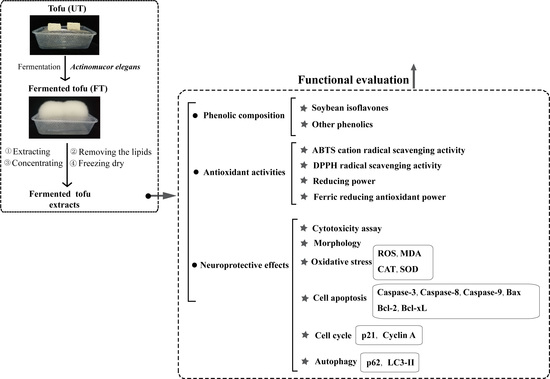
Neuroprotective Potency of Tofu Bio-Processed Using Actinomucor elegans against Hypoxic Injury Induced by Cobalt Chloride in PC12 Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Content and Antioxidant Activity
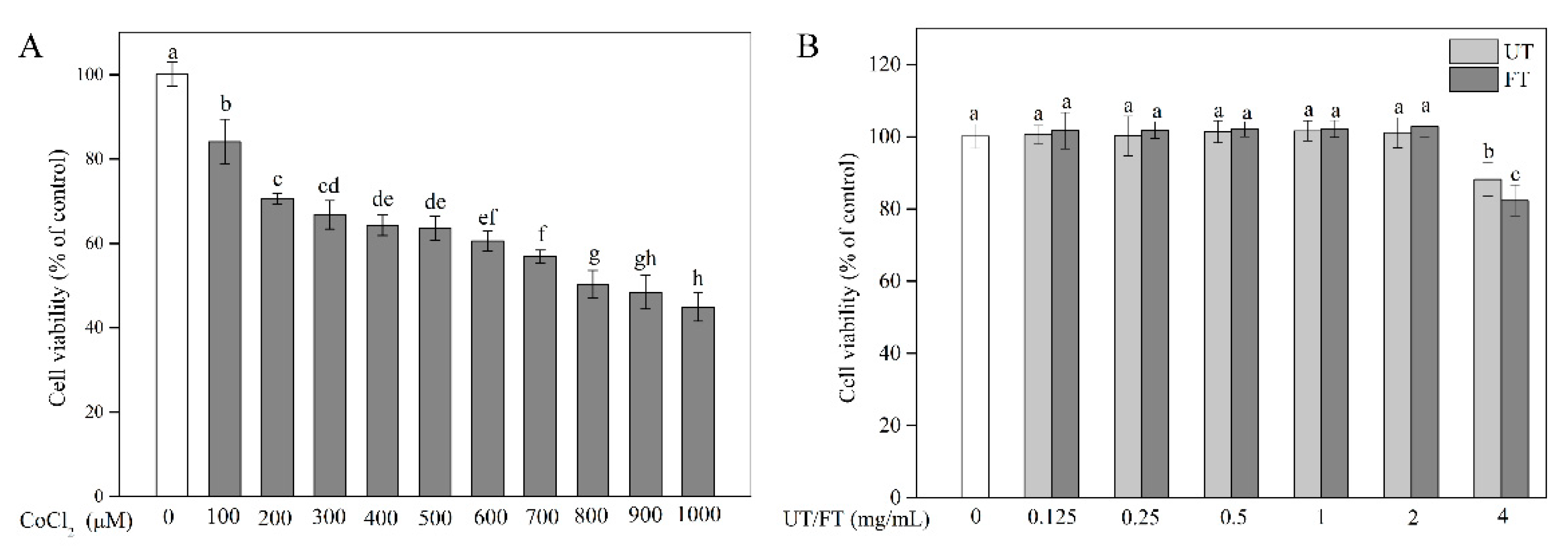
2.2. Evaluation of CoCl2, UT, and FT for Cytotoxicity
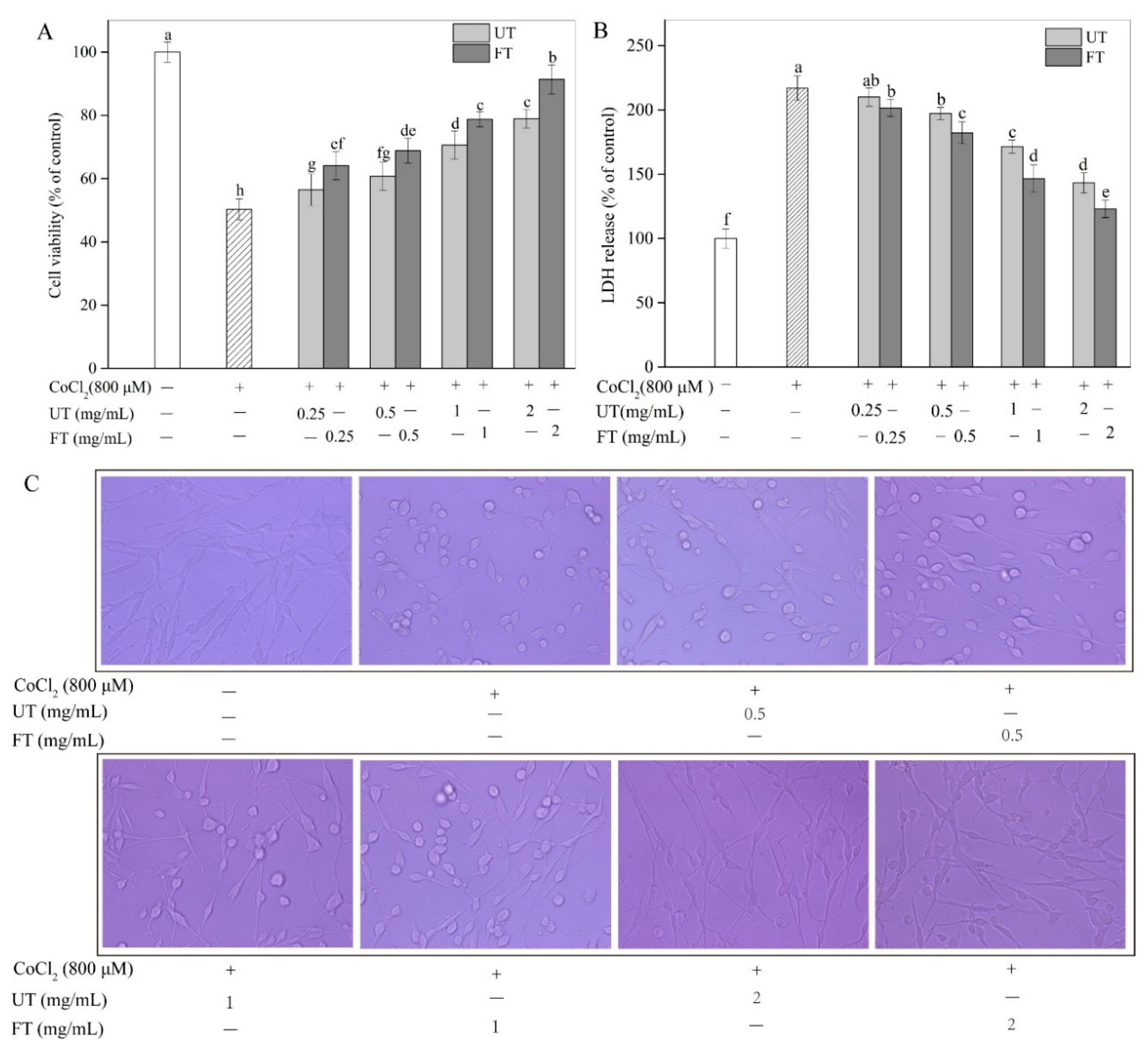
2.3. Protective Effects of UT and FT against Hypoxic Injury
2.4. Attenuation of CoCl2-Induced Oxidative Stress with UT and FT
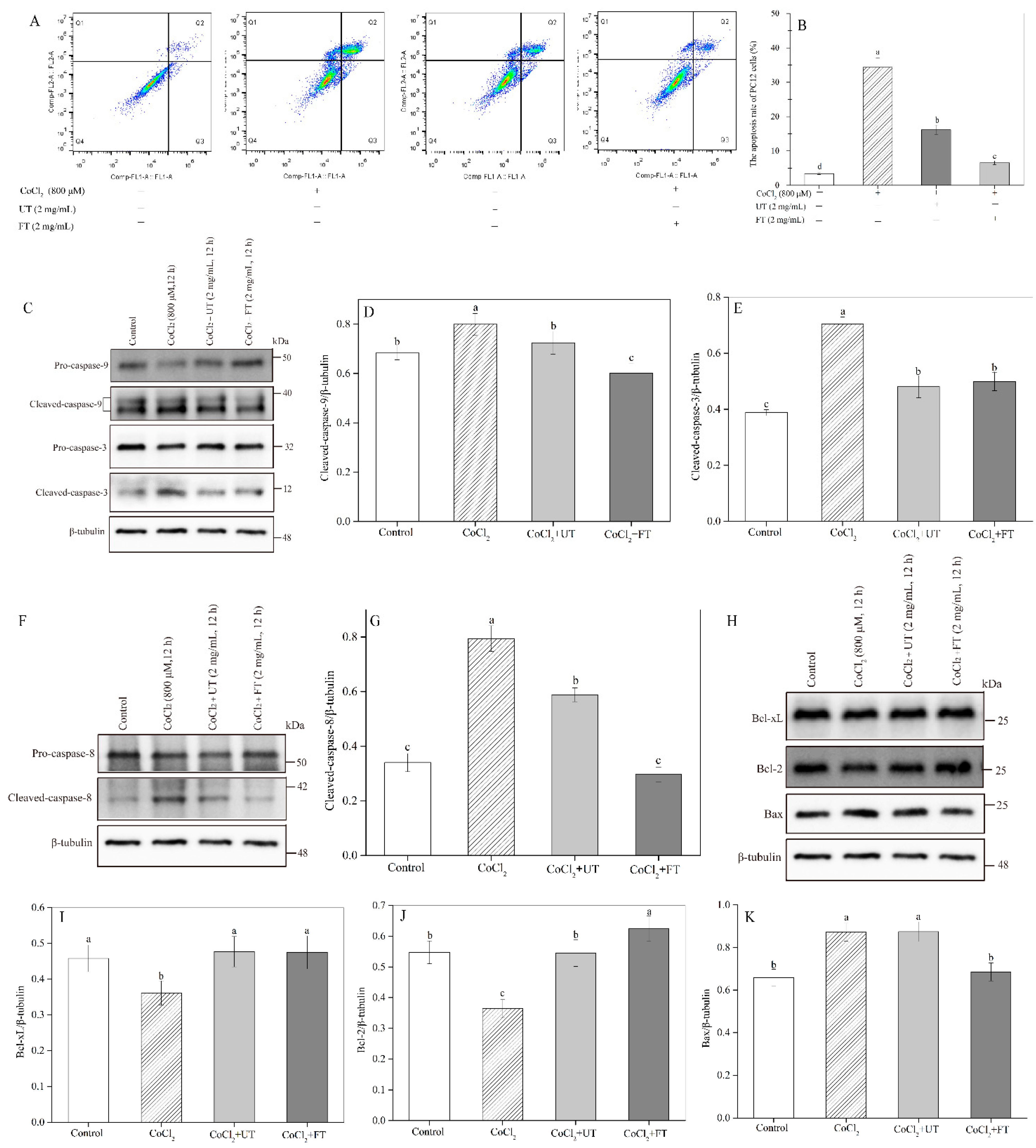
2.5. Retardation of CoCl2—Induced Cell Apoptosis by the UT and FT
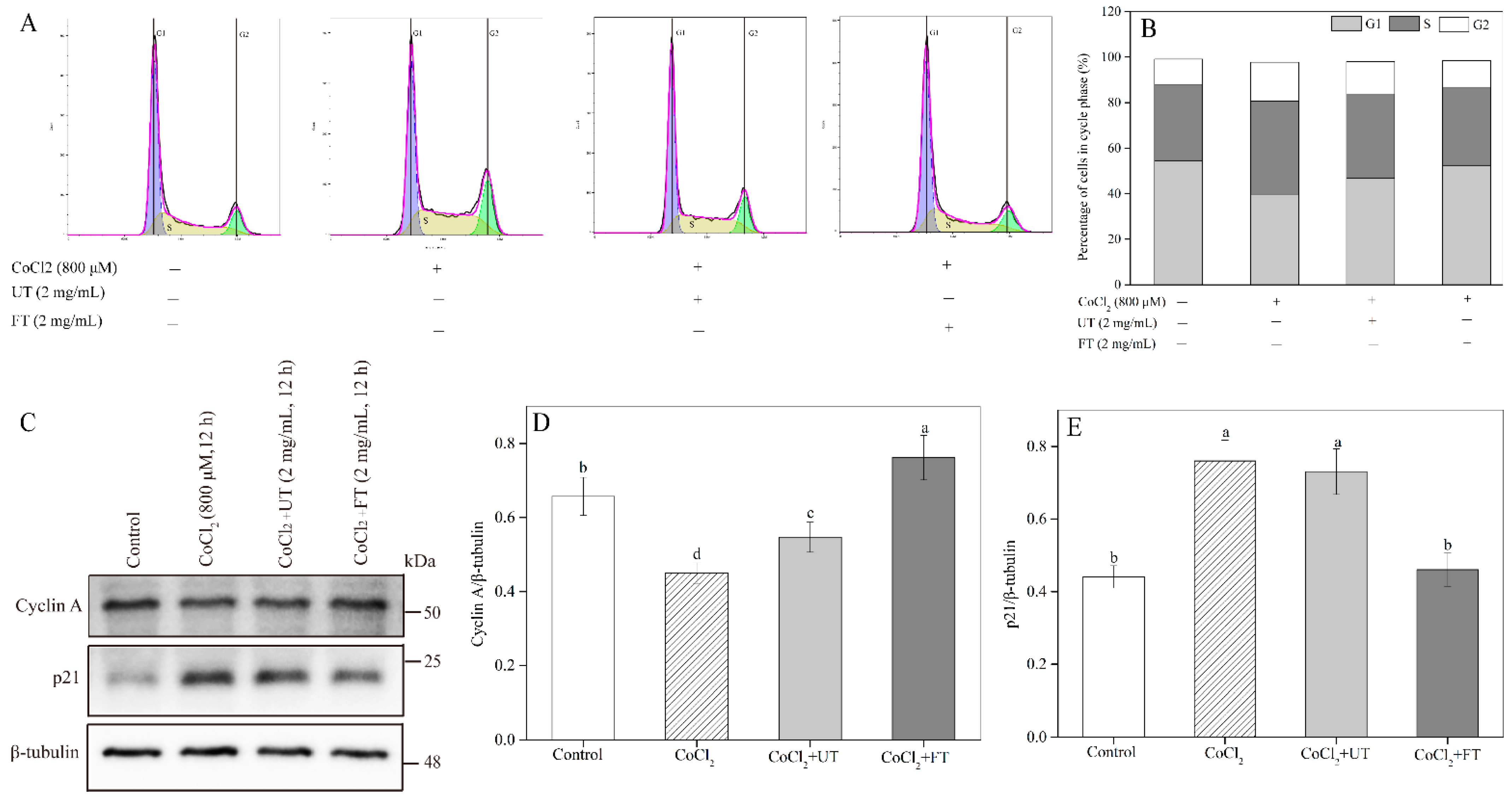
2.6. Inhibitory Effects of UT and FT on Cell Cycle in Hypoxic Injury
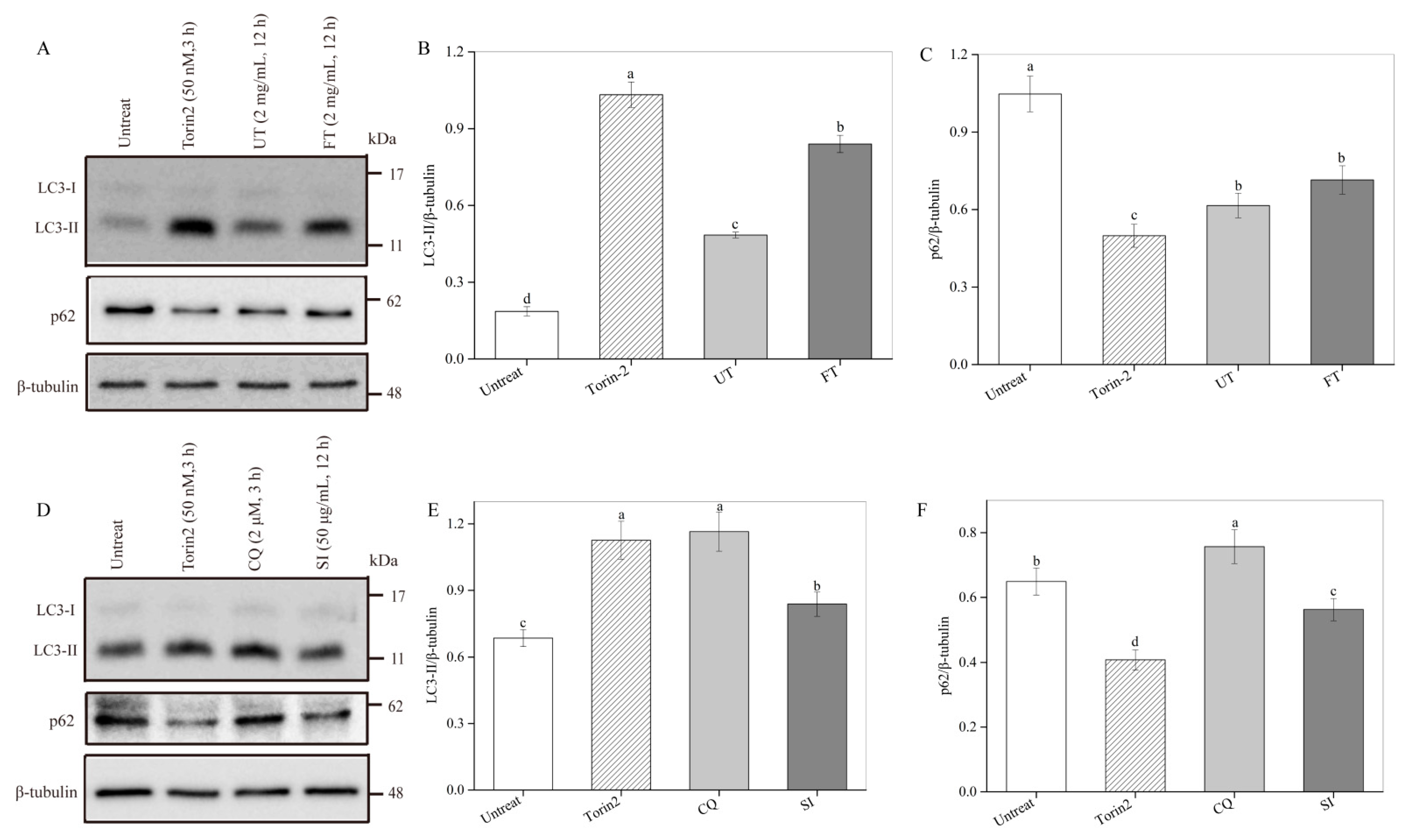
2.7. Activation of Cell Autophagy by the UT and FT
3. Materials and Methods
3.1. Materials and Microorganism
3.2. Tofu Fermentation and Sample Preparation
3.3. Analysis for Phenolic Content and Antioxidant Activity
3.4. Cell Culture
3.5. Cytotoxicity Assay
3.6. Cell Viability Assay
3.7. Effects of UT and FT on the LDH Release under Hypoxic Injury
3.8. Effects of UT and FT on ROS Formation during Hypoxic Injury
3.9. Effects of UT and FT on the MDA Level under Hypoxic Injury
3.10. Effects of UT and FT on Antioxidant Enzyme Activities during Hypoxic Injury
3.11. Effects of UT and FT on Cell Apoptosis during Hypoxic Injury
3.12. Effects of UT and FT on Cell Cycle during Hypoxic Injury
3.13. Effects of UT and FT on Autophagy
3.14. Western Blot Analysis
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, X.; Dai, Y.; Wu, H.; Liu, X.; Wang, Y.; Yin, L.; Wang, Z.; Li, X.; Zhou, J. Kombucha fermentation enhances the health-promoting properties of soymilk beverage. J. Funct. Foods 2019, 62, 103549. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, H. Characterization and comparison of phenols, flavonoids and isoflavones of soymilk and their correlations with antioxidant activity. Int. J. Food Sci. Tech. 2015, 49, 2290–2298. [Google Scholar] [CrossRef]
- Xu, L.; Du, B.; Xu, B. A systematic, comparative study on the beneficial health components and antioxidant activities of commercially fermented soy products marketed in China. Food Chem. 2015, 174, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Omoni, A.O.; Aluko, R.E. Soybean foods and their benefits: Potential mechanisms of action. Nutr. Rev. 2005, 63, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Soccol, C.R.; Costa, E.S.F.D.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; Vanden-berghe, L.P.D.S. Recent developments and innovations in solid state fermentation. Bio Technol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Shahzad, R.; Shehzad, A.; Bilal, S.; Lee, I.J. Bacillus amyloliquefaciens RWL-1 as a new potential strain for augmenting biochemical and nutritional composition of fermented soybean. Molecules 2020, 25, 2346. [Google Scholar] [CrossRef]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18. J. Funct. Foods 2014, 10, 210–222. [Google Scholar] [CrossRef]
- Ali, M.W.; Kim, I.D.; Bilal, S.; Shahzad, R.; Saeed, M.T.; Adhikari, B.; Nabi, R.B.S.; Kyo, J.R.; Shin, D.H. Effects of bacterial fermentation on the biochemical constituents and antioxidant potential of fermented and unfermented soybeans using probiotic bacillus subtilis (KCTC 13241). Molecules 2017, 22, 2200. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.H. Effect of fermentation times and extracting solvents on the in vitro immune potentials of the soluble extracts of Mucor-fermented Mao-tofu. Food Sci. Biotechnol. 2017, 26, 707–714. [Google Scholar] [CrossRef]
- Hang, M.; Zhao, X.H. Fermentation time and ethanol/water-based solvent system im-pacted in vitro ACE-inhibitory activity of the extract of Mao-tofu fermented by Mucor spp. CyTA J. Food 2012, 10, 137–143. [Google Scholar] [CrossRef]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkison’s disease, and Huntington’s disease: A mini review. Oxid. Med. Cell Longev. 2016, 8590578. [Google Scholar] [CrossRef]
- Li, M.; Xu, T.; Zhou, F.; Wang, M.; Song, H.; Xiao, X.; Lu, B. Neuroprotective effects of four phenylthanoid glycosides on H2O2-induced apoptosis on PC12 cells via the Nrf2/ARE pathway. Int. J. Mol. Sci. 2018, 19, 1135. [Google Scholar] [CrossRef]
- Choi, W.Y.; Kang, D.H.; Lee, H.Y. Enhancement of neuroprotective effects of spirulina maxima by a low-temperature extraction process with ultrasonic pretreatment. Biotechnol. Bioprocess Eng. 2018, 23, 415–423. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, Y.; Wu, Y.; Liu, Y.; Wu, Z. Fermentation and complex enzyme hydrolysis for improving the total soluble phenolic contents, flavonoid aglycones contents and bioactivities of guava leaves tea. Food Chem. 2018, 264, 189–198. [Google Scholar] [CrossRef]
- Bei, Q.; Chen, G.; Lu, F.; Wu, F.; Wu, Z. Enzymatic action mechanism of phenolic mobilization in oats (Avena sativa L.) during solid-state fermentation with Monascus anka. Food Chem. 2018, 245, 297–304. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Regitano-d’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Enzyme-assisted extraction of phenolics from winemaking by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem. 2016, 212, 395–402. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, Y.; Wu, H.; Wang, Z.; Dai, Y.; Zhou, J.; Liu, X.; Dong, M.; Xia, X. Improvement of the phenolic content, antioxidant activity, and nutritional quality of tofu fermented with Actinomucor elegans. LWT Food Sci. Technol. 2020, 133, 110087. [Google Scholar] [CrossRef]
- Tang, J.; Yan, Y.; Ran, L.; Mi, J.; Sun, Y.; Lu, L.; Gao, Y.; Zeng, X.; Cao, Y. Isolation, antioxidant property and protective effect on PC12 cell of the main anthocyanin in fruit of Lycium ruthenicum Murray. J. Funct. Foods. 2017, 30, 97–107. [Google Scholar] [CrossRef]
- Azi, F.; Tu, C.; Meng, L.; Li, Z.; Tekliye, M.; Ahmadullah, Z.; Dong, M. Metabolite dynamics and phytochemistry of a soy whey-based beverage bio-transformed by water kefir consortium. Food Chem. 2020, 342, 128225. [Google Scholar] [CrossRef]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Yin, T.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer’s disease. J. Colloid Interface Sci. 2019, 552, 388–400. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Kao, S.T.; Lee, Y.C. Ferulic acid exerts anti-apoptotic effects against ischemic injury by activating HSP70/Bcl-2- and HSP70/autophagy-mediated signaling after permanent focal cerebral ischemia in rats. Am. J. Chin. Med. 2019, 47, 39–61. [Google Scholar] [CrossRef]
- Khan, I.; Kang, S.C. Apoptotic activity of Lactobacillus plantarum DGK-17-fermented soybean seed extract in human colon cancer cells via ROS-JNK signaling pathway. J. Food Sci. 2017, 82, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, Y.; Yang, C.; Xu, X.; Meng, Y. Antioxidant and hypolipidemic effects of soymilk fermented via Lactococcus acidophilus MF204. Food Funct. 2017, 8, 4414–4420. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.H.; Tomita, I.; Watanabe, T.; Hirayama, T.; Fukui, S. Active oxygens generation by flavonoids. Biol. Pharm. Bull. 1998, 21, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Jang, H.; Lee, M.; Kang, H.; Heo, H.J.; Kim, D.O. Sea buckthorn (Hippophae rhamnoides L.) leaf extracts protect neuronal PC-12 cells from oxidative stress. J. Microbio. Biotechnol. 2017, 27, 1257–1265. [Google Scholar] [CrossRef]
- Qi, G.; Mi, Y.; Wang, Y.; Li, R.; Huang, S.; Li, X.; Liu, X. Neuroprotective action of tea polyphenols on oxidative stress-induced apoptosis through the activation of the TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y cells and mice brain. Food Funct. 2017, 8, 4421–4432. [Google Scholar] [CrossRef]
- Qian, Y.; Guan, T.; Huang, M.; Cao, L.; Li, Y.; Cheng, H.; Jin, H.; Yu, D. Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochem. Int. 2012, 60, 759–767. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, L.; Huang, L.; Tekliye, M.; Xia, X.; Li, J.; Dong, M. Composition, antioxidant activity, and neuroprotective effects of anthocyanin-rich extract from purple highland barley bran and its promotion on autophagy. Food Chem. 2020, 33, 127849. [Google Scholar] [CrossRef]
- Akinrinde, A.S.; Adebiyi, O.E. Neuroprotection by luteolin and gallic acid against cobalt chloride-induced behavioural, morphological and neurochemical alterations in Wistar rats. Neurotoxicology 2019, 74, 252–263. [Google Scholar] [CrossRef]
- Anwar, S.; Speciale, A.; Fratantonio, D.; Cristani, M.; Ciminoet, F. Cya-nidin-3-O-glucoside modulates intracellular redox status and prevents HIF-1 stabilization in endothelial cells in vitro exposed to chronic hypoxia. Toxicol. Lett. 2014, 226, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Marcotte, R.; Petroulakis, E. Signaling pathway for apoptosis: A racetrack for life or death. J. Cell Biochem. 1999, 32, 95–102. [Google Scholar] [CrossRef]
- Shi, Y.N.; Zhang, X.Q.; Hu, Z.Y.; Zhang, C.J.; Liao, D.F.; Huang, H.L.; Qin, L. Genistein protects H9c2 cardiomyocytes against chemical hypoxia-induced injury via inhibition of apoptosis. Pharmacology 2019, 103, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Colombel, M.; Olsson, C.A.; Ng, P.Y.; Buttyan, R. Hormone-regulated apoptosis results from reentry of differentiated prostate cells onto a defective cell cycle. Cancer Res. 1992, 52, 4313–4319. [Google Scholar] [PubMed]
- Chandrika, B.B.; Yang, C.; Ou, Y.; Feng, X.; Muhoza, D.; Holmes, A.F.; Theus, S.; Deshmukh, S.; Haun, R.S.; Kaushal, G.P. Endoplasmic reticulum stress-induced autophagy provides cytoprotection from chemical hypoxia and oxidant injury and ameliorates renal ischemia-reperfusion injury. PLoS ONE 2015, 10, e0140025. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Su, Z.; Fei, H.; Liu, X.; Pan, Q. The novel mTOR inhibitor Torin-2 induces autophagy and downregulates the expression of UHRF1 to suppress hepatocarcinoma cell growth. Oncol. Rep. 2015, 34, 1708–1716. [Google Scholar] [CrossRef]
- Palmeira-dos-Santos, C.; Pereira, G.J.S.; Barbosa, C.M.V.; Jurkiewicz, A.; Smail, S.S.; Bincoletto, C. Comparative study of autophagy inhibition by 3MA and CQ on cytarabine-induced death of leukaemia cells. J. Cancer Res. Clin. Oncol. 2014, 140, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Guo, L.; Jiang, X.; Wang, Y. Curcumin inhibits cell viability by inducing apoptosis and autophagy in human colon cancer cells. Pro. Anticancer Res. 2019, 3, 21–25. [Google Scholar] [CrossRef]
- Hamacher-Brady, A.; Brady, N.R.; Gottlieb, R.A. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J. Biol. Chem. 2006, 281, 29776–29787. [Google Scholar] [CrossRef]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.-S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, K.; Wu, H.Y.; Wu, Y.P.; Li, B.X. Isoflavones induce BEX2-dependent autophagy to prevent ATR-induced neurotoxicity in SH-SY5Y cells. Cell Physiol. Biochem. 2017, 43, 1866–1879. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.X.; Wu, D.; Wang, X.; Chen, H.L.; Chen, J.X.; Wang, X.X.; Wang, X.L.; Gan, L.; Guo, Z.Y.; Shi, G.X.; et al. Ghrelin protects against cobalt chloride-induced hypoxic injury in cardiac H9c2 cells by inhibiting oxidative stress and inducing autophagy. Peptides 2012, 38, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.X.; Feng, L.; Cao, F.R.; Pan, R.L.; Liao, Y.H.; Liu, X.M.; Chang, Q. Pharmacokinetic profiles of the five isoflavonoids from Pueraria lobata roots in the CSF and plasma of rats. J. Ethnopharmacol. 2016, 184, 22–29. [Google Scholar] [CrossRef]
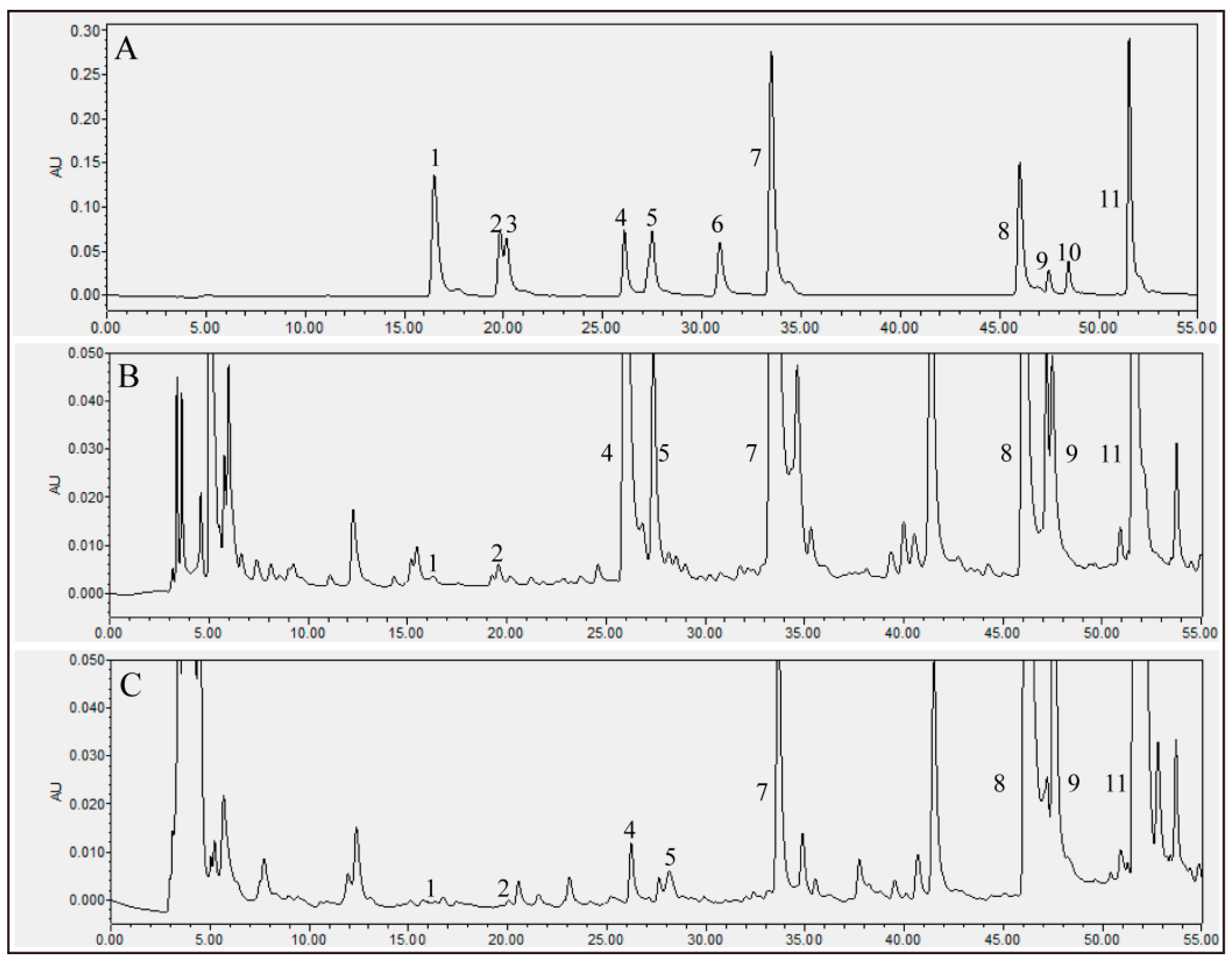


| Peak | Compounds | UT (mg/100 g) | FT (mg/100 g) |
|---|---|---|---|
| 1 | Chlorogenic acid | 1.08 ± 0.11 F,a | 0.83 ± 0.08 C,b |
| 2 | Vanillic acid | 7.87 ± 0.41 F | N.D. |
| 3 | Caffeic acid | N.D. | N.D. |
| 4 | Daidzin | 361.90 ± 27.49 A,a | 20.86 ± 2.39 C,b |
| 5 | Glycitin | 93.24 ± 3.63 E,a | 7.30 ± 0.75 C,b |
| 6 | Ferulic acid | N.D. | N.D. |
| 7 | Genistin | 207.47 ± 22.80 B,a | 30.84 ± 1.72 C,b |
| 8 | Daidzein | 106.71 ± 13.48 D,E,b | 301.16 ± 21.81 B,a |
| 9 | Glycitein | 174.39 ± 12.89 C,a | 423.69 ± 31.13 A,b |
| 10 | Quercetin | N.D. | N.D. |
| 11 | Genistein | 127.51 ± 17.40 D,b | 422.97 ± 31.85 A,a |
| TPC (GAE mg/100 g) | 1264.69 ± 54.08 b | 1835.67 ± 78.55 a |
| Samples | ABTS+ Scavenging Activity (%) | RP (OD700) | FRAP (μM FeSO4) |
|---|---|---|---|
| UT | 67.50 ± 3.17 b | 0.18 ± 0.02 b | 380.54 ± 18.23 b |
| FT | 89.97 ± 1.20 a | 0.33 ± 0.02 a | 643.08 ± 20.28 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, L.; Zhang, Y.; Azi, F.; Tekliye, M.; Zhou, J.; Liu, X.; Dong, M.; Xia, X. Neuroprotective Potency of Tofu Bio-Processed Using Actinomucor elegans against Hypoxic Injury Induced by Cobalt Chloride in PC12 Cells. Molecules 2021, 26, 2983. https://doi.org/10.3390/molecules26102983
Yin L, Zhang Y, Azi F, Tekliye M, Zhou J, Liu X, Dong M, Xia X. Neuroprotective Potency of Tofu Bio-Processed Using Actinomucor elegans against Hypoxic Injury Induced by Cobalt Chloride in PC12 Cells. Molecules. 2021; 26(10):2983. https://doi.org/10.3390/molecules26102983
Chicago/Turabian StyleYin, Liqing, Yongzhu Zhang, Fidelis Azi, Mekonen Tekliye, Jianzhong Zhou, Xiaoli Liu, Mingsheng Dong, and Xiudong Xia. 2021. "Neuroprotective Potency of Tofu Bio-Processed Using Actinomucor elegans against Hypoxic Injury Induced by Cobalt Chloride in PC12 Cells" Molecules 26, no. 10: 2983. https://doi.org/10.3390/molecules26102983
APA StyleYin, L., Zhang, Y., Azi, F., Tekliye, M., Zhou, J., Liu, X., Dong, M., & Xia, X. (2021). Neuroprotective Potency of Tofu Bio-Processed Using Actinomucor elegans against Hypoxic Injury Induced by Cobalt Chloride in PC12 Cells. Molecules, 26(10), 2983. https://doi.org/10.3390/molecules26102983