Evaluation of Motor Coordination and Antidepressant Activities of Cinnamomum osmophloeum ct. Linalool Leaf Oil in Rodent Model
Abstract
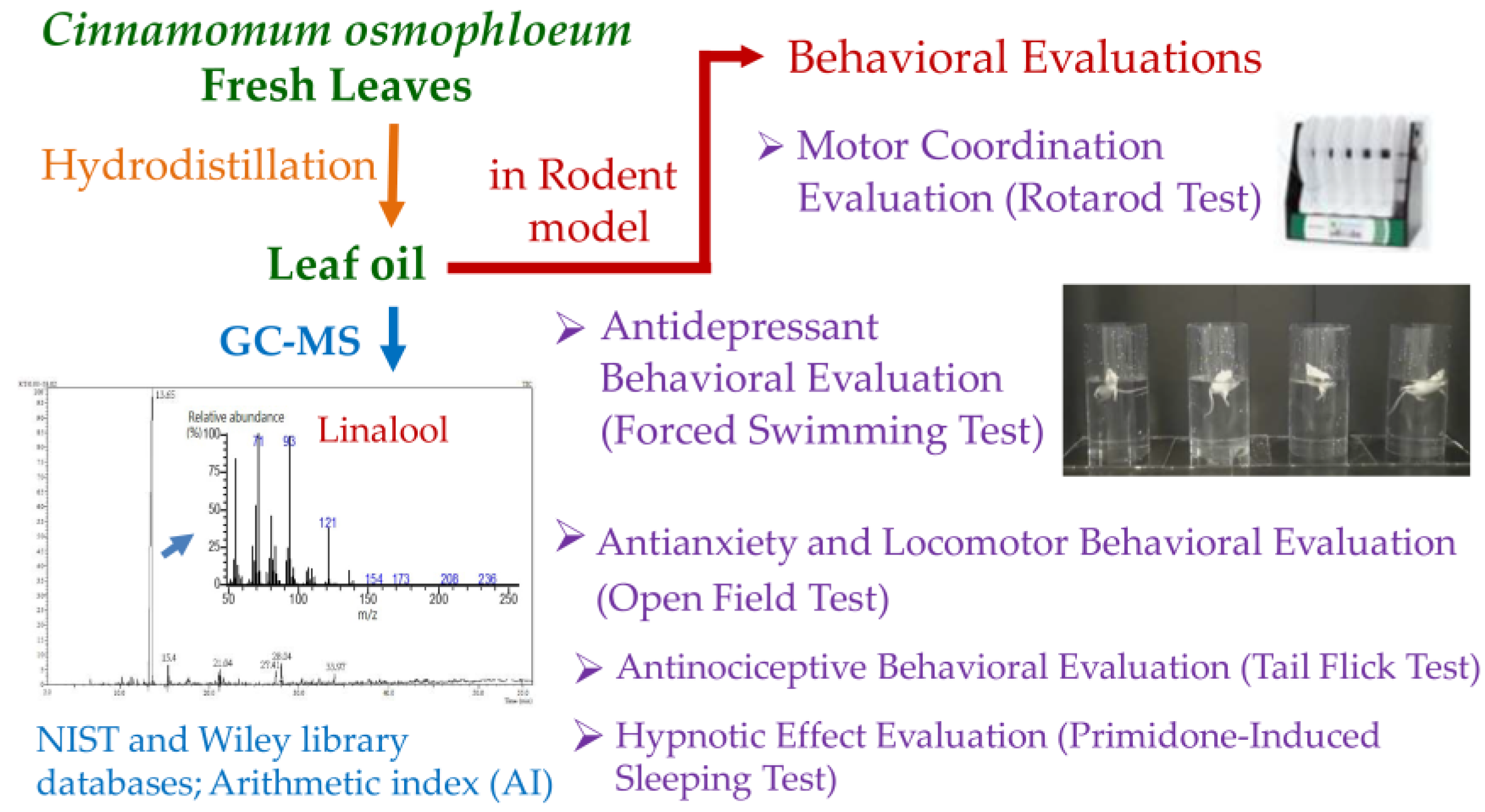
1. Introduction
2. Results and Discussion
2.1. Constituent Analysis of Cinnamomum osmophloeum ct. Linalool Leaf Oil
2.2. Effect of Leaf Oil on the Body Weight of Mice
2.3. Effect of Leaf Oil on the Antianxiety and Locomotor Behavior of Mice
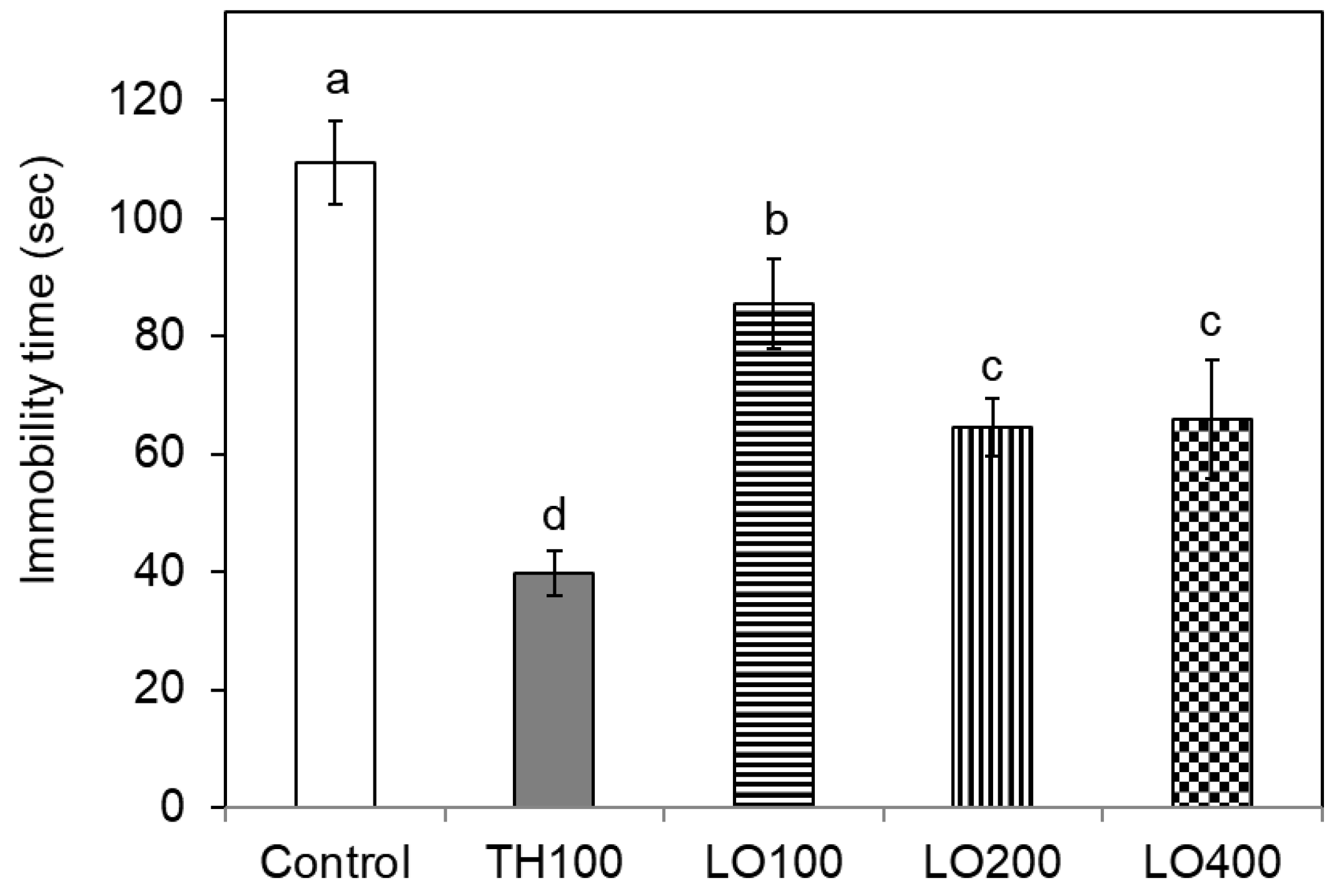
2.4. Effect of Leaf Oil on the Antidepressant Behavior of Mice
2.5. Effect of Leaf Oil on the Antinociceptive Behavior of Mice
2.6. Effect of Leaf Oil on the Hypnotic Effect of Mice
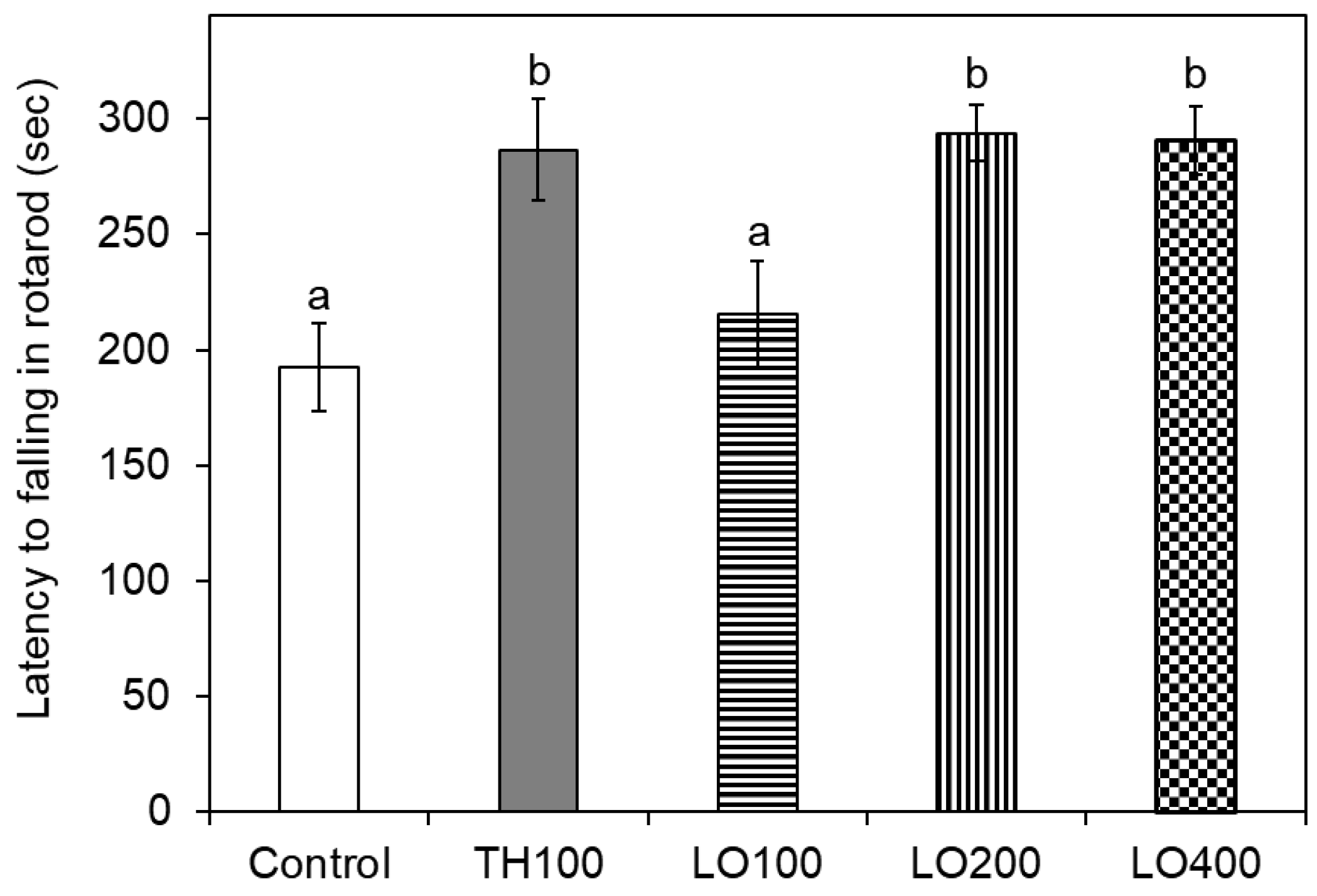
2.7. Effect of Leaf Oil on the Motor Coordination Behavior of Mice
3. Materials and Methods
3.1. Hydrodistillation of Leaf Oil
3.2. GC-MS Analysis of Leaf Oil
3.3. Animals and Treatments
3.4. Open Field Test (Antianxiety and Locomotor Behavioral Evaluation)
3.5. Forced Swimming Test (Antidepressant Behavioral Evaluation)
3.6. Tail Flick Test (Antinociceptive Behavioral Evaluation)
3.7. Primidone-Induced Sleeping Test (Hypnotic Effect Evaluation)
3.8. Rotarod Test (Motor Coordination Evaluation)
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chang, S.T.; Chen, P.F.; Chang, S.C. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J. Ethnopharmacol. 2001, 77, 123–127. [Google Scholar] [CrossRef]
- Huang, C.Y.; Yeh, T.F.; Hsu, F.L.; Lin, C.Y.; Chang, S.T.; Chang, H.T. Xanthine oxidase inhibitory activity and thermostability of cinnamaldehyde-chemotype leaf oil of Cinnamomum osmophloeum microencapsulated with β-cyclodextrin. Molecules 2018, 23, 1107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Liu, J.Y.; Huang, C.G.; Hsui, Y.R.; Chen, W.J.; Chang, S.T. Insecticidal activities of leaf essential oils from Cinnamomum osmophloeum against three mosquito species. Bioresour. Technol. 2009, 100, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Chang, H.T.; Hsui, Y.R.; Hsu, Y.W.; Liu, J.Y.; Wang, S.Y.; Chang, S.T. Antioxidant-enriched leaf water extracts of Cinnamomum osmophloeum from eleven provenances and their bioactive flavonoid glycosides. BioResources 2013, 8, 571–580. [Google Scholar] [CrossRef]
- Cheng, B.H.; Sheen, L.Y.; Chang, S.T. Evaluation of anxiolytic potency of essential oil and S-(+)-linalool from Cinnamomum osmophloeum ct. linalool leaves in mice. J. Tradit. Complement. Med. 2015, 5, 27–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, A.L. St. John’s Wort (Hypericum perforatum): Clinical effects on depression and other conditions. Altern. Med. Rev. 1998, 3, 18–26. [Google Scholar]
- Chatterjee, S.S.; Bhattacharya, S.K.; Wonnemann, M.; Singer, A.; Muller, W.E. Hyperforin as a possible antidepressant component of hypericum extracts. Life Sci. 1998, 63, 499–510. [Google Scholar] [CrossRef]
- Butterweck, V.; Schmidt, M. St. John’s wort: Role of active compounds for its mechanism of action and efficacy. Wien. Med. Wochenschr. 2007, 157, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C.P. Neuroprotective activity of Hypericum perforatum and its major components. Front. Plant Sci. 2016, 7, 1004. [Google Scholar] [CrossRef] [PubMed]
- Forsdike, K.; Pirotta, M. St John’s wort for depression: Scoping review about perceptions and use by general practitioners in clinical practice. J. Pharm. Pharmacol. 2019, 71, 117–128. [Google Scholar] [CrossRef]
- Rahmati, B.; Kiasalari, Z.; Roghani, M.; Khalili, M.; Ansari, F. Antidepressant and anxiolytic activity of Lavandula officinalis aerial parts hydroalcoholic extract in scopolamine-treated rats. Pharm. Biol. 2017, 55, 958–965. [Google Scholar] [CrossRef]
- Hritcu, L.; Noumedem, J.; Cioanca, O.; Hancianu, M.; Postu, P.; Mihasan, M. Anxiolytic and antidepressant profile of the methanolic extract of Piper nigrum fruits in beta-amyloid (1–42) rat model of Alzheimer’s disease. Behav. Brain Funct. 2015, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Mannan, A.; Abir, A.B.; Rahman, R. Antidepressant-like effects of methanolic extract of Bacopa monniera in mice. BMC Complement. Altern. Med. 2015, 15, 337. [Google Scholar] [CrossRef] [PubMed]
- Cassani, J.; Dorantes-Barron, A.M.; Novales, L.M.; Real, G.A.; Estrada-Reyes, R. Anti-depressant-like effect of kaempferitrin isolated from Justicia spicigera Schltdl (Acanthaceae) in two behavior models in mice: Evidence for the involvement of the serotonergic system. Molecules 2014, 19, 21442–21461. [Google Scholar] [CrossRef]
- Jose Bagur, M.; Alonso Salinas, G.L.; Jimenez-Monreal, A.M.; Chaouqi, S.; Llorens, S.; Martinez-Tome, M.; Alonso, G.L. Saffron: An old medicinal plant and a potential novel functional food. Molecules 2017, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Panossian, A.; Schweitzer, I.; Stough, C.; Scholey, A. Herbal medicine for depression, anxiety and insomnia: A review of psychopharmacology and clinical evidence. Eur. Neuropsychopharmacol. 2011, 21, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.L.; Akinfiresoye, L.; Nwulia, E.; Kamiya, A.; Kulkarni, A.; Tizabi, Y. Antidepressant-like effects of curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF. Behav. Brain Res. 2013, 239, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.L.; Akinfiresoye, L.; Kalejaiye, O.; Tizabi, Y. Antidepressant effects of resveratrol in an animal model of depression. Behav. Brain Res. 2014, 268, 1–7. [Google Scholar] [CrossRef]
- Williams, J.W.; Mulrow, C.D.; Chiquette, E.; Noel, P.H.; Aguilar, C.; Cornell, J. A systematic review of newer pharmacotherapies for depression in adults: Evidence report summary. Ann. Intern. Med. 2000, 132, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Ebrahimzadeh, M.A.; Abdi, M.; Arimi, Y.; Fathi, H. Antidepressant activities of Feijoa sellowiana fruit. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2510–2513. [Google Scholar]
- Mangolini, V.I.; Andrade, L.H.; Lotufo-Neto, F.; Wang, Y.P. Treatment of anxiety disorders in clinical practice: A critical overview of recent systematic evidence. Clinics 2019, 74, e1316. [Google Scholar] [CrossRef]
- Linck, V.M.; Silva, A.L.; Figueiro, M.; Caramao, E.B.; Moreno, P.R.H.; Elisabetsky, E. Effects of inhaled linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef]
- Harada, H.; Kashiwadani, H.; Kanmura, Y.; Kuwaki, T. Linalool odor-induced anxiolytic effects in mice. Front. Behav. Neurosci. 2018, 23, 241. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Reguilon, M.; Minarro, J.; De Feo, V.; Rodriguez-Arias, M. Lavandula angustifolia essential oil and linalool counteract social aversion induced by social defeat. Molecules 2018, 23, 2694. [Google Scholar] [CrossRef]
- Maria, D.B.A.; Maria, V.V.R.; Lilian, M.N.; Lucia, M.M.; Oscar, G.P.; Rosa, E.R. Neurobehavioral and toxicological effects of an aqueous extract of Turnera diffusa Willd (Turneraceae) in mice. J. Ethnopharmacol. 2019, 236, 50–62. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Askari, V.; Tajani, A.; Hosseini, A.; Rakhshandeh, H. Evaluation of the sleep-prolonging effect of Lagenaria vulgaris and Cucurbita pepo extracts on pentobarbital-induced sleep and possible mechanisms of action. Medicina 2018, 54, 55. [Google Scholar] [CrossRef] [PubMed]
- Muthaiyah, B.; Essa, M.M.; Lee, M.; Chauhan, V.; Kaur, K.; Chauhan, A. Dietary supplementation of walnuts improves memory deficits and learning skills in transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2014, 42, 1397–1405. [Google Scholar] [CrossRef]
- Rekha, K.R.; Selvakumar, G.P.; Sethupathy, S.; Santha, K.; Sivakamasundari, R.I. Geraniol ameliorates the motor behavior and neurotrophic factors inadequacy in MPTP-induced mice model of Parkinson’s disease. J. Mol. Neurosci. 2013, 51, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas. Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; pp. 6–398. ISBN 978-1932633214. [Google Scholar]
- Raghavendra, M.; Maiti, R.; Kumar, S.; Acharya, S. Role of aqueous extract of Azadirachta indica leaves in an experimental model of Alzheimer’s disease in rats. Int. J. Appl. Basic Med. Res. 2013, 3, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Wanda, G.J.M.K.; Djiogue, S.; Gamo, F.Z.; Ngitedem, S.G.; Njamen, D. Anxiolytic and sedative activities of aqueous leaf extract of Dichrocephala integrifolia (Asteraceae) in mice. J. Ethnopharmacol. 2015, 176, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Panday, D.R.; Rauniar, G.P. Effect of root-extracts of Ficus benghalensis (Banyan) in memory, anxiety, muscle coordination and seizure in animal models. BMC Complement. Altern. Med. 2016, 16, 429. [Google Scholar] [CrossRef] [PubMed]
- Vilela, F.C.; Padilha, M.M.; Alves-da-Silva, G.; Soncini, R.; Giusti-Paiva, A. Antidepressant-like activity of Sonchus oleraceus in mouse models of immobility tests. J. Med. Food 2010, 13, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Rojas, P.; Serrano-Garcia, N.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Ogren, S.O.; Rojas, C. Antidepressant-like effect of a Ginkgo biloba extract (EGb761) in the mouse forced swimming test: Role of oxidative stress. Neurochem. Int. 2011, 59, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Fajemiroye, J.O.; Galdino, P.M.; De Paula, J.A.; Rocha, F.F.; Akanmu, M.A.; Vanderlinde, F.A.; Zjawiony, J.K.; Costa, E.A. Anxiolytic and antidepressant like effects of natural food flavour (E)-methyl isoeugenol. Food Funct. 2014, 5, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Couto, V.M.; Vilela, F.C.; Dias, D.F.; Santos, M.H.; Soncini, R.; Nascimento, C.G.O.; Giusti-Paiva, A. Antinociceptive effect of extract of Emilia sonchifolia in mice. J. Ethnopharmacol. 2011, 134, 348–353. [Google Scholar] [CrossRef]
- Jokinen, V.; Lilius, T.; Laitila, J.; Niemi, M.; Kambur, O.; Kalso, E.; Rauhala, P. Do diuretics have antinociceptive actions: Studies of spironolactone, eplerenone, furosemide and chlorothiazide, individually and with oxycodone and morphine. Basic Clin. Pharmacol. Toxicol. 2017, 120, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Bashir, A.K.; Tanira, M.O. Some effects of Cassia italica on the central nervous system in mice. J. Pharm. Pharmacol. 1997, 49, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.I.; de Aquino Neto, M.R.; Teixeira Neto, P.F.; Moura, B.A.; do Amaral, J.F.; de Sousa, D.P.; Vasconcelos, S.M.; de Sousa, F.C. Central nervous system activity of acute administration of isopulegol in mice. Pharmacol. Biochem. Behav. 2007, 88, 141–147. [Google Scholar] [CrossRef]
- Shiotsuki, H.; Yoshimi, K.; Shimo, Y.; Funayama, M.; Takamatsu, Y.; Ikeda, K.; Takahashi, R.; Kitazawa, S.; Hattori, N. A rotarod test for evaluation of motor skill learning. J. Neurosci. Methods 2010, 189, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Bridi, H.; Ccana-Ccapatinta, G.V.; Stolz, E.D.; Meirelles, G.C.; Bordignon, S.A.L.; Rates, S.M.K.; von Poser, G.L. Dimeric acylphloroglucinols from Hypericum austrobrasiliense exhibiting antinociceptive activity in mice. Phytochemistry 2016, 122, 178–183. [Google Scholar] [CrossRef]
- Bisong, S.A.; Brown, R.; Osim, E.E. Comparative effects of Rauwolfia vomitoria and chlorpromazine on locomotor behaviour and anxiety in mice. J. Ethnopharmacol. 2010, 132, 334–339. [Google Scholar] [CrossRef] [PubMed]
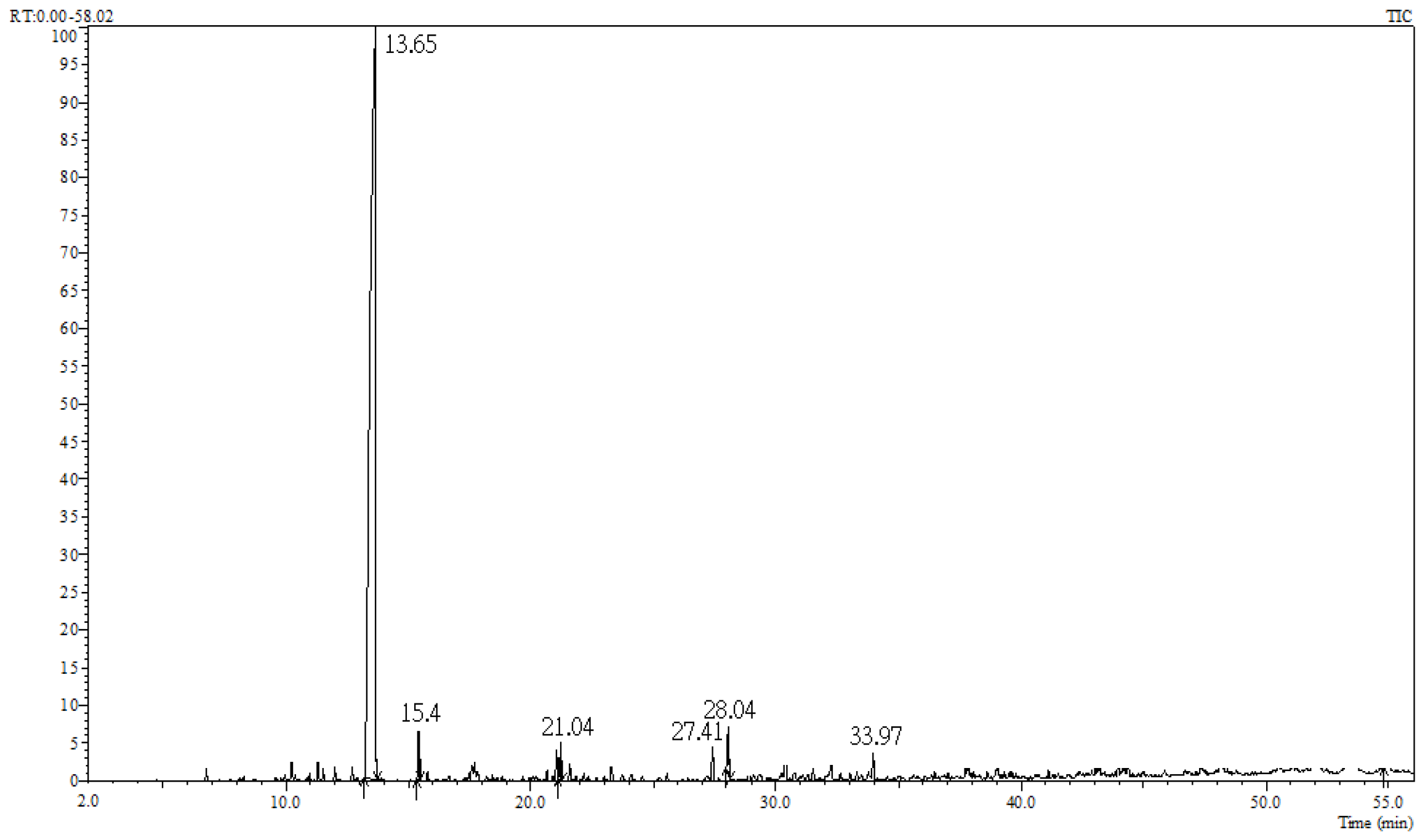
| RT | AI | Compound | Formula | Relative Content (%) | Identification Method |
|---|---|---|---|---|---|
| 13.65 | 1091 | Linalool | C10H18O | 93.2 | MS, AI |
| 15.40 | 1141 | Camphor | C10H16O | 1.5 | MS, AI |
| 21.04 | 1264 | trans-Cinnamaldehyde | C9H8O | 1.0 | MS, AI |
| 27.41 | 1418 | β-Caryophyllene | C15H24 | 1.2 | MS, AI |
| 28.04 | 1431 | Coumarin | C9H6O2 | 2.3 | MS, AI |
| 33.97 | 1580 | Caryophyllene oxide | C15H24O | 0.8 | MS, AI |
| Control | TH100 | LO100 | LO200 | LO400 | |
|---|---|---|---|---|---|
| Initial body weight (g) * | 30.1 ± 1.6 | 30.4 ± 2.1 | 30.3 ± 1.5 | 30.7 ± 1.5 | 30.1 ± 2.1 |
| Body weight after 6 weeks (g) * | 37.9 ± 2.3 | 37.2 ± 2.2 | 37.9 ± 2.1 | 38.1 ± 3.2 | 37.1 ± 3.1 |
| Weight gain (g/6 weeks) * | 7.6 ± 2.1 | 7.1 ± 1.1 | 8.2 ± 1.9 | 6.8 ± 2.1 | 7.6 ± 2.1 |
| Specimen | Number of Entries into Central Zone | Total Distance Traveled (m) |
| Control | 18.00 ± 4.47 a | 21.28 ± 2.11 A |
| TH100 * | 31.14 ± 3.76 c | 25.70 ± 2.56 B |
| LO100 | 20.71 ± 4.79 a,b | 21.58 ± 2.88 A |
| LO200 | 28.71 ± 5.22 b,c | 25.30 ± 2.62 B |
| LO400 | 31.00 ± 3.87 c | 24.39 ± 2.33 B |
| Specimen | Time (sec) in the Tail Flick Test * | Duration (min) in the Primidone-Induced Sleeping Test * |
|---|---|---|
| Control | 3.75 ± 0.38 | 55.08 ± 4.72 |
| TH100 | 3.49 ± 0.32 | 49.87 ± 5.36 |
| LO100 | 3.91 ± 0.12 | 49.91 ± 4.59 |
| LO200 | 3.81 ± 0.28 | 50.10 ± 4.22 |
| LO400 | 4.01 ± 0.19 | 52.10 ± 5.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-T.; Chang, M.-L.; Chen, Y.-T.; Chang, S.-T.; Hsu, F.-L.; Wu, C.-C.; Ho, C.-K. Evaluation of Motor Coordination and Antidepressant Activities of Cinnamomum osmophloeum ct. Linalool Leaf Oil in Rodent Model. Molecules 2021, 26, 3037. https://doi.org/10.3390/molecules26103037
Chang H-T, Chang M-L, Chen Y-T, Chang S-T, Hsu F-L, Wu C-C, Ho C-K. Evaluation of Motor Coordination and Antidepressant Activities of Cinnamomum osmophloeum ct. Linalool Leaf Oil in Rodent Model. Molecules. 2021; 26(10):3037. https://doi.org/10.3390/molecules26103037
Chicago/Turabian StyleChang, Hui-Ting, Mei-Ling Chang, Yen-Ting Chen, Shang-Tzen Chang, Fu-Lan Hsu, Chia-Chen Wu, and Cheng-Kuen Ho. 2021. "Evaluation of Motor Coordination and Antidepressant Activities of Cinnamomum osmophloeum ct. Linalool Leaf Oil in Rodent Model" Molecules 26, no. 10: 3037. https://doi.org/10.3390/molecules26103037
APA StyleChang, H.-T., Chang, M.-L., Chen, Y.-T., Chang, S.-T., Hsu, F.-L., Wu, C.-C., & Ho, C.-K. (2021). Evaluation of Motor Coordination and Antidepressant Activities of Cinnamomum osmophloeum ct. Linalool Leaf Oil in Rodent Model. Molecules, 26(10), 3037. https://doi.org/10.3390/molecules26103037






