Sweet Cherries as Anti-Cancer Agents: From Bioactive Compounds to Function
Abstract
1. Introduction
2. Nutrients, Phytochemical Composition and Bioactive Compounds
2.1. Macronutrients
2.2. Micronutrients
2.3. Phytochemical Composition and Bioactive Compounds
| Compounds | Amount (per 100 g of Sweet Cherry) | Reference | |
|---|---|---|---|
| Water | 82.25 g | [22] | |
| Macronutrients | Protein | 1.06 g | [22] |
| Fat (total lipids) | 0.20 g | [22] | |
| Carbohydrates | 16.01 g | [22] | |
| Fatty acids | Total saturated | 0.04 g | [22] |
| Total monounsaturated | 0.05 g | [22] | |
| Total polyunsaturated | 0.05 g | [22] | |
| Fiber (total dietary) | 2.10 g | [22] | |
| Amino acids | Tryptophan | 9.00 mg | [22] |
| Threonine | 22.00 mg | [22] | |
| Isoleucine | 20.00 mg | [22] | |
| Leucine | 30.00 mg | [22] | |
| Lysine | 32.00 mg | [22] | |
| Methionine | 10.00 mg | [22] | |
| Cystine | 10.00 mg | [22] | |
| Phenylalanine | 24.00 mg | [22] | |
| Tyrosine | 14.00 mg | [22] | |
| Valine | 24.00 mg | [22] | |
| Arginine | 18.00 mg | [22] | |
| Histidine | 15.00 mg | [22] | |
| Alanine | 26.00 mg | [22] | |
| Aspartic acid | 56.90 mg | [22] | |
| Glutamic acid | 83.00 mg | [22] | |
| Glycine | 23.00 mg | [22] | |
| Proline | 39.00 mg | [22] | |
| Serine | 30.00 mg | [22] | |
| Sugars | Sugars (total) | 12.82 g | [22] |
| Sucrose | 0.15 g | [22] | |
| Glucose | 6.59 g | [22] | |
| Fructose | 5.37 g | [22] | |
| Maltose | 0.12 g | [22] | |
| Galactose | 0.59 g | [22] | |
| Micronutrients: Minerals | Calcium | 13.00 mg | [22] |
| Iron | 0.36 mg | [22] | |
| Magnesium | 11.00 mg | [22] | |
| Phosphorus | 21.00 mg | [22] | |
| Potassium | 222.00 mg | [22] | |
| Zinc | 0.07 mg | [22] | |
| Copper | 0.06 mg | [22] | |
| Manganese | 0.07 mg | [22] | |
| Fluoride | 0.01 mg | [22] | |
| Micronutrients: Vitamins | Vitamin C | 7.00 mg | [22] |
| Thiamine (Vitamin B1) | 0.03 mg | [22] | |
| Riboflavin (Vitamin B2) | 0.03 mg | [22] | |
| Niacin (Vitamin B3) | 0.15 mg | [22] | |
| Pantothenic acid (Vitamin B5) | 0.20 mg | [22] | |
| Vitamin B6 | 0.05 mg | [22] | |
| Folate (Vitamin B9) | 0.01 mg | [22] | |
| Choline (Vitamin B4) | 6.10 mg | [22] | |
| Vitamin A | 0.01 mg | [22] | |
| Vitamin E | 0.07 mg | [22] | |
| Vitamin K | 0.01 mg | [22] | |
| Phenolic Compounds | 3-O-Caffeoylquinic acid | 83.00 mg | [18] |
| Catechin hexoside | 168.00 mg | [18] | |
| Gallic acid | 0.51 mg | [19] | |
| p-Coumaric acid | 2.28 mg | [19] | |
| Rutin | 10.66 mg | [19] | |
| Chlorogenic acid | 2.95 mg | [19] | |
| Cyanidin-3-O-glycoside | 22.03 mg | [23] | |
| Quercetin-3-4′-di-O-glycoside | 24.61 mg | [23] | |
| Epicatechin | 1.51 mg | [19] | |
| cis-p-Coumaroylquinic acid | 56.00 mg | [18] | |
| trans-p-Coumaroylquinic acid | 23.00 mg | [18] | |
| Taxifolin-O-deoxyhexosylhexoside | 66.00 mg | [18] | |
| Taxifolin-O-hexoside | 13.00 mg | [18] | |
| Quercetin-O-rutinoside-O-hexoside | 42.00 mg | [18] | |
| Naringenin-O-hexoside | 17.00 mg | [18] | |
| Dihydrowogonin 7-O-glucoside/sakuranetin 5-O-glucoside | 62.00 mg | [18] | |
| Phenolic acids | 162.00 mg | [18] | |
| Flavonoids (non-anthocyanins) | 396.00 mg | [18] | |
| Total phenolic compounds | 558.00 mg | [18] | |
| Cyanidin-3-O-glucoside | 219.00 mg | [18] | |
| Cyanidin-3-O-rutinoside | 1450.00 mg | [18] | |
| Peonidin-3-O-glucoside | 64.00 mg | [18] | |
| Anthocyanins | 1734.00 mg | [18] | |
| Other Bioactive Phytochemicals (Carotenoids and Melatonin) | β-Carotene | 38.00 µg | [22] |
| Lutein + zeaxanthin | 85.00 µg | [22] | |
| Melatonin | 1.60 µg | [21] | |
3. Bioaccessibility and Bioavailability of Bioactive Compounds
4. Sweet Cherries and the Hallmarks of Cancer
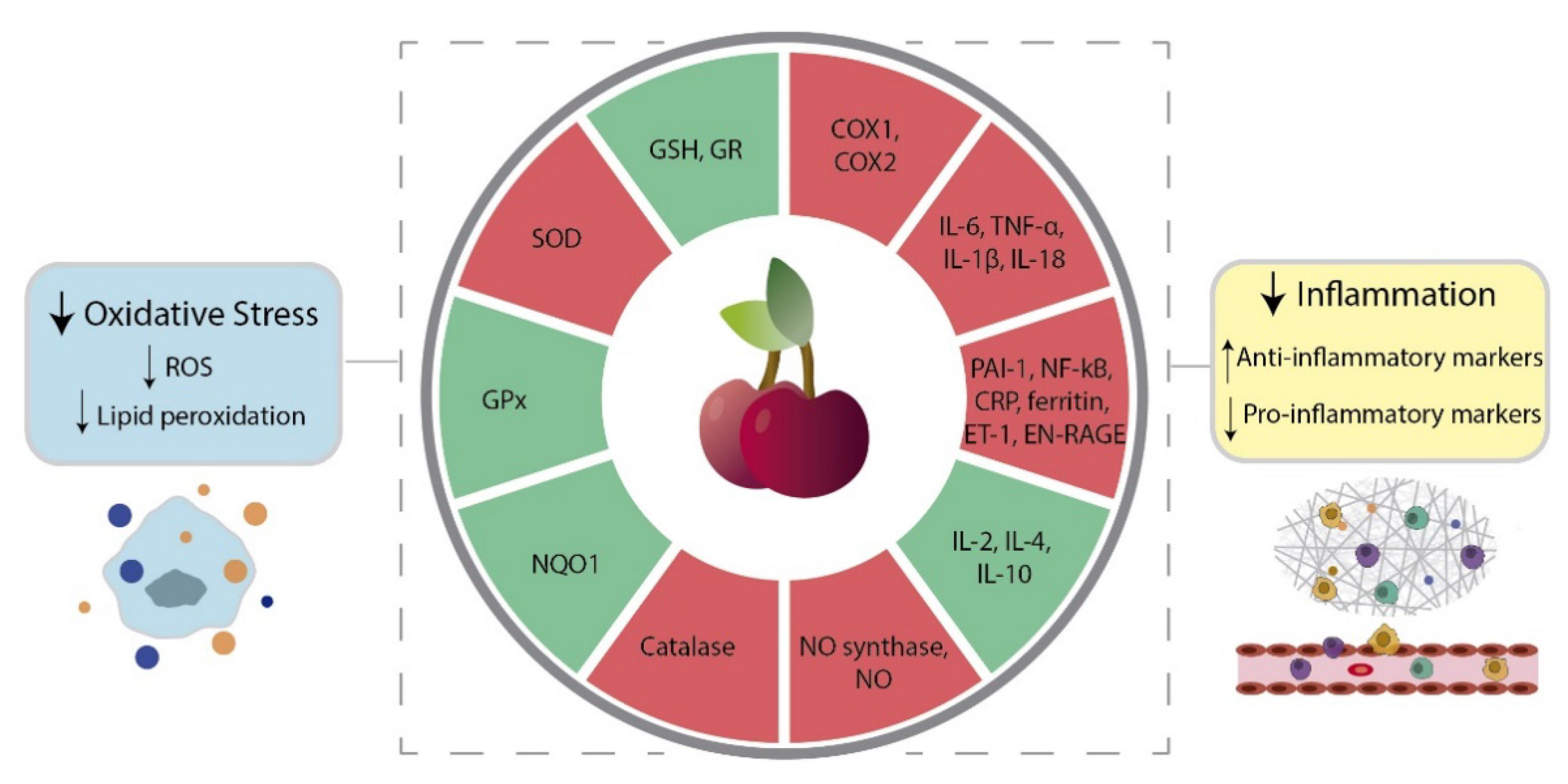
4.1. Oxidative Stress
4.2. Inflammation
4.3. Cell Death and Proliferation
4.4. Invasion and Metastization
4.5. Metabolic Reprogramming
| Hallmark of Cancer | Type of Study/Biological model | Experiment | Extract Concentration/Phenolic Content/Dose/Mass of Sweet Cherry | Time of Treatment | Effect | Reference | |
|---|---|---|---|---|---|---|---|
| Oxidative Stress | In vitro | Hep2G cells | Incubation with sweet cherry extract | High phenolic content | 90 min | ↓ Intracellular ROS | [38] |
| Pre-incubation with sweet cherry extract before H2O2 administration | 24 h | ↓ Intracellular ROS in a concentration dependent-manner | |||||
| Incubation with sweet cherry extract | Low phenolic content | 90 min | |||||
| Pre-incubation with sweet cherry extract before H2O2 administration | 24 h | ||||||
| Caco-2 cells | Pre-incubation with sweet cherry extract before H2O2 administration | 50 GAE */mL | 1 h | ↓ Intracellular ROS ↓ Carbonyl proteins Restored GSH/GSSG ratio | [42] | ||
| 10 mg dry weight/mL | 4 h | ↓ Intracellular ROS | [40] | ||||
| 25% (v/v) | 24 h | ↓ NO Inhibited LDH leakage | [52] | ||||
| Pre-incubation with sweet cherry extract before t-BHP administration | 10 mg dry weight/mL | 4 h | ↓ Intracellular ROS | [40] | |||
| Co-incubation with sweet cherry extract and H2O2 | 50 GAE/mL | 1 h | ↓ Intracellular ROS ↓ Carbonyl proteins Restored GSH/GSSG ratio | [42] | |||
| 10 mg dry weight/mL | 4 h | ↓ Intracellular ROS | [40] | ||||
| Co-incubation with sweet cherry extract and t-BHP | 10 mg dry weight/mL | ||||||
| SH-SY5Y cells | Pre-incubation with sweet cherry extract before H2O2 administration | 50 µg/mL | 24 h | ↓ Intracellular ROS ↑ GSH ↑ GR ↑ NQO1 | [49] | ||
| SK-N-MC cells | Pre-incubation with sweet cherry extract before H2O2 administration | 1 GAE/mL | 2 h | ↓ Intracellular ROS | [42] | ||
| THP-1 cells | Pre-incubation with sweet cherry extract before exposure to MSU | 1.81 mg GAE/mL | 3 h | ↓ Intracellular ROS | [56] | ||
| 2.32 mg GAE/mL | |||||||
| Pre-incubation with MSU before treatment with sweet cherry extract | 1.81 mg GAE/mL | Unknown | |||||
| 2.32 mg GAE/mL | |||||||
| LNCaP cells | Incubation with sweet cherry extract | 20 µg/mL | 72 h | ↓ Intracellular ROS Inhibited lipid peroxidation | [45] | ||
| In vivo | Wistar rats | High fructose-diet with freeze-dried sweet cherry | 50 g/kg | 12 weeks | ↑ GPx Inhibited lipid peroxidation ↓ Catalase ↓ SOD | [57] | |
| 100 g/kg | ↑ GPx ↑ GR ↓ Catalase ↓ SOD | ||||||
| Human subjects | 10 healthy men | Daily consumption of sweet cherries after overnight fasting | 280 g | 6 days | ↑ Plasma lipophilic antioxidant capacity ↑ Plasma hydrophilic antioxidant capacity | [58] | |
| 12 volunteers | Consumption of sweet cherries twice a day after lunch and diner | 200 g | 3 days | ↑ Urinary antioxidant capacity | [59] | ||
| 10 healthy women | Daily consumption of sweet cherries after overnight fasting | 280 g | 6 days | ↑ Lipophilic oxygen radical absorbance capacity ↓ Ferric reducing ability of plasma | [60] | ||
| Inflammation | In vitro | THP-1cells | Pre-incubation with sweet cherry extract before exposure to MSU | 1.81 mg GAE/mL | 3 h | ↓ IL-1β Inhibited the phagocytosis of monosodium urate crystals | [56] |
| 2.32 mg GAE/mL | |||||||
| Pre-incubation with MSU crystals before treatment with sweet cherry extract | 1.81 mg GAE/mL | Not applicable | ↓ IL-1β Inhibited phagocytosis of monosodium urate crystals | ||||
| 2.32 mg GAE/mL | |||||||
| In vivo | Wistar rats | High fructose-diet with freeze-dried sweet cherry | 50 g/kg | 12 weeks | ↓ CRP | [57] | |
| 100 g/kg | ↓ CRP ↑ IL-10 | ||||||
| Daily consumption of sweet cherry-based beverage | 75,400 µg/mL | 10 days | ↓ IL-1β in young rats during the dawn, afternoon (18 h) and the acrophase of the melatonin rhythm ↓ IL-1β in old rats during the dawn and the acrophase of the melatonin rhythm ↓TNF-α in old rats during the dawn ↑ IL-2 in young rats during the dawn and afternoon (18 h) ↑ IL-2 in old rats during the dawn ↑ IL-4 in young rats during the dawn during the dawn, afternoon (18 h) and the acrophase of the melatonin rhythm ↑ IL-4 in old rats during the dawn during the dawn, afternoon (18 h) and the acrophase of the melatonin rhythm | [70] | |||
| Ringdove birds | Daily consumption of sweet cherry-based beverage | 75,400 µg/mL | 10 days | ↓ IL-1β in young birds during the dawn and the afternoon (18 h) ↓ IL-1β in old birds during the afternoon (18 h) and the acrophase of the melatonin rhythm ↓TNF-α in young birds during the dawn ↓ TNF-α in old birds during the afternoon (18 h) and the acrophase of the melatonin rhythm ↑ IL-2 in young birds during the acrophase of the melatonin rhythm ↑ IL-2 in old birds during the dawn during the dawn and the acrophase of the melatonin rhythm ↑ IL-4 in young birds during the dawn during the dawn, afternoon (18 h) and the acrophase of the melatonin rhythm ↑ IL-4 in old birds during the dawn during the dawn, afternoon (18 h) and the acrophase of the melatonin rhythm | |||
| Obese-diabetic mice | Diet supplemented with anthocyanin-depleted cherry powder | 100 g | 12 weeks | ↓ IL-6 | [71] | ||
| Diet-induced obese mice | Diet supplemented with cyanidin-3-glucoside, cyanidin-3-rutinoside and pelargonidin-3-glucoside extracted from sweet cherries | 20 mg of anthocyanins/kg body weight | 16 weeks | ↓ IL-6 ↓ Inducible NO synthase ↓TNF-α ↓ NF-κB | [74] | ||
| Human subjects | 2 healthy men and 18 healthy women | Daily consumption of sweet cherries | 280 g | 28 days | ↓ CRP ↓ NO | [60] | |
| 2 men and 16 women | Daily consumption of sweet cherries | 280 g | 28 days | ↓ CRP ↓ EGF ↓ Endothelin 1 ↓ EN-RAGE ↓ Ferritin ↓ IL-18 ↓ PAI-1 ↑ IL-1 receptor antagonist ↓ Ferritin | [62] | ||
| 10 healthy women | Daily consumption of sweet cherries after overnight fast | 280 g | 6 days | ↓ CRP (after 3 h of sweet cherry consumption) ↓ NO (after 3 h of sweet cherry consumption) | [72] | ||
| Cell death and Proliferation | In vitro | A549 cells | Incubation with sweet cherry extract organic fraction | 15.62–250 μg/mL | 24–72 h | ↓ Cell viability | [19] |
| HeLa cells | Incubation with sweet cherry extract organic fraction | ||||||
| Incubation with sweet cherry crude extract | |||||||
| SK-B-NE (2)-C cells | Incubation with sweet cherry crude extract | ||||||
| SH-SY5Y cells | Incubation with sweet cherry crude extract | ||||||
| SW480 cells | Incubation with undigested cherry extract | 121.90 µmol/L (IC50) | 24 h | ↓ Proliferative activity | [24] | ||
| Incubation with digested cherry extract | 61.22 µmol/L | ↓ Proliferative activity (more pronounced effect compared to undigested cherry extract) | |||||
| HCT-15 cells | Incubation with digested cherry extract | 73.51 µg/mL (IC50) | 48 h | ↓ Proliferative activity | [18] | ||
| HT29 cells | Incubation with sweet cherry extract | 0.5 mg/mL | 24-96 h | ↓ Proliferative activity | [78] | ||
| 0–20 mg dried weight of cherry /mL | 96 h | [40] | |||||
| 0.5 mg/mL | 24-96 h | G1/G0 cell cycle arrest | [77] | ||||
| MKN45 cells | Incubation with sweet cherry extract | 0–20 mg dried weight of cherry /mL | 96 h | ↓ Cell viability | [40] | ||
| BT-474 cells | Incubation with sweet cherry whole extract | 80–320 μg GAE/mL | 48 h | ↓ Cell growth | [79] | ||
| Incubation with sweet cherry extract enriched in anthocyanins | 40–320 μg GAE/mL | ||||||
| MDA-MB-231 cells | Incubation with sweet cherry whole extract | 80–320 μg GAE/mL | 48 h | ↓ Cell growth | [79] | ||
| Incubation with sweet cherry extract enriched in anthocyanins | 40–320 μg GAE/mL | ||||||
| Incubation with sweet cherry extract enriched in proanthocyanins | 40–320 μg GAE/mL | ||||||
| MDA-MB-453 cells | Incubation with sweet cherry whole extract | 80–320 μg GAE/mL | 48 h | ↓ Cell growth | |||
| 83 μg GAE/mL | 8 h | ↓ AKT mRNA levels ↓mTOR mRNA levels ↓ p-38-MAPK mRNA levels ↓ Survivin mRNA levels ↓ Sirtuin 1 mRNA levels | |||||
| 83 μg GAE/mL | 24 h | ↑ AKT ↑ Phospho-p-38-MAPK/p38-MAPK protein ratio ↑ Phosphorylated ERK 1/2 ↑ Phosphorylated JNK ↑ mTOR ↓ Phosphorylated mTOR ↓ Phosphorylated mTOR/ mTOR protein ratio ↑ Bax ↑ Bcl-2 ↑ AIF ↑ Cytochrome c ↑ Cleaved caspase-9 ↑ Cleaved caspase-3 ↓ Full PARP ↑ Cleaved PARP ↓ Cleaved PARP/PARP protein ratio | [79,80] | ||||
| Incubation with sweet cherry extract enriched in anthocyanins | 40–320 μg GAE/mL | 48 h | ↓ Cell growth | [79] | |||
| 70 μg GAE/mL | 8 h | ↓ AKT mRNA levels ↓mTOR mRNA levels ↓ p-38-MAPK mRNA levels ↓ Survivin mRNA levels ↓ Sirtuin 1 mRNA levels | |||||
| 70 μg GAE/mL | 24 h | ↑ mTOR ↓ Phosphorylated mTOR ↓ Phosphorylated mTOR/ mTOR protein ratio ↓ Full PARP-1 ↑ Cleaved PARP-1 ↓ Cleaved PARP-1/PARP-1 protein ratio | |||||
| 19 µg C3G */mL | ↑ AKT ↓ Phosphorylated AKT ↑ Phospho-p-38-MAPK/p38-MAPK protein ratio ↑ Phosphorylated ERK 1/2 ↑ Phosphorylated JNK ↑ Cleaved caspase-8 ↑ Bax ↑ Bcl-2 ↑ AIF ↑ Cytochrome c ↑ Cleaved caspase-9 ↑ Cleaved caspase-3 ↓ Full PARP-1 ↑ Cleaved PARP-1 | [80] | |||||
| Incubation with sweet cherry extract enriched in proanthocyanins | 40–320 μg GAE/mL | 48 h | ↓ Cell growth | [79] | |||
| 45 μg GAE/mL | 8 h | ↓ AKT mRNA levels ↓mTOR mRNA levels ↓ p-38-MAPK mRNA levels ↓ Survivin mRNA levels ↓ Sirtuin 1 mRNA levels | |||||
| 45 μg GAE/mL | 24 h | ↑ mTOR ↓ Phosphorylated mTOR ↓ Phosphorylated mTOR/ mTOR protein ratio ↓ Full PARP ↑ Cleaved PARP ↓ Cleaved PARP/PARP protein ratio | |||||
| 22.5 μg PCN */mL | ↑ AKT ↑ Phospho-p-38-MAPK/p38-MAPK protein ratio ↑ Phosphorylated ERK 1/2 ↑ Phosphorylated JNK ↑ Bax ↑ Bcl-2 ↑ AIF ↑ Cytochrome c ↑ Cleaved caspase-9 ↑ Cleaved caspase-3 ↓ Full PARP-1 ↑ Cleaved PARP-1 | [80] | |||||
| PNT1A cells | Incubation with sweet cherry extract | 0–200 ug/mL | 72 h | ↓ Cell viability | [45] | ||
| LNCaP cells | 0–200 ug/mL | ↓ Cell viability | |||||
| 20 µg/mL | ↑ Caspase-3 activity ↓ Bcl-2 ↑ Bax/Bcl-2 protein ratio ↑ Caspase-9 | ||||||
| PC3 cells | 0–200 ug/mL | ↓ Cell viability | |||||
| 20 µg/mL | ↓ Caspase-3 activity | ||||||
| In vivo | MDA-MB-453 cells xenograft mice model | Oral administration of sweet cherry whole extract | 150 mg/kg body weight/day | 36 days | ↓ Tumor growth ↑ Phosphorylated ERK 1/2 ↓ STAT3 ↓ Ki-67 | [81] | |
| Oral administration of sweet cherry extract enriched in anthocyanins | ↓ Tumor growth ↑ Phosphorylated ERK 1/2 ↓ AKT ↓ STAT3 ↓ p38-MAPK ↓ JNK ↓ NF-κB ↓ Ki-67 | ||||||
| Oral administration of sweet cherry extract enriched in proanthocyanins | ↓ Tumor growth ↑ Phosphorylated ERK 1/2 ↓ STAT3 ↓ Ki-67 | ||||||
| Invasion and metastization | In vitro | MDA-MB-453 cells | Incubation with sweet cherry whole extract | 83 μg GAE/mL | 8 h | ↓ Sp1 mRNA levels ↓ Sp4 mRNA levels ↓ VCAM-1 mRNA levels | [79] |
| 83 μg GAE/mL | 24 h | ↓ VEGF | [80] | ||||
| 83 μg GAE/mL | 48 h | ↓ (?) Cell motility | |||||
| Incubation with sweet cherry extract enriched in anthocyanins | 70 μg GAE/mL | 8 h | ↓ Sp1 mRNA levels ↓ Sp4 mRNA levels ↓ VCAM-1 mRNA levels | [79] | |||
| 70 μg GAE/mL | 24 h | ↓ Sp1 | |||||
| 19 µg C3G/mL | ↓ Migration ↓ PLCγ-1 ↓ VEGF | [80] | |||||
| 19 µg C3G/mL | 48 h | ↓ (?) Cell motility | |||||
| Incubation with sweet cherry extract enriched in proanthocyanins | 45 μg GAE/mL | 8 h | ↓ Sp1 mRNA levels ↓ Sp4 mRNA levels ↓ VCAM-1 mRNA levels | [79] | |||
| 45 μg GAE/mL | 24 h | ↓ Sp1 | |||||
| 22.5 μg PCN/mL | ↓ VEGF | [80] | |||||
| 22.5 μg PCN/mL | 48 h | ↓ (?) Cell motility | |||||
| Metabolic reprogramming | In vitro | PNT1A cells | Incubation with sweet cherry extract | 20 µg/mL | 72 h | ↑ Lactate production ↓ GLUT1 ↓ GLUT3 ↓ PFK-1 ↑ LDH activity ↓ MCT4 | [45] |
| LNCaP cells | ↓ Glucose consumption ↓ Lactate production ↓ GLUT3 ↑ PFK-1 ↓ LDH activity ↓ MCT4 | ||||||
| PC3 cells | ↑ Glucose consumption ↓ PFK-1 ↑ Lactate production ↓ LDH activity | ||||||
| In vivo | MDA-MB-453 cells xenograft mice model | Oral administration of sweet cherry extract enriched in anthocyanins | 150 mg/kg body weight/day | 36 days | Abolished the expression of ACAT1 ↓ lipase E, hormone sensitive type | [81] | |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petrovska, B.B. Historical review of medicinal plants′ usage. Pharm. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Salamanca-Fernández, E.; Garcia-Villanova, B.; Sánchez, M.J. The Impact of Plant-Based Dietary Patterns on Cancer-Related Outcomes: A Rapid Review and Meta-Analysis. Nutrients 2020, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Agarwal, C.; Agarwal, R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J. Nutr. 2009, 139, 1806s–1812s. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Cancer and metastasis: Prevention and treatment by green tea. Cancer Metastasis Rev. 2010, 29, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.T.; Okogun, J.I.; Folk, W.R. 21st century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013, 30, 584–592. [Google Scholar] [CrossRef]
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and health: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Production Share of Cherries by Region. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 23 April 2021).
- Gonçalves, J.; Simão, A.Y.; Soares, S.; Gameiro, C.; Almeida, E.; Rosado, T.; Luis, Â.; Gallardo, E.; Duarte, A.P. Prunus avium L.: Composition, analysis and health benefits. In Prunus: Classification, Cultivation and Toxicity, 1st ed.; Schneider, W., Ed.; Nova Publisher Inc.: New York, NY, USA, 2020; Volume 1, pp. 73–125. [Google Scholar]
- Faienza, M.F.; Corbo, F.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Grano, M.; Wang, D.Q.H.; D′Amato, G.; Muraglia, M.; Franchini, C.; et al. Novel insights in health-promoting properties of sweet cherries. J. Funct. Foods 2020, 69, 103945. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A review of the health benefits of cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative stress and cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef]
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.F. The macronutrients’ interplay. Clin. Nutr. 2019, 38, 2943–2944. [Google Scholar] [CrossRef]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Di Maro, A.; Petriccione, M.; Galasso, S.; Piccolella, S.; Di Giuseppe, A.M.A.; Scortichini, M.; Monaco, P. Chemical composition, nutritional value and antioxidant properties of autochthonous Prunus avium cultivars from Campania Region. Food Res. Int. 2014, 64, 188–199. [Google Scholar] [CrossRef]
- Kim, M.; Basharat, A.; Santosh, R.; Mehdi, S.F.; Razvi, Z.; Yoo, S.K.; Lowell, B.; Kumar, A.; Brima, W.; Danoff, A.; et al. Reuniting overnutrition and undernutrition, macronutrients, and micronutrients. Diabetes Metab. Res. Rev. 2019, 35, e3072. [Google Scholar] [CrossRef]
- Rosado, T.; Henriques, I.; Gallardo, E.; Duarte, A.P. Determination of melatonin levels in different cherry cultivars by high-performance liquid chromatography coupled to electrochemical detection. Eur. Food Res. Technol. 2017, 243, 1749–1757. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Agriculture Research Service, FoodData Central (Cherries, Sweet, Raw). Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171719/nutrients (accessed on 21 December 2020).
- Gonçalves, J.; Simão, A.Y.; Soares, S.; Gameiro, C.; Almeida, E.; Rosado, T.; Luís, Â.; Duarte, A. Assessment of the Bioaccessibility and Bioavailability of the Phenolic Compounds of Prunus avium L. by in Vitro Digestion and Cell Model. ACS Omega 2019, 4, 7605–7613. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Bioactivity and cell metabolism of in vitro digested sweet cherry (Prunus avium) phenolic compounds. Int. J. Food Sci. Nutr. 2019, 70, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Fazzari, M.; Fukumoto, L.; Mazza, G.; Livrea, M.A.; Tesoriere, L.; Marco, L.D. In vitro bioavailability of phenolic compounds from five cultivars of frozen sweet cherries (Prunus avium L.). J. Agric. Food Chem. 2008, 56, 3561–3568. [Google Scholar] [CrossRef]
- Celep, E.; Charehsaz, M.; Akyüz, S.; Acar, E.T.; Yesilada, E. Effect of in vitro gastrointestinal digestion on the bioavailability of phenolic components and the antioxidant potentials of some Turkish fruit wines. Food Res. Int. 2015, 78, 209–215. [Google Scholar] [CrossRef]
- Magherini, F.; Fiaschi, T.; Marzocchini, R.; Mannelli, M.; Gamberi, T.; Modesti, P.A.; Modesti, A. Oxidative stress in exercise training: The involvement of inflammation and peripheral signals. Free Radic. Res. 2019, 53, 1155–1165. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Di Matteo, A.; Russo, R.; Graziani, G.; Ritieni, A.; Di Vaio, C. Characterization of autochthonous sweet cherry cultivars (Prunus avium L.) of southern Italy for fruit quality, bioactive compounds and antioxidant activity. J. Sci. Food Agric. 2017, 97, 2782–2794. [Google Scholar] [CrossRef]
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Kolniak-Ostek, J.; Oziembłowski, M.; Ticha, A.; Hyšpler, R.; Zadak, Z.; Židová, P.; Paprstein, F. Comparison of old cherry cultivars grown in Czech Republic by chemical composition and bioactive compounds. Food Chem. 2017, 228, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, N.; Wang, Y.; Jiang, D.; Feng, X. Characterization of phenolic compounds from early and late ripening sweet cherries and their antioxidant and antifungal activities. J. Agric. Food Chem. 2017, 65, 5413–5420. [Google Scholar] [CrossRef]
- Acero, N.; Gradillas, A.; Beltran, M.; García, A.; Muñoz Mingarro, D. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Skrzyński, J.; Leja, M.; Gonkiewicz, A.; Banach, P. Cultivar effect on the sweet cherry antioxidant and some chemical attributes. Folia Hortic. 2016, 28, 95–102. [Google Scholar] [CrossRef]
- Serra, A.T.; Duarte, R.O.; Bronze, M.R.; Duarte, C.M.M. Identification of bioactive response in traditional cherries from Portugal. Food Chem. 2011, 125, 318–325. [Google Scholar] [CrossRef]
- González-Gómez, D.; Lozano, M.; Fernández-León, M.F.; Bernalte, M.J.; Ayuso, M.C.; Rodríguez, A.B. Sweet cherry phytochemicals: Identification and characterization by HPLC-DAD/ESI-MS in six sweet-cherry cultivars grown in Valle del Jerte (Spain). J. Food Compos. Anal. 2010, 23, 533–539. [Google Scholar] [CrossRef]
- Matias, A.A.; Rosado-Ramos, R.; Nunes, S.L.; Figueira, I.; Serra, A.T.; Bronze, M.R.; Santos, C.N.; Duarte, C.M.M. Protective Effect of a (Poly)phenol-Rich Extract Derived from Sweet Cherries Culls against Oxidative Cell Damage. Molecules 2016, 21, 406. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef]
- Mulabagal, V.; Lang, G.A.; DeWitt, D.L.; Dalavoy, S.S.; Nair, M.G. Anthocyanin Content, Lipid Peroxidation and Cyclooxygenase Enzyme Inhibitory Activities of Sweet and Sour Cherries. J. Agric. Food Chem. 2009, 57, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.R.; Vaz, C.V.; Catalão, B.; Ferreira, S.; Cardoso, H.J.; Duarte, A.P.; Socorro, S. Sweet Cherry Extract Targets the Hallmarks of Cancer in Prostate Cells: Diminished Viability, Increased Apoptosis and Suppressed Glycolytic Metabolism. Nutr. Cancer 2020, 72, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.; Landbo, A.-K.; Let, M.; Silva, A.P.; Rosa, E.; Meyer, A.S. Storage affects the phenolic profiles and antioxidant activities of cherries (Prunus avium L) on human low-density lipoproteins. J. Sci. Food Agric. 2004, 84, 1013–1020. [Google Scholar] [CrossRef]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. J. Agric. Food Chem. 1998, 46, 4107–4112. [Google Scholar] [CrossRef]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef]
- Antognoni, F.; Potente, G.; Mandrioli, R.; Angeloni, C.; Freschi, M.; Malaguti, M.; Hrelia, S.; Lugli, S.; Gennari, F.; Muzzi, E. Fruit Quality Characterization of New Sweet Cherry Cultivars as a Good Source of Bioactive Phenolic Compounds with Antioxidant and Neuroprotective Potential. Antioxidants 2020, 9, 677. [Google Scholar] [CrossRef]
- Acquaviva, R.; Russo, A.; Galvano, F.; Galvano, G.; Barcellona, M.L.; Li Volti, G.; Vanella, A. Cyanidin and cyanidin 3-O-beta-D -glucoside as DNA cleavage protectors and antioxidants. Cell Biol. Toxicol. 2003, 19, 243–252. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Osawa, T. The role of anthocyanins as an antioxidant under oxidative stress in rats. Biofactors 2000, 13, 133–139. [Google Scholar] [CrossRef]
- Leong, S.Y.; Burritt, D.J.; Hocquel, A.; Penberthy, A.; Oey, I. The relationship between the anthocyanin and vitamin C contents of red-fleshed sweet cherries and the ability of fruit digests to reduce hydrogen peroxide-induced oxidative stress in Caco-2 cells. Food Chem. 2017, 227, 404–412. [Google Scholar] [CrossRef]
- Wang, S.; Melnyk, J.; Tsao, R.; Marcone, M. How natural dietary antioxidants in fruits, vegetables and legume promote vascular health. Food Res. Int. 2011, 44, 14–22. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Xia, H.; Shen, Y.; Shen, T.; Wang, X.; Zhang, X.; Hu, P.; Liang, D.; Lin, L.; Deng, H.; Wang, J.; et al. Melatonin Accumulation in Sweet Cherry and Its Influence on Fruit Quality and Antioxidant Properties. Molecules 2020, 25, 753. [Google Scholar] [CrossRef]
- Vírgen Gen, J.J.; Guzmán-Gerónimo, R.I.; Martínez-Flores, K.; Martínez-Nava, G.A.; Fernández-Torres, J.; Zamudio-Cuevas, Y. Cherry extracts attenuate inflammation and oxidative stress triggered by monosodium urate crystals in THP-1 cells. J. Food Biochem. 2020, 44, e13403. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Piątkowska, E.; Leszczyńska, T. High-Fructose Diet-Induced Metabolic Disorders Were Counteracted by the Intake of Fruit and Leaves of Sweet Cherry in Wistar Rats. Nutrients 2019, 11, 2638. [Google Scholar] [CrossRef]
- Prior, R.L.; Gu, L.; Wu, X.; Jacob, R.A.; Sotoudeh, G.; Kader, A.A.; Cook, R.A. Plasma Antioxidant Capacity Changes Following a Meal as a Measure of the Ability of a Food to Alter In Vivo Antioxidant Status. J. Am. Coll. Nutr. 2007, 26, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Paredes, S.D.; Cubero, J.; Lozano, M.; Toribio-Delgado, A.F.; Muñoz, J.L.; Reiter, R.J.; Barriga, C.; Rodríguez, A.B. Jerte Valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2010, 65, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Rasooly, R.; Jacob, R.A.; Kader, A.A.; Mackey, B.E. Consumption of Bing Sweet Cherries Lowers Circulating Concentrations of Inflammation Markers in Healthy Men and Women. J. Nutr. 2006, 136, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Russell, R.M.; Lischner, N.; Prior, R.L. Serum Antioxidant Capacity Is Increased by Consumption of Strawberries, Spinach, Red Wine or Vitamin C in Elderly Women. J. Nutr. 1998, 128, 2383–2390. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y.; Reddy, A.; Woodhouse, L.R.; Mackey, B.E.; Erickson, K.L. Sweet Bing Cherries Lower Circulating Concentrations of Markers for Chronic Inflammatory Diseases in Healthy Humans. J. Nutr. 2013, 143, 340–344. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Paixão, J.; Nunes, C.; Dinis, T.C.; Almeida, L.M. Cyanidin-3-glucoside suppresses cytokine-induced inflammatory response in human intestinal cells: Comparison with 5-aminosalicylic acid. PLoS ONE 2013, 8, e73001. [Google Scholar] [CrossRef] [PubMed]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. 2016, 9, 895. [Google Scholar] [CrossRef]
- Ghosh, N.; Chaki, R.; Mandal, V.; Mandal, S.C. COX-2 as a target for cancer chemotherapy. Pharmacol. Rep. 2010, 62, 233–244. [Google Scholar] [CrossRef]
- Beconcini, D.; Felice, F.; Zambito, Y.; Fabiano, A.; Piras, A.M.; Macedo, M.H.; Sarmento, B.; Di Stefano, R. Anti-inflammatory effect of cherry extract loaded in polymeric nanoparticles: Relevance of particle internalization in endothelial cells. Pharmaceutics 2019, 11, 500. [Google Scholar] [CrossRef]
- Corbo, F.; Brunetti, G.; Crupi, P.; Bortolotti, S.; Storlino, G.; Piacente, L.; Carocci, A.; Catalano, A.; Milani, G.; Colaianni, G.; et al. Effects of Sweet Cherry Polyphenols on Enhanced Osteoclastogenesis Associated With Childhood Obesity. Front. Immunol. 2019, 10, 1001. [Google Scholar] [CrossRef]
- Delgado, J.; del Pilar Terrón, M.; Garrido, M.; Barriga, C.; Espino, J.; Paredes, S.D.; Rodríguez, A.B. Jerte Valley cherry-based product modulates serum inflammatory markers in rats and ringdoves. J. Appl. Biomed. 2012, 10, 41–50. [Google Scholar] [CrossRef]
- Noratto, G.D.; Lage, N.N.; Chew, B.P.; Mertens-Talcott, S.U.; Talcott, S.T.; Pedrosa, M.L. Non-anthocyanin phenolics in cherry (Prunus avium L.) modulate IL-6, liver lipids and expression of PPARδ and LXRs in obese diabetic (db/db) mice. Food Chem. 2018, 266, 405–414. [Google Scholar] [CrossRef]
- Jacob, R.A.; Spinozzi, G.M.; Simon, V.A.; Kelley, D.S.; Prior, R.L.; Hess-Pierce, B.; Kader, A.A. Consumption of Cherries Lowers Plasma Urate in Healthy Women. J. Nutr. 2003, 133, 1826–1829. [Google Scholar] [CrossRef]
- Peiffer, D.S.; Zimmerman, N.P.; Wang, L.S.; Ransom, B.W.; Carmella, S.G.; Kuo, C.T.; Siddiqui, J.; Chen, J.H.; Oshima, K.; Huang, Y.W.; et al. Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev. Res. 2014, 7, 574–584. [Google Scholar] [CrossRef]
- Wu, T.; Yin, J.; Zhang, G.; Long, H.; Zheng, X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 687–694. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Serra, A.T.; Matias, A.A.; Almeida, A.P.C.; Bronze, M.R.; Alves, P.M.; de Sousa, H.C.; Duarte, C.M.M. Processing cherries (Prunus avium) using supercritical fluid technology. Part 2. Evaluation of SCF extracts as promising natural chemotherapeutical agents. J. Supercrit. Fluids 2011, 55, 1007–1013. [Google Scholar] [CrossRef]
- Serra, A.T.; Seabra, I.J.; Braga, M.E.M.; Bronze, M.R.; de Sousa, H.C.; Duarte, C.M.M. Processing cherries (Prunus avium) using supercritical fluid technology. Part 1: Recovery of extract fractions rich in bioactive compounds. J. Supercrit. Fluids 2010, 55, 184–191. [Google Scholar] [CrossRef]
- Lage, N.N.; Layosa, M.A.A.; Arbizu, S.; Chew, B.P.; Pedrosa, M.L.; Mertens-Talcott, S.; Talcott, S.; Noratto, G.D. Dark sweet cherry (Prunus avium) phenolics enriched in anthocyanins exhibit enhanced activity against the most aggressive breast cancer subtypes without toxicity to normal breast cells. J. Funct. Foods 2020, 64, 103710. [Google Scholar] [CrossRef]
- Layosa, M.A.A.; Lage, N.N.; Chew, B.P.; Atienza, L.; Mertens-Talcott, S.; Talcott, S.; Noratto, G.D. Dark Sweet Cherry (Prunus avium) Phenolics Enriched in Anthocyanins Induced Apoptosis in MDA-MB-453 Breast Cancer Cells through MAPK-Dependent Signaling and Reduced Invasion via Akt and PLCγ-1 Downregulation. Nutr. Cancer 2020, 1–13. [Google Scholar] [CrossRef]
- Noratto, G.; Layosa, M.A.; Lage, N.N.; Atienza, L.; Ivanov, I.; Mertens-Talcott, S.U.; Chew, B.P. Antitumor potential of dark sweet cherry sweet (Prunus avium) phenolics in suppressing xenograft tumor growth of MDA-MB-453 breast cancer cells. J. Nutr. Biochem. 2020, 84, 108437. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Li, Z.; Wu, Y.; Li, X.; Li, Y.; Wei, J.; Li, J.; Zhang, Y.; Li, L. Euscaphic acid and Tormentic acid protect vascular endothelial cells against hypoxia-induced apoptosis via PI3K/AKT or ERK 1/2 signaling pathway. Life Sci. 2020, 252, 117666. [Google Scholar] [CrossRef]
- Cao, Z.; Liao, Q.; Su, M.; Huang, K.; Jin, J.; Cao, D. AKT and ERK dual inhibitors: The way forward? Cancer Lett. 2019, 459, 30–40. [Google Scholar] [CrossRef]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef]
- Morris, G.; Walker, A.J.; Berk, M.; Maes, M.; Puri, B.K. Cell Death Pathways: A Novel Therapeutic Approach for Neuroscientists. Mol. Neurobiol. 2018, 55, 5767–5786. [Google Scholar] [CrossRef]
- Solinas, G.; Marchesi, F.; Garlanda, C.; Mantovani, A.; Allavena, P. Inflammation-mediated promotion of invasion and metastasis. Cancer Metastasis Rev. 2010, 29, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-N.; Chu, S.-C.; Chiou, H.-L.; Kuo, W.-H.; Chiang, C.-L.; Hsieh, Y.-S. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006, 235, 248–259. [Google Scholar] [CrossRef]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef]
- Chen, P.-N.; Kuo, W.-H.; Chiang, C.-L.; Chiou, H.-L.; Hsieh, Y.-S.; Chu, S.-C. Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u-PA expression. Chem. Biol. Interact. 2006, 163, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, R.; Piantelli, M.; Falasca, M. Role of phospholipase C in cell invasion and metastasis. Adv. Biol. Regul. 2013, 53, 309–318. [Google Scholar] [CrossRef]
- Zhang, D.; Bi, J.; Liang, Q.; Wang, S.; Zhang, L.; Han, F.; Li, S.; Qiu, B.; Fan, X.; Chen, W.; et al. VCAM1 Promotes Tumor Cell Invasion and Metastasis by Inducing EMT and Transendothelial Migration in Colorectal Cancer. Front. Oncol. 2020, 10, 1066. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell. Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Bikfalvi, A. Chapter 3—Angiogenesis and invasion in cancer. In Handbook of Clinical Neurology; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 104, pp. 35–43. [Google Scholar]
- Flamini, V.; Jiang, W.G.; Lane, J.; Cui, Y.X. Significance and therapeutic implications of endothelial progenitor cells in angiogenic-mediated tumour metastasis. Crit. Rev. Oncol. Hematol. 2016, 100, 177–189. [Google Scholar] [CrossRef]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)-Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar] [PubMed]
- Pradeep, C.R.; Sunila, E.S.; Kuttan, G. Expression of Vascular Endothelial Growth Factor (VEGF) and VEGF Receptors in Tumor Angiogenesis and Malignancies. Integr. Cancer Ther. 2005, 4, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Vizcaíno, C.; Mansilla, S.; Portugal, J. Sp1 transcription factor: A long-standing target in cancer chemotherapy. Pharmacol. Ther. 2015, 152, 111–124. [Google Scholar] [CrossRef]
- Wang, L.S.; Hecht, S.S.; Carmella, S.G.; Yu, N.; Larue, B.; Henry, C.; McIntyre, C.; Rocha, C.; Lechner, J.F.; Stoner, G.D. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev. Res. 2009, 2, 84–93. [Google Scholar] [CrossRef]
- Soni, S.; Padwad, Y.S. HIF-1 in cancer therapy: Two decade long story of a transcription factor. Acta Oncol. 2017, 56, 503–515. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Ohshima, K.; Morii, E. Metabolic Reprogramming of Cancer Cells during Tumor Progression and Metastasis. Metabolites 2021, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.M.A.; Cardoso, H.J.; Figueira, M.I.; Vaz, C.V.; Socorro, S. The peculiarities of cancer cell metabolism: A route to metastasization and a target for therapy. Eur. J. Med. Chem. 2019, 171, 343–363. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, H.J.; Carvalho, T.M.A.; Fonseca, L.R.S.; Figueira, M.I.; Vaz, C.V.; Socorro, S. Revisiting prostate cancer metabolism: From metabolites to disease and therapy. Med. Res. Rev. 2020. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. THE METABOLISM OF TUMORS IN THE BODY. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, H.J.; Figueira, M.I.; Vaz, C.V.; Carvalho, T.M.A.; Brás, L.A.; Madureira, P.A.; Oliveira, P.J.; Sardão, V.A.; Socorro, S. Glutaminolysis is a metabolic route essential for survival and growth of prostate cancer cells and a target of 5α-dihydrotestosterone regulation. Cell. Oncol. 2021, 44, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Alzaid, F.; Cheung, H.M.; Preedy, V.R.; Sharp, P.A. Regulation of glucose transporter expression in human intestinal Caco-2 cells following exposure to an anthocyanin-rich berry extract. PLoS ONE 2013, 8, e78932. [Google Scholar] [CrossRef] [PubMed]
- de Sales, N.F.F.; Silva da Costa, L.; Carneiro, T.I.A.; Minuzzo, D.A.; Oliveira, F.L.; Cabral, L.M.C.; Torres, A.G.; El-Bacha, T. Anthocyanin-Rich Grape Pomace Extract (Vitis vinifera L.) from Wine Industry Affects Mitochondrial Bioenergetics and Glucose Metabolism in Human Hepatocarcinoma HepG2 Cells. Molecules 2018, 23, 611. [Google Scholar] [CrossRef]
- Guo, H.; Li, D.; Ling, W.; Feng, X.; Xia, M. Anthocyanin inhibits high glucose-induced hepatic mtGPAT1 activation and prevents fatty acid synthesis through PKCζ. J. Lipid Res. 2011, 52, 908–922. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, L.R.S.; Silva, G.R.; Luís, Â.; Cardoso, H.J.; Correia, S.; Vaz, C.V.; Duarte, A.P.; Socorro, S. Sweet Cherries as Anti-Cancer Agents: From Bioactive Compounds to Function. Molecules 2021, 26, 2941. https://doi.org/10.3390/molecules26102941
Fonseca LRS, Silva GR, Luís Â, Cardoso HJ, Correia S, Vaz CV, Duarte AP, Socorro S. Sweet Cherries as Anti-Cancer Agents: From Bioactive Compounds to Function. Molecules. 2021; 26(10):2941. https://doi.org/10.3390/molecules26102941
Chicago/Turabian StyleFonseca, Lara R. S., Gonçalo R. Silva, Ângelo Luís, Henrique J. Cardoso, Sara Correia, Cátia V. Vaz, Ana P. Duarte, and Sílvia Socorro. 2021. "Sweet Cherries as Anti-Cancer Agents: From Bioactive Compounds to Function" Molecules 26, no. 10: 2941. https://doi.org/10.3390/molecules26102941
APA StyleFonseca, L. R. S., Silva, G. R., Luís, Â., Cardoso, H. J., Correia, S., Vaz, C. V., Duarte, A. P., & Socorro, S. (2021). Sweet Cherries as Anti-Cancer Agents: From Bioactive Compounds to Function. Molecules, 26(10), 2941. https://doi.org/10.3390/molecules26102941









