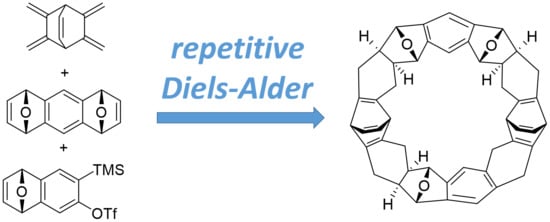
Synthesis of the [11]Cyclacene Framework by Repetitive Diels–Alder Cycloadditions
Abstract
1. Introduction
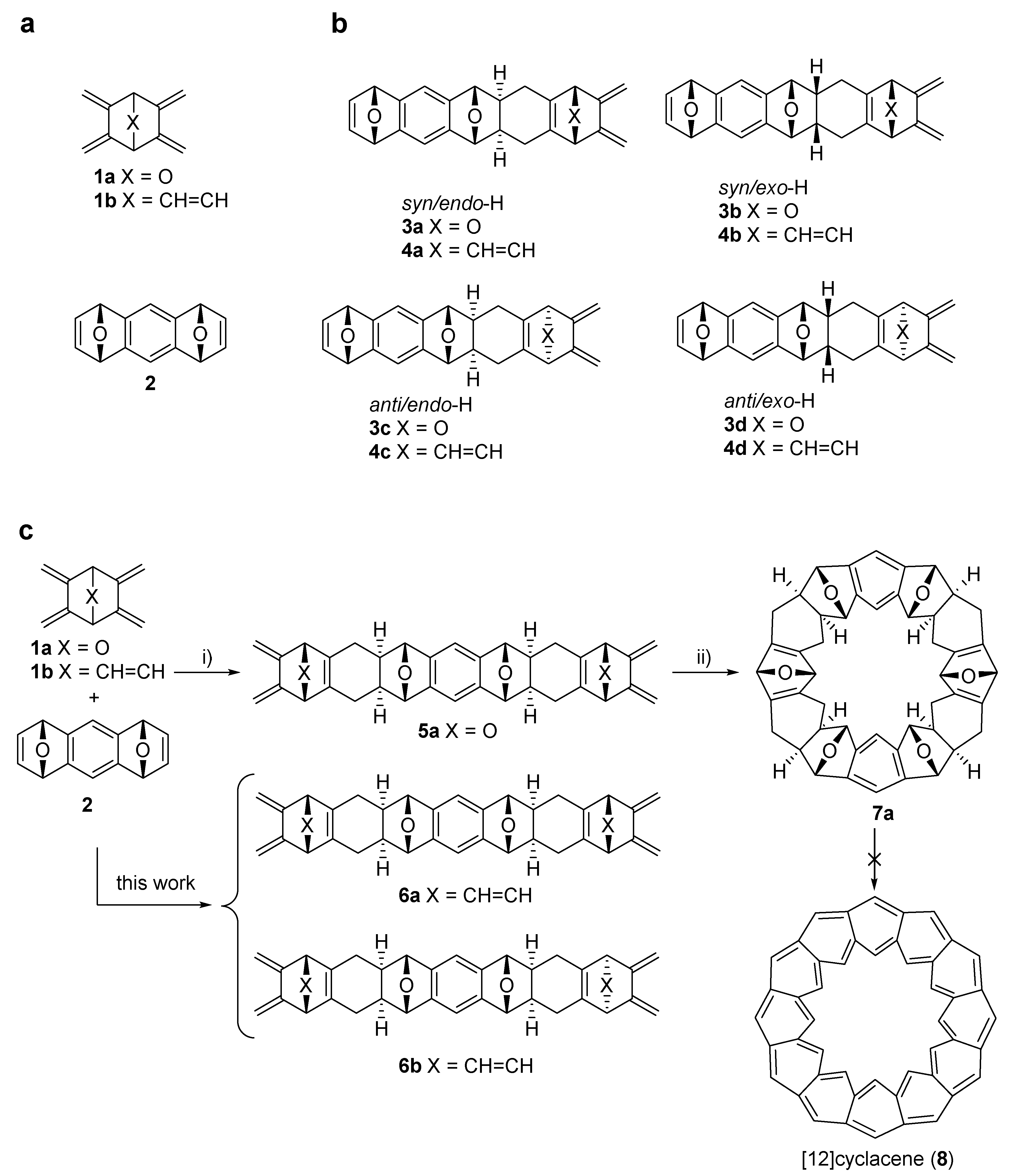
2. Results and Discussion
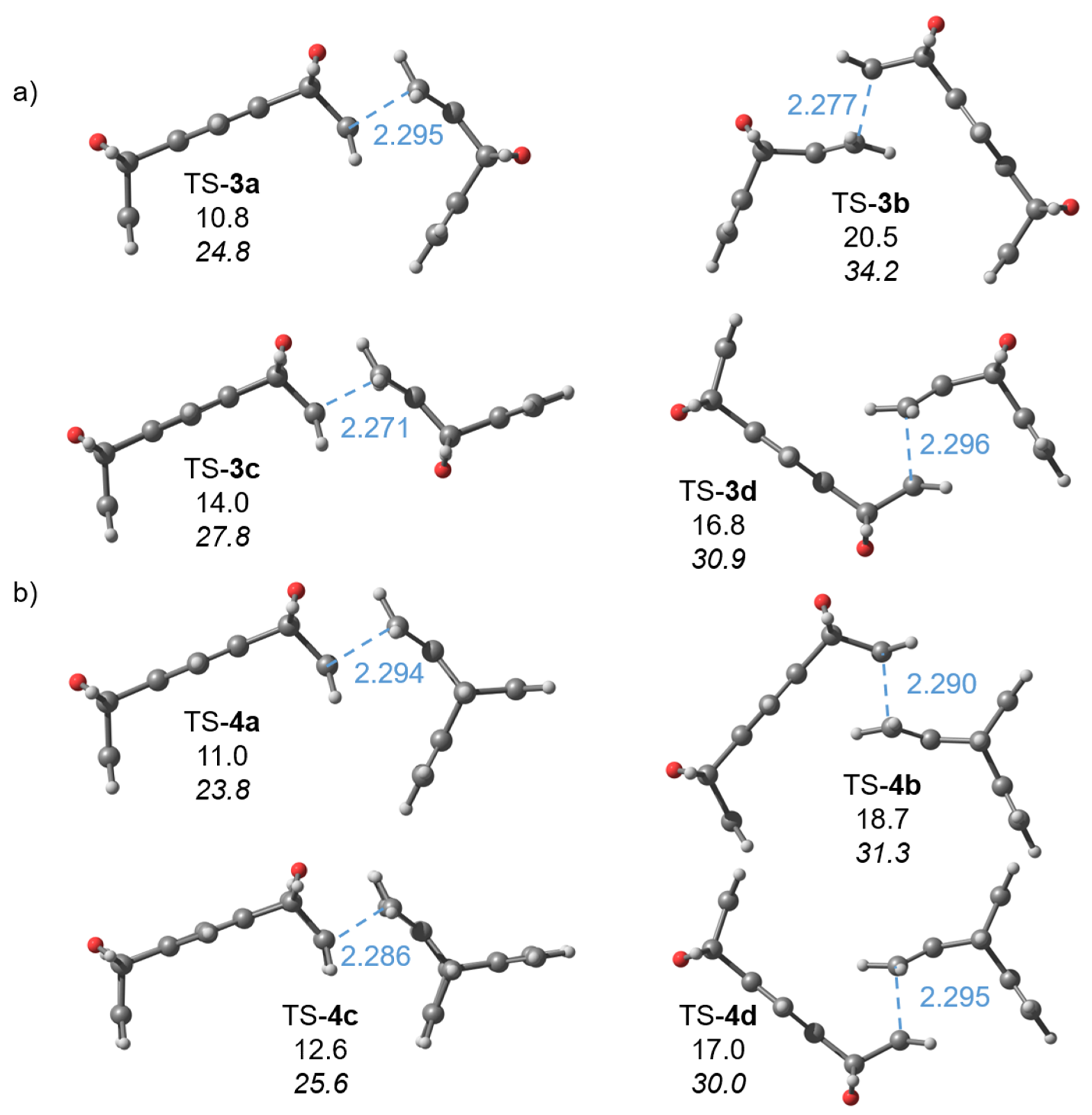
2.1. Computations
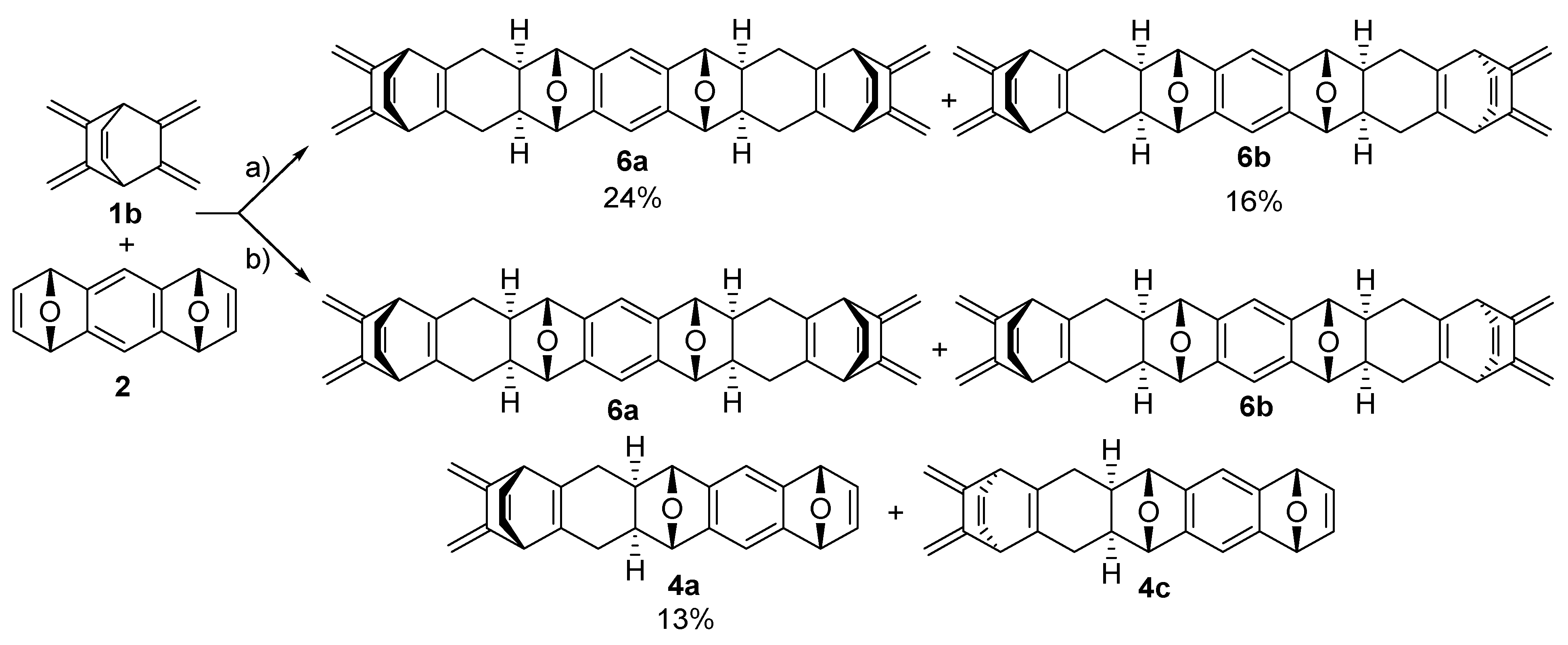
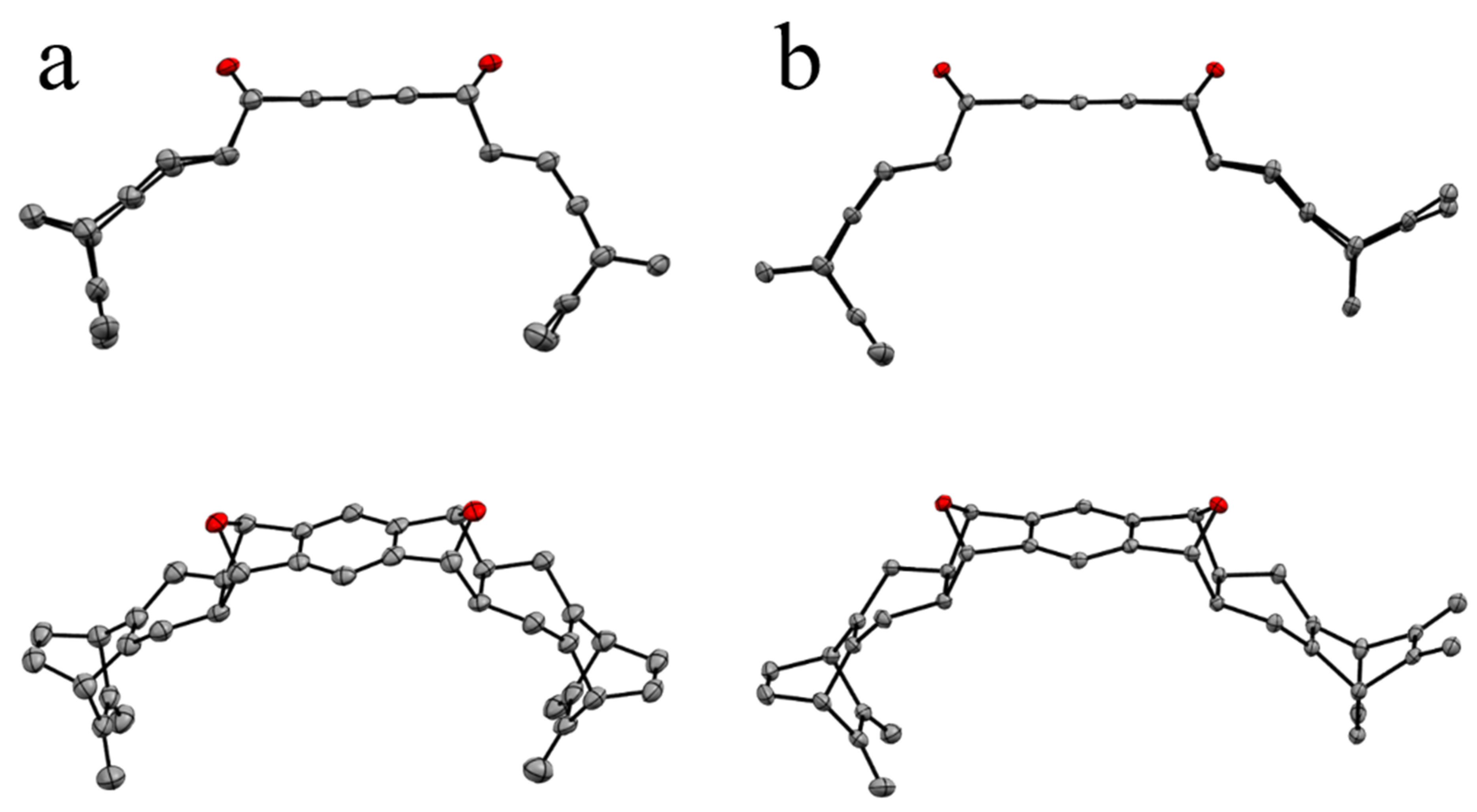
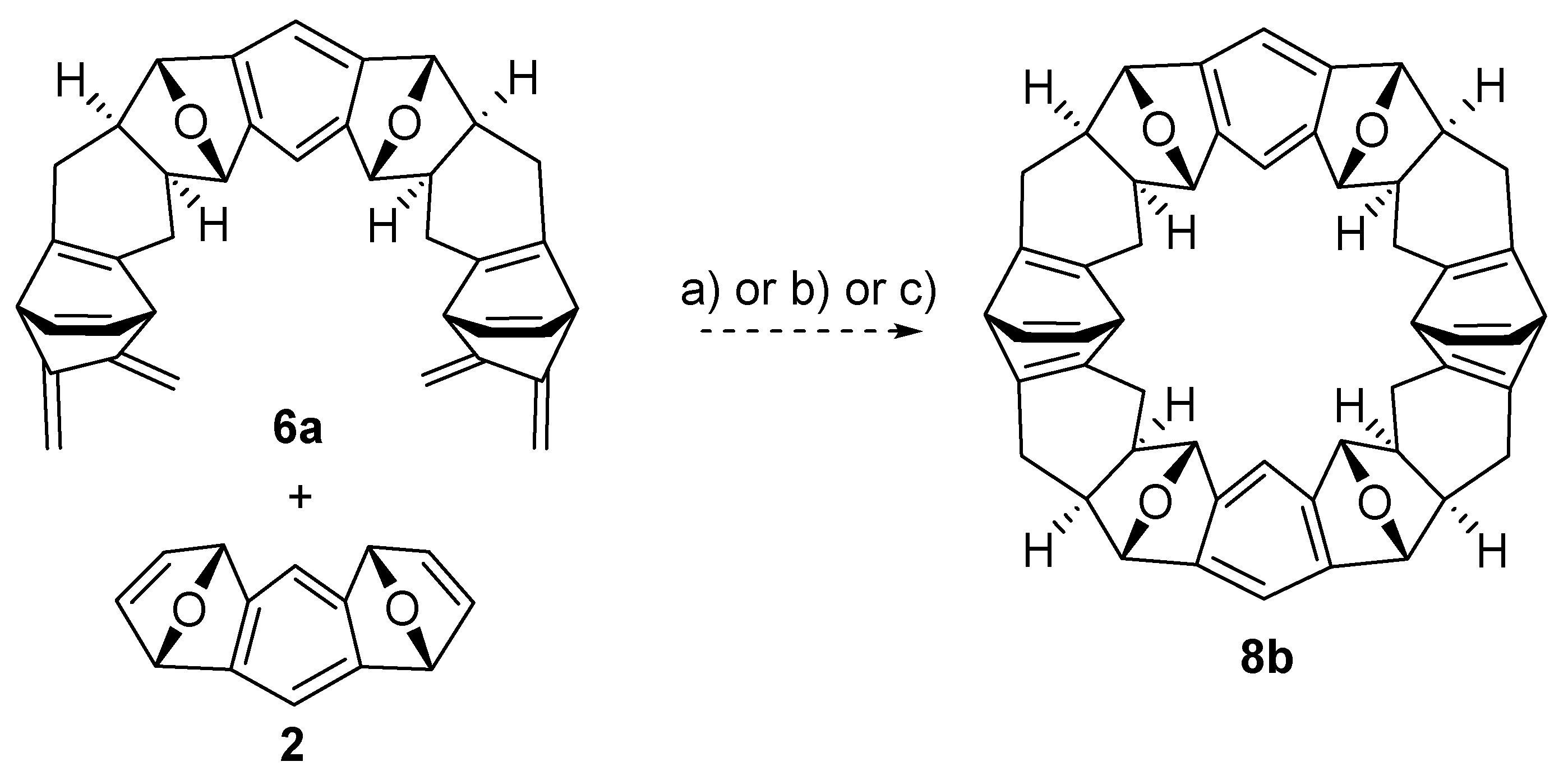
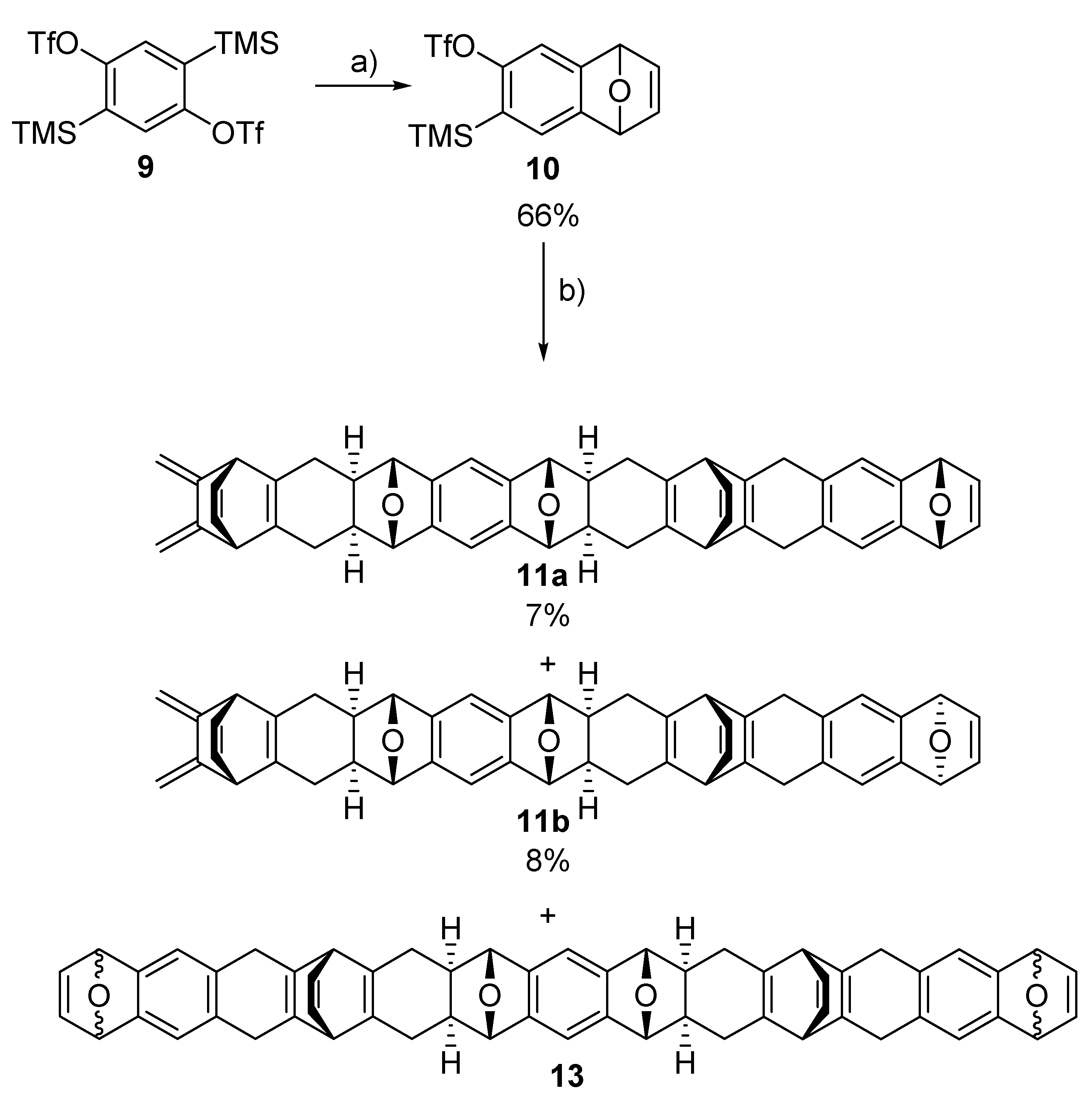
2.2. Synthesis
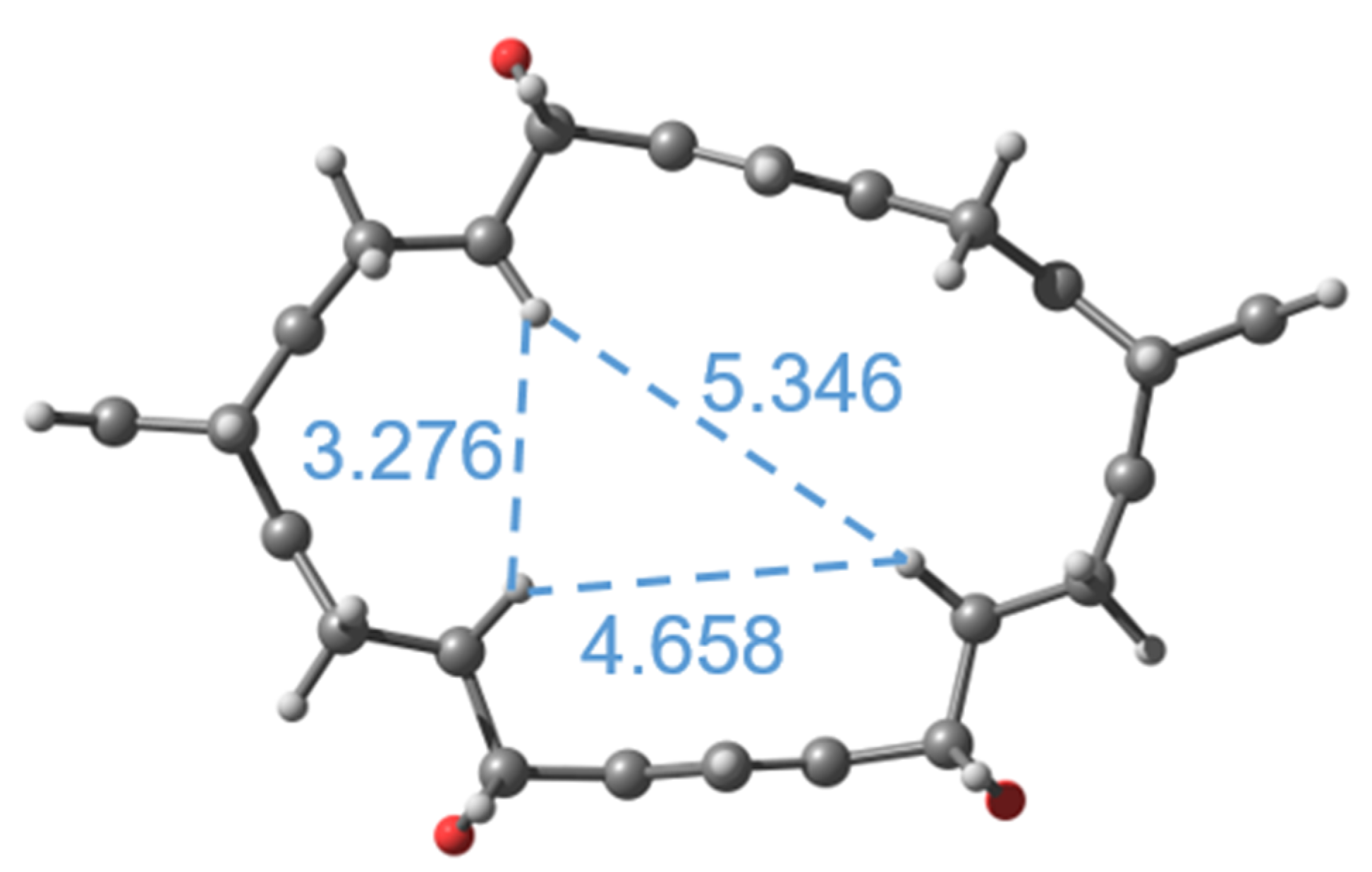
2.3. Properties of Macrocycle 12
3. Materials and Methods
3.1. General Methods
3.2. Synthetic Procedures
3.3. X-ray Crystallographic Data
3.4. Computational Chemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe. Liebigs Ann. Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe, III. Liebigs Ann. Chem. 1929, 470, 62–103. [Google Scholar] [CrossRef]
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe, IV. Ber. Dtsch. Chem. Ges. 1929, 62, 2081–2087. [Google Scholar] [CrossRef]
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe, V. Ber. Dtsch. Chem. Ges. 1929, 62, 2087–2090. [Google Scholar] [CrossRef]
- Ichihara, A. Retro-Diels–Alder strategy in natural product synthesis. Synthesis 1987, 1987, 207–222. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels–Alder reaction in total synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Ashton, P.R.; Brown, G.R.; Isaacs, N.S.; Giuffrida, D.; Kohnke, F.H.; Mathias, J.P.; Slawin, A.M.Z.; Smith, D.R.; Stoddart, J.F.; Williams, D.J. Molecular LEGO. 1. Substrate-directed synthesis via stereoregular Diels–Alder oligomerizations. J. Am. Chem. Soc. 1992, 114, 6330–6353. [Google Scholar] [CrossRef]
- Cory, R.M.; McPhail, C.L.; Dikmans, A.J.; Vittal, J.J. Macrocyclic cyclophane belts via double Diels–Alder cycloadditions: Macroannulation of bisdienes by bisdienophiles. Synthesis of a key precursor to an [8]cyclacene. Tetrahedron Lett. 1996, 37, 1983–1986. [Google Scholar] [CrossRef]
- Neudorff, W.D.; Lentz, D.; Anibarro, M.; Schlüter, A.D. The carbon skeleton of the belt region of fullerene C84 (D2). Chem. Eur. J. 2003, 9, 2745–2757. [Google Scholar] [CrossRef]
- Schulz, F.; García, F.; Kaiser, K.; Pérez, D.; Guitián, E.; Gross, L.; Peña, D. Exploring a route to cyclic acenes by on-surface synthesis. Angew. Chem. Int. Ed. 2019, 58, 9038–9042. [Google Scholar] [CrossRef]
- Ashton, P.R.; Isaacs, N.S.; Kohnke, F.H.; Slawin, A.M.Z.; Spencer, C.M.; Stoddart, J.F.; Williams, D.J. Towards the making of [12]collarene. Angew. Chem. Int. Ed. Engl. 1988, 27, 966–969. [Google Scholar] [CrossRef]
- Girreser, U.; Giuffrida, D.; Kohnke, F.H.; Mathias, J.P.; Philip, D.; Stoddart, J.F. The structure-directed synthesis of cyclacene and polyacene derivatives. Pure Appl. Chem. 1993, 65, 119–125. [Google Scholar] [CrossRef]
- Kohnke, F.H.; Mathias, J.P.; Stoddart, J.F. Structure-directed synthesis of new organic materials. Angew. Chem. Int. Ed. Engl. 1989, 28, 1103–1110. [Google Scholar] [CrossRef]
- Kohnke, F.H.; Slawin, A.M.Z.; Stoddart, J.F.; Williams, D.J. Molecular belts and collars in the making: A hexaepoxyoctacosahydro [12]cyclacene derivative. Angew. Chem. Int. Ed. Engl. 1987, 26, 892–894. [Google Scholar] [CrossRef]
- Kohnke, F.H.; Stoddart, J.F. The evolution of molecular belts and collars. Pure Appl. Chem. 1989, 61, 1581–1586. [Google Scholar] [CrossRef]
- Heilbronner, E. Molecular Orbitals in homologen Reihen mehrkerniger aromatischer Kohlenwasserstoffe: I. Die Eigenwerte von LCAO-MO’s in homologen Reihen. Helv. Chim. Acta 1954, 37, 921–935. [Google Scholar] [CrossRef]
- Gleiter, R.; Hellbach, B.; Gath, S.; Schaller, R.J. From superphanes to beltenes. Pure Appl. Chem. 2006, 78, 699–706. [Google Scholar] [CrossRef]
- Hellbach, B.; Rominger, F.; Gleiter, R. Synthesis of beltenes by reactions of 5,6,11,12-tetradehydrodibenzo[a,e]cyclooctene with [CpCo(CO)2] derivatives. Angew. Chem. Int. Ed. 2004, 43, 5846–5849. [Google Scholar] [CrossRef]
- Takaba, H.; Omachi, H.; Yamamoto, Y.; Bouffard, J.; Itami, K. Selective synthesis of [12]cycloparaphenylene. Angew. Chem. Int. Ed. 2009, 48, 6112–6116. [Google Scholar] [CrossRef]
- Stuparu, M.; Lentz, D.; Rüegger, H.; Schlüter, A.D. Exploring the chemistry of a double-stranded cycle with the carbon skeleton of the belt region of the C84 fullerene. Eur. J. Org. Chem. 2007, 2007, 88–100. [Google Scholar] [CrossRef]
- Cory, R.M.; McPhail, C.L. Transformations of a macrocyclic cyclophane belt into advanced [8]cyclacene and [8]cyclacene triquinone precursors. Tetrahedron Lett. 1996, 37, 1987–1990. [Google Scholar] [CrossRef]
- Chen, H.; Miao, Q. Recent advances and attempts in synthesis of conjugated nanobelts. Phys. Org. Chem. 2020, 33, 1–20. [Google Scholar] [CrossRef]
- Cheung, K.Y.; Segawa, Y.; Itami, K. Synthetic strategies of carbon nanobelts and related belt-shaped polycyclic aromatic hydrocarbons. Chem. Eur. J. 2020, 26, 14791–14801. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.-H.; Wang, M.-X. Zigzag hydrocarbon belts. CCS Chem. 2020, 2, 916–931. [Google Scholar] [CrossRef]
- Gleiter, R.; Esser, B.; Kornmayer, S.C. Cyclacenes: Hoop-shaped systems composed of conjugated rings. Acc. Chem. Res. 2009, 42, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Tobe, Y. Molecular loops and belts. Chem. Rev. 2006, 106, 5274–5290. [Google Scholar] [CrossRef]
- Gupta, D.; Omont, A.; Bettinger, H.F. Energetics of formation of cyclacenes from 2,3-didehydroacenes and implications for astrochemistry. Chem. Eur. J. 2021, 27, 4605–4616. [Google Scholar] [CrossRef]
- Sadowsky, D.; McNeill, K.; Cramer, C.J. Electronic structures of [n]-cyclacenes (n = 6–12) and short, hydrogen-capped, carbon nanotubes. Faraday Discuss. 2010, 145, 507–521. [Google Scholar] [CrossRef]
- Segawa, Y.; Yagi, A.; Ito, H.; Itami, K. A theoretical study on the strain energy of carbon nanobelts. Org. Lett. 2016, 18, 1430–1433. [Google Scholar] [CrossRef]
- Zuzak, R.; Dorel, R.; Kolmer, M.; Szymonski, M.; Godlewski, S.; Echavarren, A.M. Higher acenes by on-surface dehydrogenation: From heptacene to undecacene. Angew. Chem. Int. Ed. 2018, 57, 10500–10505. [Google Scholar] [CrossRef]
- Krüger, J.; García, F.; Eisenhut, F.; Skidin, D.; Alonso, J.M.; Guitián, E.; Pérez, D.; Cuniberti, G.; Moresco, F.; Peña, D. Decacene: On-surface generation. Angew. Chem. Int. Ed. 2017, 56, 11945–11948. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.; Eisenhut, F.; Skidin, D.; Lehmann, T.; Ryndyk, D.A.; Cuniberti, G.; García, F.; Alonso, J.M.; Guitián, E.; Pérez, D.; et al. Electronic Resonances and Gap Stabilization of Higher Acenes on a Gold Surface. ACS Nano 2018, 12, 8506–8511. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, F.; Kühne, T.; García, F.; Fernández, S.; Guitián, E.; Pérez, D.; Trinquier, G.; Cuniberti, G.; Joachim, C.; Peña, D.; et al. Dodecacene generated on surface: Reopening of the energy gap. ACS Nano 2020, 14, 1011–1017. [Google Scholar] [CrossRef]
- Urgel, J.I.; Mishra, S.; Hayashi, H.; Wilhelm, J.; Pignedoli, C.A.; Di Giovannantonio, M.; Widmer, R.; Yamashita, M.; Hieda, N.; Ruffieux, P.; et al. On-surface light-induced generation of higher acenes and elucidation of their open-shell character. Nat. Commun. 2019, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Tönshoff, C.; Bettinger, H.F. Pushing the limits of acene chemistry: The recent surge of large acenes. Chem. Eur. J. 2021, 27, 3193–3212. [Google Scholar] [CrossRef]
- Geiger, T.; Schundelmeier, S.; Hummel, T.; Ströbele, M.; Leis, W.; Seitz, M.; Zeiser, C.; Moretti, L.; Maiuri, M.; Cerullo, G.; et al. Modulating the electronic and solid-state structure of organic semiconductors by site-specific substitution: The case of tetrafluoropentacenes. Chem. Eur. J. 2020, 26, 3420–3434. [Google Scholar] [CrossRef]
- Wegener, S.; Müllen, K. 5,6,7,8-Tetramethylenebicyclo [2.2.2]oct-2-ene as “Bis(diene)” in repetitive Diels–Alder reactions. Chem. Ber. 1991, 124, 2101–2103. [Google Scholar] [CrossRef]
- Wegener, S.; Müllen, K. New ladder polymers via repetitive Diels–Alder reaction under high pressure. Macromolecules 1993, 26, 3037–3040. [Google Scholar] [CrossRef]
- Gabioud, R.; Vogel, P. Synthesis and Diels–Alder reactivity of 5,6,7,8-tetramethylidene-2-bicyclo [2.2.2]octanol and -octanone. Selective oxidations of the corresponding bis(irontricarbonyl) complexes. Helv. Chim. Acta 1983, 66, 1134–1147. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Krüger, J.; Eisenhut, F.; Alonso, J.M.; Lehmann, T.; Guitian, E.; Perez, D.; Skidin, D.; Gamaleja, F.; Ryndyk, D.A.; Joachim, C.; et al. Imaging the electronic structure of on-surface generated hexacene. Chem. Commun. 2017, 53, 1583–1586. [Google Scholar] [CrossRef] [PubMed]
- Bruker AXS Inc. COSMO v. 1.61; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Bruker AXS Inc. APEX 2 v. 2012.10_0; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Bruker AXS Inc. SAINT v. 8.34A; Bruker AXS Inc.: Madison: WI, USA, 2013. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bruker AXS Inc. APEX 3 V. 2017.3-0; Bruker AXS Inc.: Madison, WI, USA, 2017. [Google Scholar]
- Bruker AXS Inc. SAINT v. 8.38A; Bruker AXS Inc.: Madison, WI, USA, 2017. [Google Scholar]
- Sheldrick, G. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian: Wallingford, CT, USA, 2016. [Google Scholar]

| No. | 11a | 12 | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| 1 | 101.2, CH2 | Ha: 4.70, Hb: 4.86 | 31.16a, CH2 or 31.19a, CH2 | Ha/Hb: 2.57–2.48/2.20–2.04 |
| 2 | 144.4, C | - | 147.5, C or 147.4, C | - |
| 3 | 53.6, CH | 3.88–3.86 | 57.0, CH | 4.14–4.12 |
| 4 | 133.8, CH | 6.39–6.37 | 139.8, CH | 6.75–6.73 |
| 5 | 137.5, C | - | 147.5, C or 147.4, C | - |
| 6 | 30.7, CH2 | Ha/Hb: 2.60–2.55/2.18–2.12 | 31.16a, CH2 or 31.19a, CH2 | Ha/Hb: 2.57–2.48/2.20–2.04 |
| 7 | 43.1, CH | 1.36–1.31 or 1.31–1.25 or 1.25–1.20 | 46.3, CH or 45.8, CH | 1.36–1.31 or 1.31–1.25 or 1.25–1.20 |
| 8 | 85.19 a, CH or 85.24 a, CH | 4.87 | 84.3, CH or 84.0, CH | 4.79 |
| 9 | 144.8, C or 144.7, C | - | 145.2, C or 145.1, C | - |
| 10 | 110.2, CH | 6.95 | 110.0, CH | 6.85 |
| 11 | 144.8, C or 144.7, C | - | 145.2, C or 145.1, C | - |
| 12 | 85.19 a, CH or 85.24 a, CH | 4.85 | 84.3, CH or 84.0, CH | 4.82 |
| 13 | 42.9, CH | 1.77–1.71 | 47.2, CH | 1.36–1.31 or 1.31–1.25 or 1.25–1.20 |
| 14 | 31.4, CH2 | Ha/Hb: 2.70–2.65/2.12–2.06 | 31.7, CH2 | Ha/Hb: 2.57–2.48/2.23–2.17 |
| 15 | 143.8, C | - | 147.8, C | - |
| 16 | 55.3, CH | 4.15–4.13 | 56.3, CH | 4.23–4.21 |
| 17 | 139.5, CH | 6.77–6.75 | 139.0, CH | 6.82–6.80 |
| 18 | 140.8, C | - | 143.3, C | - |
| 19 | 33.7, CH2 | Ha, Hb: 3.49–3.33 | 34.3, CH2 | Ha/Hb: 3.53–3.46/3.26–3.19 |
| 20 | 130.7, C | - | 133.5, C | - |
| 21 | 120.9, CH | 6.93 | 118.8, CH | 6.77 |
| 22 | 146.6, C | - | 143.7, C | - |
| 23 | 82.2, CH | 5.61 | 84.1, CH | 4.81 |
| 24 | 143.0, CH | 6.91 | 46.3, CH or 45.8, CH | 1.36–1.31 or 1.31–1.25 or 1.25–1.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, J.B.; Diab, F.; Maichle-Mössmer, C.; Schubert, H.; Bettinger, H.F. Synthesis of the [11]Cyclacene Framework by Repetitive Diels–Alder Cycloadditions. Molecules 2021, 26, 3047. https://doi.org/10.3390/molecules26103047
Bauer JB, Diab F, Maichle-Mössmer C, Schubert H, Bettinger HF. Synthesis of the [11]Cyclacene Framework by Repetitive Diels–Alder Cycloadditions. Molecules. 2021; 26(10):3047. https://doi.org/10.3390/molecules26103047
Chicago/Turabian StyleBauer, John B., Fatima Diab, Cäcilia Maichle-Mössmer, Hartmut Schubert, and Holger F. Bettinger. 2021. "Synthesis of the [11]Cyclacene Framework by Repetitive Diels–Alder Cycloadditions" Molecules 26, no. 10: 3047. https://doi.org/10.3390/molecules26103047
APA StyleBauer, J. B., Diab, F., Maichle-Mössmer, C., Schubert, H., & Bettinger, H. F. (2021). Synthesis of the [11]Cyclacene Framework by Repetitive Diels–Alder Cycloadditions. Molecules, 26(10), 3047. https://doi.org/10.3390/molecules26103047