Abstract
Diabetes mellitus (DM) is a chronic disorder and has affected a large number of people worldwide. Insufficient insulin production causes an increase in blood glucose level that results in DM. To lower the blood glucose level, various drugs are employed that block the activity of the α-glucosidase enzyme, which is considered responsible for the breakdown of polysaccharides into monosaccharides leading to an increase in the intestinal blood glucose level. We have synthesized novel 2-(3-(benzoyl/4-bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-arylacetamides and have screened them for their in silico and in vitro α-glucosidase inhibition activity. The derivatives 11c, 12a, 12d, 12e, and 12g emerged as potent inhibitors of the α-glucosidase enzyme. These compounds exhibited good docking scores and excellent binding interactions with the selected residues (Asp203, Asp542, Asp327, His600, Arg526) during in silico screening. Similarly, these compounds also showed good in vitro α-glucosidase inhibitions with IC50 values of 30.65, 18.25, 20.76, 35.14, and 24.24 μM, respectively, which were better than the standard drug, acarbose (IC50 = 58.8 μM). Furthermore, a good agreement was observed between in silico and in vitro modes of study.
1. Introduction
Diabetes mellitus (DM) is an endocrine system illness and causes metabolic ailment that leads to hyperglycemia. DM is characterized by increased blood sugar levels either due to the body’s reduced production of insulin or ineffective utilization of available insulin by the body. Chronic hyperglycemia results in neuron oxidative damage while prolonged hyperglycemia causes the production of lower caloric content of sugar in different organs [1,2]. Although genetic predisposition can be a factor in the development of DM, other major causing factors may include sedentariness, smoking, and intake of high-saturated fatty meals [3,4]. Change in lifestyle can decrease the risk of type 2 diabetes by 58%, for example, overweight people can avoid it by losing weight, a fact explained experimentally by the Finnish prevention trial [5] and the US diabetes prevention program [6]. DM also enhances the chances of cardiovascular diseases [7]. People suffering with hypertension are already leading a risky life due to high blood pressure [8] but they often develop DM and this combination of risks increases cardiovascular transience. The rate of DM is growing rapidly worldwide especially in developing countries [9]. The International Diabetes Federation (IDF) has observed that 371 million humans are infected with T2DM (type 2 diabetes mellitus) while an increase rate of 2% annually has been estimated. α-Amylase and α-glucosidase are the two enzymes that have a key role in the breakdown of polysaccharides to monosaccharides [10]. α-Glucosidase enzyme, released from mucosal cells, usually converts polysaccharides into monosaccharides causing a gradual increase in the absorption of glucose and hence increases intestinal blood glucose level [11]. These enzymes have role in breakdown and absorption of polysaccharides, so by inhibiting the activity of these enzymes, glucose levels in diabetic patients can be controlled even after consumption of carbohydrate-rich diets [12].
Different α-glucosidase and α-amylase inhibitors are being used for delaying glucose absorption in the blood, such as acarbose, voglibose, miglitol, etc., but this medication has many side effects [13,14,15,16,17], such as diarrhea, gastrointestinal distress (flatulence, pain), and liver disorders (hepatic injury, hepatitis, and renal tumors) [18,19,20,21,22]. Hence, there is a need in the present era to develop more potent antidiabetic agents with minimum side effects.
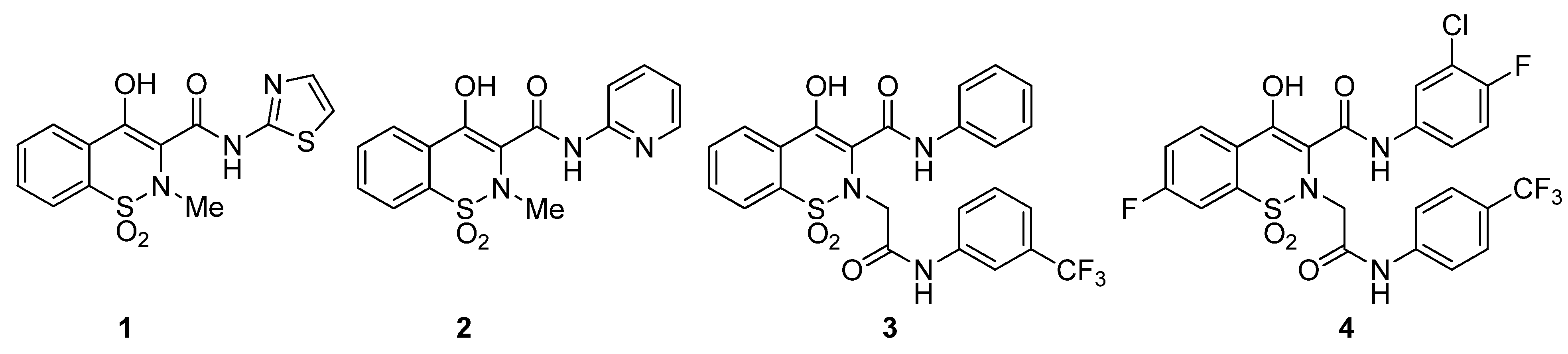
Benzothiazines are well-known heterocycles due to their wide spectrum of pharmacological activities. Among the various types of benzothiazines, 1,2-benzothiazine 1,1-dioxides and their derivatives have broadly been studied. Prominently, 1,2-benzothiazine 1,1-dioxide carboxamides have a major footprint in clinical therapy due to their anti-inflammatory activity. As revealed by the literature, sudoxicam 1 (Figure 1) is an important oxicam having an extended plasma half-life [23], piroxicam 2 (Figure 1), another potent oxicam, is used to cure rheumatoid arthritis, osteoarthritis, and other inflammations [24]. Similarly, various 1,2-benzothiazine 1,1-dioxides have been reported as antileukemic [25,26,27], 11β-HSD1 inhibitors [28], endotheline receptor antagonists [29], antimalarial agents [30], MAO inhibitors [31], antiallergic agents [32], antithrombotics [33], antiparasitic [34], antimicrobials [35,36], antioxidants [37], inhibitors of calpain I [38], and aldose reductase enzymes [39,40].
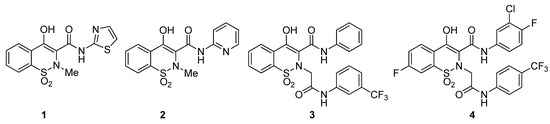
Figure 1.
Structures of biologically important 1,2-benzothiazine 1,1-dioxides.
Our research group is involved in the search for novel and more effective antidiabetic agents [41,42,43,44,45], and in this context we have synthesized novel 1,2-benzothiazine-N-arylacetamides and screened them for their in silico and in vitro α-glucosidase inhibitory potential.
2. Results and Discussion
2.1. Synthetic Chemistry
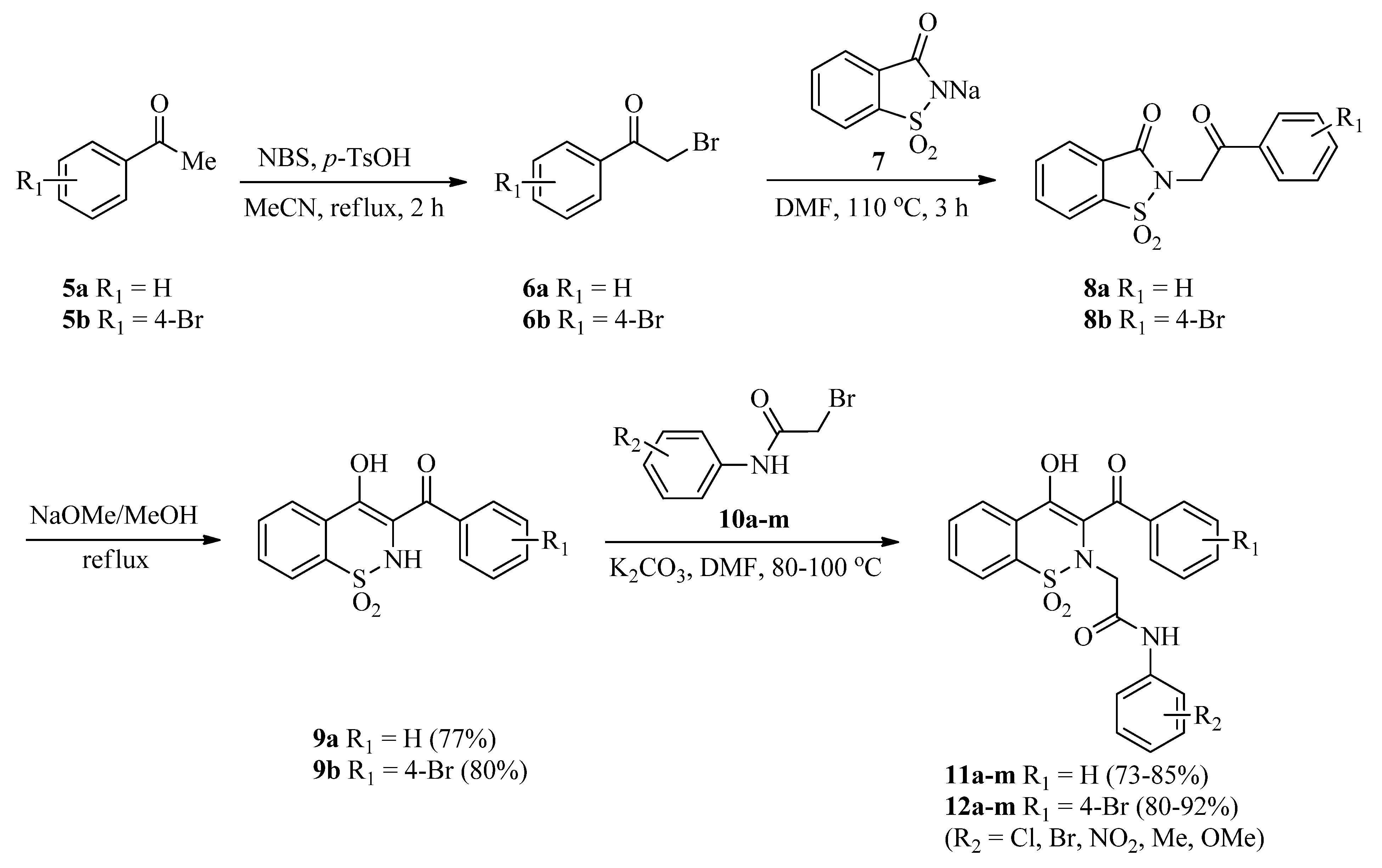
The precursors, 3-(benzoyl/4-bromobenzoyl)-1,2-benzothazines 9a,b, were synthesized via reported methods involving Gabriel–Colman rearrangement [46,47]. Later on, 3-(benzoyl/4-bromobenzoyl)-1,2-benzothazines 9a,b were treated with a library of 2-bromo-N-arylacetamides 10a–m (synthesized as described previously [48]) in the presence of a potassium carbonate base in a DMF solvent. Reactions were completed in 2–2.5 h (as indicated by TLC) and then dilution with distilled water followed by acidification with 20% HCl afforded the targeted 1,2-benzothiazine-N-arylacetamides 11a–m and 12a–m good to excellent yields (73–92%) (Scheme 1). Increase in reaction time was not helpful regarding the yields of products. Similarly, use of low boiling solvents like acetonitrile also resulted in good yields but in a longer reaction time (4 h).
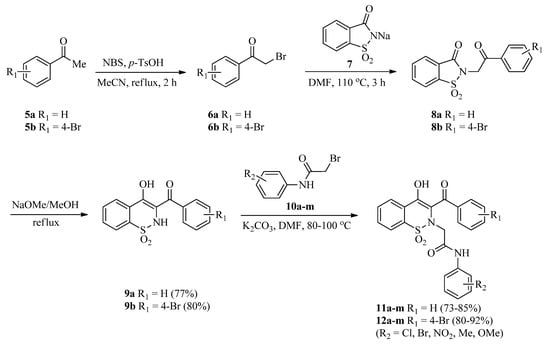
Scheme 1.
Synthesis of 1,2-benzothiazine-N-arylacetamides 11a–m and 12a–m.
2.2. Spectroscopic Characterization
All the synthesized derivatives were well characterized with 1H-NMR, 13C-NMR, and MS spectroscopic techniques. In the 1H-NMR spectra, the methylene (CH2) protons appeared as a singlet at 3.95–4.20 ppm and the observation was consistent with literature values of similar compounds [46,49]. The methyl protons (CH3) and methoxy protons appeared as singlets at 1.96–2.17 and 3.64–3.86 ppm, respectively. The NH and enolic–OH protons were observed as singlets at 9.16–10.54 and 14.92–15.18 ppm, respectively [37,49,50]. Peaks for enolic–OH protons were not observed in some spectra due to the exchangeable nature of OH protons [49]. All the peak values from 6.50–8.50 ppm were assigned to aromatic protons. In MS spectra, the observed molecular ion peaks were found in accordance with the calculated values.
2.3. Biological Activity
Diabetes mellitus is a severe chronic metabolic syndrome that is illustrated by hyperglycemia and escorted by different severe vascular complications. The main therapy of diabetes is to control the postprandial blood-glucose level, however, limited uptake of carbohydrates after meals is also considered as a therapy. α-Amylase and α-glucosidase enzymes are responsible for the breakdown of polysaccharides to monosaccharides. Consequently, inhibition of these enzymes leads to treatment postprandial hyperglycemia by interrupting glucose absorption levels. As described above, the 1,2-benzothiazine 1,1-dioxides are valuable heterocycles and possess various biological activities. In particular, 1,2-benzothiazine 1,1-dioxide based carboxamides are the most biologically active templates. In 2011, Kim and colleagues synthesized different 1,2-benzothiazine-N-arylacetamides and screened them for their in vitro inhibition toward human 11β-HSD1. The derivative 3 (Figure 1) showed the most promising activity with an IC50 value of 0.041 μM [49]. Recently, Shin and coworkers reported novel 1,2-benzothiazine-N-arylacetamides as SARS-CoV-2 inhibitors and among them compound 4 (Figure 1) showed robust activity with an IC50 value of 0.88 μM [51]. Furthermore, 2-(2-(4-bromo-2-fluorobenzyl)-1,1-dioxido-2H-benzo[e][1,2]thiazin-4(3H)-ylidene)acetic acid was proved to be a good aldose-reductase inhibitor with an IC50 value of 0.11 μM [39]. Similarly, 4-hydroxy-2-methyl-N’-(1-phenylethylidene)-2H-benzo[e][1,2]thiazine-3-carbohydrazide 1,1-dioxide and 4-hydroxy-N’-(1-phenylethylidene)-2H-benzo[e][1,2]thiazine-3-carbohydrazide 1,1-dioxide showed excellent inhibitions of α-glucosidase enzymes with IC50 values of 4.2 and 3.9, respectively [41,45]. Encouraged by these results and to explore further biological potentials, the present study highlighted the antidiabetic activity of novel 1,2-benzothiazine-N-arylacetamides 11a–m and 12a–m.
2.3.1. Molecular Docking (In Silico Analysis)
In silico screening is an excellent approach for the screening of libraries of compounds in a short time and thus it minimizes the laborious synthetic work [52,53,54,55]. Molecular docking is a widely used method to predict binding interactions between the 3D conformations of various ligands and receptor proteins that helps in optimization and leads toward drug development [56,57,58].
Regarding the effectiveness of this tool, we executed molecular docking studies of all the synthesized 1,2-benzothiazine derivatives. Molecular docking was performed via MOE software to screen out the compounds having the best residue binding interactions with the receptor protein (α-glucosidase). These compounds were ranked on the basis of root-mean-square deviation (rmsd) values, bindings with the selected residues (Asp203, Asp542, Asp327, His600, Arg526) (Figure S1), and docking scores [59].
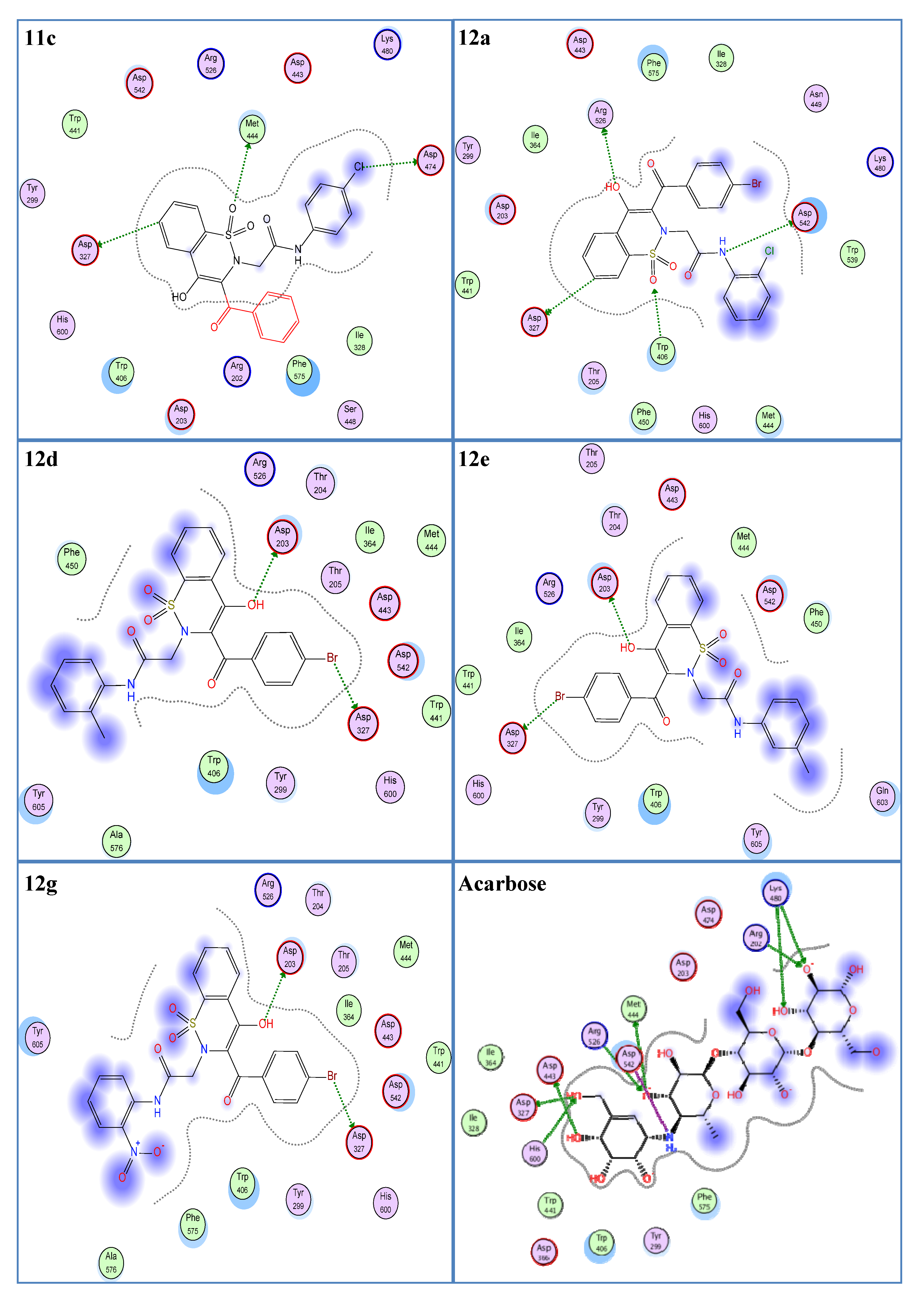
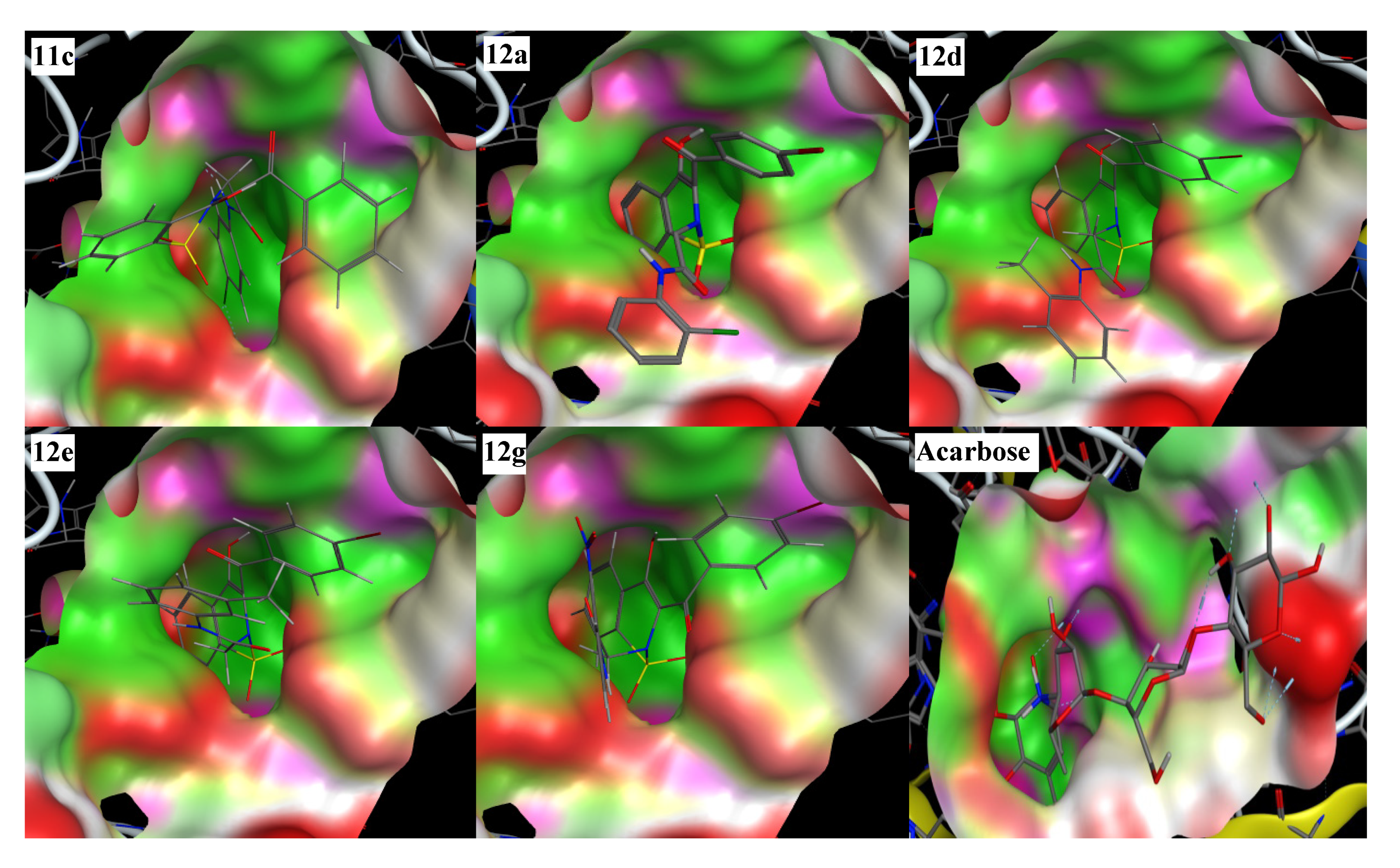
In the molecular docking experiment, most of the compounds showed good binding scores and binding interactions with the selected residues (Asp203, Asp542, Asp327, His600, Arg526) (Table 1, Figure 2). In silico studies of salacinol having a cyclic thiosugar sulfonium and a sulfate group in its structure and its derivatives found it blocked the α-glucosidase activity via bindings with the Asp203, Asp327, and Asp542 residues [60]. Recently, in silico analysis of anthocyanidins and anthocyanins was carried out that showed these scaffolds to be a good inhibitor of α-glucosidase enzymes. These compounds also showed their inhibitory action by interacting with the selected residues, Asp203, Asp327, and Asp542 [61]. Similarly, aglycone of curculigoside A and its derivatives also exhibited good in silico inhibition of α-glucosidase enzymes via interactions with Asp203, Asp327, and Asp542 residues [62]. The ligands 12a, 12d, 12e, and 12g having docking scores from −13.47 to −14.23 Kcal/mol were ranked the most effective antidiabetic scaffolds. Moreover, compounds 11c, 11h, 11i, and 12m were also considered important due to good binding energy values in the range of −12.13 to −12.80 Kcal/mol. These above-described ligands showed binding energy values close to the standard drug, acarbose (−16.18 Kcal/mol) and were tightly packed in the selected pocket of the receptor enzyme as shown in 3D maps (Figure 3). The 2D interaction modes revealed that most of the ligands bind with Asp203, Asp327, and Asp542 among the selected residues and were supposed to be responsible for the α-glucosidase inhibitory activity (Figure 2, Table S1). Acarbose (control) also showed good interactions with the selected residues, His600, Asp542, Arg526, and Asp327, in the re-docking experiment. The docking score and rmsd value for acarbose were found as −16.18 Kcal/mol and 2.00 Å, respectively.

Table 1.
Structural parameters, in silico (docking scores, binding residues, and rmsd values) and in vitro (IC50 values) inhibitory potential of 1,2-benzothiazines 9a,b, 1,2-benzothiazine-N-arylacetamides 11a–m, and 12a–m against α-glucosidase enzymes.
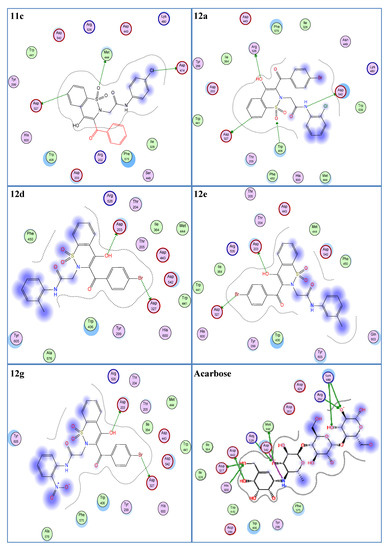
Figure 2.
Two-dimensional interaction modes of the most active compounds (11c, 12a, 12d, 12e, 12g) and the standard drug, acarbose. Blue color indicates ligand exposure and a green color dotted line indicates the interactions of receptor enzymes with ligands.
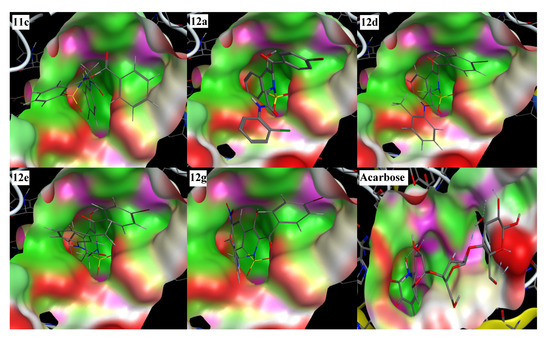
Figure 3.
Three-dimensional interaction modes of the most active compounds (11c, 12a, 12d, 12e, 12g) and the standard drug, acarbose. Color portions show pocket selected and red portions indicate the ligand exposure points.
As a whole, 1,2-Benzothiazine-N-arylacetamides 11a–m were observed to be less effective compared to derivatives 12a–m regarding their in silico α-glucosidase inhibitory potential (Table 1). However, compounds 11c, 11h, and 11i showed comparatively better in silico enzyme inhibitions as revealed from their low binding energies and rmsd values (Table 1). The compound 11c was observed as the most effective derivative in terms of binding energy and rmsd value, which were found to be −12.80 Kcal/mol and 1.08 Å, respectively. This ligand showed interactions with Asp327, Asp474, and Met444 residues. Asp327 interacted with the benzene ring of the benzothiazine nucleus. Asp474 formed a hydrogen bond with the chloro and Met444 with the oxygen atom of the SO2 group. The ligand 11h exhibited a good docking score (−12.13 Kcal/mol) and low rmsd value (1.21 Å). A hydrogen bonding was observed between the NH group and the Asp542 residue while Thr205 interacted with the oxygen of the CO group. Similarly, a low docking score (−12.25 Kcal/mol) and rmsd value (1.11 Å) were found for the compound 11i. This is explained by the strong hydrogen bonding of this compound with the selected residue, Asp542, via NH functionality.
1,2-Benzothiazine-N-arylacetamides 12a–m preferentially exhibited good binding energies and better interactions with the selected residues compared to 1,2-benzothiazine-N-arylacetamides 11a–m (Table 1). Compound 12a was observed as the most potent compound as it showed a low docking score (−14.23 Kcal/mol), small rmsd value (1.21 Å), and good interactions with selected residues (Arg526, Asp542, Asp327). This ligand exhibited a hydrogen bond with Arg526 via the oxygen atom of the OH group. A second hydrogen interaction was found between the nitrogen atom of the NH group and the Asp542 residue. Similarly, Asp327 interacted with the benzene ring of the benzothiazine nucleus of ligand 12a. In addition, the present ligand also formed an interaction with Trp406 using the oxygen atom of the SO2 group. These interactions and smaller rmsd value are responsible for the low binding energy value of ligand 12a. The compounds 12d, 12e, and 12g also showed good docking scores (−13.91, −13.47, and −13.52 Kcal/mol, respectively) due to dipole–dipole interactions with Asp203 and Asp327 via their OH and Br functionalities, respectively (Figure 2). These ligands (12d, 12e, and 12g) also exhibited low rmsd values of 1.13, 1.27, and 1.01 Å, respectively. In the ligand 12m, the bromo group showed an interaction with the selected residue, Asp327. Due to this interaction and a low rmsd value of 1.19 Å, ligand 12m showed a good binding energy value of −12.32 Kcal/mol.
The compound 12k was found somewhat less effective as an inhibitor as revealed from its docking score and rmsd value (Table 1). The compound 12k showed interactions with Asp327 and Tyr605 via both the bromo groups individually. The other members of this series showed high rmsd values and no interactions with the selected residues. In addition, the 1,2-benzothiazines 9a,b were also docked into the receptor pocket but these proved to be noninhibitors of the α-glucosidase enzyme (Table 1 and Table S1).
It is concluded from the above discussion that not only the benzothiazine ring system but also the presence of the acetamide group was found indispensable for the in silico inhibition of α-glucosidase enzymes. Furthermore, it was noticed that the presence of polar as well as nonpolar groups in the structures of scaffolds played a crucial role toward the inhibition of enzymes by interacting with the selected residue. It is clear from the 2D diagrams of molecules that the interactions with the selected residues groups, such as CO, CONH, NH, SO2, Cl, Br, OH, phenyl, and aryl, all participated equally. Compounds with low rmsd values having good binding energies and interactions with the selected residues were of our interest.
2.3.2. α-Glucosidase Inhibition (In Vitro Analysis)
In order to assess the in silico results, all the compounds were also subjected to the in vitro inhibition of α-glucosidase enzymes, which was investigated using the p-nitrophenyl-α-d-glucopyranoside (PNPG) substrate. Acarbose was employed as the reference drug. In vitro analysis of all the synthesized compounds was performed in order to justify the validity of in silico outcomes. Results were found in good agreement with the in silico study. Initially, percentage inhibition values were determined and then the compounds having 50% inhibition values or above were subjected to the micro dilution experiment (Table 1). As previously stated, 1,2-benzothiazine-N-arylacetamides 12a–m were witnessed as the most effective antidiabetic agents during the in silico analysis. Herein, during in vitro screening these derivatives were also proved effective α-glucosidase inhibitors compared to 1,2-benzothiazine-N-arylacetamides 11a–m. Among all the compounds, 11c, 12a, 12d, 12e, and 12g showed the most promising α-glucosidase inhibition with IC50 values of 30.65, 18.25, 20.76, 35.14, and 24.24 μM, respectively (Table 1). These inhibitors have IC50 values even less than the standard drug, acarbose (58.8 μM [42,43]). These compounds showed more potent α-glucosidase inhibitions compared to the previously reported sulfonamides [63], dihydroxy pyrrolidines [64], and thiobarbituric acid enamine derivatives [65]. The compounds 11h, 11i, and 12m having IC50 values 47.13, 40.41, and 58.45 μM, respectively, were witnessed as the moderate antidiabetic agents while other derivatives failed to show any promising inhibitory effects against α-glucosidase enzymes and were categorized as weak inhibitors or in-active compounds.
Derivatives 11a–m with 3-benzoyl functionality exhibited low antidiabetic activity compared to derivatives 12a–m, which was also observed during in silico studies. In this series, the presence of deactivating groups (like Cl and NO2) in the structure resulted in better enzyme inhibitions. However, herein, the presence of EWGs at the para-position of the N-phenyl ring was proved more effective compared to other sites as indicated by the IC50 values of compounds 11c (30.65 μM), 11h (47.13 μM), and 11i (40.41 μM). Similarly, for the derivatives 12a–m, the presence of electron withdrawing groups (weak or strong) and weak electron donating groups (like CH3) at the ortho-position of the N-phenyl group resulted in better antidiabetic activity as indicated by low IC50 values of derivatives 12a (18.25 μM), 12d (20.76 μM), and 12g (24.24 μM). The derivatives 12a and 12d were found to be roughly three-fold more effective as inhibitors of α-glucosidase enzymes compared to acarbose. The increase in bioactivity with the introduction of EWGs in the benzoyl and N-phenyl moieties was also witnessed during the evaluation of previously reported 1,2-benzothiazine-N-arylacetamides as SARS-CoV-2 inhibitors [51]. In the series 12a–m, the variation in the position of nitro, chloro, and bromo substituents failed to enhance the enzymatic inhibition potential of the compounds.
3. Materials and Methods
3.1. General
Different instruments were used for the identification of structural features of synthesized compounds. For the determination of melting points, Stuart apparatus (Open capillary SMP3) was employed. 1H and 13C-NMR spectra were recorded with the help of an NMR-spectrophotometer (Bruker/Hazimin) at 600 MHz using a deuterated DMSO solvent. Chemical shift values (δ) were given in ppm (parts per million) relative to tetramethylsilane (TMS) as an internal standard. Coupling constants (J) were given in Hertz. For the progress of chemical reactions, silica-coated TLC-plates (Merk, 0.2 mm 60 HF254) were used. Spots were visualized under a UV lamp (254 nm).
3.2. General Procedure for the Synthesis of 3-(Benzoyl/4-bromobenzoyl)-1,2-benzothazines (9a,b)
Acetophenones (5a,b) (1.0 mmol) and p-toluenesulfonic acid monohydrate (285 mg, 1.5 mmols) were dissolved in acetonitrile (10 mL). Subsequently, N-bromosuccinamide (196 mg, 1.10 mmols) was added in portions and the mixture was refluxed for 2 h. Reaction mixture was cooled to room temperature and solvent was evaporated under vacuum. The residue obtained was dissolved in dichloromethane (DCM) and washed with distilled water followed by the saturated solution of sodium bicarbonate. After separating the DCM layer from aqueous layer using a separating funnel, the DCM was evaporated with the help of a rotary evaporator to get α-bromoacetophenones (6a,b). α-Bromoacetophenones (6a,b) (1.0 mmol) were added to a sodium saccharin 7 (226 mg, 1.10 mmol) suspension made in DMF (5 mL) and the mixture was heated at 110 °C for 3 h to get the N-alkylated saccharin derivatives (8a,b). The dried N-alkylated saccharin derivatives (8a,b) (1.0 mmol) were refluxed with sodium methoxide (270 mg, 5.0 mmols) in dry methanol for 30 min. The mixture was cooled and treated with 15% cold HCl to get the 3-(benzoyl/4-bromobenzoyl)-1,2-benzothiazines (9a,b) [46,47] in good isolated yields (given below). The products were also re-crystallized with methanol.
(4-Hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-3-yl)(phenyl)methanone (9a): Yellow solid; yield: (232 mg, 77%); m.p. 156–157 °C; 1H-NMR (DMSO-d6, 600 MHz) δ: 7.61 (t, J = 7.2 Hz, 2H, ArH), 7.68 (t, J =7.2 Hz, 1H, ArH), 7.92–7.96 (m, 3H, ArH), 8.01 (d, J = 7.2 Hz, 2H, ArH), 8.19–8.21 (m, 1H, ArH), 9.91 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 128.2, 128.3, 128.4, 129.3, 130.2, 132.6, 132.8, 133.0, 133.4, 133.5, 134.1, 134.8, 135.2, 148.8, 164.7. MS (ESI+): m/z calcd. for C15H11NO4NaS [M + Na]+ 324.02; found 324.03.
(4-Bromophenyl)(4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-3-yl)methanone (9b): Light yellow solid; yield: (304 mg, 80%); m.p. 144–146 °C; 1H-NMR (DMSO-d6, 600 MHz) δ: 7.60 (d, J = 7.9 Hz, 3H, ArH), 7.69 (t, J = 7.6 Hz, 1H, ArH), 7.93 (d, J = 7.5 Hz, 2H, ArH), 8.12–8.17 (m, 1H, ArH), 8.23 (t, J = 7.8 Hz, 1H, ArH), 9.93 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 127.4, 127.7, 128.0, 128.3 (2C), 130.2, 130.6 (2C), 131.8, 132.6, 133.2, 134.7, 136.3, 149.2, 165.7. MS (ESI+): m/z calcd. for C15H10BrNO4NaS [M + Na]+ 401.93; found 401.93.
3.3. General Procedure for the Synthesis of 2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-arylacetamides (11a–m)
3-Benzoyl-1,2-benzothazine (9a) (301 mg, 1.0 mmol), 2-bromo-N-arylacetamides 10a–m (1.0 mmol), and K2CO3 (207 mg, 1.5 mmols) were added in DMF (5 mL) and heated for 2-2.5 h at 80-100 °C. Afterward, the reaction mixture was cooled to room temperature and diluted with thrice amount of cold distilled water. Acidification with cold dilute HCl (20%) furnished the colored precipitates of 2-(3-benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-arylacetamides (11a–m) (Table 1). Precipitates were filtered, washed with excess distilled water, and finally dried. All the products were obtained in good isolated yields (given below) and were also re-crystallized with methanol.
2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(2-chlorophenyl)acetamide (11a): Light yellow solid; yield: (347 mg, 74%); m.p. 141–142 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.43 (s, 2H, CH2), 7.01–7.13 (m, 2H, ArH), 7.19 (dd, J = 8.2, 2.0 Hz, 2H, ArH), 7.32–7.39 (m, 1H, ArH), 7.63 (t, J = 8.2 Hz, 1H,), 7.90–7.99 (m, 3H, ArH), 8.04 (d, J = 7.6 Hz, 2H, ArH), 8.23–8.28 (m, 2H, ArH), 10.03 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 53.9, 116.9, 118.6, 119.4, 120.0, 121.7, 122.3, 126.0 (2C), 126.4 (2C), 127.6, 129.0 (2C), 132.6, 133.7, 134.9, 136.2, 138.0, 139.6, 164.9, 167.9, 188.9. MS (ESI+): m/z calcd. for C23H17ClN2O5NaS [M + Na]+ 491.03; found 491.02.
2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(3-chlorophenyl)acetamide (11b): Light yellow solid; yield: (366 mg, 78%); m.p. 182–184 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.02 (s, 2H, CH2), 7.03 (dd, J = 7.9, 2.1 Hz, 1H, ArH), 7.12 (dd, J = 8.2, 2.0 Hz, 1H, ArH), 7.22 (t, J = 8.0 Hz, 1H, ArH), 7.36–7.37 (m, 1H, ArH), 7.63 (t, J = 7.5, 2H, ArH), 7.89–7.97 (m, 4H, ArH), 8.06 (d, J = 7.7 Hz, 2H, ArH), 8.20–8.22 (m, 1H, ArH), 10.08 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 53.7, 117.0, 118.3, 118.8, 119.0, 119.8, 121.9, 123.8, 127.8, 128.7 (3C), 131.6 (2C), 134.2 (2C), 134.9, 135.2, 139.0, 139.9, 165.1, 168.1, 189.1. MS (ESI+): m/z calcd. for C23H17ClN2O5NaS [M + Na]+ 491.03; found 491.03.
2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(4-chlorophenyl)acetamide (11c): Yellow solid; yield: (347 mg, 73%); m.p. 146-148 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.00 (s, 2H, CH2), 7.20–7.26 (m, 4H, ArH), 7.63 (t, 2H, J = 7.6 Hz, ArH), 7.70 (t, J = 7.3 Hz, 1H, ArH), 7.87–7.90 (m, 1H, ArH), 7.90–7.94 (m, 2H, ArH), 8.05 (d, J = 7.6 Hz, 2H, ArH), 8.18–8.19 (m, 1H, ArH), 10.01 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 53.8, 116.2, 120.3, 121.8, 126.9, 127.0, 128.4, 128.5 (3C), 128.6 (2C), 128.7 (2C), 132.7, 132.9, 133.8, 135.2, 137.0, 138.5, 164.9, 168.0, 189.1. MS (ESI+): m/z calcd. for C23H17ClN2O5NaS [M + Na]+ 491.0397; found 491.0295.
2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(o-tolyl)acetamide (11d): Light Brown powder; yield: (359 mg, 80%); m.p. 165-167 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 2.20 (s, 3H, CH3), 3.94 (s, 2H, CH2), 6.96 (t, J = 7.8 Hz, 2H, ArH), 7.05 (dd, J = 7.4, 2.4 Hz, 2H ArH), 7.65 (d, J = 7.8 Hz, 3H, ArH), 7.70–7.76 (m, 2H, ArH), 7.87 (d, 1H, ArH), 7.94–8.01 (m, 1H, ArH), 8.04 (t, J = 7.9 Hz, 1H, ArH), 8.16–8.21 (m, 1H, ArH), 9.73 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 21.2, 54.0, 115.1, 116.6, 119.3, 121.4, 124.2 (2C), 127.6, 128.4 (2C), 128.6 (2C), 128.7, 128.8, 132.2, 132.8, 133.9, 134.4, 137.2, 139.0, 164.4, 167.0, 187.9. MS (ESI+): m/z calcd. for C24H20N2O5NaS [M + Na]+ 471.09; found 471.09.
2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(m-tolyl)acetamide (11e): Light orange powder; yield: (350 mg, 78%); m.p. 177–178 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 2.16 (s, 3H, CH3), 4.00 (s, 2H, CH2), 6.78 (d, J = 7.2 Hz, 1H, ArH), 7.02 (d, J = 8.3 Hz, 2H, ArH), 7.05 (t, J = 7.7 Hz, 2H, ArH), 7.63 (t, J = 7.7 Hz, 2H, ArH), 7.70 (t, J = 7.4 Hz, 1H, ArH), 7.88–7.93 (m, 2H, ArH), 8.07 (d, J = 7.6 Hz, 2H, ArH), 8.19–8.21 (m, 1H, ArH), 9.78 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 21.0, 53.7, 115.2, 116.2, 119.4, 121.8, 124.1, 127.1 (2C), 128.4, 128.5, 128.6 (2C), 128.7 (2C), 132.6, 132.9, 133.8, 134.1, 137.0, 138.9, 164.6, 166.3, 187.8. MS (ESI+): m/z calcd. for C24H20N2O5NaS [M + Na]+ 471.09; found 471.08.
2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(p-tolyl)acetamide (11f): Yellow powder; yield: (341 mg, 76%); m.p. 136-137 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 2.16 (s, 3H, CH3), 3.97 (s, 2H, CH2), 6.97 (d, J = 8.2 Hz, 2H, ArH), 7.09 (d, J = 7.9 Hz, 2H, ArH), 7.63 (t, J = 7.2 Hz, 2H, ArH), 7.70 (t, J = 7.2 Hz, 1H, ArH), 7.86–7.89 (m, 1H, ArH), 7.90–7.93 (m, 2H, ArH), 8.05 (d, J = 7.2 Hz, 2H, ArH), 8.17–8.19 (m, 1H, ArH), 9.77 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 20.3, 53.7, 116.2, 118.8, 121.8, 127.0, 128.5, 128.5 (2C), 128.6 (2C), 128.7 (2C), 128.9, 132.3, 132.6, 132.9, 133.8, 135.2, 135.6, 138.6, 164.5, 168.2, 188.9. MS (ESI+): m/z calcd. for C24H20N2O5NaS [M + Na]+ 471.09; found 471.09.
2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(2-nitrophenyl)acetamide (11g): Light brown solid; yield: (398 mg, 83%); m.p. 157-158 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.06 (s, 2H, CH2), 7.23–7.34 (m, 2H, ArH), 7.37 (d, J = 8.2 Hz, 2H, ArH), 7.55 (d, J = 8.2 Hz, 2H, ArH), 7.53–7.58 (m, 1H, ArH), 7.62 (d, J = 7.8 Hz, 1H, ArH), 7.84–7.87 (m, 2H, ArH), 7.88–7.91 (m, 2H, ArH), 8.14–8.12 (m, 1H, ArH), 9.87 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 53.8, 113.1, 116.1, 118.0, 121.8, 124.8, 127.2 (2C), 128.3 (2C), 128.7, 128.8, 130.1, 132.6, 132.7, 133.9, 135.3, 138.3, 139.3, 146.9, 165.5, 168.2, 188.9. MS (ESI+): m/z calcd. for C23H17N3O7NaS [M + Na]+ 502.06; found 502.06.
2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(3-nitrophenyl)acetamide (11h): Light yellow powder; yield: (403 mg, 84%); m.p. 170-172 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.04 (s, 2H, CH2), 7.49 (t, J = 8.1 Hz, 1H, ArH), 7.56 (d, J = 8.1 Hz, 1H, ArH), 7.63 (t, J = 7.5 Hz, 2H, ArH), 7.69 (t, J = 7.3 Hz, 1H, ArH), 7.84 (dd, J = 8.3, 4.2 Hz, 1H, ArH), 7.89–7.97 (m, 3H, ArH), 8.05 (d, J = 7.6 Hz, 2H, ArH), 8.09 (s, 1H, ArH), 8.15–8.22 (m, 1H, ArH), 10.38 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 53.9, 113.0, 116.2, 117.9, 121.9, 124.9, 127.1, 128.4, 128.6 (2C), 128.7 (2C), 130.1, 132.8, 132.9, 133.9, 135.1, 138.4, 139.1, 147.7, 165.5, 167.9, 189.1. MS (ESI+): m/z calcd. for C23H17N3O7NaS [M + Na]+ 502.0697; found 502.0531.
2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(4-nitrophenyl)acetamide (11i): Light brown solid; yield: (369 mg, 77%); m.p. 194–195 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.08 (s, 2H, CH2), 7.44–7.47 (m, 2H, ArH), 7.63 (td, J = 7.2, 1.6 Hz, 2H, ArH), 7.68–7.71 (m, 1H, ArH), 7.89–7.95 (m, 3H, ArH), 8.05 (d, J = 7.2 Hz, 2H, ArH), 8.09–8.11 (m, 2H, ArH), 8.20–8.21 (m, 1H, ArH), 10.48 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 53.9, 116.1, 118.6 (2C), 121.8, 124.8 (2C), 127.1, 128.3, 128.6 (2C), 128.7 (2C), 132.8, 132.9, 133.9, 135.2, 138.5, 142.2, 144.2, 165.9, 167.7, 189.4. MS (ESI+): m/z calcd. for C23H17N3O7NaS [M + Na]+ 502.0697; found 502.0675.
2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(3-bromophenyl)acetamide (11j): Yellow solid; yield: (416 mg, 81%); m.p. 163-166 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.04 (s, 2H, CH2), 7.04 (d, J = 8.5 Hz, 2H, ArH), 7.21 (t, J = 7.6 Hz, 2H, ArH), 7.56–7.59 (m, 3H, ArH), 7.68 (t, J = 7.5 Hz, 1H, ArH), 7.88–7.90 (m, 3H, ArH), 7.98 (d, J = 7.2 Hz, 1H, ArH), 8.12 (s, 1H, ArH), 9.46 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 53.9, 116.4, 119.3, 119.7, 122.0, 126.4, 127.3 (2C), 128.3 (3C), 128.5, 130.6, 132.0, 134.5, 136.6 (2C), 138.6, 139.2, 140.5, 165.2, 167.2, 189.2. MS (ESI+): m/z calcd. for C23H17BrN2O5NaS [M + Na]+ 534.98; found 534.98.
2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(4-bromophenyl)acetamide (11k): Light yellow solid; yield: (421 mg, 82%); m.p. 150-153 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.09 (s, 2H, CH2), 7.05 (d, J = 7.9 Hz, 2H, ArH), 7.22–7.35 (m, 2H, ArH), 7.56 (d, J = 7.9 Hz, 3H, ArH), 7.69–7.74 (m, 2H, ArH), 7.95 (t, J = 7.9 Hz, 2H, ArH), 8.09–8.11 (m, 1H, ArH), 8.12–8.14(m, 1H, ArH), 9.48 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 54.0, 116.9, 119.4, 119.8, 120.2, 126.4, 126.9 (2C), 127.2 (2C), 128.3 (2C), 128.6, 130.5, 132.1, 134.4, 136.4 (2C), 138.5, 139.9, 140.2, 165.3, 167.4, 188.9. MS (ESI+): m/z calcd. for C23H17BrN2O5NaS [M + Na]+ 534.98; found 534.97.
2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(2-methoxyphenyl)acetamide (11l): Light brown solid; yield: (372 mg, 80%); m.p. 199-200 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 3.72 (s, 3H, CH3), 4.09 (s, 2H, CH2), 6.74 (t, J = 7.6 Hz, 1H, ArH), 6.93 (d, J = 8.0 Hz, 1H, ArH), 6.98 (t, J = 8.1 Hz, 1H, ArH), 7.49 (d, J = 7.9 Hz, 1H, ArH), 7.63 (t, J = 7.6 Hz, 2H, ArH), 7.70 (t, J = 7.4 Hz, 1H, ArH), 7.86–7.91 (m, 3H, ArH), 8.06 (d, J = 7.5 Hz, 2H, ArH), 8.16–8.18 (m, 1H, ArH), 9.17 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 53.8, 55.5, 111.0, 116.4, 120.0, 121.4, 121.8, 124.4, 126.3, 127.0, 128.5, 128.6 (2C), 128.9 (2C), 132.6, 132.9, 133.7, 135.1, 138.4, 149.2, 165.0, 168.2, 188.8. MS (ESI+): m/z calcd. for C24H20N2O6NaS [M + Na]+ 487.08; found 487.07.
2-(3-Benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(4-methoxyphenyl)acetamide (11m): Yellow solid; yield: (367 mg, 79%); m.p. 164-165 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 3.64 (s, 3H, OCH3), 3.95 (s, 2H, CH2), 6.75 (d, J = 7.8 Hz, 2H, ArH), 7.11 (d, J = 7.8 Hz, 2H, ArH), 7.63 (t, J = 7.2 Hz, 2H, ArH), 7.70 (t, J = 7.2 Hz, 1H, ArH), 7.86–7.92 (m, 3H, ArH), 8.04 (d, J = 7.2 Hz, 2H, ArH), 8.17 (d, J = 7.8 Hz, 1H, ArH), 9.71 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 53.7, 55.0, 113.7 (3C), 116.2, 120.4 (3C), 121.8, 127.0, 128.6, 128.7 (2C), 131.2, 132.6, 132.9, 133.7, 135.1, 138.6, 155.2, 164.1, 168.3, 188.8. MS (ESI+): m/z calcd. for C24H20N2O6NaS [M + Na]+ 487.08; found 487.07.
3.4. General Procedure for the Synthesis of 2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-arylacetamides (12a–m)
3-(4-Bromobenzoyl)-1,2-benzothazine (9b) (380 mg, 1.0 mmol), 2-bromo-N-arylacetamides 10a–m (1.0 mmol), and K2CO3 (207 mg, 1.5 mmols) were added in DMF (5 mL) and heated for 2-2.5 h at 80–100 °C. Afterward, the reaction mixture was cooled to room temperature and diluted with thrice amount of cold distilled water. Acidification with cold dilute HCl (20%) furnished the colored precipitates of 2-(3-(4-bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-aryl acetamides (12a–m) (Table 1). Precipitates were filtered, washed with excess distilled water and then dried. The products were re-crystallized from methanol and were dried.
2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(2-chlorophenyl)acetamide (12a): Light yellow solid; yield: (438 mg, 80%); m.p. 180-181 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.12 (s, 2H, CH2), 7.12 (t, J = 7.2 Hz, 1H, ArH), 7.19 (t, J = 7.5 Hz, 1H, ArH), 7.34 (d, J = 7.8 Hz, 1H, ArH), 7.39 (d, J = 7.9 Hz, 1H, ArH), 7.85–7.90 (m, 5H, ArH), 7.96 (d, J = 8.1 Hz, 2H, ArH), 8.13–8.15 (m, 1H, ArH), 9.53 (s, 1H, NH), 14.99 (s, 1H, OHenolic). 13C-NMR (DMSO-d6, 150 MHz) δ: 53.5, 116.3, 122.0, 125.4, 126.0, 126.3, 126.9, 127.1, 127.3, 128.5, 129.4, 130.7 (2C), 131.8 (2C), 132.7, 133.8, 133.9, 134.3, 138.3, 165.4, 168.1, 187.8. MS (ESI+): m/z calcd. for C23H16BrClN2O5NaS [M(81Br) + Na]+ 570.9497; found 570.9286.
2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(3-chlorophenyl)acetamide (12b): Yellow powder; yield: (460 mg, 84%); m.p. 148-150 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.00 (s, 2H, CH2), 7.12–7.17 (m, 3H, ArH), 7.27 (dd, J = 7.8, 2.7 Hz, 1H, ArH), 7.59–7.60 (m, 2H, ArH), 7.74 (d, J = 7.6 Hz, 2H, ArH), 7.88–7.95 (m, 3H, ArH), 8.17–8.23 (m, 1H, ArH), 9.55 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 53.8, 116.3, 121.9, 126.0, 126.2, 126.2, 126.9, 127.1, 127.4 (2C), 128.3 (2C), 129.8, 130.6 (2C), 132.8, 133.6, 133.9, 134.3, 138.4, 165.1, 168.7, 188.0. MS (ESI+): m/z calcd. for C23H16BrClN2O5NaS [M + Na]+ 568.94; found 568.94.
2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(4-chlorophenyl)acetamide (12c): Yellow solid; yield: (477 mg, 87%); m.p. 181-184 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.08 (s, 2H, CH2), 7.24–7.30 (m, 1H, ArH), 7.44–7.48 (m, 2H, ArH), 7.54–7.57 (m, 2H, ArH), 7.74 (d, J = 7.6 Hz, 1H, ArH), 7.88–7.95 (m, 3H, ArH), 8.08–8.13 (m, 2H, ArH), 8.19–8.21 (m, 1H, ArH), 9.59 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 54.0, 116.8, 116.1, 118.6 (2C), 124.9 (2C), 126.3 (2C), 126.8, 127.1 (2C), 128.2 (2C), 129.8, 132.8, 133.7, 133.9, 136.4, 138.5, 165.0, 167.4, 188.3. MS (ESI+): m/z calcd. for C23H16BrClN2O5NaS [M + Na]+ 568.94; found 568.93.
2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(o-tolyl)acetamide (12d): Light orange powder; yield: (464 mg, 88%); m.p. 193-194 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 1.96 (s, 3H, CH3), 4.07 (s, 2H, CH2), 6.98–7.02 (m, 3H, ArH), 7.10 (d, J = 6.9 Hz, 1H, ArH), 7.86–7.92 (m, 5H, ArH), 7.98 (d, J = 8.2 Hz, 2H, ArH), 8.13–8.15 (m, 1H, ArH), 9.29 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 17.4, 53.5, 116.3, 121.9, 124.5, 125.3, 125.8, 127.0, 127.5, 128.5, 130.2, 130.7 (2C), 131.5, 131.8 (2C), 132.7, 133.8, 134.2, 135.2, 138.4, 164.9, 168.3, 187.2. MS (ESI+): m/z calcd. for C24H19BrN2O5NaS [M + Na]+ 551.0097; found 550.9858.
2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(m-tolyl)acetamide (12e): Light yellow solid; yield: (427 mg, 81%); m.p. 143-144 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 2.17 (s, 3H, CH3), 3.99 (s, 2H, CH2), 6.79 (d, J = 7.3 Hz, 1H, ArH), 7.01 (d, J = 8.9 Hz, 2H, ArH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 7.83–7.95 (m, 7H, ArH), 8.16–8.19 (m, 1H, ArH), 9.37 (s, 1H, NH), 14.92 (s, 1H, OHenolic). 13C-NMR (DMSO-d6, 150 MHz) δ: 21.0, 53.9, 116.1, 116.3, 119.3, 122.0, 124.1, 126.9, 127.1, 128.4 (2C), 130.7 (2C), 131.7 (2C), 132.8, 133.9, 134.3, 137.8, 138.0, 138.4, 164.6, 168.0, 187.6. MS (ESI+): m/z calcd. for C24H19BrN2O5NaS [M + Na]+ 549.0097; found 550.0000.
2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(p-tolyl)acetamide (12f): Light brown solid; yield: (454 mg, 86%); m.p. 190-192 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 2.43 (s, 3H, CH3), 3.98 (s, 2H, CH2), 6.98 (d, J = 8.3 Hz, 2H, ArH), 7.09 (d, J = 7.9 Hz, 1H, ArH), 7.70 (t, J = 7.2 Hz, 1H, ArH), 7.74 (t, J = 7.2 Hz, 2H, ArH), 7.87–7.90 (m, 1H, ArH), 7.91–7.94 (m, 2H, ArH), 8.05 (d, J = 7.2 Hz, 2H, ArH), 8.17–8.19 (m, 1H, ArH), 9.48 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 21.0, 53.8, 116.2, 118.8, 121.8, 127.0, 128.3 (2C), 128.5, 128.6 (2C), 128.7 (2C), 128.9, 132.3, 132.6, 132.9, 133.8, 135.2, 135.6, 138.6, 164.4, 168.1, 188.0. MS (ESI+): m/z calcd. for C24H19BrN2O5NaS [M + Na]+ 549.00; found 549.01.
2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(2-nitrophenyl)acetamide (12g): Light brown powder; yield: (497 mg, 89%); m.p. 177–178 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.08 (s, 2H, CH2), 7.29 (q, J = 7.2, 1H, ArH), 7.40 (t, J = 8.4, 1H, ArH), 7.58 (q, J = 7.2 Hz, 1H, ArH), 7.83–7.90 (m, 6H, ArH), 7.98 (t, J = 8.4 Hz, 2H, ArH), 8.11–8.15 (m, 1H, ArH), 10.17 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 53.7, 116.2, 122.0, 124.8, 125.1, 125.4, 126.9, 127.2 (2C), 128.4, 130.2, 130.6 (2C), 131.8 (2C), 132.8, 133.9, 134.0, 134.5, 138.2, 165.5, 168.3, 187.7. MS (ESI+): m/z calcd. for C23H16BrN3O7NaS [M + Na]+ 579.97; found 579.98.
2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(3-nitrophenyl)acetamide (12h): Light yellow solid; yield: (469 mg, 84%); m.p. 208-209 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.05 (s, 2H, CH2), 7.50 (t, J = 7.8 Hz, 1H, ArH), 7.58 (dd, J = 6.0, 2.0 Hz, 1H, ArH), 7.82–7.87 (m, 3H, ArH), 7.91–8.07 (m, 5H, ArH), 8.16–8.17 (m, 1H, ArH), 8.19–8.21 (m, 1H, ArH), 10.39 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 54.1, 112.9, 116.3, 118.0, 126.2, 126.5, 127.4 (2C), 127.9 (2C), 128.4 (2C), 130.0, 131.7, 132.9, 134.0, 134.2, 138.2, 139.1, 147.7, 165.5, 167.9, 188.1. MS (ESI+): m/z calcd. for C23H16BrN3O7NaS [M + Na]+ 579.97; found 579.98.
2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(4-nitrophenyl)acetamide (12i): Yellow powder; yield: (491 mg, 88%); m.p. 210-212 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.08 (s, 2H, CH2), 7.47 (d, J = 7.8 Hz, 1H, ArH), 7.84 (d, J = 7.8 Hz, 2H, ArH), 7.88–7.95 (m, 5H, ArH), 8.11 (d, J = 7.8 Hz, 3H, ArH), 8.19 (d, J = 7.0 Hz, 1H, ArH), 10.04 (s, 1H, NH), 15.18 (s, 1H, OHenolic). 13C-NMR (DMSO-d6, 150 MHz), δ: 53.8, 114.1, 116.2, 118.5, 122.0, 124.9, 127.2 (2C), 130.0 (2C), 130.2, 131.5, 131.7, 132.9, 133.9, 136.9, 138.4, 142.2, 144.2, 148.2, 165.9, 167.9, 188.7. MS (ESI+): m/z calcd. for C23H16BrN3O7NaS [M + Na]+ 579.97; found 579.97.
2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(3-bromophenyl)acetamide (12j): Light orange solid; yield: (527 mg, 89%); m.p. 145–147 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.00 (s, 2H, CH2), 7.13–7.17 (m, 3H, ArH), 7.48 (s, 1H, ArH), 7.55–7.59 (m, 3H, ArH), 7.84 (d, J = 7.8 Hz, 2H, ArH), 7.88–7.94 (m, 2H, ArH), 8.17–8.19 (m, 1H, ArH), 10.05 (s, 1H, NH), 14.97 (s, 1H, OHenolic). 13C-NMR (DMSO-d6, 150 MHz) δ: 54.0, 116.3, 117.6, 121.1, 121.3 (2C), 122.0, 126.0, 127.2 (2C), 130.7 (2C), 130.7 (3C), 131.7 (2C), 132.8, 133.9, 138.3, 139.6, 165.4, 168.7, 186.9. MS (ESI+): m/z calcd. for C23H16Br2N2O5NaS [M + Na]+ 612.89; found 612.89.
2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(4-bromophenyl)acetamide (12k): Yellow solid; yield: (545 mg, 92%); m.p. 200-203 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 4.00 (s, 2H, CH2), 7.19 (d, J = 8.0 Hz, 3H, ArH), 7.37 (dt, J = 9.0, 3.0 Hz, 2H, ArH), 7.83–7.94 (m, 3H, ArH), 8.16–8.18 (m, 3H, ArH), 10.12 (s, 1H, NH). 13C-NMR (DMSO-d6, 150 MHz) δ: 54.0, 114.9, 116.2, 120.7 (3C), 121.1, 127.9, 130.7 (3C), 131.4 (3C), 131.7 (2C), 132.8, 133.9, 137.4, 138.0, 165.2, 167.9, 187.0. MS (ESI+): m/z calcd. for C23H16Br2N2O5NaS [M + Na]+ 612.89; found 612.88.
2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(2-methoxyphenyl)acetamide (12l): Yellow solid; yield: (484 mg, 89%); m.p. 186–187 °C. 1H-NMR (DMSO-d6, 600 MHz) δ: 3.73 (s, 3H, OCH3), 4.11 (s, 2H, CH2), 6.75 (t, J = 7.8 Hz, 1H, ArH), 6.94 (dd, J = 7.8, 1.4 Hz, 1H, ArH), 6.98 (t, J = 7.8 Hz, 1H, ArH), 7.51 (d, J = 7.8 Hz, 1H, ArH), 7.85 (d, J = 7.8 Hz, 1H, ArH), 7.86–7.91 (m, 4H, ArH), 7.95 (d, J = 7.8 Hz, 2H, ArH), 8.15–8.18 (m, 1H, ArH), 9.17 (s, 1H, NH), 14.92 (s, 1H, OHenolic). 13C-NMR (DMSO-d6, 150 MHz) δ: 53.7, 55.1, 111.7, 116.2, 120.4 (2C), 121.3, 121.8, 124.6, 126.5, 127.0, 127.6, 128.7 (2C), 131.2, 132.6, 132.9, 133.7, 135.1, 138.6, 149.2, 164.9, 167.6, 187.8. MS (ESI+): m/z calcd. for C24H19BrN2O6NaS [M + Na]+ 564.99; found 564.98.
2-(3-(4-Bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-N-(4-methoxyphenyl)acetamide (12m): Yellow powder; yield: (489 mg, 90%); m.p. 198-199 °C. 1H NMR (DMSO-d6, 600 MHz) δ: 3.73 (s, 3H, OCH3), 4.01 (s, 2H, CH2), 6.75 (t, J = 7.8 Hz, 1H, ArH), 6.93 (dd, J = 7.8, 1.4 Hz, 1H, ArH), 6.98 (t, J = 7.8 Hz, 1H, ArH), 7.50 (d, J = 7.8 Hz, 1H, ArH), 7.84 (d, J = 7.8 Hz, 1H, ArH), 7.86–7.91 (m, 4H, ArH), 7.96 (d, J = 7.8 Hz, 2H, ArH), 8.15–8.18 (m, 1H, ArH), 9.17 (s, 1H, NH), 14.92 (s, 1H, OHenolic). 13C-NMR (DMSO-d6, 150 MHz) δ: 54.0, 55.5, 111.0, 116.4, 120.0, 121.2, 121.9, 124.4, 126.4, 126.9, 127.1, 128.4, 130.7 (2C), 131.7 (2C), 132.7, 133.8, 134.3, 138.4, 149.1, 165.0, 168.0, 187.8. MS (ESI+): m/z calcd. for C24H19BrN2O6NaS [M + Na]+ 566.99; found 564.98.
3.5. In Silico α-Glucosidase Inhibition
The Molecular Operating Environment (MOE) Ver.2014.0901 software [66,67,68] was utilized for the in silico analysis of all the 1,2-benzothiazine derivatives along with the reference acarbose as follows.
3.5.1. Ligand Preparation
Structures of all these compounds were designed with the help of ChemDraw Ultra 12.02. In order to display these files in the MOE, structures of these compounds were saved in an MDL file (“.sdf”). The 2D structure of the reference acarbose in the SDF file was retrieved from NCBI Pubchem. After this, 3D protonation and energy minimizations of all these compounds along with the reference acarbose were executed under default parameters in the MOE.
3.5.2. Protein Preparation
The 3D structure of Saccharomyces cerevisiae α-glucosidase (PDB ID: 2QMJ) was downloaded from the Protein Data Bank (http://www.rcsb.org/pdb; accessed on 10 October 2020). Binding interactions of the ligand (acarbose) with the protein residues were checked (Asp203, Asp542, Asp327, His600, Arg526) and then the ligand, solvent molecules, and unknown atoms from the receptor protein were removed. With the help of the MOE site finder module, the active site from the 3D structure of the protein was computed. The ligand site was found surrounded by Asp203, Tyr299, Asp327, Ile328, Asp366, Trp406, Trp441, Asp443, Met444, Arg526, Trp539, Gly541, Asp542, Asp571, Phe575, Arg598, and His600 residues. Later on, the site was occupied by dummy atoms and the protein was 3D-protonated and energy minimized with default parameters set in the MOE software (Ver.2014.0901).
3.5.3. Molecular Docking
To access the binding modes of all the ligands with α-glucosidase enzymes, ten conformations of all the ligands along with acarbose were docked into the selected site of enzymes with the help of the MOE dock module under the parameters fixed as, placement: triangle matcher, rescoring: London dG, refinement: forcefield and retain: 10. Finally, 2D and 3D maps of ligands were obtained. Binding modes of ligands were studied using rmsd values and docking scores of their top-ranked conformations. Results of molecular docking are depicted in Table 1, Table S1, and Figure 2, Figure 3, and Figure S1.
3.6. In Vitro α-Glucosidase Inhibition
A general spectrophotometric technique was used to perform the α-glucosidase inhibition assay with slight modifications [69]. All the compounds were dissolved in a dimethyl sulfoxide (DMSO) solvent while α-glucosidase enzymes (obtained from Saccharomyces cerevisiae (Sigma-Aldrich, Munich, Germany) were dissolved in a phosphate buffer (pH 6.8, 100 mM). Twelve and a half microliters of each test compound (dissolved in DMSO), 40 μL of enzymes (0.5 U/mL), and 120 μL of phosphate buffer (100 mM) were added in 96-well microliter plates. The plates were incubated for 5 min at 37 °C. After incubation, 40 μL of 5 mM p-nitrophenyl-α-D-glucopyranoside (Sigma Aldrich, Munich, Germany) was added in each sample solution and incubated for a further 30 min. Afterward, absorbance of released 4-nitrophenol was measured at 405 nm and 37 °C using a microplate reader. DMSO and acarbose (Sigma-Aldrich, Munich, Germany) were used as a negative control and reference inhibitor, respectively. The experiment was repeated thrice and inhibition percentages were calculated using the following formula.
where ‘A’ stands for absorbance.
% Inhibition = [(A control − A sample) / A control] × 100
Finally, IC50 values were also determined for the compounds having 50% or above inhibition values.
4. Conclusions
Novel 1,2-benzothiazine-N-arylacetamides have been discovered as inhibitors of α-glucosidase enzymes for the first time. The 1,2-benzothiazine-N-arylacetamides 12a–m proved better inhibitors compared to derivatives 11a–m. The compounds 11c, 11h, 11i, 12a, 12d, 12e, 12g, and 12m exhibited low IC50 values compared to acarbose (IC50 = 58.8 μM). However, the derivatives 11c, 12a, 12d, 12e, and 12g were envisioned as the most potent inhibitors as these templates showed good docking scores, low rmsd values and significant interactions with the targeted receptor pockets during in silico experiments. Theses derivatives also showed low IC50 values of 30.65, 18.25, 20.76, 35.14, and 24.24 μM, respectively, during in vitro α-glucosidase inhibition. We hope the present work will benefit researchers to develop more active antidiabetic agents with no side effects.
Author Contributions
Conceptualization, S.A., M.A., U.A.A.; methodology, F.A.S., S.T., M.H.; synthesis, F.A.S., S.A., M.A.; characterization, F.A.S., S.A., M.A., S.S., M.H., D.S.L., M.E.A.Z.; enzyme inhibition studies, F.A.S., U.A.A., S.T., M.M.; manuscript writing, review and editing, F.A.S., M.A., M.M., D.S.L., M.E.A.Z.; supervision, M.A., M.E.A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The research work was supported by the ‘Higher Education Commission of Pakistan’ through Research Grant NRPU-3715.
Data Availability Statement
he data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Kim, J.S.; Yang, J.; Kim, M.J. Alpha glucosidase inhibitory effect, anti-microbial activity and UPLC analysis of Rhus verniciflua under various extract conditions. J. Med. Plants Res. 2011, 5, 778–783. [Google Scholar]
- Kador, P.F.; Robison, W.G., Jr.; Kinoshita, J.H. Inhibitors. Ann. Rey. Pharmacol. Toxicol. 1985, 25, 691–714. [Google Scholar] [CrossRef]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Dam, R.M.V.; Liu, S. Diet and risk of type II diabetes: The role of types of fat and carbohydrate. Diabetologia 2001, 44, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Knowler, W.C.; Connor, E.B.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or pharmetformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular risk factors in the Framingham study. Circulation 1979, 59, 8–13. [Google Scholar] [CrossRef]
- Stamler, J.; Vaccaro, O.; Neaton, J.D.; Wentworth, D. Diabetes, other risk factors and 12-year cardiovascular mortality for men screened in Multiple Risk Factor Intervention Trial. Diabetes Care 1993, 16, 434–444. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and societal implications of the diabetic epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Dowaidar, A.; Kamal, H. Inhibitory effect of dates-extract on α-Amylase and β-glucosidase enzymes relevant to non-insulin dependent diabetes mellitus. J. Biochem. Technol. 2010, 2, 158–160. [Google Scholar]
- Al-Malki, A.L. Inhibition of α-glucosidase by thiosulfinate as a target for glucose modulation in diabetic rats. Evid. Based Complement. Alternat. Med. 2016. [Google Scholar] [CrossRef]
- Abirami, A.; Nagarani, G.; Siddhuraju, P. In vitro antioxidant, anti-diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C. maxima fruits. Food Sci. Hum. Wellness 2014, 3, 16–25. [Google Scholar] [CrossRef]
- Hiroyuki, F.; Tomohide, Y.; Kazunori, O. Efficacy and safety of Touchi extract, an α-glucosidase inhibitor derived from fermented soybeans, in non-insulin dependent diabetic mellitus. J. Nutr. Biochem. 2001, 12, 351–356. [Google Scholar] [CrossRef]
- Matsui, T.; Ueda, T.; Oki, T.; Sugita, K.; Terhara, N.; Matsumoto, K. α-Glucosidase inhibitory action of natural acylated anthocyanins: 1. Survey of natural pigments with potent inhibitory activity. J. Agric. Food Chem. 2001, 49, 1948–1951. [Google Scholar] [CrossRef]
- Wansi, J.D.; Lallemand, M.C.; Chiozem, D.D.; Toze, F.A.A.; Mbaze, L.M.; Naharkhan, S.; Iqbal, M.C.; Tillequin, F.; Wandji, J.; Fomum, Z.T. α-Glucosidase inhibitory constituents from stem bark of Terminalia superba (Combretaceae). Phytochemistry 2007, 68, 2096–2100. [Google Scholar] [CrossRef]
- Lee, S.S.; Lin, H.C.; Chen, C.K. Acylated flavonol monorhamnosides, α-glucosidase inhibitors, from Machilus philippinensis. Phytochemistry 2008, 69, 2347–2353. [Google Scholar] [CrossRef]
- Kim, K.Y.; Nama, K.A.; Kurihara, H.; Kim, S.M. Potent α-glucosidase inhibitors purified from the red alga Grateloupia elliptica. Phytochemistry 2008, 69, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Madar, Z. The effect of acarbose and miglitol (BAY-M-1099) on postprandial glucose levels following ingestion of various sources of starch by non-diabetic and streptozotocin-induced diabetic rats. J. Nutr. 1989, 119, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Murai, A.; Iwamura, K.; Takada, M.; Ogawa, K.; Usui, T.; Okumura, J. Control of postprandial hyperglycaemia by galactosyl maltobionolactone and its novel anti-amylase effect in mice. Life Sci. 2002, 71, 1405–1415. [Google Scholar] [CrossRef]
- Amarowicz, R.; Troszyńska, A.; Shahidi, F. Antioxidant activity of almond seed extract and its fractions. J. Food Lipids 2005, 12, 344–358. [Google Scholar] [CrossRef]
- Fujisawa, T.; Ikegami, H.; Inoue, K.; Kawabata, Y.; Ogihara, T. Effect of two alpha-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism 2005, 54, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Shobana, S.; Sreerama, Y.N.; Malleshi, N.G. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009, 115, 1268–1273. [Google Scholar] [CrossRef]
- Lombardino, J.G.; Wiseman, E.H.; Chiaini, J. Potent Antiinflammatory N-Heterocyclic 3-Carboxamides of 4-Hydroxy-2-methyl-2H- 1,2-benzothiazine 1-dioxide. J. Med. Chem. 1973, 16, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Lombardino, J.G.; Wiseman, E.H.; Mclamore, W. Synthesis and antiinflammatory activity of some 3-carboxamides of 2-alkyl-4-hydroxy-2H-1, 2-benzothiazine 1, 1-dioxide. J. Med. Chem. 1971, 14, 1171–1175. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsuri, T.; Jyoyama, H.; Ono, T.; Yamada, K.; Kobayashi, M.; Hori, Y.; Arimura, A.; Yasui, K.; Ohno, K.; et al. Novel antiarthritic agents with 1,2-isothiazolidine-1,1-dioxide (γ-sultam) skeleton: Cytokine suppressive dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase. J. Med. Chem. 2000, 43, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Lebegue, N.; Gallet, S.; Flouquet, N.; Carato, P.; Pfeiffer, B.; Renard, P.; Leonce, S.; Pierre, A.; Chavatte, P.; Berthelot, P. Novel benzopyridothiadiazepines as potential active antitumor agents. J. Med. Chem. 2005, 48, 7363–7373. [Google Scholar] [CrossRef]
- Wells, G.J.; Tao, M.; Josef, K.A.; Bihovsky, R. 1,2-Benzothiazine 1,1-dioxide P2-P3 peptide mimetic aldehyde calpain I inhibitors. J. Med. Chem. 2001, 44, 3488–3503. [Google Scholar] [CrossRef]
- Kim, S.H.; Ramu, R.; Kwon, S.W.; Lee, S.-H.; Kim, C.H.; Kang, S.K.; Rhee, S.D.; Bae, M.A.; Ahn, S.H.; Ha, D.C.; et al. Discovery of cyclicsulfonamide derivatives as 11β-hydroxysteroid dehydrogenase 1 inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1065–1069. [Google Scholar] [CrossRef]
- Berryman, K.A.; Edmunds, J.J.; Bunker, A.M.; Haleen, S.; Bryant, J.; Welchb, K.M.; Doherty, A.M. Endothelin Receptor Antagonists: Synthesis and structure-activity relationships of substituted benzothiazine-1,1-dioxides. Bioorg. Med. Chem. Lett. 1998, 6, 1447–1456. [Google Scholar] [CrossRef]
- Barazarte, A.; Lobo, G.; Gamboa, N.; Rodrigues, J.R.; Capparelli, M.V.; Alvarez-Larena, A.; Lopez, S.E.; Charris, J.E. Synthesis and antimalarial activity of pyrazolo and pyrimido benzothiazine dioxide derivatives. Eur. J. Med. Chem. 2009, 44, 1303–1310. [Google Scholar] [CrossRef]
- Saddique, F.A.; Zaib, S.; Jalil, S.; Aslam, S.; Ahmada, M.; Sultan, S.; Naz, H.; Iqbal, M.; Iqbal, J. Synthesis, monoamine oxidase inhibition activity and molecular docking studies of novel 4-hydroxy-N’-[benzylidene or 1-phenylethylidene]-2-H/methyl/benzyl-1,2-benzothiazine-3-carbohydrazide 1,1-dioxides. Eur. J. Med. Chem. 2018, 143, 1373–1386. [Google Scholar] [CrossRef]
- Ikeda, T.; Kakegawa, H.; Miyataka, H.; Matsumoto, H.; Satoht, T. Anti-allergic and anti-inflammatory actions of 2′-(tetrazole-5-yl)-4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxanilide 1,1-dioxide. Biorg. Med. Chem. Lett. 1992, 2, 709–714. [Google Scholar] [CrossRef]
- Constantine, J.W. Aggregation and adhesion of rat platelets. Nature 1967, 214, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Jantova, S.; Greif, G.; Spirkova, K.; Stankovsky, S.; Oravcova, M. Antibacterial effects of trisubstituted quinazoline derivatives. Folia Microbiol. 2000, 45, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Zia-ur-Rehman, M.; Choudary, J.A.; Ahmad, S.; Siddiqui, H.L. Synthesis of potential biologically active 1,2-benzothiazin-3-yl-quinazolin-4(3H)-ones. Chem. Pharm. Bull. 2006, 54, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Bassin, J.P.M.; Flye, J.; Hunter, A.P.; Martin, L.; Goyal, M. Synthesis and Antimicrobial Activity of 1,2-Benzothiazine Derivatives. Molecules 2016, 21, 861. [Google Scholar] [CrossRef] [PubMed]
- Zia-ur-Rehmana, M.; Choudary, J.A.; Elsegood, M.R.J.; Siddiqui, H.L.; Khan, K.M. A facile synthesis of novel biologically active 4-hydroxy-N’-(benzylidene)-2H-benzo[e][1,2]thiazine-3-carbohydrazide 1,1-dioxides. Eur. J. Med. Chem. 2009, 44, 1311–1316. [Google Scholar] [CrossRef]
- Bihovsky, R.; Tao, M.; Mallamo, J.P.; Wells, G.J. 1,2-Benzothiazine 1,1-dioxide α-ketoamide analogues as potent calpain I inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 1035–1038. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, S.; Yang, Y.; Hussain, S.; He, M.; Gui, D.; Ma, B.; Jing, C.; Qiao, Z.; Zhu, C.; et al. 1,2-Benzothiazine 1,1-dioxide carboxylate derivatives as novel potent inhibitors of aldose reductase. Bioorg. Med. Chem. 2011, 19, 7262–7269. [Google Scholar] [CrossRef]
- Parveen, S.; Hussain, S.; Qin, X.; Hao, X.; Zhu, S.; Rui, M.; Zhang, S.; Fu, F.; Ma, B.; Yu, Q.; et al. Copper-catalyzed asymmetric synthesis and comparative aldose reductase inhibition activity of (+)/(−)-1,2-benzothiazine-1,1-dioxide, acetic acid derivatives. J. Org. Chem. 2014, 79, 4963–4972. [Google Scholar] [CrossRef]
- Saddique, F.A.; Ahmad, M.; Ashfaq, U.A.; Aslam, A.; Khan, S.G. Alpha-glucosidase inhibition and molecular docking studies of 4-hydroxy-N’-[benzylidene/1-phenylethylidene]-2H-1,2-benzothiazine-3-carbohydrazide 1,1-dioxides. Chiang Mai J. Sci. 2021, 48, 460–469. [Google Scholar]
- Ibraheem, F.; Ahmad, M.; Ashfaq, U.A.; Aslam, S.; Khan, Z.A.; Sultan, S. Synthesis, molecular docking and anti-diabetic studies of novel benzimidazole-pyrazoline hybrid molecules. Pak. J. Pharm. Sci. 2020, 33, 847–854. [Google Scholar] [PubMed]
- Taj, S.; Ashfaq, U.A.; Aslam, S.; Ahmad, M.; Bhatti, S.H. Alpha-glucosidase activity of novel pyrazolobenzothiazine 5, 5-dioxide derivatives for the treatment of diabetes mellitus. Invitro combined with molecular docking approach. Biologia 2019, 74, 1523–1530. [Google Scholar] [CrossRef]
- Javaid, A.; Ashfaq, U.A.; Zafar, Z.; Akmal, A.; Taj, S.; Khalid, H. Phytochemical Analysis and Antidiabetic Potential of Armoracia Rusticana: Pharmacological and Computational Approach. Comb. Chem. High Throughput Screen. 2021, 24, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Saddique, F.A.; Ahmad, M.; Ashfaq, U.A.; Ahmad, M.N.; Anjum, M.N.; Mohsin, N.U.A.; Aslam, S. Alpha-glucosidase inhibition and molecular docking studies of 1, 2-benzothiazine 1, 1-dioxide based carbohydrazides. Pak. J. Pharm. Sci. 2019, 32, 2829–2834. [Google Scholar] [PubMed]
- Dudek-Wicher, R.K.D.; Szczęśniak-Sięga, B.M.; Wiglusz, R.J.; Janczak, J.; Bartoszewicz, M.; Junka, A.F. Evaluation of 1, 2-benzothiazine 1, 1-dioxide derivatives in vitro activity towards clinical-relevant microorganisms and fibroblasts. Molecules 2020, 25, 3503. [Google Scholar] [CrossRef]
- Ahmad, M.; Aslam, S.; Bukhari, M.H.; Montero, C.; Detorio, M.; Parvez, M.; Schinazi, R.F. Synthesis of novel pyrazolobenzothiazine 5, 5-dioxide derivatives as potent anti-HIV-1 agents. Med. Chem. Res. 2014, 23, 1309–1319. [Google Scholar] [CrossRef]
- Henderson, B.J.; Carper, D.J.; Gonzalez-Cestari, T.F.; Yi, B.; Mahasenan, V.; Pavlovicz, R.E.; Dalefield, M.L.; Coleman, R.S.; Li, C.; McKay, D.B. Structure–activity relationship studies of sulfonylpiperazine analogues as novel negative allosteric modulators of human neuronal nicotinic receptors. J. Med. Chem. 2011, 54, 8681–8692. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwon, S.W.; Chu, S.Y.; Lee, J.H.; Narsaiah, B.; Kim, C.H.; Kang, S.K.; Kang, N.S.; Rhee, S.D.; Bae, M.A.; et al. Identification of Cyclicsulfonamide Derivatives with an Acetamide Group as 11β-Hydroxysteroid Dehydrogenase 1 Inhibitors. Chem. Pharm. Bull. 2011, 59, 46–52. [Google Scholar] [CrossRef][Green Version]
- Ahmad, N.; Zia-ur-Rehman, M.; Siddiqui, H.L.; Ullah, M.F.; Parvez, M. Microwave assisted synthesis and structure-activity relationship of 4-hydroxy-N′-[1-phenylethylidene]-2H/2-methyl-1,2-benzothiazine-3-carbohydrazide 1,1-dioxides as anti-microbial agents. Eur. J. Med. Chem. 2011, 46, 2368–2377. [Google Scholar] [CrossRef]
- Shin, Y.S.; Lee, J.Y.; Noh, S.; Kwak, Y.; Jeon, S.; Kwon, S.; Jin, Y.H.; Jang, M.S.; Kim, S.; Song, J.H.; et al. Discovery of cyclic sulfonamide derivatives as potent inhibitors of SARS-CoV-2. Bioorg. Med. Chem. Lett. 2021, 31, 127667. [Google Scholar] [CrossRef]
- Sabbah, D.A.; Haroon, R.A.; Bardaweel, S.K.; Hajjo, R.; Sweidan, K. N-phenyl-6-chloro-4-hydroxy-2-quinolone-3-carboxamides: Molecular Docking, Synthesis, and Biological Investigation as Anticancer Agents. Molecules 2021, 26, 73. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Alshamrani, F.J.; Rahim, F.; Hayat, S.; Ullah, H.; Zaman, K.; Imran, S.; Khan, K.M.; Naz, F. Synthesis of novel triazinoindole-based thiourea hybrid: A study on α-glucosidase inhibitors and their molecular docking. Molecules 2019, 24, 3819. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, I.H.; El-Sheshtawy, H.S.; Bakr, R.B.; Elkanzi, N. New 1,2,3-Triazole-Containing Hybrids as Antitumor Candidates: Design, Click Reaction Synthesis, DFT Calculations, and Molecular Docking Study. Molecules 2021, 26, 708. [Google Scholar] [CrossRef]
- Munir, R.; Zia-ur-Rehman, M.; Murtaza, S.; Zaib, S.; Javid, N.; Awan, S.J.; Iftikhar, K.; Athar, M.M.; Khan, I. Microwave-Assisted Synthesis of (Piperidin-1-yl) quinolin-3-yl) methylene) hydrazinecarbothioamides as Potent Inhibitors of Cholinesterases: A Biochemical and In Silico Approach. Molecules 2021, 26, 656. [Google Scholar] [CrossRef]
- Lavecchia, A.; Giovanni, C.D. Virtual screening strategies in drug discovery: A critical review. Curr. Med. Chem. 2013, 20, 2839–2860. [Google Scholar] [CrossRef]
- Ruyck, J.D.; Brysbaert, G.; Blossey, R.; Lensink, M.F. Molecular docking as a popular tool in drug design, an in silico travel. Adv. Appl. Bioinform. Chem. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Liebeschuetz, J.W.; Cole, J.C.; Korb, O. Pose prediction and virtual screening performance of GOLD scoring functions in a standardized test. J. Comput. Aided Mol. Des. 2012, 26, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Takahira, K.; Tanabe, G.; Morikawa, T.; Sakano, M.; Ninomiya, K.; Yoshikawa, M.; Muraoka, O.; Nakanishi, I. Docking and SAR studies of salacinol derivatives as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 4420–4423. [Google Scholar] [CrossRef] [PubMed]
- Promyos, N.; Temviriyanukul, P.; Suttisansanee, U. Investigation of anthocyanidins and anthocyanins for targeting α-glucosidase in diabetes mellitus. Prev. Nutr. Food Sci. USA 2020, 25, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Nursamsiar, N.; Mangande, M.M.; Awaluddin, A.; Nur, S.; Asnawi, A. In Silico study of aglycon curculigoside A and its derivatives as α-amylase inhibitors. Indones. J. Pharm. Sci. Technol. 2020, 7, 29–37. [Google Scholar] [CrossRef]
- Abbasi, M.A.; Siddiqui, S.Z.; Mumtaz, A.; Shah, S.A.A.; Ashraf, M.; Abbasi, G.H. Synthesis of some new N-(alkyl/aralkyl)-N-(2,3-dihydro-1,4-benzodioxan-6-yl)-4-chlorobenzenesulfonamides as possible therapeutic agents for Alzheimer’s disease and Type-2 Diabetes. Pak. J. Pharm. Sci. 2019, 32, 61–68. [Google Scholar] [PubMed]
- Kasturi, S.; Surarapu, S.; Uppalanchi, S.; Anireddy, J.S.; Dwivedi, S.; Anantaraju, H.S.; Perumal, Y.; Sigalapalli, D.K.; Babu, B.N.; Ethiraj, K.S. Synthesis and α-glucosidase inhibition activity of dihydroxy pyrrolidines. Bioorg. Med. Chem. Lett. 2017, 27, 2818–2823. [Google Scholar] [CrossRef]
- Ali, M.; Barakat, A.; El-Faham, A.; Al-Rasheed, H.H.; Dahlous, K.; Al-Majid, A.M.; Sharma, A.; Yousuf, S.; Sanam, M.; Ul-Haq, Z.; et al. Synthesis and characterization of thiobarbituric acid enamine derivatives and evaluation of their α-glucosidase inhibitory and anti-glycation activity. J. Enzym. Inhib. Med. Chem. 2020, 35, 692–701. [Google Scholar] [CrossRef]
- Salar, U.; Taha, M.; Khan, K.M.; Ismail, N.H.; Imran, S.; Perveen, S.; Gul, S.; Wadood, A. Syntheses of new 3-thiazolyl coumarin derivatives, in vitro α-glucosidase inhibitory activity, and molecular modeling studies. Eur. J. Med. Chem. 2016, 122, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Ismail, N.H.; Imran, S.; Wadood, A.; Rahim, F.; Saad, S.M.; Khan, K.M.; Nasir, A. Synthesis, molecular docking and α-glucosidase inhibition of 5-aryl-2-(6’-nitrobenzofuran-2’-yl)-1,3,4-oxadiazoles. Bioorg. Chem. 2016, 1, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Zawawi, N.K.; Taha, M.; Ahmat, N.; Wadood, A.; Ismail, N.H.; Rahim, F.; Azam, S.S.; Abdullah, N. Benzimidazole derivatives as new α-glucosidase inhibitors and in silico studies. Bioorg. Chem. 2016, 1, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mosihuzzman, M.; Naheed, S.; Hareem, S.; Talib, S.; Abbas, G.; Khan, S.N.; Israr, M. Studies on α-glucosidase inhibition and anti-glycation potential of Iris loczyi and Iris unguicularis. Life Sci. 2013, 92, 187–192. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).