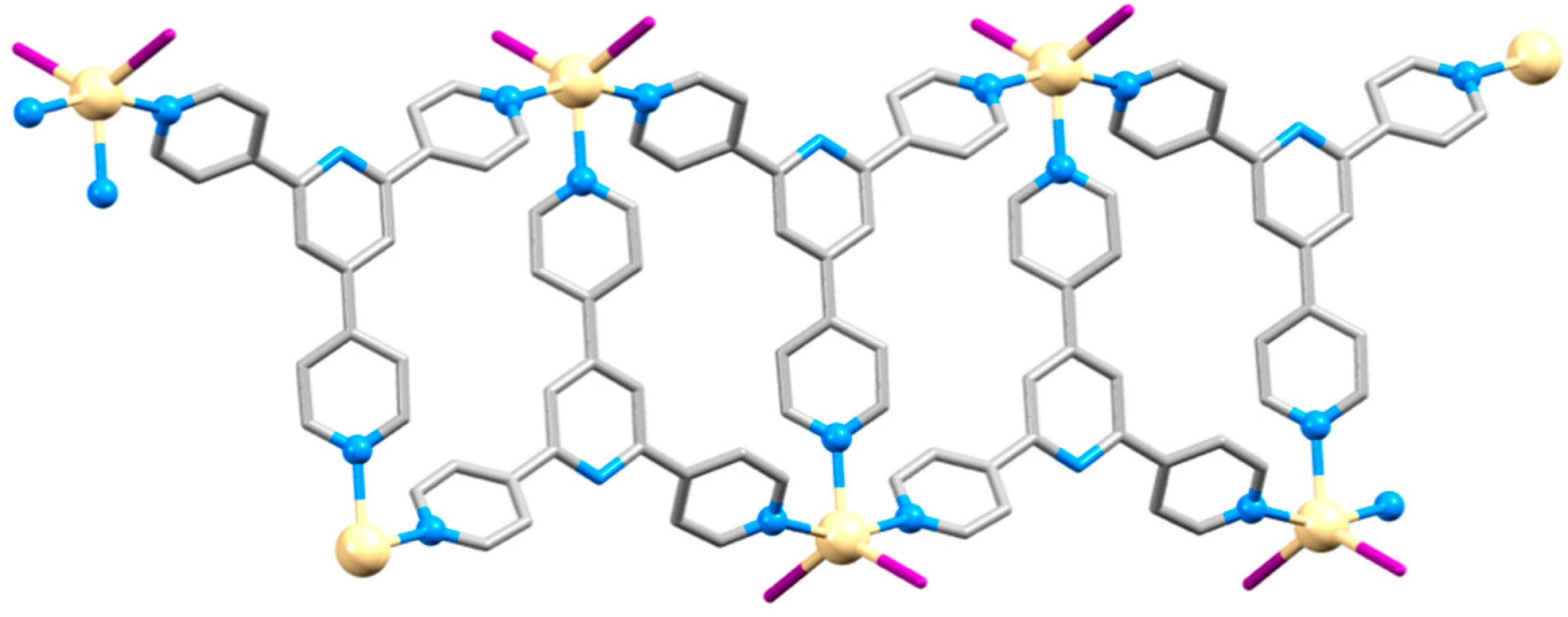
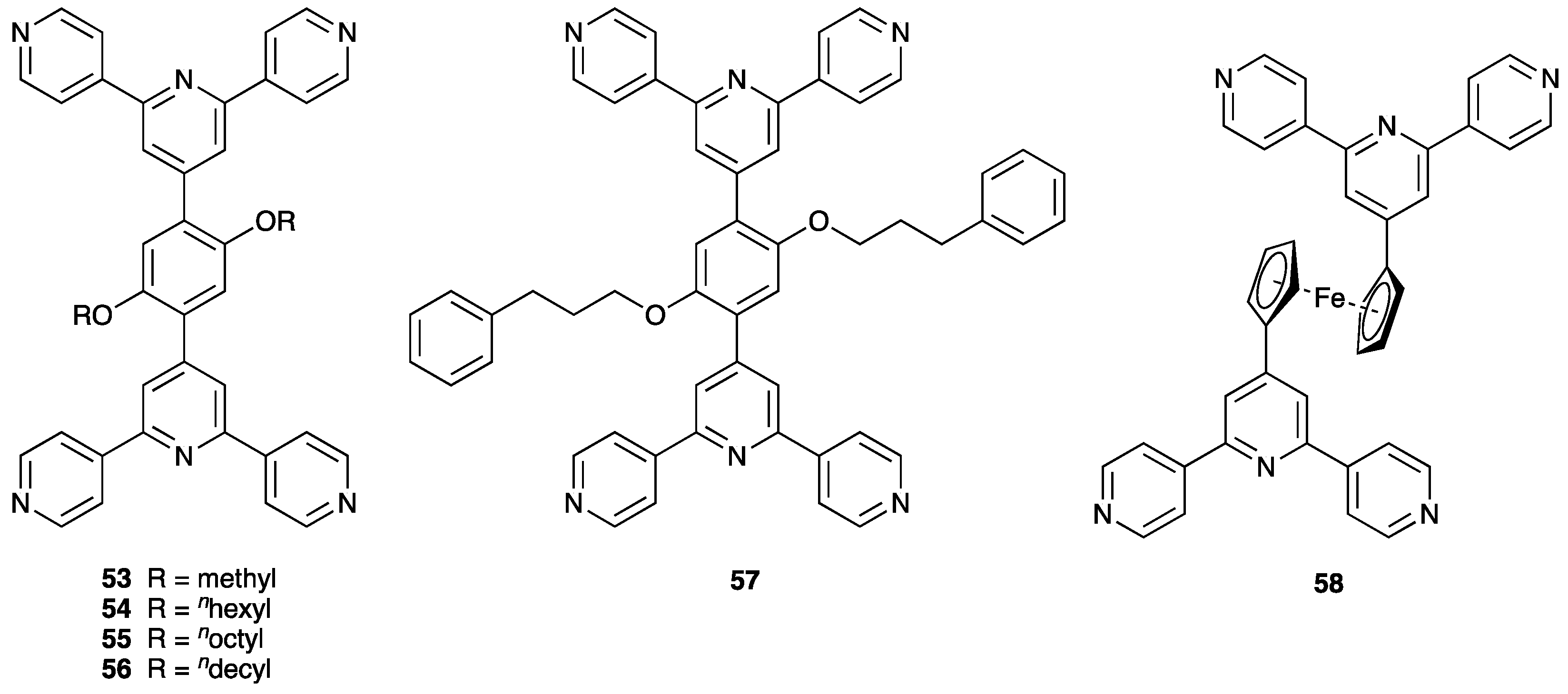
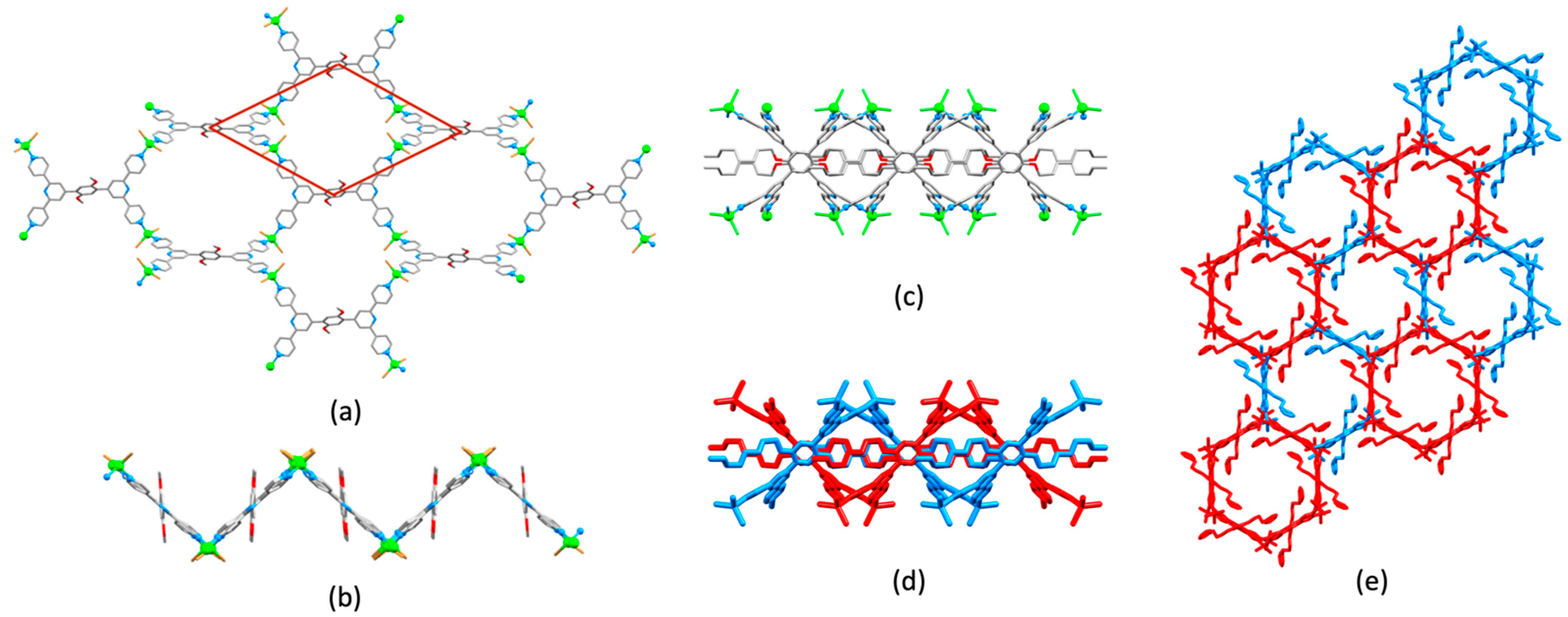
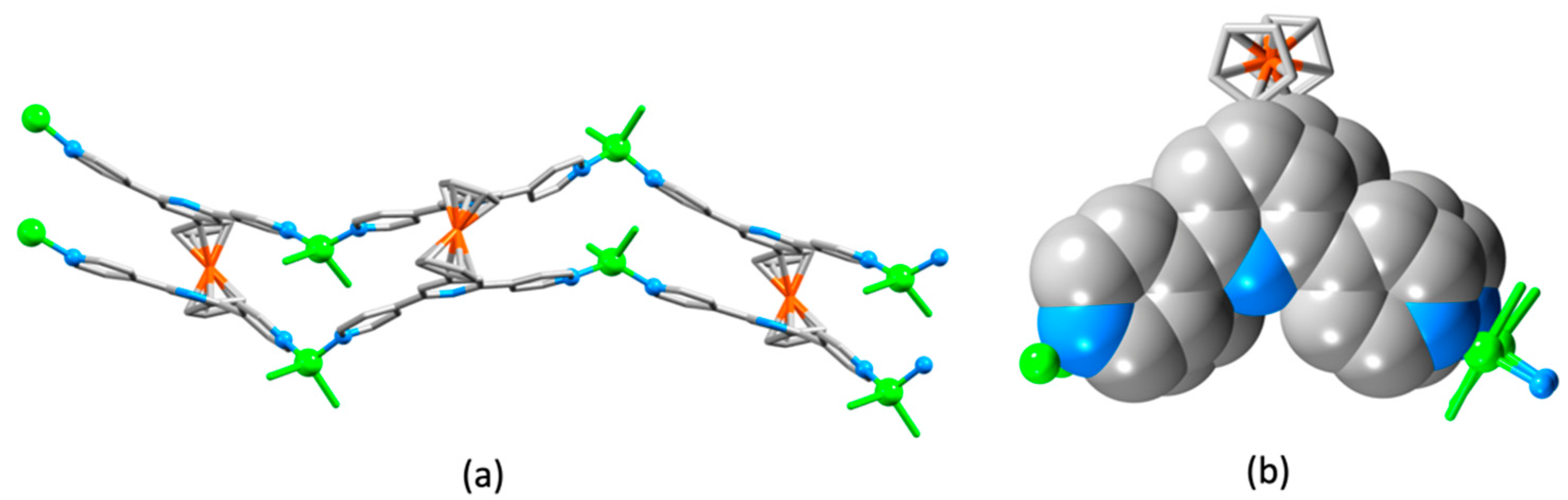
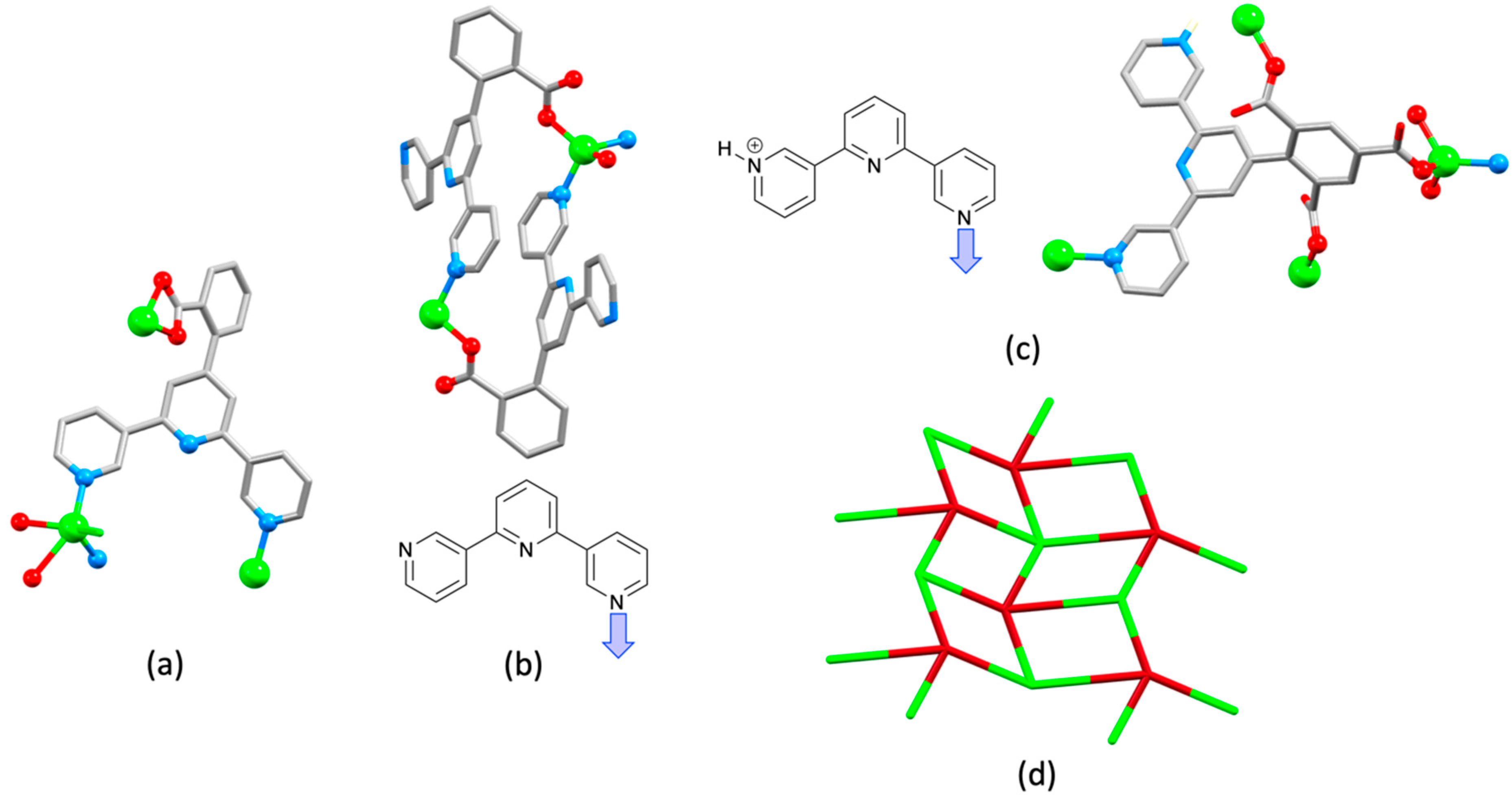
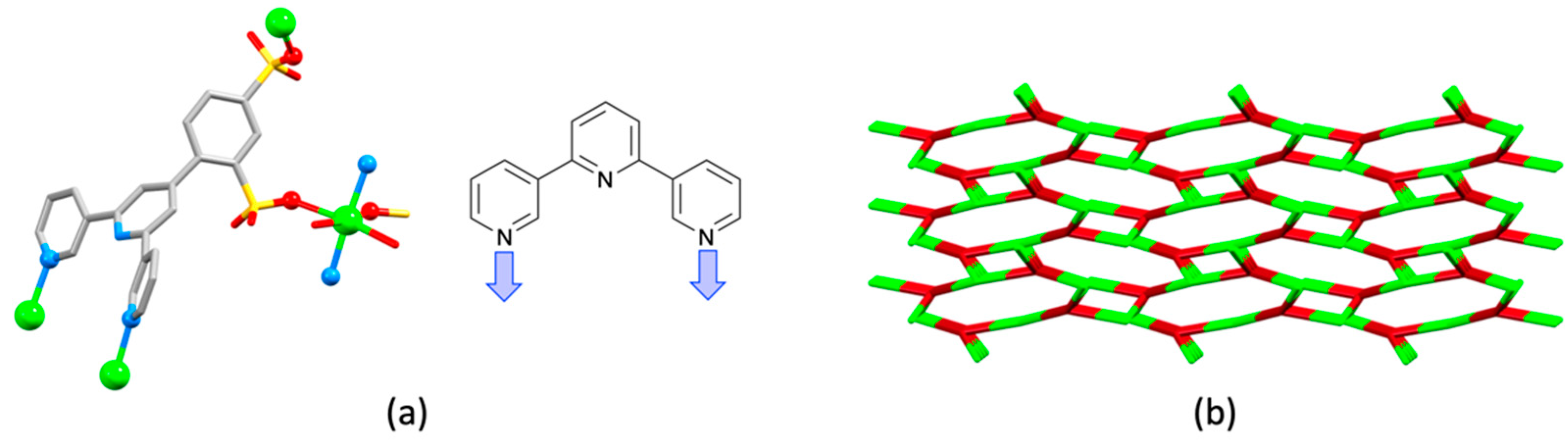
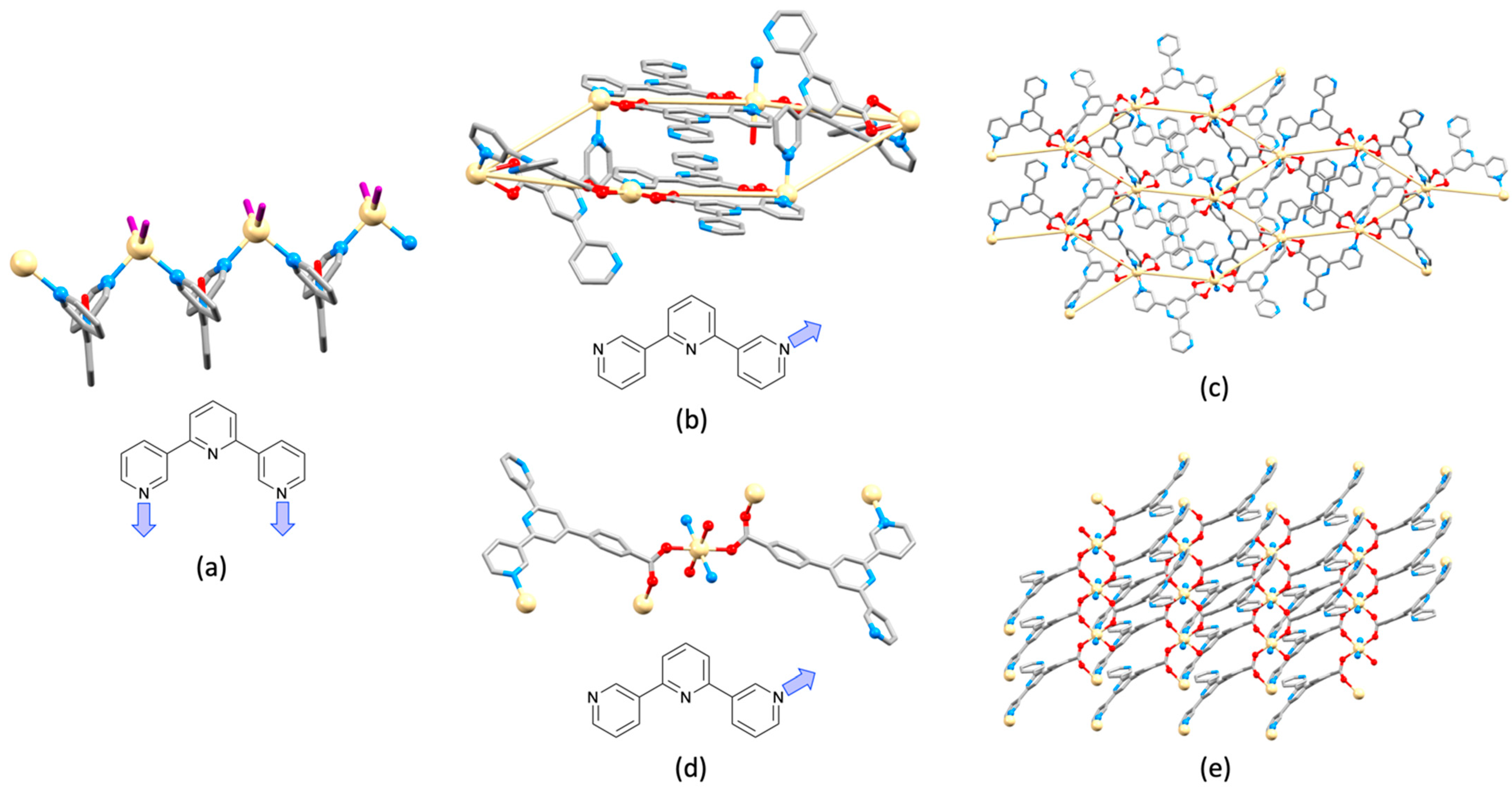
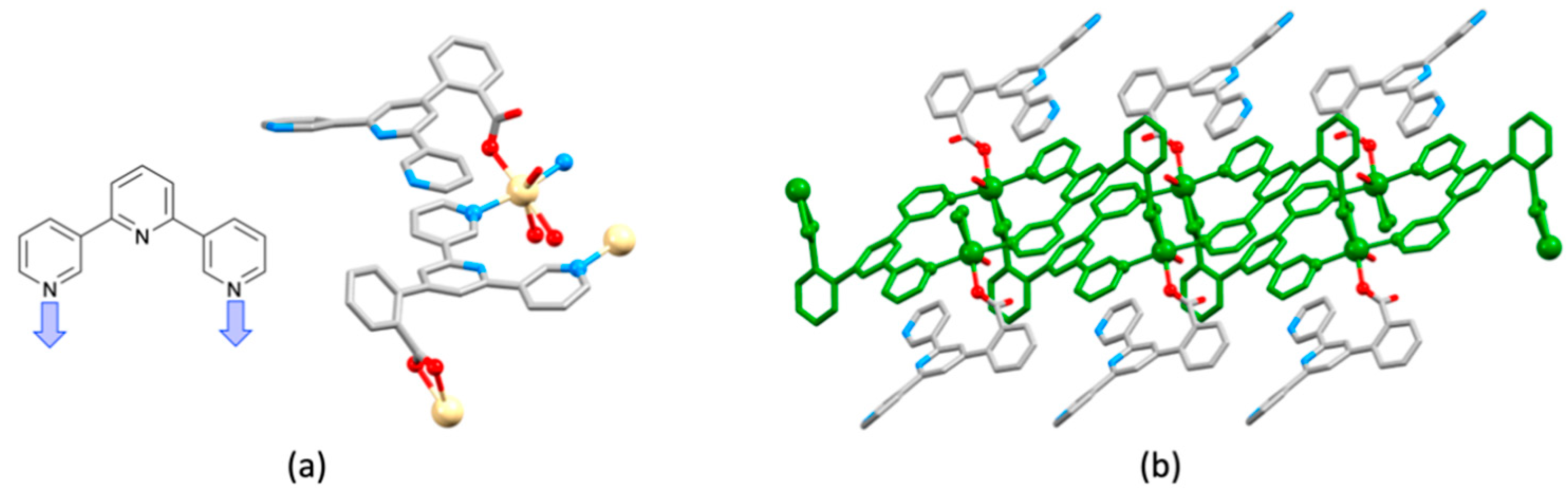
| Compound | CSD Refcode | Space Group | N–Zn–N/° | Zn…Zn/Å | Ref. |
|---|---|---|---|---|---|
| [Zn(4,2′:6′,4″-tpy)Cl2]n | GAQYUS | P21/n | 98.6(2) | 12.528(4) | [44] |
| [Zn(14)Cl2]n | FEPRUO | P21/n | 105.96(11) | 13.040(1) | [46] |
| [Zn(15)Cl2]n | LOCTED | P21/n | 99.5(1) | 12.992(1) | [47] |
| [Zn(15)Cl2]n a | NOGFIZ | P3121 | 107.9; 100.3(2) | 12.564; 13.029(2) | [47] |
| [Zn(16)Cl2]n·nEtOH·n/2H2O | NOGFOF | P21/n | 93.5(1) | 12.379(2) | [47] |
| [Zn2(H17)Cl2]n·2nEtOH·nH2O | DIRMEY | Pnna | 101.1; 97.6 | 12.626 | [48] |
| [Zn(18)Cl2]n | DOMBIS | P21/n | 100.06(1) | 13.037(1) | [49] |
| [Zn(19)Cl2]n·nH2O | SEBRID | Pna21 | 100.3(2) | 12.706(1) | [50] |
| [Zn(20)Cl2]n | AJURIG | P21/c | 104.01(10) | 12.8502(6) | [51] |
| [Zn(21)Cl2]n | ELAMOV | P21/c | 103.88(8) | 12.523(2) | [14] |
| [Zn(22)Cl2]n | QOJBOI | P–1 | 97.4(1), 98.1(1), 102.8(1), 104.4(1) | 12.400(1), 12.325(2), 12.863(2) | [52] |
| [Zn(23)Cl2]n | QOJBAU | P–1 | 104.6(1), 94.3(2), 100.2(1), 103.0(1) | 12.899(1), 12.4525(9), 13.062(1), 12.3425(9) | [52] |
| Compound | CSD Refcode | Space Group | N–Zn–N/° | Zn…Zn/Å | Ref. |
|---|---|---|---|---|---|
| [Zn(14)(OAc)2]n | CUXDOP | P21/n | 106.99(5) | 12.583(2) | [54] |
| [Zn(21)I2]n·nH2O | ELAMEL | P21/c | 98.1(1) | 12.583(2) | [14] |
| [Zn(24)I2]n·nC6H4Cl2 | FAKRUG | P21/c | 105.0(1) | 12.6858(7) | [55] |
| [Zn(25)I2]n·2nCHCl3 | TUNKEU | P21/c | 104.5(1) | 12.771(2) | [56] |
| [Zn(25)(OAc)2]n·2nH2O | TUNCIQ | P21/n | 106.8(2) | 12.516(5) | [56] |
| [Zn(25)(NCS)2]n·nCHCl3 | TUNNIB | P21/n | 111.7(1) a | 12.416(3) | [56] |
| [Zn(25)(OAc)2]n·nMeOH·nH2O | NEFWED | Pbca | 110.06(9) | 12.1240(9) | [57] |
| (M)-[Zn(26)(OAc)2]n | OFIVOQ | P212121 | 103.2(2) | 12.5757(9) | [58] |
| [2{Zn(26)(OAc)2}]n·nCHCl3·5nMeOH·2nH2O | OFIWEH | Pnna | 114.29 | 13.194 | [58] |
Publisher′s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Housecroft, C.E.; Constable, E.C. Isomers of Terpyridine as Ligands in Coordination Polymers and Networks Containing Zinc(II) and Cadmium(II). Molecules 2021, 26, 3110. https://doi.org/10.3390/molecules26113110
Housecroft CE, Constable EC. Isomers of Terpyridine as Ligands in Coordination Polymers and Networks Containing Zinc(II) and Cadmium(II). Molecules. 2021; 26(11):3110. https://doi.org/10.3390/molecules26113110
Chicago/Turabian StyleHousecroft, Catherine E., and Edwin C. Constable. 2021. "Isomers of Terpyridine as Ligands in Coordination Polymers and Networks Containing Zinc(II) and Cadmium(II)" Molecules 26, no. 11: 3110. https://doi.org/10.3390/molecules26113110
APA StyleHousecroft, C. E., & Constable, E. C. (2021). Isomers of Terpyridine as Ligands in Coordination Polymers and Networks Containing Zinc(II) and Cadmium(II). Molecules, 26(11), 3110. https://doi.org/10.3390/molecules26113110