Photodegradation of Ciprofloxacin, Clarithromycin and Trimethoprim: Influence of pH and Humic Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Photodegradation Experiments
3. Results and Discussion
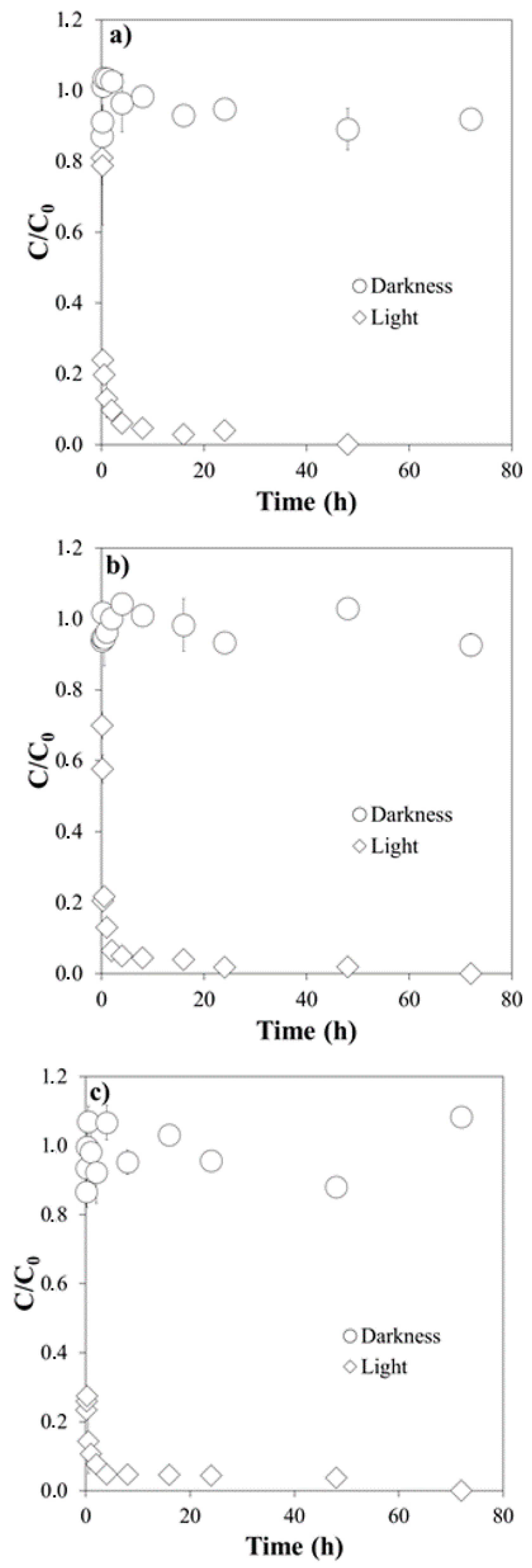
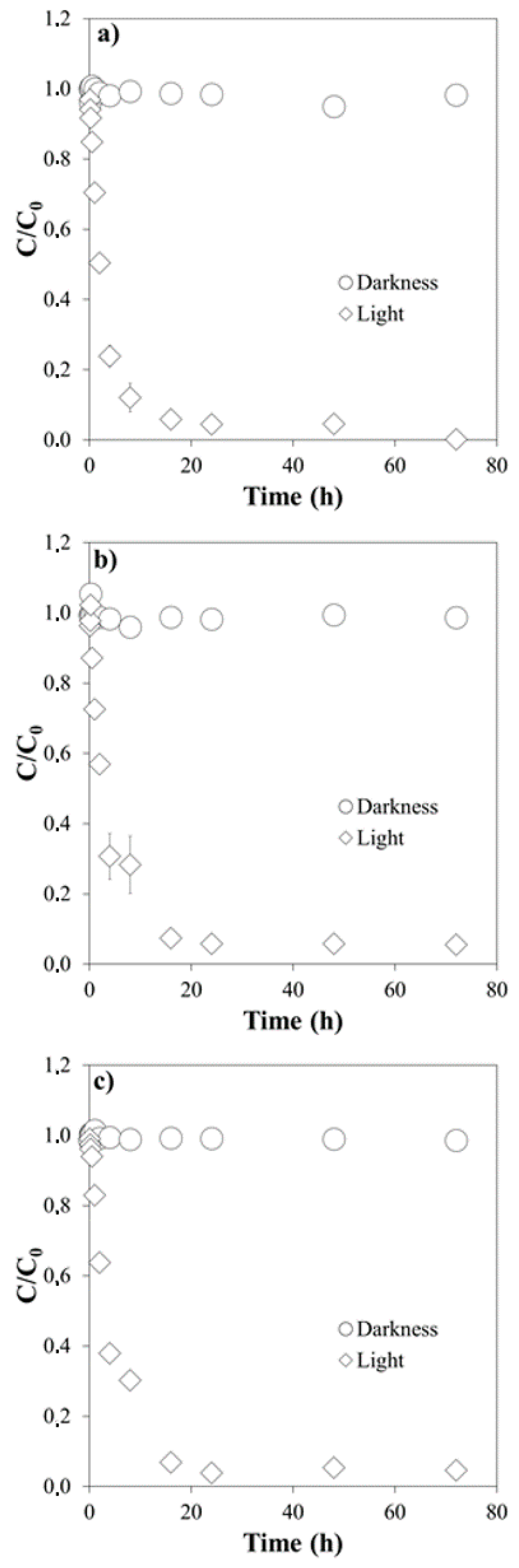
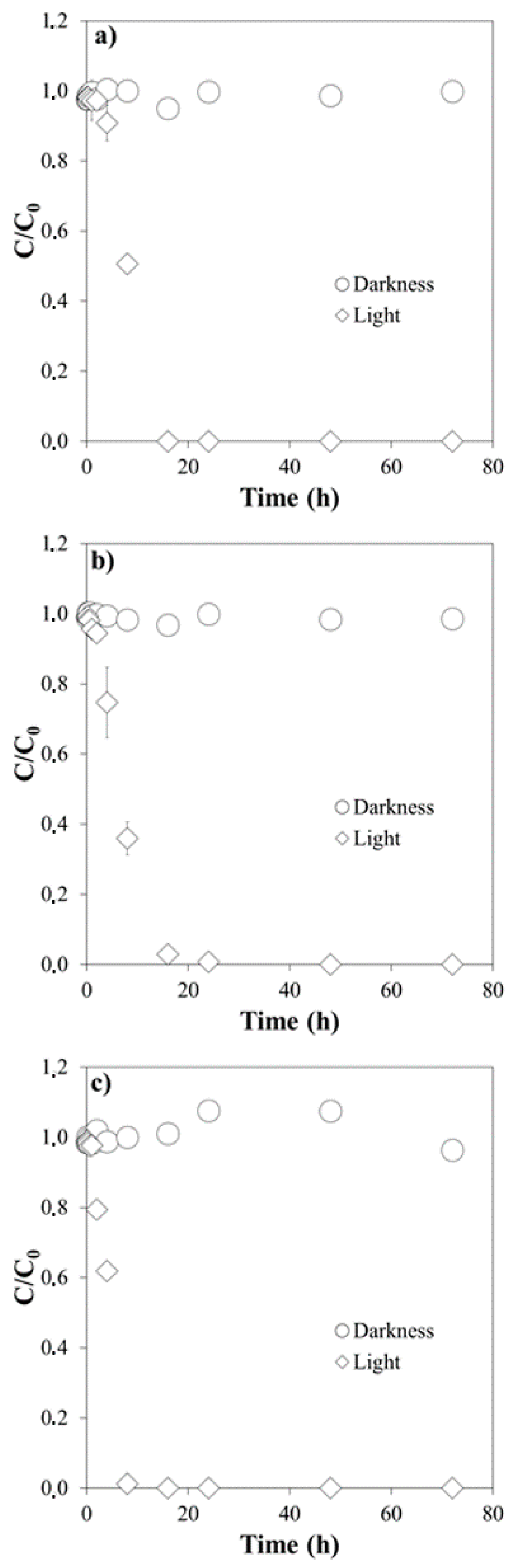
3.1. Influence of pH
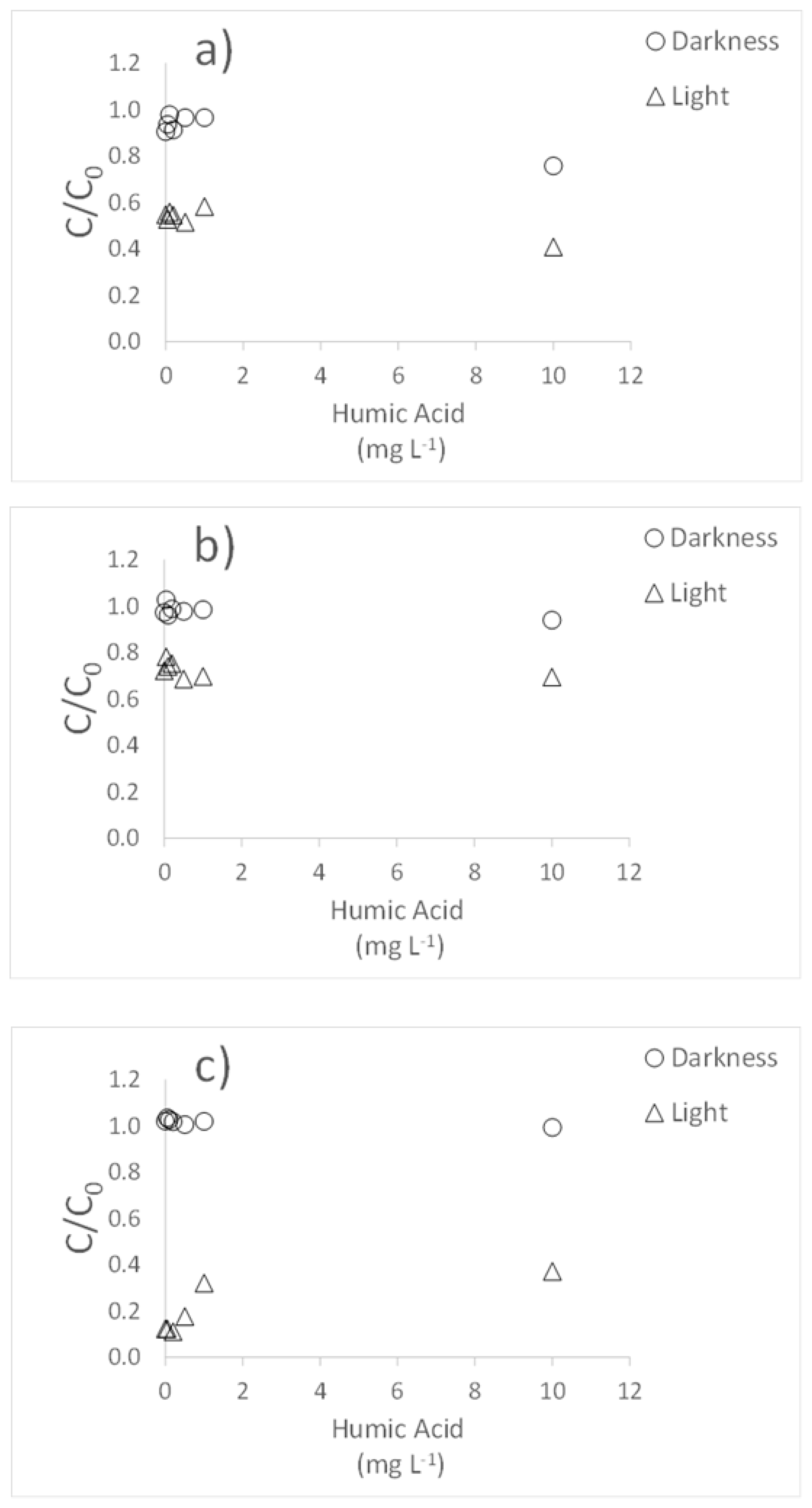
3.2. Influence of Humic Acids
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cully, M. Public health: The politics of antibiotics. Nature 2014, 509, S16–S17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Perez-Cuasquer, G.J.; Li, Z.; Mang, H.P.; Lv, Y. Occurrence of typical antibiotics, representative antibiotic-resistant bacteria, and genes in fresh and stored source-separated human urine. Environ. Int. 2021, 146, 106280. [Google Scholar] [CrossRef] [PubMed]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Castiglioni, S.; Bagnati, R.; Fanelli, R.; Pomati, F.; Calamari, D.; Zuccato, E. Removal of Pharmaceuticals in Sewage Treatment Plants in Italy. Environ. Sci. Technol. 2006, 40, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Della-Giustina, S.V.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef]
- Binh, V.N.; Dang, N.; Anh, N.T.K.; Ky, L.X.; Yhai, P.K. Antibiotics in the aquatic environment of Vietnam: Sources, concentrations, risk and control strategy. Chemosphere 2018, 197, 438–450. [Google Scholar] [CrossRef]
- Van, T.T.H.; Yidana, Z.; Smooker, P.M.; Coloe, P.J. Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. J. Glob. Antimicrob. Resist. 2020, 20, 170–177. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Gao, H.; Zhan, L.X.; Lu, Z.H.; He, C.M.; Li, Q.W.; Na, G.S. Complex migration of antibiotic resistance in natural aquatic environments. Environ. Poll. 2018, 232, 1–9. [Google Scholar] [CrossRef]
- Thai, P.K.; Ky, L.X.; Bing, V.N.; Nhung, P.H.; Nhan, P.T.; Hieu, N.Q.; Dang, N.T.T.; Tam, N.K.B.; Anh, N.T.K. Occurrence of antibiotic residues and antibiotic-resistant bacteria in effluents of pharmaceutical manufacturers and other sources around Hanoi, Vietnam. Sci. Total Environ. 2018, 645, 393–400. [Google Scholar] [CrossRef]
- Kümmerer, K.; Al-Ahmad, A.; Mersch-Sundermann, V. Biodegradability of some antibiotics, elimination of the genotoxicity and affection of wastewater bacteria in a simple test. Chemosphere 2000, 40, 701–710. [Google Scholar] [CrossRef]
- Alexy, R.; Kümpel, T.; Kümmerer, K. Assesment of degradation of 18 antibiotics in the Closed Bottle Test. Chemosphere 2004, 57, 505–512. [Google Scholar] [CrossRef]
- Batchu, S.R.; Panditi, V.R.; O’Shea, K.E.; Gardinali, P.R. Photodegradation of antibiotics under simulated solar radiation: Implications for their environmental fate. Sci. Total Environ. 2014, 470–471, 299–310. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; Wei, G.; Khan, E.; Ng, Y.H.; Ok, Y.S. Recent advances in photodegradation of antibiotic residues in water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Díaz-Raviña, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E. Degradation of sulfadiazine, sulfachloropyridazine and sulfamethazine in aqueous media. J. Environ. Manag. 2018, 228, 239–248. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Díaz-Raviña, M.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez-Rodríguez, E. Biotic and abiotic dissipation of tetracyclines using simulated sunlight and in the dark. Sci. Total Environ. 2018, 635, 1520–1529. [Google Scholar] [CrossRef]
- Biošić, M.; Mitrevski, M.; Babić, S. Environmental behavior of sulfadiazine, sulfamethazine, and their metabolites. Environ. Sci. Pollut. Res. 2017, 24, 9802–9812. [Google Scholar] [CrossRef]
- Xenopoulos, M.A.; Barnes, R.T.; Boodoo, K.S.; Butman, D.; Catalán, N.; D’Amario, S.C.; Fasching, C.; Kothawala, D.N.; Pisani, O.; Solomon, C.T.; et al. How humans alter dissolved organic matter composition in freshwater: Relevance for the Earth’s biogeochemistry. Biogeochemistry 2021. [Google Scholar] [CrossRef]
- Chen, C.E.; Chen, W.; Ying, G.G.; Jones, K.C.; Zhang, H. In situ measurement of solution concentrations and fluxes of sulfonamides and trimethoprim antibiotics in soils using o-DGT. Talanta 2015, 132, 902–908. [Google Scholar] [CrossRef]
- Leifeld, J.; von Lützow, M. Chemical and microbial activation energies of soil organic matter decomposition. Biol. Fertil. Soils 2014, 50, 147–153. [Google Scholar] [CrossRef]
- Schnitzer, M. Humic Substances: Chemistry and Reactions. In Soil Organic Matter; Schnitzer, M., Khan, S.U., Eds.; Elsevier Publishing: Amsterdam, The Netherlands, 1978; pp. 1–64. [Google Scholar]
- Schnitzer, M. Binding of humic substances by soil mineral colloids. In Interactions of Soil Minerals with Natural Organics and Microbes; Soil Science Society of America: Madison, WI, USA, 1986. [Google Scholar]
- Schnitzer, M.; Khan, S.U. Humic Substances in the Environment; Marcel Dekker: New York, USA, 1972. [Google Scholar]
- Gad-Allah, T.A.; Ali, M.E.M.; Badawy, M.I. Photocatalytic oxidation of ciprofloxacin under simulated sunlight. J. Hazard. Mater. 2011, 186, 751–755. [Google Scholar] [CrossRef]
- Ghirardini, A.; Grillini, V.; Verlicchi, P. A review of the occurrence of selected micropollutants and microorganisms in different raw and treated manure—Environmental risk due to antibiotics after application to soil. Sci. Total Environ. 2020, 707, 136118. [Google Scholar] [CrossRef]
- Baumann, M.; Weiss, K.; Maletzki, D.; Schussler, W.; Schudoma, D.; Kopf, W.; Kuhnen, U. Aquatic toxicity of the macrolide antibiotic clarithromycin and its metabolites. Chemosphere 2015, 120, 192–198. [Google Scholar] [CrossRef]
- Batt, A.L.; Kim, S.; Aga, D.S. Comparison of the occurrence of antibiotics in four full-scale wastewater treatment plants with varying designs and operations. Chemosphere 2007, 68, 428–435. [Google Scholar] [CrossRef]
- Arias, M.; Barral, M.T.; Mejuto, J.C. Enhancement of copper and cadmium adsorption on kaolin by the presence of humic acids. Chemosphere 2002, 48, 1081–1088. [Google Scholar] [CrossRef]
- Belden, J.B.; Maul, J.D.; Lydy, M.J. Partitioning and photodegradation of ciprofloxacin in aqueous systems in the presence of organic matter. Chemosphere 2007, 66, 1390–1395. [Google Scholar] [CrossRef]
- Bermúdez-Couso, A.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Calviño, D. Influence of different abiotic and biotic factor on the metalaxyl and carbofuran dissipation. Chemosphere 2013, 90, 2526–2533. [Google Scholar] [CrossRef]
- De Levie, R. Aqueous Acid-Base Equilibria and Titration; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Enke, C.G. The Art and Science of Chemical Analysis; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Torniainen, K.; Tammilehto, S.; Ulvi, V. The effect of pH, buffer type and drug concentration on the photodegradation of ciprofloxacin. Int. J. Pharm. 1996, 132, 53–61. [Google Scholar] [CrossRef]
- Guo, H.G.; Gao, N.Y.; Chu, W.H.; Li, L.; Zhang, Y.J.; Gu, J.S.; Gu, Y.L. Photochemical degradation of ciprofloxacin in UV and UV/H2O2 process: Kinetics, parameters, and products. Environ. Sci. Pollut. Res. 2013, 20, 3202–3213. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.G.; Henriques, D.M.; Konig, A.; Martins, A.F.; Kümmerer, K. Photo-degradation of the antimicrobial ciprofloxacin at hight pH. Identification and biodegradability assessment of the primary by-products. Chemosphere 2009, 76, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, X.; Duan, X.; Fu, Y.; Dionysiou, D.D. Photochemical degradation of oxytetracycline: Influence of pH and role of carbonate radical. Chem. Eng. J. 2015, 276, 113–121. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, C.; Qu, J.; Yang, M. Photodegradation of tetracycline and formation of reactive oxygen species in aqueous tetracycline solution under simulated sunlight irradiation. J. Photochem. Photobiol. A Chem. 2008, 197, 81–87. [Google Scholar] [CrossRef]
- Jiao, S.; Zheng, S.; Yin, D.; Wang, L.; Chen, L. Aqueous oxytetracycline degradation and the toxicity change of degradation compounds in photoirradiation process. J. Environ. Sci. 2008, 20, 806–813. [Google Scholar] [CrossRef]
- López-Peñalver, J.J.; Sánchez-Polo, M.; Gómez-Pacheco, C.V.; Rivera-Utrilla, J. Photodegradation of tetracyclines in aqueous solution by using UV and UV/H2O2 oxidation processes. J. Chem. Technol. Biotechnol. 2010, 85, 1325–1333. [Google Scholar] [CrossRef]
- Wang, J.M.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices- A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef]
- Vione, D.; Feitosa-Felizzola, J.; Minero, C.; Chiron, S. Phototransformation of selected human-used macrolides in Surface water: Kinetics, model predictions and degradation pathways. Water Res. 2009, 43, 1959–1967. [Google Scholar] [CrossRef]
| Common Name | Chemical Formula | Molecular Weight (g mol−1) | Log KOW 1 | pKa 2 | Water Solubility (mg L−1) 1 |
|---|---|---|---|---|---|
| Ciprofloxacin 1 | C17H18FN3O3 | 331.34 | 0.28 | 6.09–8.74 | 36,000 |
| Clarithromycin 2 | C38H69NO13 | 748.0 | 3.16 | 9.00–12.46 | 2 |
| Trimethoprim 3 | C14H18N4O3 | 290.32 | 0.91 | 6.16–7.16 | 400 |
| k (h−1) | t1/2 (h) | R2 | |
|---|---|---|---|
| Ciprofloxacin | |||
| pH 4.0 | 3.13 ± 0.48 | 0.22 | 0.966 |
| pH 5.5 | 4.14 ± 0.47 | 0.17 | 0.981 |
| pH 7.0 | 10.37 ± 2.07 | 0.07 | 0.906 |
| Clarithromycin | |||
| pH 4.0 | 0.34 ± 0.02 | 2.06 | 0.999 |
| pH 5.5 | 0.25 ± 0.03 | 2.82 | 0.991 |
| pH 7.0 | 0.19 ± 0.01 | 3.57 | 0.991 |
| Trimethoprim | |||
| pH 4.0 | 0.09 ± 0.02 | 7.35 | 0.978 |
| pH 5.5 | 0.12 ± 0.02 | 5.81 | 0.989 |
| pH 7.0 | 0.18 ± 0.03 | 3.95 | 0.984 |
| A+ | A+/− | A− | |
|---|---|---|---|
| Ciprofloxacin | |||
| pH 4.0 | 99.19 | 0.81 | 0.00 |
| pH 5.5 | 79.54 | 20.45 | 0.01 |
| pH 7.0 | 10.78 | 87.63 | 2.04 |
| Clarithromycin | |||
| pH 4.0 | 100.00 | 0.00 | 0.00 |
| pH 5.5 | 99.97 | 0.03 | 0.00 |
| pH 7.0 | 99.01 | 0.99 | 0.00 |
| Trimethoprim | |||
| pH 4.0 | 99.31 | 0.69 | 0.00 |
| pH 5.5 | 81.73 | 17.88 | 0.39 |
| pH 7.0 | 7.87 | 54.46 | 43.86 |
| Time | |||||
|---|---|---|---|---|---|
| 0 h | 2 h | 16 h | 48 h | 72 h | |
| Ciprofloxacin | |||||
| pH 4.0 | 10.1 | 10.0 | 9.8 | 8.7 | 7.3 |
| pH 5.5 | 10.1 | 9.4 | 8.0 | 7.7 | 7.3 |
| pH 7.0 | 10.2 | 9.6 | 8.9 | 7.6 | 6.4 |
| Trimethoprim | |||||
| pH 4.0 | 10.6 | 8.7 | 6.8 | 5.4 | 5.1 |
| pH 5.5 | 10.7 | 9.0 | 5.9 | 5.5 | 4.9 |
| pH 7.0 | 10.6 | 9.2 | 6.2 | 5.3 | 4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-López, L.; Cela-Dablanca, R.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Fernández-Calviño, D.; Arias-Estévez, M. Photodegradation of Ciprofloxacin, Clarithromycin and Trimethoprim: Influence of pH and Humic Acids. Molecules 2021, 26, 3080. https://doi.org/10.3390/molecules26113080
Rodríguez-López L, Cela-Dablanca R, Núñez-Delgado A, Álvarez-Rodríguez E, Fernández-Calviño D, Arias-Estévez M. Photodegradation of Ciprofloxacin, Clarithromycin and Trimethoprim: Influence of pH and Humic Acids. Molecules. 2021; 26(11):3080. https://doi.org/10.3390/molecules26113080
Chicago/Turabian StyleRodríguez-López, Lucía, Raquel Cela-Dablanca, Avelino Núñez-Delgado, Esperanza Álvarez-Rodríguez, David Fernández-Calviño, and Manuel Arias-Estévez. 2021. "Photodegradation of Ciprofloxacin, Clarithromycin and Trimethoprim: Influence of pH and Humic Acids" Molecules 26, no. 11: 3080. https://doi.org/10.3390/molecules26113080
APA StyleRodríguez-López, L., Cela-Dablanca, R., Núñez-Delgado, A., Álvarez-Rodríguez, E., Fernández-Calviño, D., & Arias-Estévez, M. (2021). Photodegradation of Ciprofloxacin, Clarithromycin and Trimethoprim: Influence of pH and Humic Acids. Molecules, 26(11), 3080. https://doi.org/10.3390/molecules26113080











