Synthetic Iowaite Can Effectively Remove Inorganic Arsenic from Marine Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instrumentation
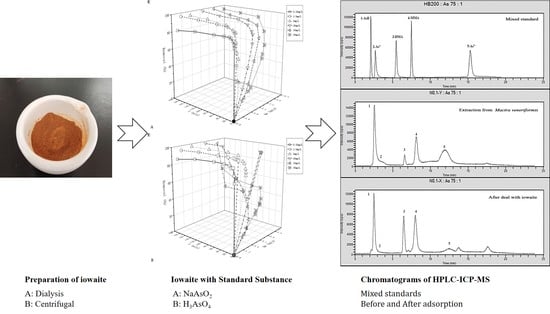
2.2. Preparation of Iowaite
2.3. Materials Modifactions
3. Batch Sorption Experiments
3.1. Influence of pH
3.2. Kinetics of Sorption
3.3. Adsorption to the Mactra veneriformis Extract
4. Results
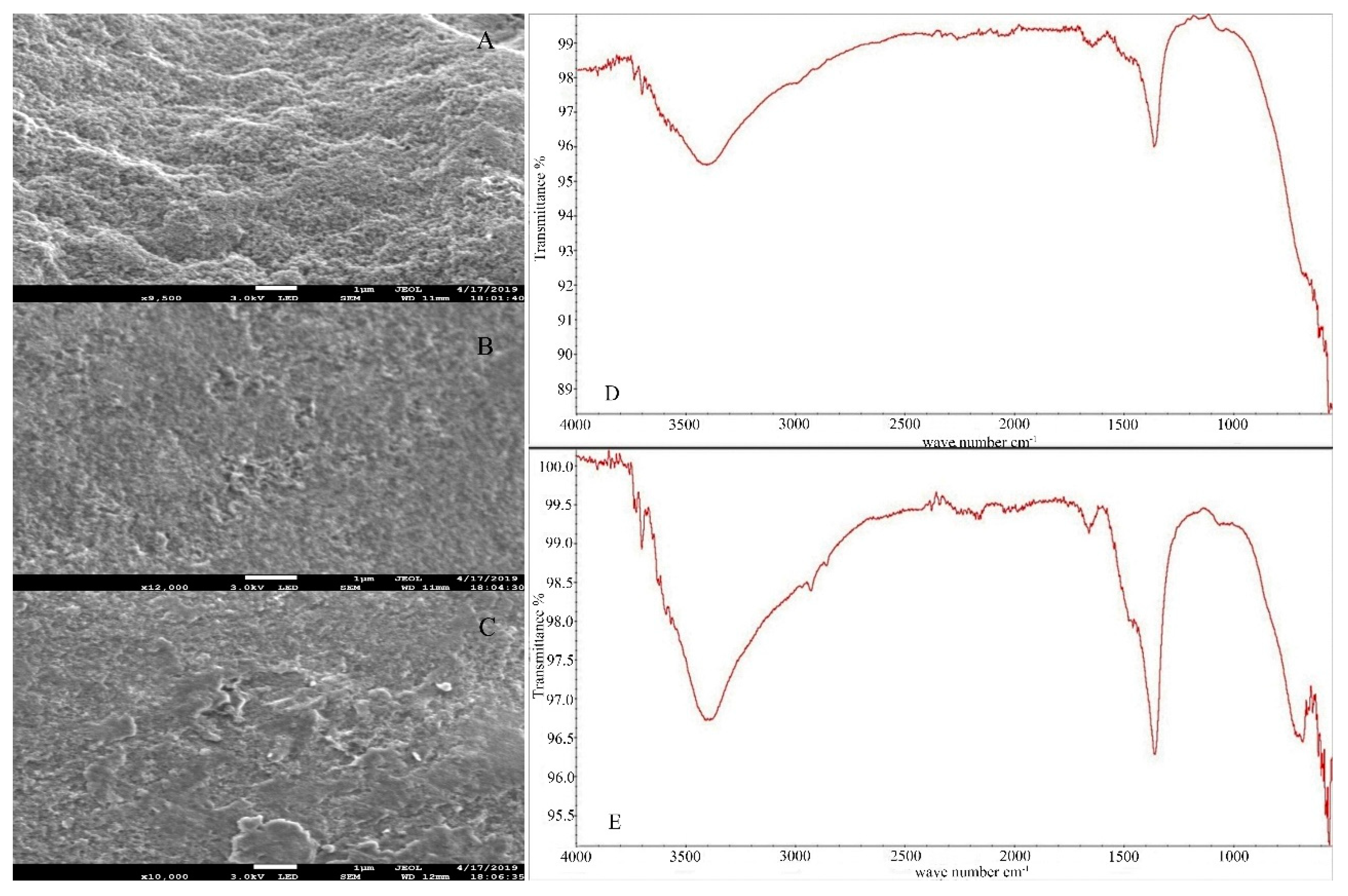
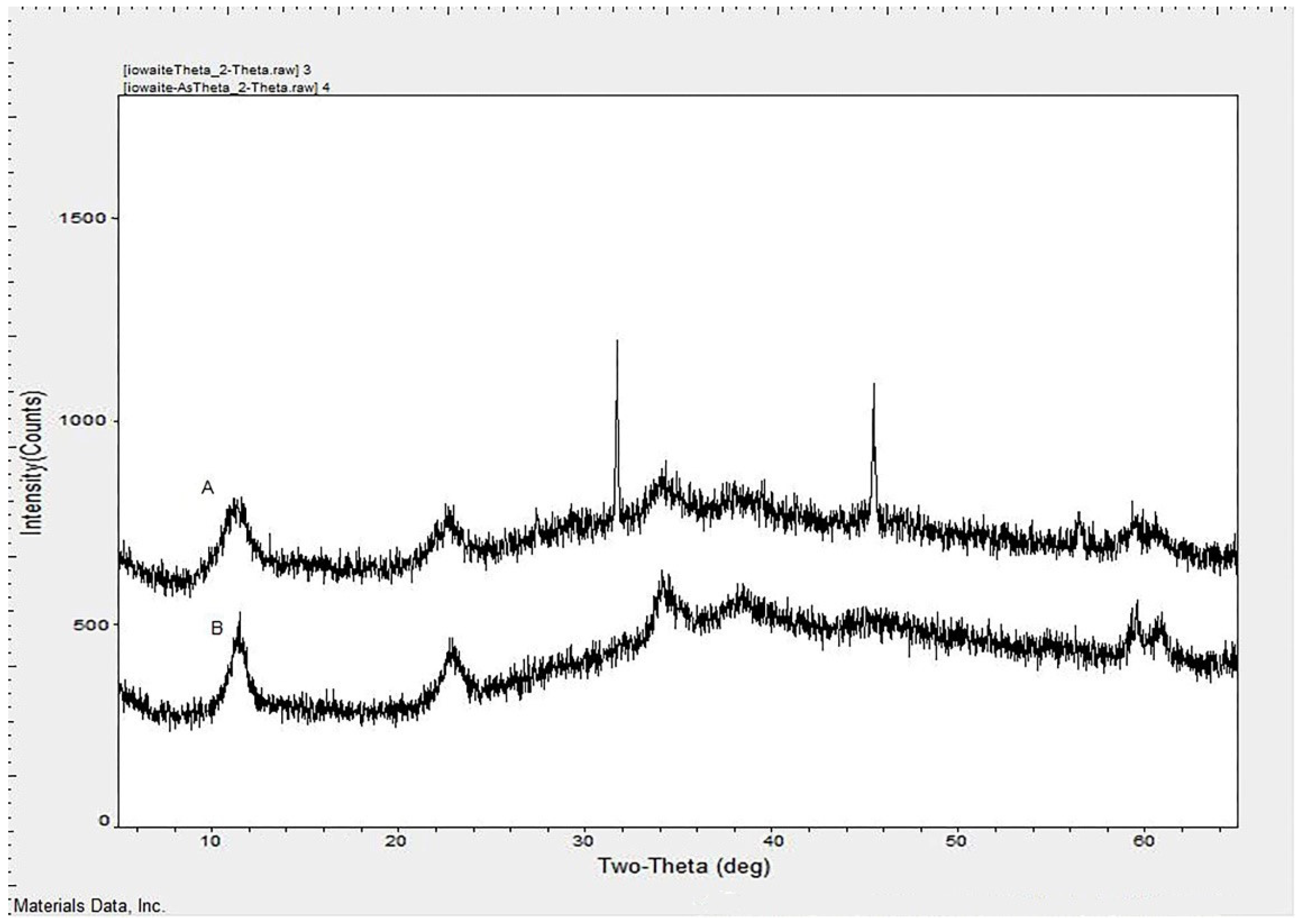
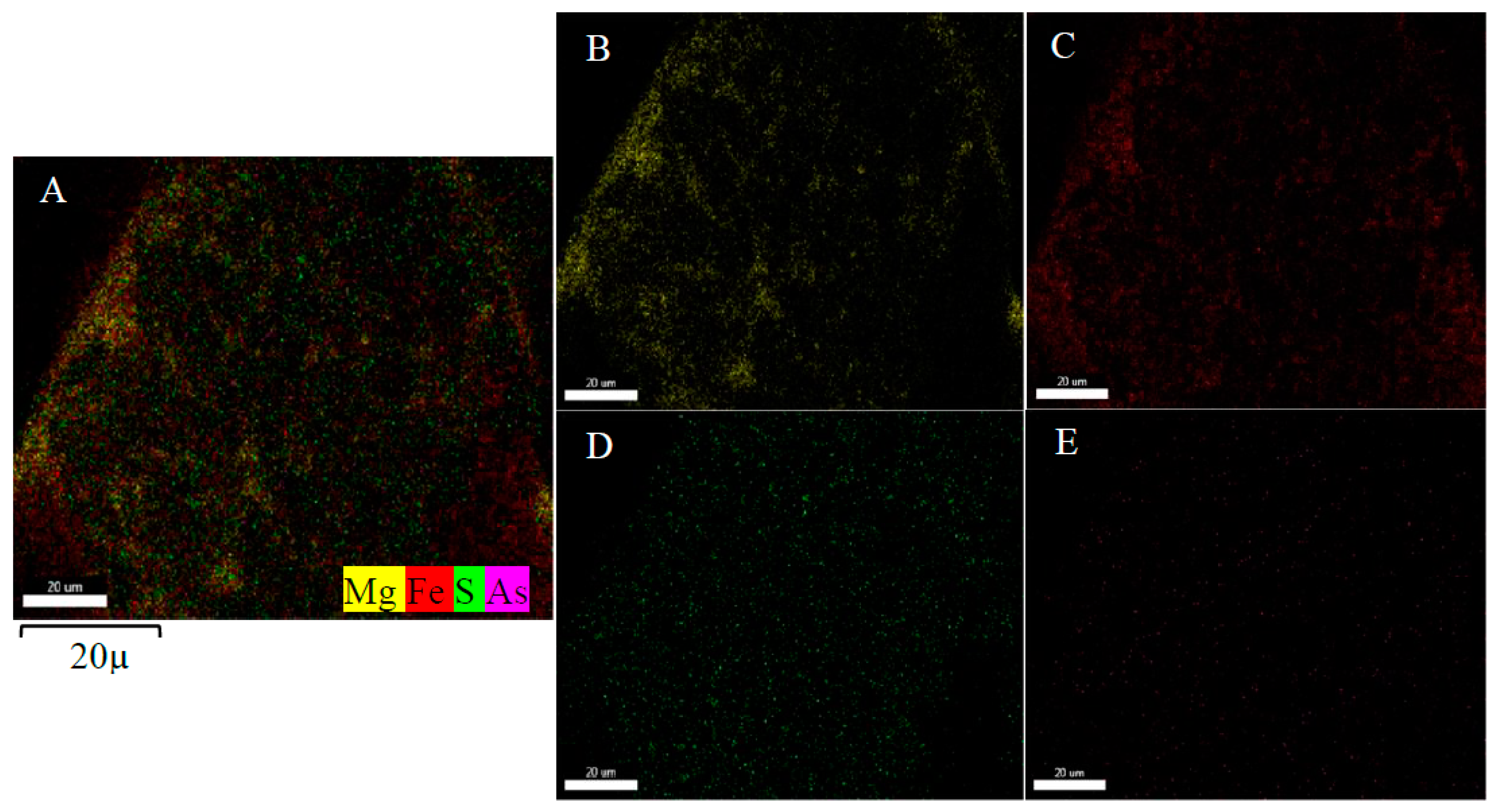
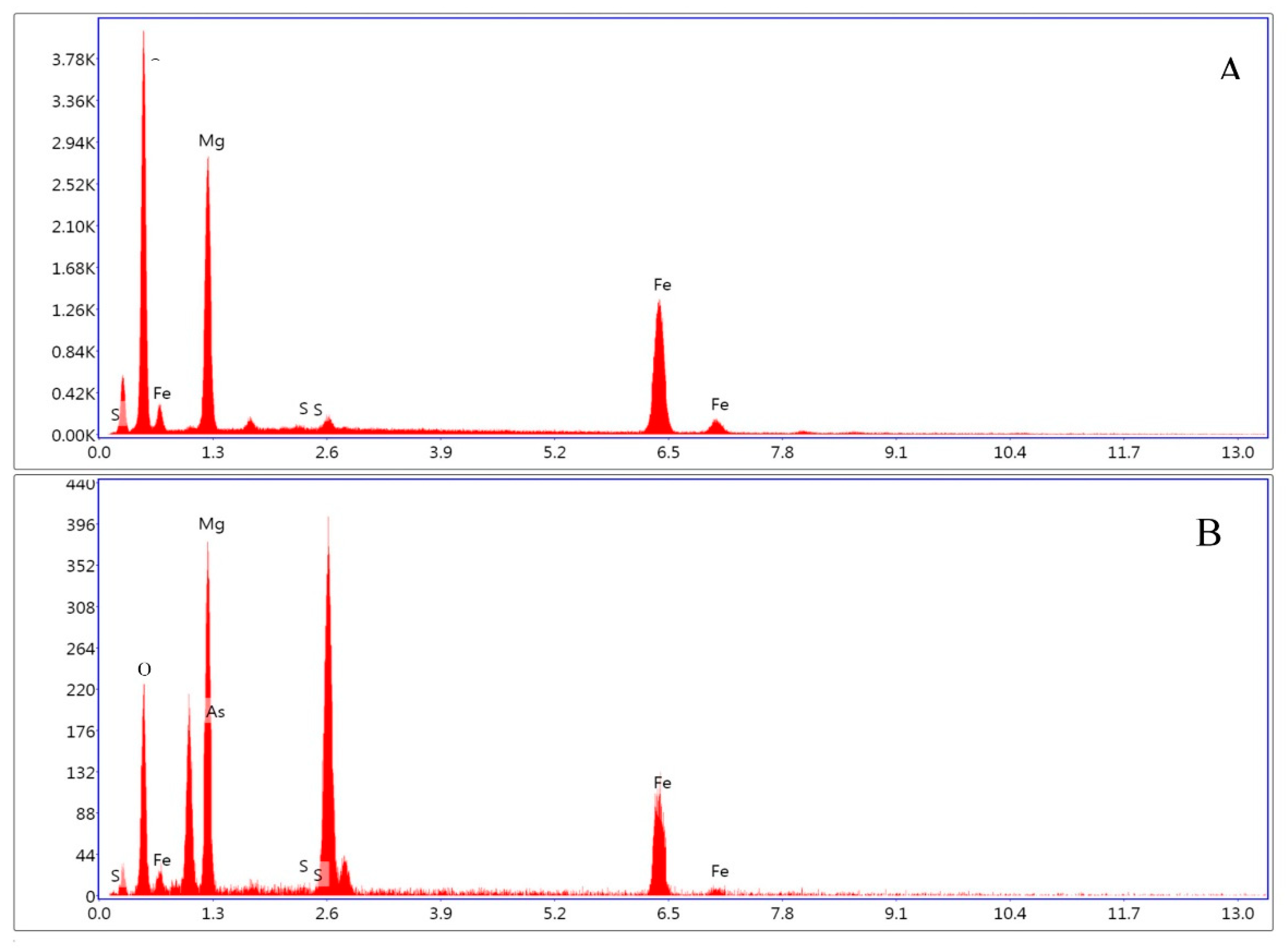
4.1. Characterization of Synthetic Iowaites
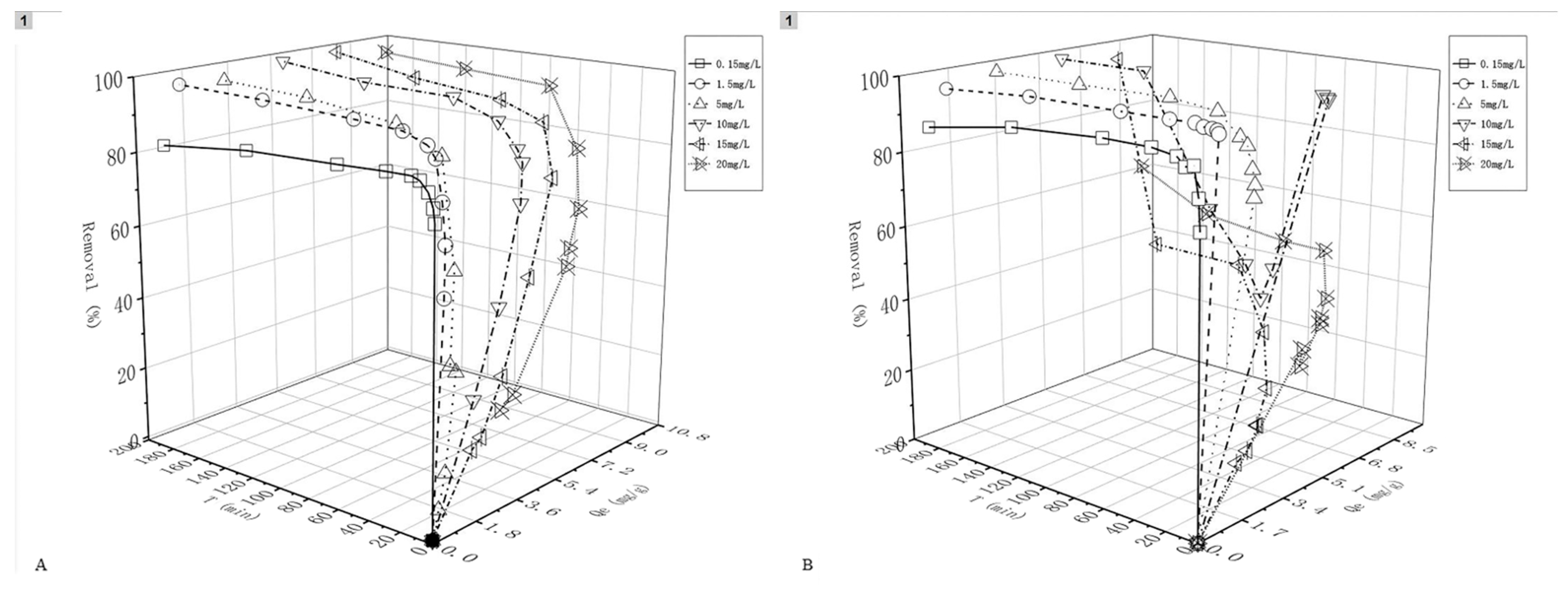
4.2. Influence of pH on the Arsenic Removing with Iowaites
4.3. Kinetics of Sorption
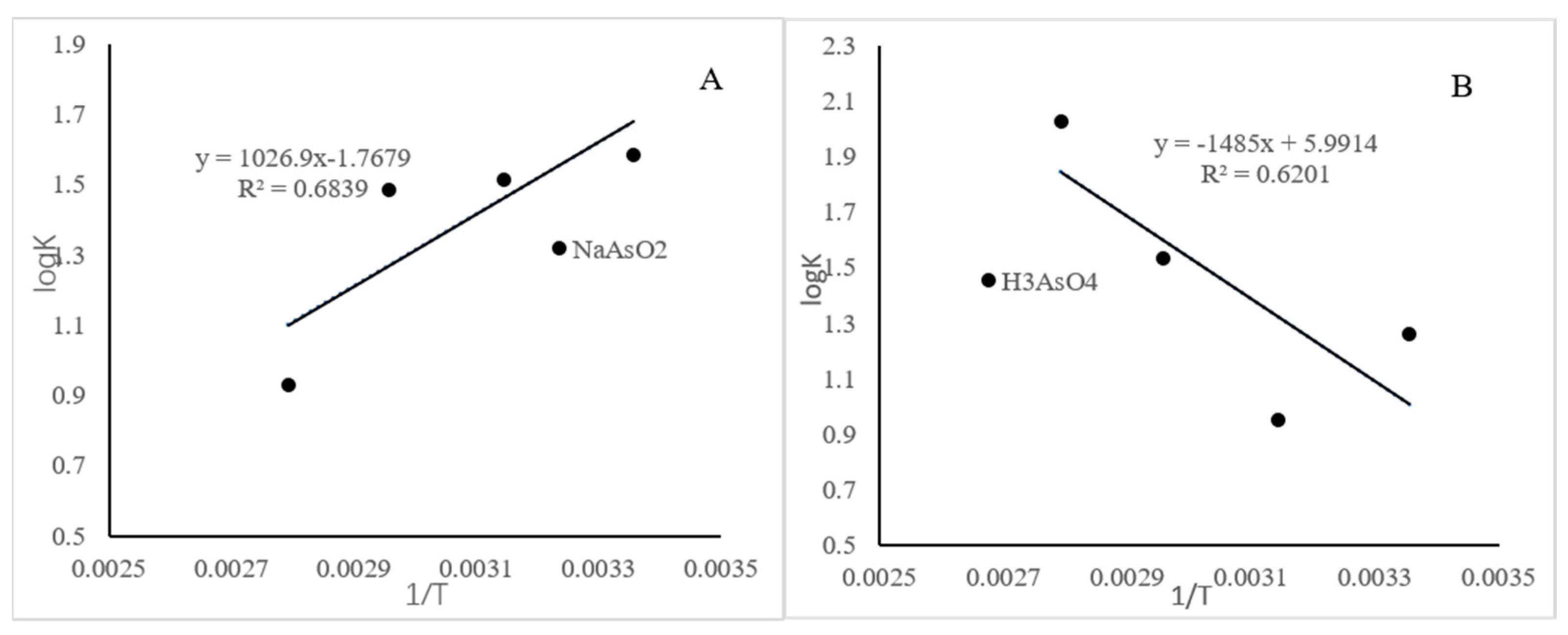
4.4. Thermodynamic Fitting for the Sorption
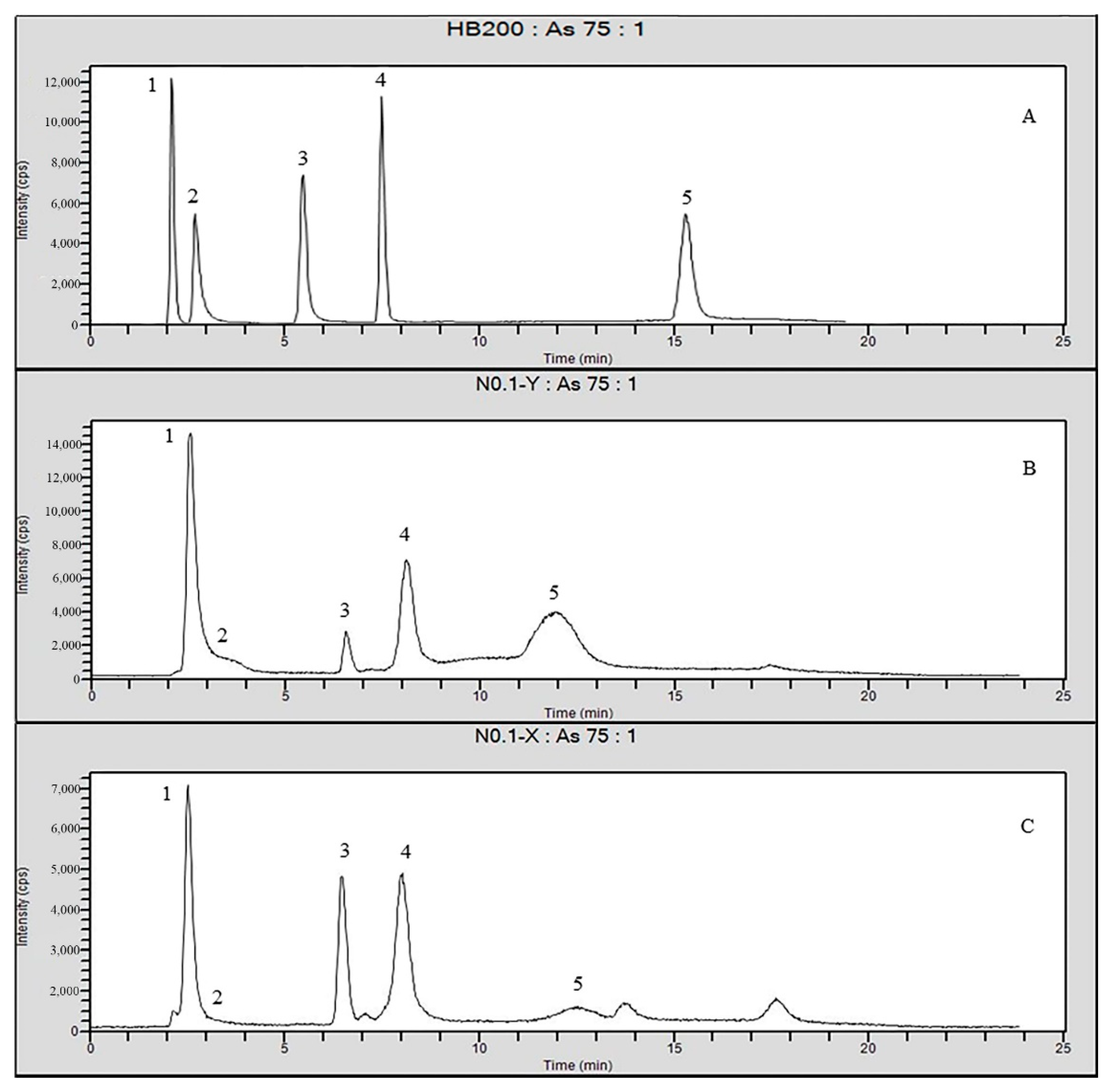
4.5. Adsorption of Different Forms of Arsenic
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, C.-T.; Chen, T.-H.; Lo, K.-F.; Chiu, C.-Y. Effects of proline on copper transport in rice seedlings under excess copper stress. Plant Sci. 2004, 166, 103–111. [Google Scholar] [CrossRef]
- Balaji, S.; Kalaivani, T.; Rajasekaran, C. Biosorption of Zinc and Nickel and Its Effect on Growth of Different Spirulina Strains. Clean-Soil Air Water 2013, 42, 507–512. [Google Scholar] [CrossRef]
- Harvey, C.F.; Swartz, C.H.; Badruzzaman, A.B.M.; Keon-Blute, N.; Yu, W.; Ali, M.A.; Jay, J.; Beckie, R.; Niedan, V.; Brabander, D.; et al. Arsenic Mobility and Groundwater Extraction in Bangladesh. Science 2002, 298, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Dudka, S.; Miller, W.P. Accumulation of potentially toxic elements in plants and their transfer to human food chain. J. Environ. Sci. Health Part B 1999, 34, 681–708. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Ali, I. Arsenic: Occurrence, toxicity and speciation techniques. Water Res. 2000, 34, 4304–4312. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Natural attenuation processes for remediation of arsenic contaminated soils and groundwater. J. Hazard. Mater. 2006, 138, 459–470. [Google Scholar] [CrossRef]
- Magos, L.; Hutchinson, T.C.; Meema, K.M. Lead, Mercury, Cadmium and Arsenic in the Environment; John Wiley & Sons: Chichester, UK, 1987; p. 360. [Google Scholar]
- Simsek, C.S.; Ergin, S.A.; Basa, D.S. Arsenic problem and arsenic treatment systems of haskoy and dambaslar in hayrabolu district of tekirdag province. J. Environ. Prot. Ecol. 2017, 18, 1100–1108. [Google Scholar]
- Kundu, S.; Kavalakatt, S.S.; Pal, A.; Ghosh, S.K.; Mandal, M.; Pal, T. Removal of arsenic using hardened paste of Portland cement: Batch adsorption and column study. Water Res. 2004, 38, 3780–3790. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Saha, K.C. Arsenical dermatosis from tube well water in West Bengal. Indian J. Med. Res. 1987, 85, 326–334. [Google Scholar]
- Terlecka, E. Arsenic Speciation Analysis in Water Samples: A Review of the Hyphenated Techniques. Environ. Monit. Assess. 2005, 107, 259–284. [Google Scholar] [CrossRef]
- Shalat, S.L.; Walker, D.B.; Finnell, R.H. Role of arsenic as a reproductive toxin with particular attention to neural tube defects. J. Toxicol. Environ. Health Part A 1996, 48, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczykab, B.; Spiegelsteinc, O.; Waesad, J.-V.; Vorce, R.L.; Luf, X.; Le, C.X.; Finnellcd, R.H. Arsenic-Induced Congenital Malformations in Genetically Susceptible Folate Binding Protein-2 Knockout Mice. Toxicol. Appl. Pharmacol. 2001, 177, 238–246. [Google Scholar] [CrossRef]
- Rangsayatorn, N.; Pokethitiyook, P.; Upatham, E.S.; Lanza, G.R. Cadmium biosorption by cells of Spirulina platensis TISTR 8217 im-mobilized in alginate and silica gel. Environ. Int. 2004, 30, 57–63. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A. Biosorption of lead(II) from aqueous solutions by non-living algal biomass Oedogonium sp. and Nostoc sp.—A comparative study. Colloids Surf. B Biointerfaces 2008, 64, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, A. Layered Double Hydroxides: Present and Future; Nova Science Publishers: New York, NY, USA, 2002; Volume 22, pp. 75–76. [Google Scholar]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Basu, D.; Das, A.; Stöckelhuber, K.W.; Wagenknecht, U.; Heinrich, G. Advances in layered double hydroxide (LDH)-based elastomer composites. Prog. Polym. Sci. 2014, 39, 594–626. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, Q.; Shu, Z.; Zhuang, Y.; Yu, Z.; Guo, W.; Zhang, C.; Zhu, M.; Zhao, Q.; Ren, T. Application of calcined iowaite in arsenic removal from aqueous solution. Appl. Clay Sci. 2016, 126, 313–321. [Google Scholar] [CrossRef]
- Martemianov, D.; Xie, B.-B.; Yurmazova, T.; Khaskelberg, M.; Wang, F.; Wei, C.-H.; Preis, S. Cellular concrete-supported cost-effective adsorbents for aqueous arsenic and heavy metals abatement. J. Environ. Chem. Eng. 2017, 5, 3930–3941. [Google Scholar] [CrossRef]
- Guo, Q.; Cao, Y.; Yin, Z.; Yu, Z.; Zhao, Q.; Shu, Z. Enhanced Removal of Arsenic from Water by Synthetic Nanocrystalline Iowaite. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Acharya, S.P.; Johnson, J.; Weidhaas, J. Adsorption kinetics of the herbicide safeners, benoxacor and furilazole, to activated carbon and agricultural soils. J. Environ. Sci. 2020, 89, 23–34. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, J.; Huang, K.; Hou, X.; Zheng, C. Facile electrochemical synthesis of nano iron porous coordination polymer using scrap iron for simultaneous and cost-effective removal of organic and inorganic arsenic. Chin. Chem. Lett. 2018, 29, 456–460. [Google Scholar] [CrossRef]
- Smedley, P.; Kinniburgh, D. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Goldberg, S.; Johnston, C.T. Mechanisms of Arsenic Adsorption on Amorphous Oxides Evaluated Using Macroscopic Measurements, Vibrational Spectroscopy, and Surface Complexation Modeling. J. Colloid Interface Sci. 2001, 234, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Puls, R.W. Arsenate and Arsenite Sorption on Magnetite: Relations to Groundwater Arsenic Treatment Using Zerovalent Iron and Natural Attenuation. Water Air Soil Pollut. 2008, 193, 65–78. [Google Scholar] [CrossRef]
- Si, Y.; Huo, J.; Hengbo, Y.; Wang, A. Adsorption Kinetics, Isotherms, and Thermodynamics of Cr(III), Pb(II), and Cu(II) on Porous Hydroxyapatite Nanoparticles. J. Nanosci. Nanotechnol. 2018, 18, 3484–3491. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Gitari, W.M.; Izuagie, A.A.; Gumbo, J.R. Synthesis, characterization and batch assessment of groundwater fluoride removal capacity of tri-metal Mg/Ce/Mn oxide-modified diatomaceous earth. Arab. J. Chem. 2020, 13, 1–16. [Google Scholar] [CrossRef]
- Pordel, S.; Schrage, B.R.; Ziegler, C.J.; White, J.K. Impact of steric bulk on photoinduced ligand exchange reactions in Mn(I) photoCORMs. Inorg. Chim. Acta 2020, 511, 119845. [Google Scholar] [CrossRef]
- Ho, Y.-S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]

| ICP-MS Parameters | |
| Plasma power | 1650 W |
| Nebulizer gas flow | 0.85 L/min |
| Auxiliary gas flow | 1.2 L/min |
| Plasma gas flow | 18 L/min |
| Monitored ion | m/z 75 (75As) |
| Reaction Mode | KDC |
| Dwell Time | 250 ms |
| Acquisition Rate | 4 pt/s |
| HPLC Parameters | |
| Column | Hamilton PRX-X100 (250 mm × 4.1 mm, 10 μm) |
| Column temperature | 30 °C |
| Mobile phase A | 25 mmol/L NH4HCO3 adjusted to pH 8 with NH4OH |
| Mobile phase B | Water |
| Gradient program | 0–15 min: 0–100% A; 16–19 min: 100–0% A; 20–24 min: 0% A |
| Flow rate | 1 mL/min |
| Injected volume | 50 µL |
| Initial pH | Equilibrium Concentration/(mg/L) | Dosage of Adsorbent/mg | Qe/(mg/g) | Removal/% |
|---|---|---|---|---|
| 3.01 | 0.0046 | 10.14 | 7.37 | 99.69% |
| 5.13 | 0.0039 | 10.74 | 6.96 | 99.74% |
| 7.14 | 0.0047 | 10.82 | 6.91 | 99.69% |
| 9.17 | 0.0051 | 10.43 | 7.17 | 99.66% |
| 11.08 | 0.0121 | 10.57 | 7.04 | 99.19% |
| Initial pH | Equilibrium Concentration/(mg/L) | Dosage of Adsorbent/mg | Qe/(mg/g) | Removal/% |
|---|---|---|---|---|
| 3.13 | 0.0636 | 10.17 | 7.06 | 95.76% |
| 5.05 | 0.0607 | 10.25 | 7.02 | 95.95% |
| 6.80 | 0.0807 | 10.01 | 7.09 | 94.62% |
| 9.08 | 0.0835 | 10.22 | 6.93 | 94.43% |
| 11.24 | 0.2154 | 10.02 | 6.41 | 85.64% |
| Kinetic Parameter | Co/(mg/L) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| k1 | qe | R2 | k2 | qe | R2 | ||
| NaAsO2 | 0.150 | 1.922 | 0.065 | 0.991 | 82.036 | 0.066 | 0.999 |
| 1.500 | 0.897 | 0.683 | 0.976 | 2.196 | 0.710 | 0.998 | |
| 5.000 | 0.106 | 2.370 | 0.977 | 0.058 | 2.574 | 0.986 | |
| 10.000 | 0.428 | 4.597 | 0.987 | 0.128 | 4.880 | 0.997 | |
| 15.000 | 0.132 | 6.951 | 0.980 | 0.026 | 7.492 | 0.985 | |
| 20.000 | 0.212 | 8.741 | 0.936 | 0.032 | 9.431 | 0.986 | |
| H3AsO4 | 0.150 | 1.612 | 0.065 | 0.994 | 62.743 | 0.067 | 0.999 |
| 1.500 | 4.131 | 0.707 | 1.000 | 72.370 | 0.708 | 0.999 | |
| 5.000 | 1.706 | 2.399 | 0.976 | 1.611 | 2.457 | 0.992 | |
| 10.000 | 196.645 | 4.372 | 0.768 | − | 4.372 | 0.768 | |
| 15.000 | 0.023 | 5.872 | 0.830 | − | 3.920 | 0.242 | |
| 20.000 | 0.527 | 6.856 | 0.817 | 1.949 | 6.142 | 0.598 | |
| Solution | T/°C | Freundlich Model | Langmuir Model | ||||
|---|---|---|---|---|---|---|---|
| 1/n | k4 | R2 | Qm | k3 | R2 | ||
| NaAsO2 | 25 | 0.268 | 0.073 | 0.996 | 2.17 | 38.38 | 0.996 |
| 45 | 0.275 | 0.069 | 0.976 | 2.45 | 32.47 | 0.996 | |
| 65 | 0.342 | 0.117 | 0.891 | 1.81 | 30.48 | 0.946 | |
| 85 | 0.374 | 0.108 | 0.944 | 8.62 | 8.53 | 0.999 | |
| H3AsO4 | 25 | 0.068 | 2.78 | 0.468 | 5.20 | 18.12 | 0.929 |
| 45 | 0.177 | 2.87 | 0.594 | 1.99 | 8.92 | 0.981 | |
| 65 | 0.486 | 146.96 | 0.951 | 1.20 | 34.30 | 0.982 | |
| 85 | 0.294 | 20.85 | 0.784 | 3.66 | 21.49 | 0.571 | |
| T/K | k3 | ΔG0 (J/mol) | ΔH0 (J/mol) | ΔS0 (J/mol) | |
|---|---|---|---|---|---|
| NaAsO2 | 298 | 38.38 | −9.04 | −8.54 | −14.70 |
| 318 | 32.47 | −9.20 | |||
| 338 | 30.48 | −9.60 | |||
| 358 | 8.53 | −6.38 | |||
| H3AsO4 | 298 | 18.12 | −7.18 | 12.68 | 49.81 |
| 318 | 8.92 | −5.79 | |||
| 338 | 34.30 | −9.93 | |||
| 358 | 21.49 | −13.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Huang, W.; Wang, L.; Chen, L.; Wei, Y.; Liu, R.; Cheng, J.; Wu, H. Synthetic Iowaite Can Effectively Remove Inorganic Arsenic from Marine Extract. Molecules 2021, 26, 3052. https://doi.org/10.3390/molecules26103052
Ji J, Huang W, Wang L, Chen L, Wei Y, Liu R, Cheng J, Wu H. Synthetic Iowaite Can Effectively Remove Inorganic Arsenic from Marine Extract. Molecules. 2021; 26(10):3052. https://doi.org/10.3390/molecules26103052
Chicago/Turabian StyleJi, Jing, Wenwen Huang, Lingchong Wang, Lu Chen, Yuanqing Wei, Rui Liu, Jianming Cheng, and Hao Wu. 2021. "Synthetic Iowaite Can Effectively Remove Inorganic Arsenic from Marine Extract" Molecules 26, no. 10: 3052. https://doi.org/10.3390/molecules26103052
APA StyleJi, J., Huang, W., Wang, L., Chen, L., Wei, Y., Liu, R., Cheng, J., & Wu, H. (2021). Synthetic Iowaite Can Effectively Remove Inorganic Arsenic from Marine Extract. Molecules, 26(10), 3052. https://doi.org/10.3390/molecules26103052