Jasmonate Compounds and Their Derivatives in the Regulation of the Neoplastic Processes
Abstract
1. Introduction
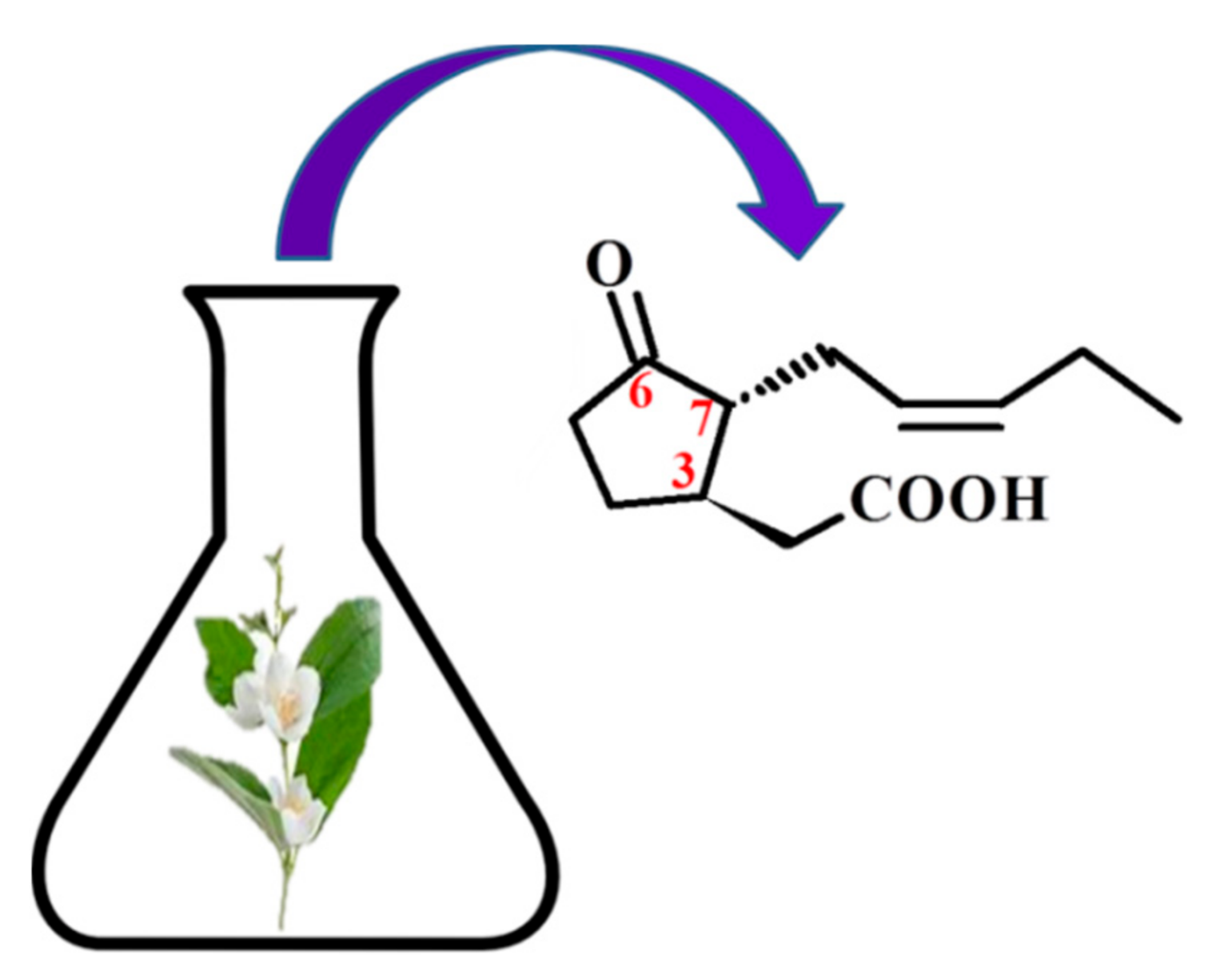
2. Jasmonic Acid and Its Derivatives Occurring in Plants
3. Chemical Structure of Jasmonates
4. Pharmacological Activity of Jasmonates
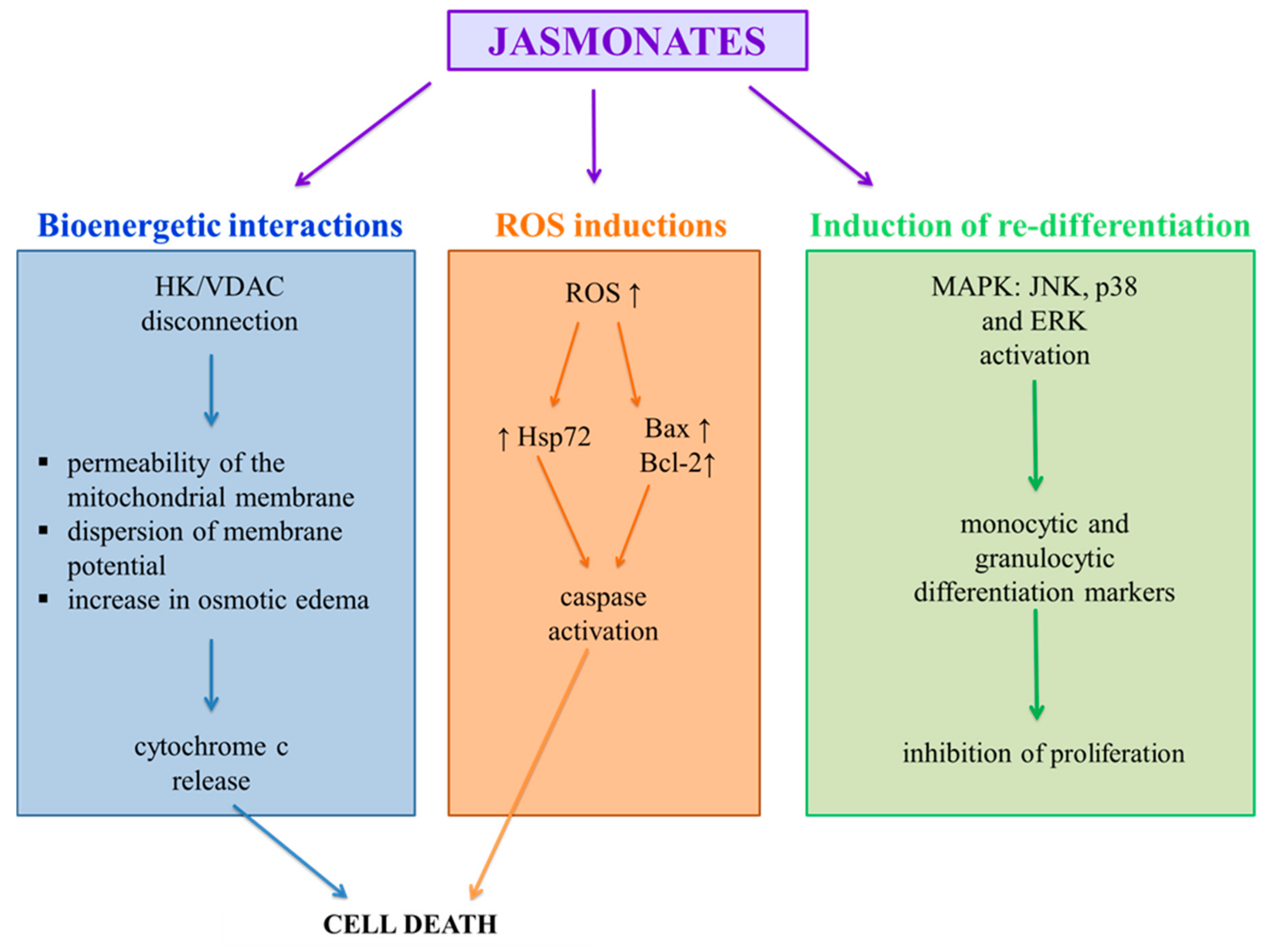
5. Antitumor Mechanisms of Action of Jasmonates Compounds
5.1. Bioenergetic Interactions
5.2. Induction of Re-Differentiation
5.3. Induction of Apoptosis by ROS
6. Therapeutic Benefits of Combining Jasmonates with Anti-Cancer Drugs
7. New Synthetic Derivatives of Jasmonates
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choudhary, D.; Goykar, H.; Karanwad, T.; Kannaujia, S.; Gadekar, V.; Misra, M. An Understanding of Mitochondria and Its Role in Targeting Nanocarriers for Diagnosis and Treatment of Cancer. Asian J. Pharm. Sci. 2020. [Google Scholar] [CrossRef]
- Kuruppu, A.I.; Paranagama, P.; Goonasekara, C.L. Medicinal Plants Commonly Used against Cancer in Traditional Medicine Formulae in Sri Lanka. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2019, 27, 565–573. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Qaradakhi, T.; Zulli, A.; Smejkal, K.; Kajo, K.; Jakubikova, J.; Behzadi, P.; Pec, M.; et al. Genoprotective Activities of Plant Natural Substances in Cancer and Chemopreventive Strategies in the Context of 3P Medicine. EPMA J. 2020, 11, 261–287. [Google Scholar] [CrossRef] [PubMed]
- Wilmowicz, E.; Frankowski, K.; Sidłowska, M.; Kućko, A.; Kesy, J.; Gasiorowski, A.; Glazińska, P.; Kopcewicz, J. [Jasmonate biosynthesis—The latest discoveries]. Postepy Biochem. 2012, 58, 26–33. [Google Scholar]
- Ghasemi Pirbalouti, A.; Sajjadi, S.E.; Parang, K. A Review (Research and Patents) on Jasmonic Acid and Its Derivatives. Arch. Pharm. 2014, 347, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Umukoro, S.; Alabi, A.O.; Eduviere, A.T.; Ajayi, A.M.; Oluwole, O.G. Anti-Inflammatory and Membrane Stabilizing Properties of Methyl Jasmonate in Rats. Chin. J. Nat. Med. 2017, 15, 202–209. [Google Scholar] [CrossRef]
- Kramell, R.; Atzorn, R.; Schneider, G.; Miersch, O.; Brückner, C.; Schmidt, J.; Sembdner, G.; Parthier, B. Occurrence and Identification of Jasmonic Acid and Its Amino Acid Conjugates Induced by Osmotic Stress in Barley Leaf Tissue. J. Plant Growth Regul. 1995, 14, 29. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Wasternack, C.; Kombrink, E. Jasmonates: Structural Requirements for Lipid-Derived Signals Active in Plant Stress Responses and Development. ACS Chem. Biol. 2010, 5, 63–77. [Google Scholar] [CrossRef]
- Staswick, P.E.; Tiryaki, I. The Oxylipin Signal Jasmonic Acid Is Activated by an Enzyme That Conjugates It to Isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Suza, W.P.; Staswick, P.E. The Role of JAR1 in Jasmonoyl-L: -Isoleucine Production during Arabidopsis Wound Response. Planta 2008, 227, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Heitz, T.; Smirnova, E.; Widemann, E.; Aubert, Y.; Pinot, F.; Ménard, R. The Rise and Fall of Jasmonate Biological Activities. In Lipids in Plant and Algae Development; Subcellular Biochemistry; Nakamura, Y., Li-Beisson, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 405–426. ISBN 978-3-319-25979-6. [Google Scholar]
- Swiatek, A.; Van Dongen, W.; Esmans, E.L.; Van Onckelen, H. Metabolic Fate of Jasmonates in Tobacco Bright Yellow-2 Cells. Plant Physiol. 2004, 135, 161–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koch, T.; Bandemer, K.; Boland, W. Biosynthesis of Cis-Jasmone: A Pathway for the Inactivation and the Disposal of the Plant Stress Hormone Jasmonic Acid to the Gas Phase? Helv. Chim. Acta 1997, 80, 838–850. [Google Scholar] [CrossRef]
- Han, Y.; Bai, Y.; Xiao, Y.; Du, F.; Liang, Y.; Tan, Z.; Zhao, M.; Liu, H. Simultaneous Discrimination of Jasmonic Acid Stereoisomers by CE-QTOF-MS Employing the Partial Filling Technique. Electrophoresis 2011, 32, 2693–2699. [Google Scholar] [CrossRef]
- Krumm, T.; Bandemer, K.; Boland, W. Induction of Volatile Biosynthesis in the Lima Bean (Phaseolus Lunatus) by Leucine- and Isoleucine Conjugates of 1-Oxo- and 1-Hydroxyindan-4-Carboxylic Acid: Evidence for Amino Acid Conjugates of Jasmonic Acid as Intermediates in the Octadecanoid Signalling Pathway. FEBS Lett. 1995, 377, 523–529. [Google Scholar] [CrossRef]
- Koda, Y.; Kikuta, Y.; Tazaki, H.; Tsujino, Y.; Sakamura, S.; Yoshihara, T. Potato Tuber-Inducing Activities of Jasmonic Acid and Related Compounds. Phytochemistry 1991, 30, 1435–1438. [Google Scholar] [CrossRef]
- Sá-Nakanishi, A.B.; Soni-Neto, J.; Moreira, L.S.; Gonçalves, G.A.; Silva, F.M.S.; Bracht, L.; Bersani-Amado, C.A.; Peralta, R.M.; Bracht, A.; Comar, J.F. Anti-Inflammatory and Antioxidant Actions of Methyl Jasmonate Are Associated with Metabolic Modifications in the Liver of Arthritic Rats. Oxid. Med. Cell. Longev. 2018, 2018, 2056250. [Google Scholar] [CrossRef]
- Raviv, Z.; Cohen, S.; Reischer-Pelech, D. The Anti-Cancer Activities of Jasmonates. Cancer Chemother. Pharmacol. 2013, 71, 275–285. [Google Scholar] [CrossRef]
- Fingrut, O.; Flescher, E. Plant Stress Hormones Suppress the Proliferation and Induce Apoptosis in Human Cancer Cells. Leukemia 2002, 16, 608–616. [Google Scholar] [CrossRef]
- Cesari, I.M.; Carvalho, E.; Figueiredo Rodrigues, M.; Mendonça, B.D.S.; Amôedo, N.D.; Rumjanek, F.D. Methyl Jasmonate: Putative Mechanisms of Action on Cancer Cells Cycle, Metabolism, and Apoptosis. Int. J. Cell Biol. 2014, 2014, 572097. [Google Scholar] [CrossRef]
- Pérez-Salamó, I.; Krasauskas, J.; Gates, S.; Díaz-Sánchez, K.E.; Devoto, A. An Update on Core Jasmonate Signalling Networks, Physiological Scenarios, and Health Applications. Annu. Plant Rev. 2019, 2. [Google Scholar] [CrossRef]
- Kniazhanski, T.; Jackman, A.; Heyfets, A.; Gonen, P.; Flescher, E.; Sherman, L. Methyl Jasmonate Induces Cell Death with Mixed Characteristics of Apoptosis and Necrosis in Cervical Cancer Cells. Cancer Lett. 2008, 271, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Bömer, M.; Pérez-Salamó, I.; Florance, H.V.; Salmon, D.; Dudenhoffer, J.-H.; Finch, P.; Cinar, A.; Smirnoff, N.; Harvey, A.; Devoto, A. Jasmonates Induce Arabidopsis Bioactivities Selectively Inhibiting the Growth of Breast Cancer Cells through CDC6 and MTOR. New Phytol. 2021, 229, 2120–2134. [Google Scholar] [CrossRef] [PubMed]
- Klippel, S.; Jakubikova, J.; Delmore, J.; Ooi, M.; McMillin, D.; Kastritis, E.; Laubach, J.; Richardson, P.G.; Anderson, K.C.; Mitsiades, C.S. Methyljasmonate Displays in vitro and in vivo Activity against Multiple Myeloma Cells. Br. J. Haematol. 2012, 159, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Fingrut, O.; Reischer, D.; Rotem, R.; Goldin, N.; Altboum, I.; Zan-Bar, I.; Flescher, E. Jasmonates Induce Nonapoptotic Death in High-Resistance Mutant P53-Expressing B-Lymphoma Cells. Br. J. Pharmacol. 2005, 146, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.Y.; Oh, S.Y.; Han, S.I.; Park, H.G.; Yoo, M.-A.; Kang, H.S. Methyl Jasmonate Induces Apoptosis through Induction of Bax/Bcl-XS and Activation of Caspase-3 via ROS Production in A549 Cells. Oncol. Rep. 2004, 12, 1233–1238. [Google Scholar] [CrossRef]
- Kansara, S.; Parabia, F.M.; Badgujar, N.; Mistry, K. Effect of Methyl Jasmonate on A-549 Lung Cancer Cell Line and Docking with GLUT 1 and HK2 Targeted Proteins. Int. J. Cancer Tremnt. 2018, 1, 1–9. [Google Scholar]
- Ezekwudo, D.; Shashidharamurthy, R.; Devineni, D.; Bozeman, E.; Palaniappan, R.; Selvaraj, P. Inhibition of Expression of Anti-Apoptotic Protein Bcl-2 and Induction of Cell Death in Radioresistant Human Prostate Adenocarcinoma Cell Line (PC-3) by Methyl Jasmonate. Cancer Lett. 2008, 270, 277–285. [Google Scholar] [CrossRef]
- Yeruva, L.; Elegbede, J.A.; Carper, S.W. Methyl Jasmonate Decreases Membrane Fluidity and Induces Apoptosis through Tumor Necrosis Factor Receptor 1 in Breast Cancer Cells. Anticancer. Drugs 2008, 19, 766–776. [Google Scholar] [CrossRef]
- Tong, Q.-S.; Jiang, G.-S.; Zheng, L.-D.; Tang, S.-T.; Cai, J.-B.; Liu, Y.; Zeng, F.-Q.; Dong, J.-H. Methyl Jasmonate Downregulates Expression of Proliferating Cell Nuclear Antigen and Induces Apoptosis in Human Neuroblastoma Cell Lines. Anticancer. Drugs 2008, 19, 573–581. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Yehia, R.; Hathout, R.M.; Attia, D.A.; Elmazar, M.M.; Mortada, N.D. Anti-Tumor Efficacy of an Integrated Methyl Dihydrojasmonate Transdermal Microemulsion System Targeting Breast Cancer Cells: In vitro and in vivo Studies. Colloids Surf. B Biointerfaces 2017, 155, 512–521. [Google Scholar] [CrossRef]
- Flescher, E. Jasmonates in Cancer Therapy. Cancer Lett. 2007, 245, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, E.; Pedersen, P.L. High Aerobic Glycolysis of Rat Hepatoma Cells in Culture: Role of Mitochondrial Hexokinase. Proc. Natl. Acad. Sci. USA 1977, 74, 3735–3739. [Google Scholar] [CrossRef]
- Goldin, N.; Arzoine, L.; Heyfets, A.; Israelson, A.; Zaslavsky, Z.; Bravman, T.; Bronner, V.; Notcovich, A.; Shoshan-Barmatz, V.; Flescher, E. Methyl Jasmonate Binds to and Detaches Mitochondria-Bound Hexokinase. Oncogene 2008, 27, 4636–4643. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10. [Google Scholar] [CrossRef]
- Li, J.; Chen, K.; Wang, F.; Dai, W.; Li, S.; Feng, J.; Wu, L.; Liu, T.; Xu, S.; Xia, Y.; et al. Methyl Jasmonate Leads to Necrosis and Apoptosis in Hepatocellular Carcinoma Cells via Inhibition of Glycolysis and Represses Tumor Growth in Mice. Oncotarget 2017, 8, 45965–45980. [Google Scholar] [CrossRef]
- Rotem, R.; Heyfets, A.; Fingrut, O.; Blickstein, D.; Shaklai, M.; Flescher, E. Jasmonates: Novel Anticancer Agents Acting Directly and Selectively on Human Cancer Cell Mitochondria. Cancer Res. 2005, 65, 1984–1993. [Google Scholar] [CrossRef]
- Ishii, Y.; Kiyota, H.; Sakai, S.; Honma, Y. Induction of Differentiation of Human Myeloid Leukemia Cells by Jasmonates, Plant Hormones. Leukemia 2004, 18, 1413–1419. [Google Scholar] [CrossRef][Green Version]
- Cohen, S.; Flescher, E. Methyl Jasmonate: A Plant Stress Hormone as an Anti-Cancer Drug. Phytochemistry 2009, 70, 1600–1609. [Google Scholar] [CrossRef]
- Khokon, M.A.R.; Salam, M.A.; Jammes, F.; Ye, W.; Hossain, M.A.; Okuma, E.; Nakamura, Y.; Mori, I.C.; Kwak, J.M.; Murata, Y. MPK9 and MPK12 Function in SA-Induced Stomatal Closure in Arabidopsis Thaliana. Biosci. Biotechnol. Biochem. 2017, 81, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.; Logsdon, C.D. S100P: A Novel Therapeutic Target for Cancer. Amino Acids 2011, 41, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Francisco-Marquez, M.; Galano, A. The Reactions of Plant Hormones with Reactive Oxygen Species: Chemical Insights at a Molecular Level. J. Mol. Model. 2018, 24, 255. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hong, X.; Gao, X.; Gu, X.; Xiong, W.; Zhao, J.; Yu, H.; Cui, M.; Xie, M.; Bai, Y.; et al. Methyl Jasmonate Enhances the Radiation Sensitivity of Esophageal Carcinoma Cells by Inhibiting the 11-Ketoprostaglandin Reductase Activity of AKR1C3. Cancer Manag. Res. 2018, 10, 3149–3158. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, J.H.; Park, M.J.; Kim, S.M.; Yoon, C.S.; Joo, Y.M.; Park, J.S.; Han, S.I.; Park, H.G.; Kang, H.S. Induction of Heat Shock Protein 72 in C6 Glioma Cells by Methyl Jasmonate through ROS-Dependent Heat Shock Factor 1 Activation. Int. J. Mol. Med. 2005, 16, 833–839. [Google Scholar] [CrossRef]
- Besson, J.C.F.; de Carvalho Picoli, C.; Matioli, G.; Natali, M.R.M. Methyl Jasmonate: A Phytohormone with Potential for the Treatment of Inflammatory Bowel Diseases. J. Pharm. Pharmacol. 2018, 70, 178–190. [Google Scholar] [CrossRef]
- Yeruva, L.; Pierre, K.J.; Carper, S.W.; Elegbede, J.A.; Toy, B.J.; Wang, R.C. Jasmonates Induce Apoptosis and Cell Cycle Arrest in Non-Small Cell Lung Cancer Lines. Exp. Lung Res. 2006, 32, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of Reactive Oxygen Species Levels and Radioresistance in Cancer Stem Cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Zhang, M.; Su, L.; Xiao, Z.; Liu, X.; Liu, X. Methyl Jasmonate Induces Apoptosis and Pro-Apoptotic Autophagy via the ROS Pathway in Human Non-Small Cell Lung Cancer. Am. J. Cancer Res. 2016, 6, 187–199. [Google Scholar]
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants 2021, 10, 569. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Darvishi, P.; Yousefi, Z.; Pourfathollah, A.A. Effect of Methyl Jasmonate and 3-Bromopyruvate Combination Therapy on Mice Bearing the 4 T1 Breast Cancer Cell Line. J. Bioenerg. Biomembr. 2020, 52, 103–111. [Google Scholar] [CrossRef]
- Heyfets, A.; Flescher, E. Cooperative Cytotoxicity of Methyl Jasmonate with Anti-Cancer Drugs and 2-Deoxy-D-Glucose. Cancer Lett. 2007, 250, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, L.; Hall, C.; Elegbede, J.A.; Carper, S.W. Perillyl Alcohol and Methyl Jasmonate Sensitize Cancer Cells to Cisplatin. Anticancer. Drugs 2010, 21, 1–9. [Google Scholar] [CrossRef]
- Elia, U.; Flescher, E. PI3K/Akt Pathway Activation Attenuates the Cytotoxic Effect of Methyl Jasmonate toward Sarcoma Cells. Neoplasia 2008, 10, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, P.P.G.; Gaglione, S.; Sewastianik, T.; Carrasco, R.D.; Langer, R.; Mitchell, M.J. Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy. ACS Nano 2018, 12, 912–931. [Google Scholar] [CrossRef]
- Raviv, Z.; Zilberberg, A.; Cohen, S.; Reischer-Pelech, D.; Horrix, C.; Berger, M.; Rosin-Arbesfeld, R.; Flescher, E. Methyl Jasmonate Down-Regulates Survivin Expression and Sensitizes Colon Carcinoma Cells towards TRAIL-Induced Cytotoxicity. Br. J. Pharmacol. 2011, 164, 1433–1444. [Google Scholar] [CrossRef]
- Twomey, J.D.; Kim, S.-R.; Zhao, L.; Bozza, W.P.; Zhang, B. Spatial Dynamics of TRAIL Death Receptors in Cancer Cells. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2015, 19, 13–21. [Google Scholar] [CrossRef]
- Jiang, G.; Zhao, J.; Xiao, X.; Tao, D.; Gu, C.; Tong, Q.; Luo, B.; Wang, L.; Zeng, F. AN N-Terminal Smac Peptide Sensitizes Human Prostate Carcinoma Cells to Methyl Jasmonate-Induced Apoptosis. Cancer Lett. 2011, 302, 37–46. [Google Scholar] [CrossRef]
- Xiao, X.; Jiang, G.; Wang, L.; Lv, L.; Zeng, F.-Q. Predominant Enhancement of Apoptosis Induced by Methyl Jasmonate in Bladder Cancer Cells: Therapeutic Effect of the Antp-Conjugated Smac Peptide. Anticancer. Drugs 2011, 22, 853–863. [Google Scholar] [CrossRef]
- Milrot, E.; Jackman, A.; Flescher, E.; Gonen, P.; Kelson, I.; Keisari, Y.; Sherman, L. Enhanced Killing of Cervical Cancer Cells by Combinations of Methyl Jasmonate with Cisplatin, X or Alpha Radiation. Invest. New Drugs 2013, 31, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Conti, M. Cyclopentenone: A Special Moiety for Anticancer Drug Design. Anticancer. Drugs 2006, 17, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Maeng, K.; Dang, H.-T.; Kang, G.-J.; Ryou, C.; Jung, J.H.; Kang, H.-K.; Prchal, J.T.; Yoo, E.-S.; Yoon, D. Anti-Inflammatory Effect of Methyl Dehydrojasmonate (J2) Is Mediated by the NF-ΚB Pathway. J. Mol. Med. Berl. Ger. 2011, 89, 83–90. [Google Scholar] [CrossRef]
- Reischer, D.; Heyfets, A.; Shimony, S.; Nordenberg, J.; Kashman, Y.; Flescher, E. Effects of Natural and Novel Synthetic Jasmonates in Experimental Metastatic Melanoma. Br. J. Pharmacol. 2007, 150, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.T.; Lee, H.J.; Yoo, E.S.; Hong, J.; Bao, B.; Choi, J.S.; Jung, J.H. New Jasmonate Analogues as Potential Anti-Inflammatory Agents. Bioorg. Med. Chem. 2008, 16, 10228–10235. [Google Scholar] [CrossRef]
- Dang, H.T.; Lee, Y.M.; Kang, G.J.; Yoo, E.S.; Hong, J.; Lee, S.M.; Lee, S.K.; Pyee, Y.; Chung, H.-J.; Moon, H.R.; et al. In vitro Stability and in vivo Anti-Inflammatory Efficacy of Synthetic Jasmonates. Bioorg. Med. Chem. 2012, 20, 4109–4116. [Google Scholar] [CrossRef]
- Sucu, B.O.; Ipek, O.S.; Kurtulus, S.O.; Yazici, B.E.; Karakas, N.; Guzel, M. Synthesis of Novel Methyl Jasmonate Derivatives and Evaluation of Their Biological Activity in Various Cancer Cell Lines. Bioorganic Chem. 2019, 91, 103146. [Google Scholar] [CrossRef]
- Biernacki, K.; Daśko, M.; Ciupak, O.; Kubiński, K.; Rachon, J.; Demkowicz, S. Novel 1,2,4-Oxadiazole Derivatives in Drug Discovery. Pharmaceuticals 2020, 13, 111. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Zaprutko, L. Microwave Assisted Synthesis of Unsaturated Jasmone Heterocyclic Analogues as New Fragrant Substances. Eur. J. Med. Chem. 2009, 44, 3032–3039. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Sowa-Kasprzak, K.; Michalak, J.; Kędzia, B.; Zaprutko, L. Ocena Aktywności Antybiotycznej Z-Jasmonu Oraz Jego Pochodnych Heterocyklicznych. Postępy Fitoter. 2017, 18, 171–177. [Google Scholar] [CrossRef]
- Alexiades, M. Jasmonates and Tetrahydrojasmonic Acid: A Novel Class of Anti-Aging Molecules. J. Drugs Dermatol. JDD 2016, 15, 206–207. [Google Scholar]
- Tran, C.; Michelet, J.F.; Simonetti, L.; Fiat, F.; Garrigues, A.; Potter, A.; Segot, E.; Watson, R.E.B.; Griffiths, C.E.M.; Lacharrière, O. de In Vitro and in Vivo Studies with Tetra-Hydro-Jasmonic Acid (LR2412) Reveal Its Potential to Correct Signs of Skin Ageing. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 415–423. [Google Scholar] [CrossRef]
- Kapuscinska, A.; Olejnik, A.; Nowak, I. The Conjugate of Jasmonic Acid and Tetrapeptide as a Novel Promising Biologically Active Compound. New J. Chem. 2016, 40, 9007–9011. [Google Scholar] [CrossRef]
| Jasmonates/Drug | Cancer | Concentration Range | Action/Effects | References |
|---|---|---|---|---|
| MJ + BCNU (in vitro) MJ + taxol (in vitro) | Pancreatic cell: PaCa-2 BCL1 MCF-7 DA-3 D-122 | MJ: 0.1 mM BCNU: 1, 10, 25 µg/mL-PaCa-2 2.5, 5 µg/mL-BCL1 taxol: 1, 2.5, 5, 10 μg/ml | mitochondriotoxic synergic cytotoxicity IC50 ↓ | [54] |
| MJ + POH or MJ + cisplatin or MJ + cisplatin + POH (in vitro) | Breast cancer cell lines: MDA-MB-231 MDA-MB-435 MCF7 | IC2O (POH) MDA-MB-231: 0.76 mM MDA-MB-435: 0.6 mM MCF7: 0.8 mM | MJ + POH: cytotoxicity ↑ apoptosis ↑ TNFR1 ↑ MJ + POH: apoptosis ↑ | [55] |
| MJ + 2DG 2-deoxyglucose (in vitro) | Sarcoma: SaOS-2 MCA-105 | MJ: 0.5–3 mM 2DG: 1 and 2 mM | synergic cytotoxicity ↑ ATP glycolysis ↑ | [56] |
| MJ + TRAIL (in vitro) | CRC cell lines: SW480, HT29, LS180, HCT116 | MJ: 0.5 mM TRAIL: 100–200 ng/ml | IAP (survivin) ↓ caspase activity ↑ TRAIL-induced apoptosis ↑ | [58] |
| MJ + Smac7N (in vitro) | prostate carcinoma cells: DU145, PC-3 proximal tubular epithelial cells: HK-2 | MJ: 0.5–2 mM | Smac7N: MJ-induced cytotoxicity ↑ ran caspase-9 dependent and independent pathways | [60] |
| MJ + 3-BrP (in vitro) | Mice breast carcinoma cell line: 4 T1 | MJ: 0.5–3 mM 3-BrP: 12.5, 25, 50, 100, 200, 400 μM | ALT ↑ AST ↑ tumor growth ↓ antitumor activity ↑ | [53] |
| MJ + cisplatin MJ + X-rays MJ + α-rays | Cervical cancer cells: SiHa, CaSki, HeLa C33A | MJ: 0.1–1 mM Cisplatin: 0.1–0.5 μM X-rays: 0 25–3 Gy | cell survival ↓ IC50 radiation dose ↓ cell viability ↓ | [62] |
| Name and Structure of the Synthetic Derivatives of MJ | Tumor Cells | IC50 | References | |
|---|---|---|---|---|
| MJ | Derivative | |||
methyl 4,5-didehydrojasmonate DHJM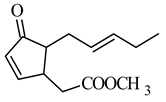 | human myeloid leukemia HL-60 | 347 μM | 12 μM | [41] |
methyl 5,7,9,10-tetrabromojasmonate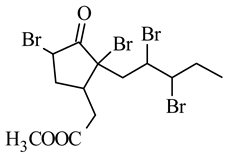 | murine melanoma B16-F10 | 2.6 mM | 0.042 mM | [65] |
| human lymphoblastic leukemia Molt-4 | 0.5 mM | 0.009 mM | ||
| human breast cancer MCF7 | 1.5 mM | 0.015 mM | ||
| human pancreatic cancer MIA PaCa-2 | 1.4 mM | 0.09 mM | ||
| murine lung cancer D122 | 1.8 mM | 0.25 mM | ||
3-((3-methyl-1,2,4-oxadiazol-5-yl) methyl)-2-(pent-2-en-1-yl)cyclo-pentanol | lung carcinoma A549 | 6.38 mM | 4.25 mM | [68] |
| ovarian carcinoma SKOV-3 | 4.17 mM | 1.772 mM | ||
| Synthetic Derivatives of MJ | Biological Activity | References |
|---|---|---|
DHJM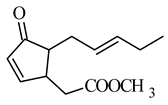 |
| [41] |
| [64] | |
methyl 5,7,9,10-tetrabromojasmonate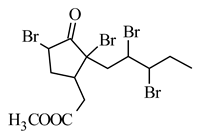 |
| [65] |
methyl 5-chloro-4,5-didehydrodihydro-jasmonate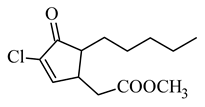 |
| [66] |
t-butyl 5-chloro-4,5-didehydrodihydro-jasmonate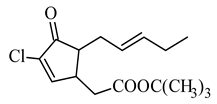 |
| [67] |
3-((3-methyl-1,2,4-oxadiazol-5-yl) methyl)-2-(pent-2-en-1-yl)cyclo-pentanol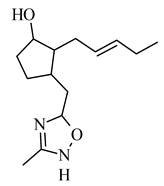 |
| [68] |
4-methyl-3-(2-pentenyl)-2-oxazolidinone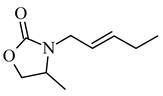 |
| [71] |
(3-hydroxy-2-pentylcyclopentyl) acetic acid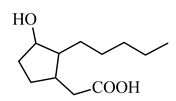 |
| [72,73] |
JA–YPFF–NH2 |
| [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarocka-Karpowicz, I.; Markowska, A. Jasmonate Compounds and Their Derivatives in the Regulation of the Neoplastic Processes. Molecules 2021, 26, 2901. https://doi.org/10.3390/molecules26102901
Jarocka-Karpowicz I, Markowska A. Jasmonate Compounds and Their Derivatives in the Regulation of the Neoplastic Processes. Molecules. 2021; 26(10):2901. https://doi.org/10.3390/molecules26102901
Chicago/Turabian StyleJarocka-Karpowicz, Iwona, and Agnieszka Markowska. 2021. "Jasmonate Compounds and Their Derivatives in the Regulation of the Neoplastic Processes" Molecules 26, no. 10: 2901. https://doi.org/10.3390/molecules26102901
APA StyleJarocka-Karpowicz, I., & Markowska, A. (2021). Jasmonate Compounds and Their Derivatives in the Regulation of the Neoplastic Processes. Molecules, 26(10), 2901. https://doi.org/10.3390/molecules26102901






