Silver Catalyzed Decarbonylative [3 + 2] Cycloaddition of Cyclobutenediones and Formamides
Abstract
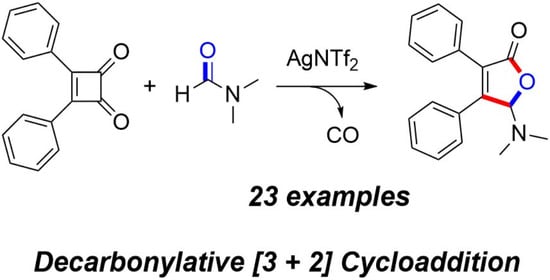
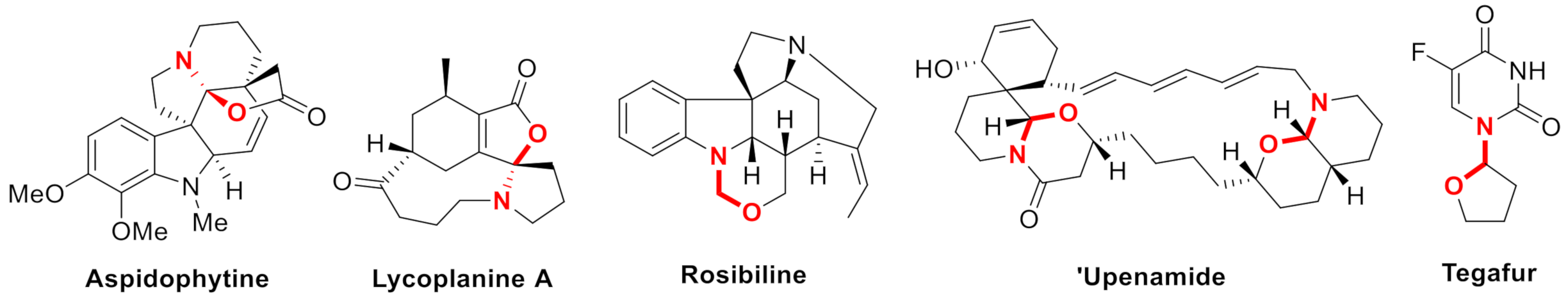
:1. Introduction
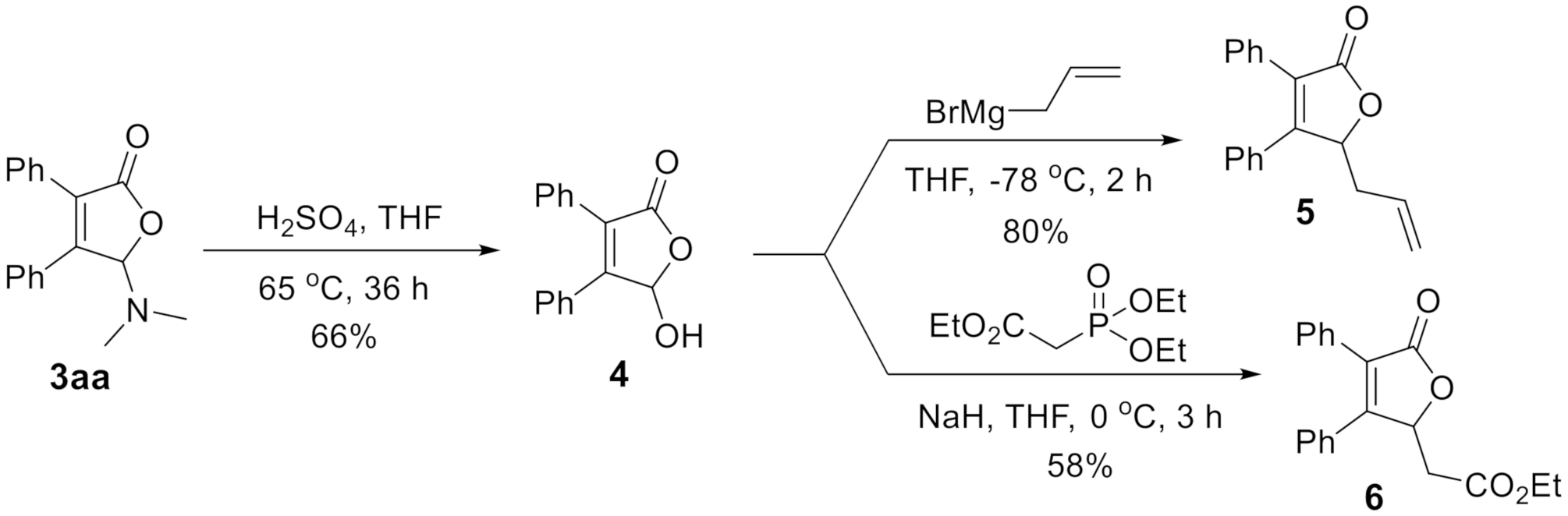
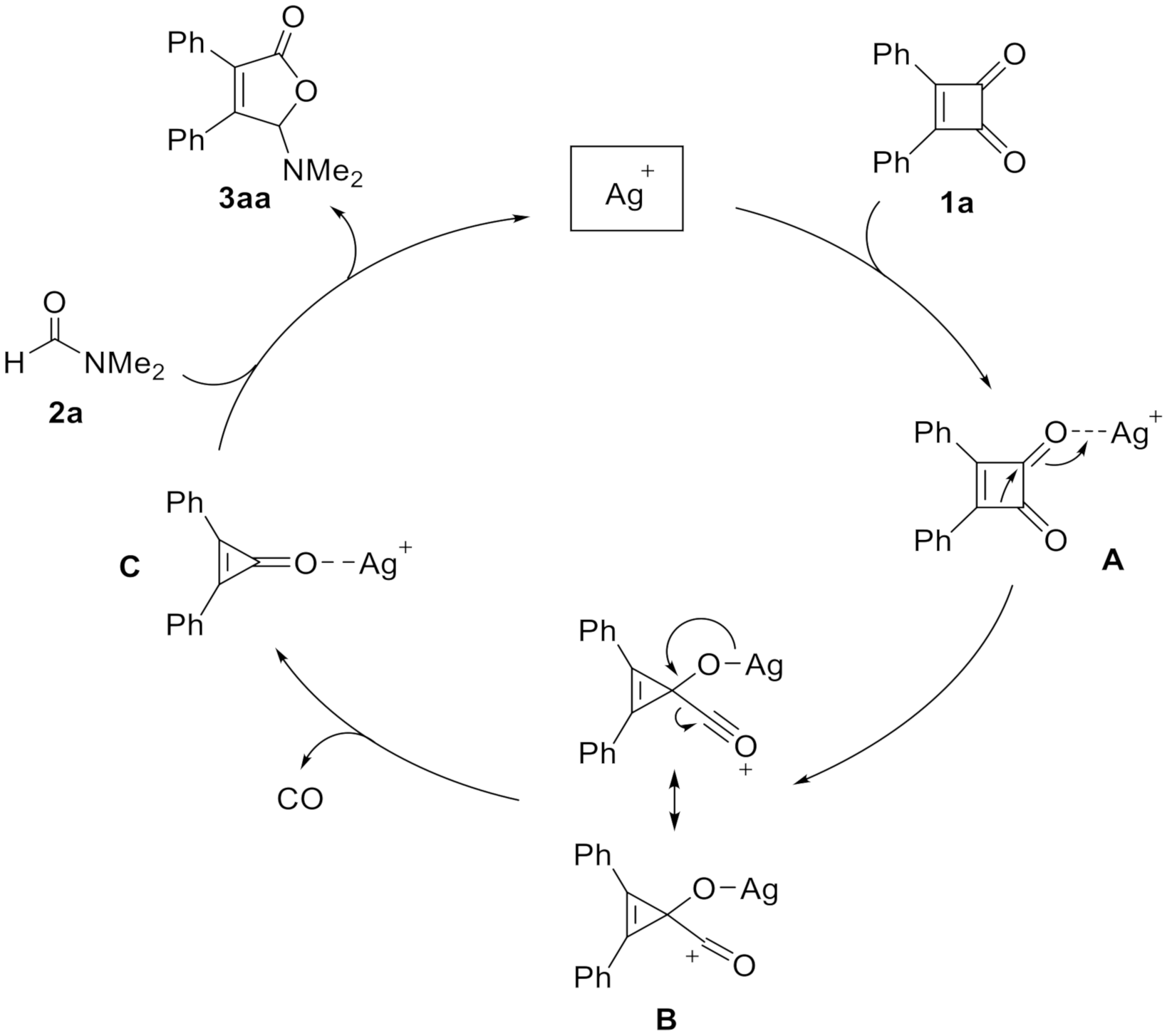
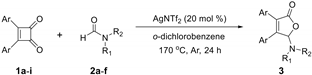
2. Results
3. Materials and Methods
3.1. General Methods
3.2. Experiment Procedures
3.3. Characterization of the Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ricci, A. Amino Group Chemistry, From Synthesis to the Life Sciences; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Zhang, P.; Wei, Q.; Yuan, X.; Xu, K. Newly reported alkaloids produced by marine-derived penicillium species (covering 2014–2018). Bioorg. Chem. 2020, 99, 103840. [Google Scholar] [CrossRef] [PubMed]
- Maiti, M.; Kumar, G.S. Biophysical aspects and biological implications of the interaction of benzophenanthridine alkaloids with DNA. Biophys. Rev. 2009, 1, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, E.F.; Snyder, H.R.; Fischer, R.F. Plant insecticides. II. the alkaloids of Haplophyton cimicidum. J. Am. Chem. Soc. 1952, 74, 1987–1989. [Google Scholar] [CrossRef]
- Rae, I.D.; Rosenberger, M.; Szabo, A.G.; Willis, C.R.; Yates, P.; Zacharias, D.E.; Jeffrey, G.A.; Douglas, B.; Kirkpatrick, J.L.; Weisbach, J.A. Haplophytine. J. Am. Chem. Soc. 1967, 89, 3061–3062. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Nian, Y.; Zhu, Q.-F.; Li, X.-N.; Su, J.; Wu, X.-D.; Yang, J.; Zhao, Q.-S. Lycoplanine A, a C16N Lycopodium alkaloid with a 6/9/5 tricyclic skeleton from Lycopodium complanatum. Org. Lett. 2017, 19, 4668–4671. [Google Scholar] [CrossRef]
- Tits, M.; Angenot, L. 12’-Hydroxystrychnobiline, nouvel alcaloïde bisindolinique du Strychnos variabilis. J. Nat. Prod. 1983, 46, 638–645. [Google Scholar] [CrossRef]
- Jiménez, J.I.; Goetz, G.; Mau, C.M.S.; Yoshida, W.Y.; Scheuer, P.J.; Williamson, R.T.; Kelly, M. ‘Upenamide: An unprecedented macrocyclic alkaloid from the indonesian sponge Echinochalina sp. J. Org. Chem. 2000, 65, 8465–8469. [Google Scholar] [CrossRef]
- Unsworth, W.P.; Gallagher, K.A.; Jean, M.; Schmidt, J.P.; Diorazio, L.J.; Taylor, R.J.K. Direct imine acylation: Synthesis of the proposed structures of ‘upenamide. Org. Lett. 2013, 15, 262–265. [Google Scholar] [CrossRef]
- Engel, D.; Nudelman, A.; Tarasenko, N.; Levovich, I.; Makarovsky, I.; Sochotnikov, S.; Tarasenko, I.; Rephaeli, A. Novel prodrugs of tegafur that display improved anticancer activity and antiangiogenic properties. J. Med. Chem. 2008, 51, 314–323. [Google Scholar] [CrossRef]
- Sazlóki, G.; Alévêque, Q.; Pozzo, J.-L.; Hadji, R.; Levillain, E.; Sanguinet, L. Indolinooxazolidine: A versatile switchable unit. J. Phys. Chem. B 2015, 119, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Sears, J.E.; Boger, D.L. Tandem intramolecular Diels-Alder/1,3-Dipolar cycloaddition cascade of 1,3,4-Oxadiazoles: Initial scope and applications. Acc. Chem. Res. 2016, 49, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Dian, L.; Xing, Q.; Zhang-Negrerie, D.; Du, Y. Direct functionalization of alkyl ethers to construct Hemiaminal Ether Skeletons (HESs). Org. Biomol. Chem. 2018, 16, 4384–4389. [Google Scholar] [CrossRef]
- Dian, L.; Wang, S.; Zhang-Negrerie, D.; Du, Y.; Zhang, K. Organocatalytic amination of alkyl ethers via n-Bu4NI/t-BuOOH-Mediated intermolecular oxidative C(sp3)-N bond formation: Novel synthesis of hemiaminal ethers. Chem. Commun. 2014, 50, 11738–11741. [Google Scholar] [CrossRef]
- Yang, Q.; Choy, P.Y.; Fu, W.C.; Fan, B.; Kwong, F.Y. Copper-catalyzed oxidative C-H amination of tetrahydrofuran with indole/carbazole derivatives. J. Org. Chem. 2015, 80, 11193–11199. [Google Scholar] [CrossRef]
- Viuf, C.; Bols, M. Radical azidonation of benzylic positions with iodonium azide. Angew. Chem. Int. Ed. 2001, 40, 623–625. [Google Scholar] [CrossRef]
- Albone, D.P.; Challenger, S.; Derrick, A.M.; Fillery, S.M.; Irwin, J.L.; Parsons, C.M.; Takada, H.; Taylor, P.C.; Wilson, D.J. Amination of ethers using chloramine-t hydrate and a copper(I) catalyst. Org. Biomol. Chem. 2005, 3, 107–111. [Google Scholar] [CrossRef]
- Fructos, M.R.; Trofimenko, S.; Díaz-Requejo, M.M.; Pérez, P.J. Facile amine formation by intermolecular catalytic amidation of carbon-hydrogen bonds. J. Am. Chem. Soc. 2006, 128, 11784–11791. [Google Scholar] [CrossRef]
- He, L.; Yu, J.; Zhang, J.; Yu, X.-Q. α-Amidation of cyclic ethers catalyzed by simple copper salt and a mild and efficient preparation method for α, ϖ-amino alcohols. Org. Lett. 2007, 9, 2277–2280. [Google Scholar] [CrossRef]
- Ochiai, M.; Yamane, S.; Hoque, M.M.; Saito, M.; Miyamoto, K. Metal-Free α-CH amination of ethers with hypervalent sulfonylimino-λ3-bromane that acts as an active nitrenoid. Chem. Commun. 2012, 48, 5280–5282. [Google Scholar] [CrossRef]
- Campos, J.; Goforth, S.K.; Crabtree, R.H.; Gunnoe, T.B. Metal-free amidation of ether sp3 c-h bonds with sulfonamides using PhI(OAc)2. RSC Adv. 2014, 4, 47951–47957. [Google Scholar] [CrossRef]
- Mazzocchi, P.H.; Somich, C.; Edwards, M.; Morgan, T.; Ammon, H.L. Electron transfer photochemistry of aromatic imides and phenylcyclopropane. Radical anion-radical cation cycloaddition. J. Am. Chem. Soc. 1986, 108, 6828–6829. [Google Scholar] [CrossRef]
- Padwa, A.; Hertzog, D.L. Bimolecular cycloaddition reactions of isomünchnones derived from the rhodium (II) catalyzed cyclization of diazo pyrrolidinones. Tetrahedron 1993, 49, 2589–2600. [Google Scholar] [CrossRef]
- Ren, J.-T.; Wang, J.-X.; Tian, H.; Xu, J.-L.; Hu, H.; Aslam, M.; Sun, M. Ag(Ⅰ)-Catalyzed [3 + 2]-Annulation of cyclopropenones and formamides via C-C bond cleavage. Org. Lett. 2018, 20, 6636–6639. [Google Scholar] [CrossRef]
- Matsuda, T.; Tabata, Y.; Suzuki, H. Silver-catalyzed ring-opening [3 + 2] annulation of cyclopropenones with amides. New J. Chem. 2018, 42, 19178–19182. [Google Scholar] [CrossRef]
- Dehmlow, E.V.; Neuhaus, R.; Schell, H.G. 2-Alkoxy-3-alkylcyclopropenone. Chem. Ber. 1988, 121, 569–571. [Google Scholar] [CrossRef]
- Simon, J.G.G.; Schweig, A. 1H and 13C NMR spectra of benzocyclopropenone in liquid solutions at 193 K. Chem. Phys. Lett. 1993, 201, 377–382. [Google Scholar] [CrossRef]
- Fu, N.; Allen, A.D.; Kobayashi, S.; Tidwell, T.T.; Vukovic, S. Structural effects on interconversion of oxygen-substituted bisketenes and cyclobutenediones. J. Org. Chem. 2008, 73, 1768–1773. [Google Scholar] [CrossRef]
- Piech, K.; Bally, T. The bisketene radical cation and its formation by oxidative ring-opening of cyclobutenedione. J. Org. Chem. 2013, 78, 2908–2913. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Liebeskind, L.S. tert-Butyl substituent as a regiodirecting and novel C-H protecting group in cyclobutenedione-based benzannulation chemistry. J. Org. Chem. 1998, 63, 2835–2844. [Google Scholar] [CrossRef]
- Nguyen, T.V. Convenient access to hydroquinone and quinone derivatives from cyclobutenedione units. Aust. J. Chem. 2010, 63, 1309–1310. [Google Scholar] [CrossRef]
- Beesu, M.; Periasamy, M. Reactive iron carbonyl reagents via reaction of metal alkoxides with Fe(CO)5 or Fe2(CO)9: synthesis of cyclobutenediones via double carbonylation of alkynes. J. Org. Chem. 2011, 76, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yu, T.-Y.; Xu, P.-F.; Wei, H. Selective decarbonylation via transition-metal-catalyzed carbon-carbon bond cleavage. Chem. Rev. 2021, 121, 365–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Nishihara, Y. Nickel or palladium-catalyzed decarbonylative transformations of carboxylic acid derivatives. Chem. Asian J. 2020, 15, 1234–1247. [Google Scholar] [CrossRef] [PubMed]
- Jette, C.I.; Tong, Z.J.; Hadt, R.G.; Stoltz, B.M. Copper-catalyzed enantioselective allylic alkylation with a γ-butyrolactone- derived silyl ketene acetal. Angew. Chem. Int. Ed. 2020, 59, 2033–2038. [Google Scholar] [CrossRef]
- Jayamani, M.; Pillai, C.N. Reactions of benzoin and benzil over alumina: Decarbonylation of α-diketones. J. Catal. 1985, 92, 422–425. [Google Scholar] [CrossRef]
- Aguilar-Aguilar, A.; Liebeskind, L.S.; Peña-Cabrera, E. Pd-Catalyzed, Cu(I)-Mediated coss-couplings of bisarylthiocyclobutenediones with boronic acids and organostannanes. J. Org. Chem. 2007, 72, 8539–8542. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Y.; Suzzarini, L. Novel reactive cyclobutenedione in poly (arylene ether) synthesis. Macromolecules 1996, 29, 1073–1075. [Google Scholar] [CrossRef]
- Bouancheau, C.; Rudler, M.; Chelain, E.; Rudler, H.; Vaissermann, J.; Daran, J.-C. Reaction of aminocarbene complexes of chromium with alkynes IV. New transformations of the nitrogen yield complexes derived thereform. J. Organomet. Chem. 1995, 496, 127–135. [Google Scholar] [CrossRef]
- Zhang, J.; Blazecka, P.G.; Belmont, D.; Davidson, J.G. Reinvestigation of mucohalic acid, versatile and useful building blocks for highly functionalized α,β-unsaturated γ-butyrolactones. Org. Lett. 2002, 4, 4559–4561. [Google Scholar] [CrossRef]

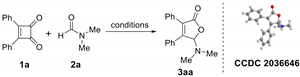 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Solvent | Time/h | Yield/% b |
| 1 | none | o-Dichlorobenzene | 24 | NR |
| 2 | [Cp*RhCl2]2 | o-Dichlorobenzene | 24 | 17 |
| 3 | Rh(PPh3)3Cl | o-Dichlorobenzene | 24 | 5 |
| 4 | Pd(PPh3)4 | o-Dichlorobenzene | 24 | 12 |
| 5 | Pd(OAc)2 | o-Dichlorobenzene | 24 | 0 |
| 6 | Cu(OTf)2 | o-Dichlorobenzene | 24 | trace |
| 7 | AgSbF6 | o-Dichlorobenzene | 24 | 21 |
| 8 | AgOTf | o-Dichlorobenzene | 24 | 29 |
| 9 | AgBF4 | o-Dichlorobenzene | 24 | 43 |
| 10 | AgNTf2 | o-Dichlorobenzene | 24 | 60 |
| 11 | AgNTf2 | o-Dichlorobenzene | 48 | 15 |
| 12 | AgNTf2 | o-Dichlorobenzene | 24 | NR c |
| 13 | AgNTf2 | Chlorobenzene | 24 | 52 |
 | ||||
 |  |  |  |  |
| 3aa, 60% | 3ab, 36% | 3ac, trace | 3ad, 52% | 3ae, 55% |
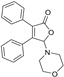 | 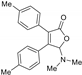 | 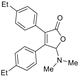 | 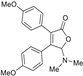 | 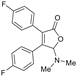 |
| 3af, 43% | 3ba, 59% | 3ca, 52% | 3da, 55% | 3ea, 45% |
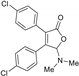 |  |  |  | |
| 3fa, 40% | 3ga, 34% | 3ha, 54% | 3ia, 51% | |
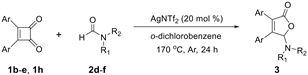 | |||
 | 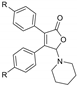 | 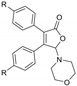 | 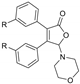 |
| 3bd, R = Me, 45% | 3be, R = Me, 54% | 3bf, R = Me, 49% | 3hf, R = Me, 42% |
| 3dd, R = OMe, 43% | 3ce, R = Et, 62% | 3cf, R = Et, 52% | |
| 3de, R = OMe, 46% | 3df, R = OMe, 55% | ||
| 3ee, R = F, 36% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Yu, R.; Ali, S.; Wang, Z.; Liu, Z.; Gao, J.; Zheng, H. Silver Catalyzed Decarbonylative [3 + 2] Cycloaddition of Cyclobutenediones and Formamides. Molecules 2021, 26, 2974. https://doi.org/10.3390/molecules26102974
Wang P, Yu R, Ali S, Wang Z, Liu Z, Gao J, Zheng H. Silver Catalyzed Decarbonylative [3 + 2] Cycloaddition of Cyclobutenediones and Formamides. Molecules. 2021; 26(10):2974. https://doi.org/10.3390/molecules26102974
Chicago/Turabian StyleWang, Pengcheng, Ruirui Yu, Sajjad Ali, Zhengshen Wang, Zhigang Liu, Jinming Gao, and Huaiji Zheng. 2021. "Silver Catalyzed Decarbonylative [3 + 2] Cycloaddition of Cyclobutenediones and Formamides" Molecules 26, no. 10: 2974. https://doi.org/10.3390/molecules26102974
APA StyleWang, P., Yu, R., Ali, S., Wang, Z., Liu, Z., Gao, J., & Zheng, H. (2021). Silver Catalyzed Decarbonylative [3 + 2] Cycloaddition of Cyclobutenediones and Formamides. Molecules, 26(10), 2974. https://doi.org/10.3390/molecules26102974