Synthesis and Nano-Sized Characterization of Bioactive Oregano Essential Oil Molecule-Loaded Small Unilamellar Nanoliposomes with Antifungal Potentialities
Abstract
1. Introduction
2. Results and Discussion
2.1. Size Distribution of OEO Nanoliposomes
2.2. Zeta Potential Values of OEO Nanoliposomes
2.3. Scanning Electron Microscopy (SEM) Analysis
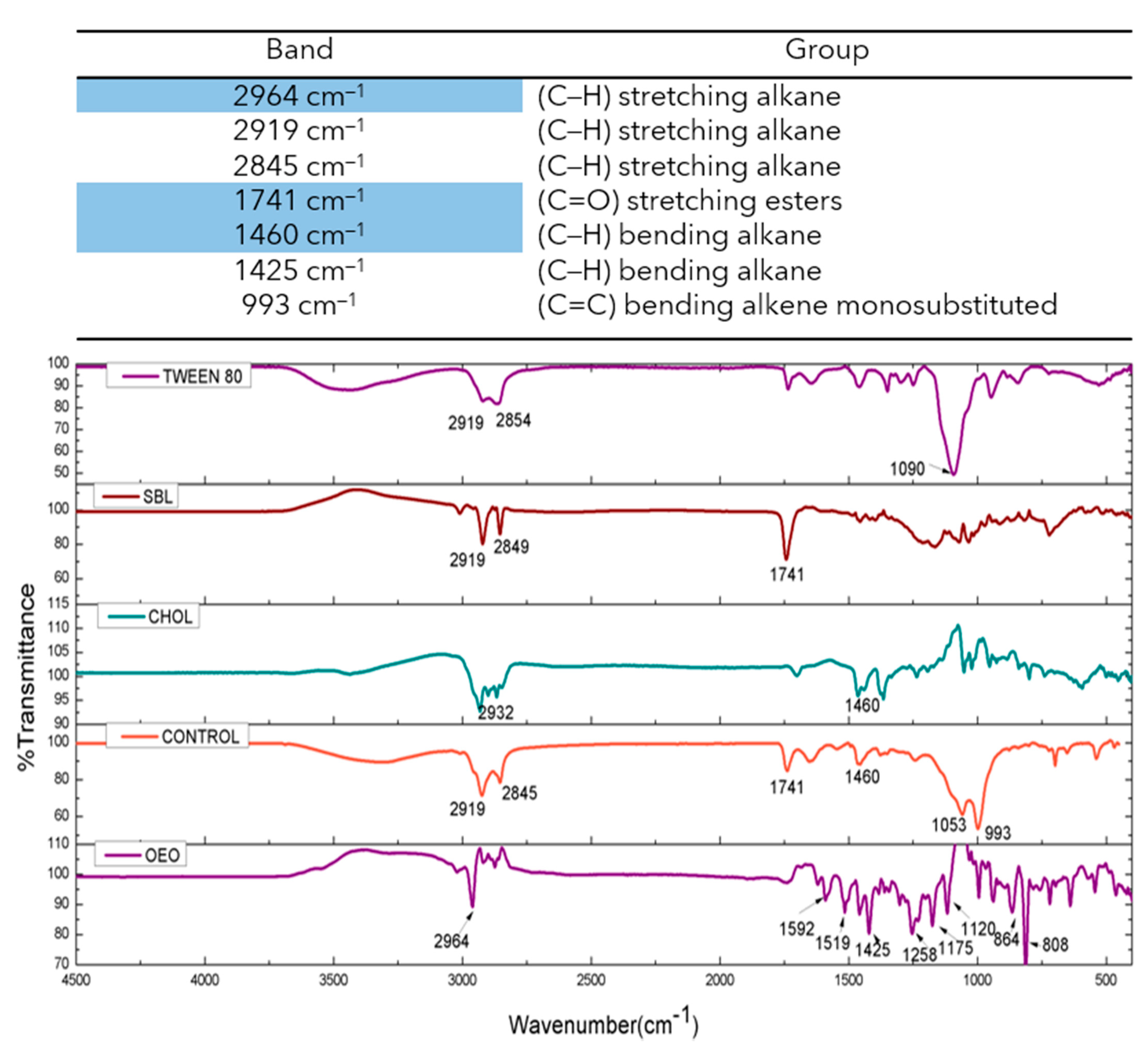
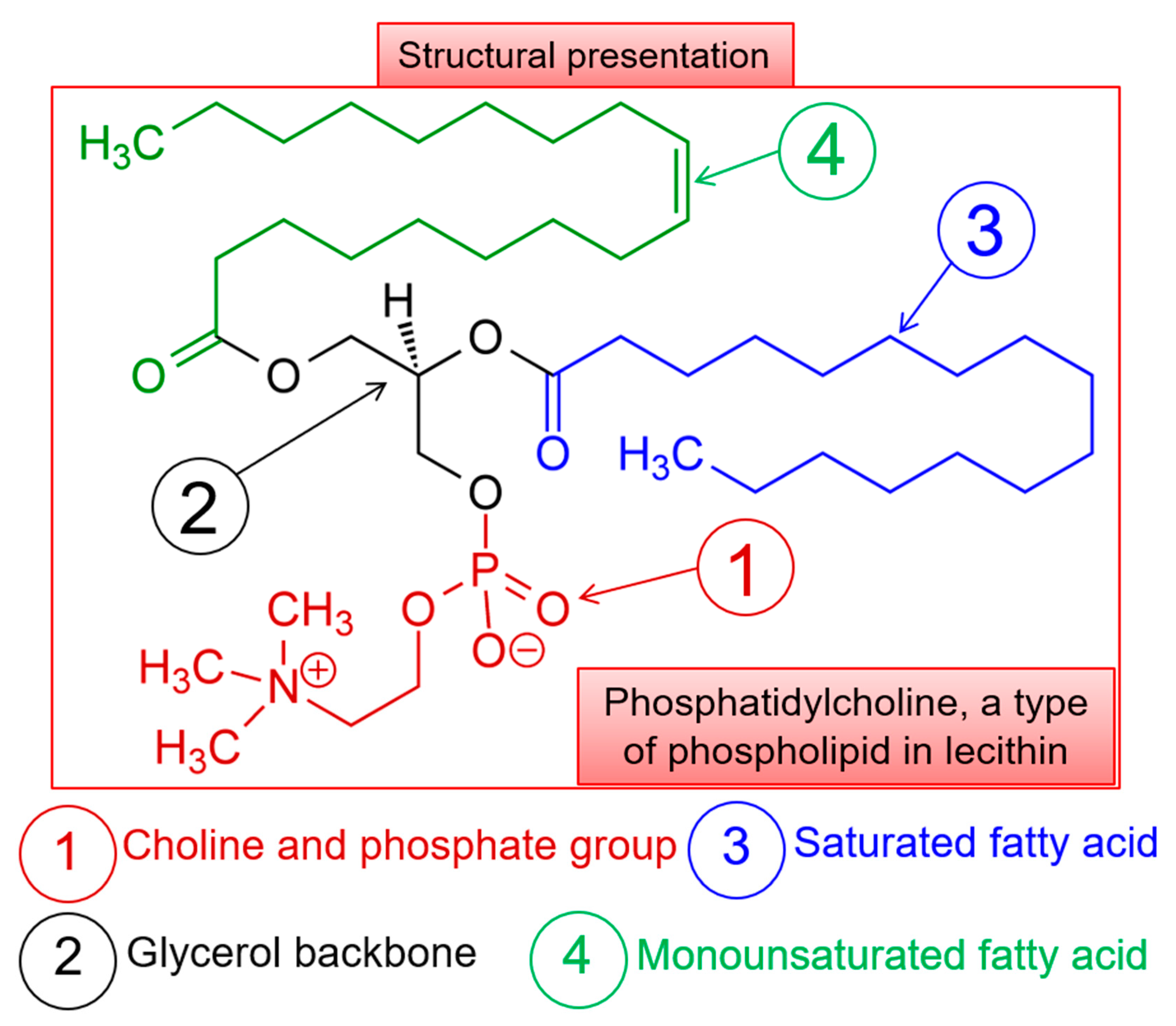
2.4. ATR-FTIR Studies of OEO Nanoliposomes
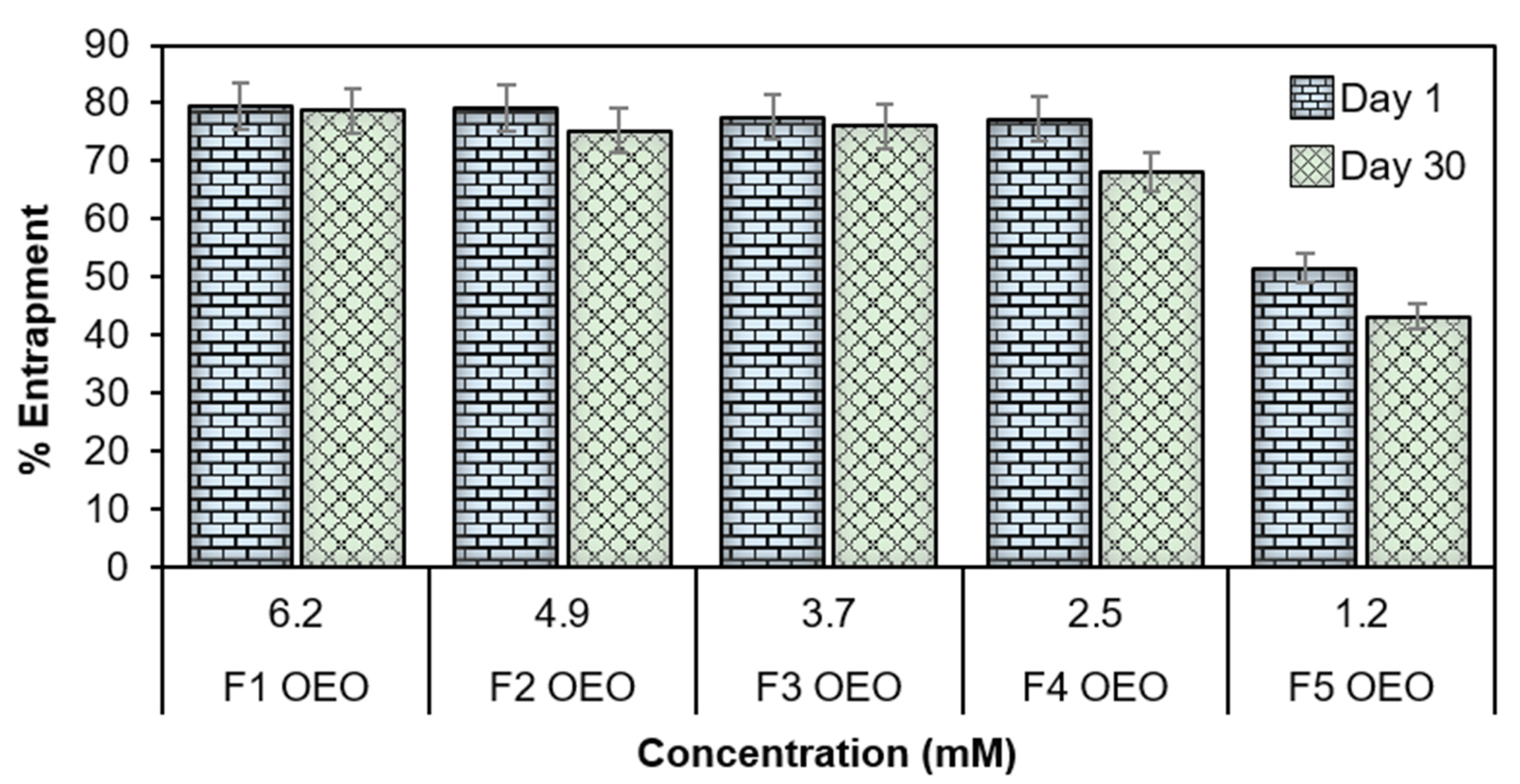
2.5. Entrapment Efficiency (EE%)
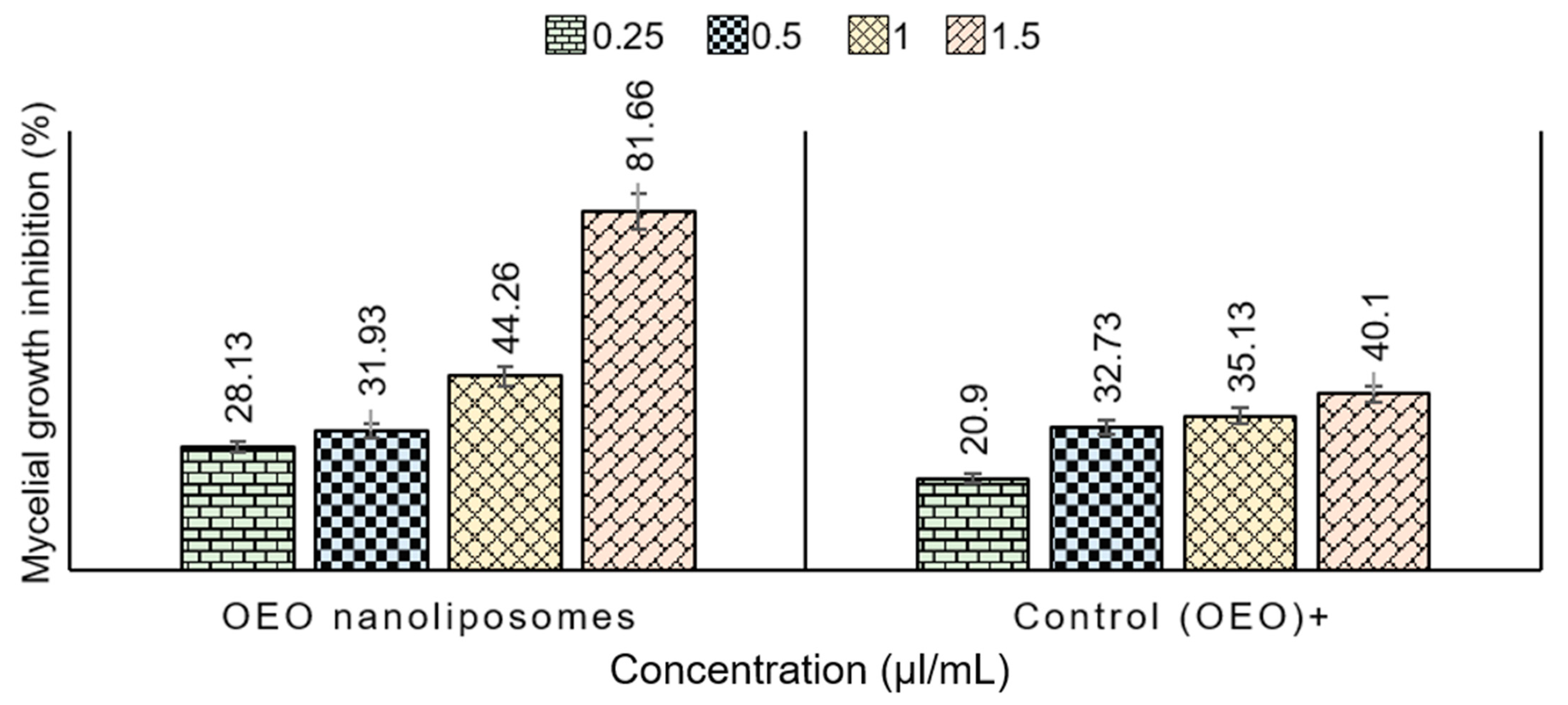
2.6. In Vitro Antifungal Activity of Nanoliposomes—Mycelial Growth Test
3. Materials and Methods
3.1. Materials/Chemicals/Reagents
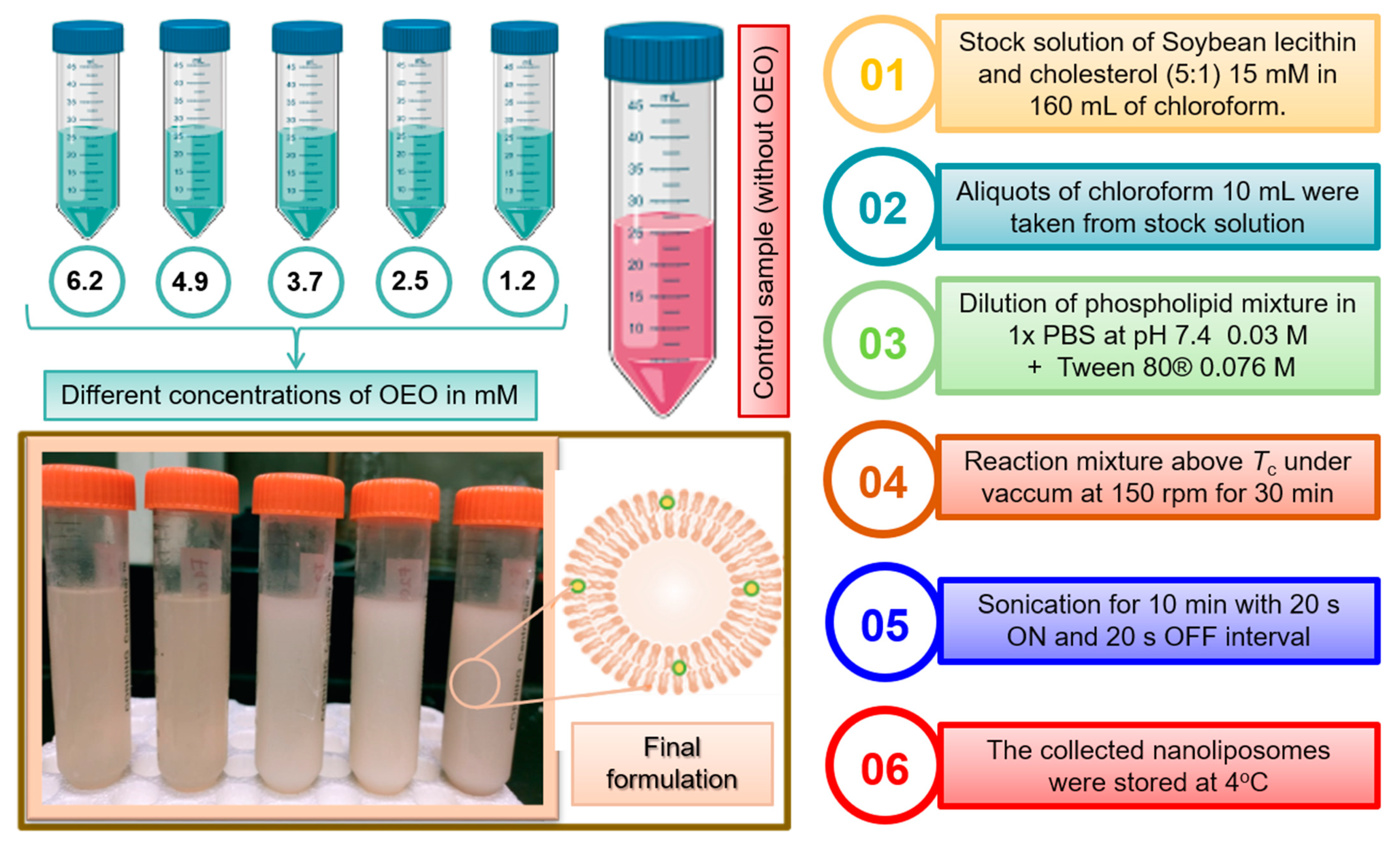
3.2. Preparation of Nanoliposomes in the Presence/Absence of OEO
3.3. Characterization of Nanoliposomes
3.3.1. Dynamic Light Scattering (DLS)
3.3.2. Scanning Electron Microscopy (SEM) Analysis
3.3.3. Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
3.3.4. Determination of Entrapment Efficiency
3.4. Isolation of Dermatophyte Fungi
In Vitro Antifungal Activity of Nanoliposomes—Mycelial Growth Test
3.5. Statistical Analysis
4. Concluding Remarks and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vallesi, A.; Pucciarelli, S.; Buonanno, F.; Fontana, A.; Mangiagalli, M. Bioactive molecules from protists: Perspectives in biotechnology. Eur. J. Protistol. 2020, 75, 125720. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E. Stimuli-responsive polymer-modified liposomes and their application to DDS. Stimuli. Responsive Polym. Nanocarriers Drug Deliv. Appl. 2019, 2, 305–319. [Google Scholar] [CrossRef]
- Verma, S.; Utreja, P. Vesicular nanocarrier based treatment of skin fungal infections: Potential and emerging trends in nanoscale pharmacotherapy. Asian J. Pharm. Sci. 2019, 14, 117–129. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.M.; Avilés-Castrillo, J.I.; Medina, D.I.; Parra-Saldivar, R.; Iqbal, H.M.N. Insight Into Nanoliposomes as Smart Nanocarriers for Greening the Twenty-First Century Biomedical Settings. Front. Bioeng. Biotechnol. 2020, 8, 1441. [Google Scholar] [CrossRef] [PubMed]
- Khosravi-Darani, K.; Mozafari, M.R. Nanoliposome potentials in nanotherapy: A concise overview. Int. J. Nanosci. Nanotechnol. 2010, 6, 3–13. [Google Scholar]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface. 2014, 11, 459. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Shishir, M.R.I.; Chen, W. Surface decoration of neohesperidin-loaded nanoliposome using chitosan and pectin for improving stability and controlled release. Int. J. Biol. Macromol. 2020, 164, 2903–2914. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.K.H.; Delgado-Charro, B.; Bolhuis, A. A microtiter plate-based quantitative method to monitor the growth rate of dermatophytes and test antifungal activity. J. Microbiol. Method. 2019, 165, 105722. [Google Scholar] [CrossRef]
- Michalczyk, A.; Ostrowska, P. Essential oils and their components in combating fungal pathogens of animal and human skin. J. Med. Mycol. 2021, 31, 101118. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Li, X.J.; Qin, Q.F.; Li, Y.S.; Zhang, W.K.; Tang, H.B. Anti-inflammatory and antinociceptive effects of active ingredients in the essential oils from Gynura procumbens, a traditional medicine and a new and popular food material. J. Ethnopharmacol. 2019, 239, 111916. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Hanlidou, E.; Lanaras, T. Essential oil composition of Greek (Origanum vulgare ssp. hirtum) and Turkish (O. onites) oregano: A tool for their distinction. J. Essent. Oil Res. 2004, 16, 334–338. [Google Scholar] [CrossRef]
- Giannenas, I.; Bonos, E.; Christaki, E.; Florou-Paneri, P. Oregano: A feed additive with functional properties. Ther. Foods. 2018, 8, 179–208. [Google Scholar] [CrossRef]
- Nagaraju, P.G.; Sengupta, P.; Chicgovinda, P.P.; Rao, P.J. Nanoencapsulation of clove oil and study of physicochemical properties, cytotoxic, hemolytic, and antioxidant activities. J. Food Pro. Eng. 2021, 44, 13645. [Google Scholar]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.A.; Casabianca, H.; Elaissari, A. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Unalan, I.; Boccaccini, A.R. Essential oils in biomedical applications: Recent progress and future opportunities. Current Opin. Biomed. Eng. 2021, 17, 100261. [Google Scholar] [CrossRef]
- Yahyazadeh, M.; Omidbaigi, R.; Zare, R.; Taheri, H. Effect of some essential oils on mycelial growth of Penicillium digitatum Sacc. World J. Microbiol. Biotechnol. 2008, 24, 1445–1450. [Google Scholar] [CrossRef]
- Malakouti-Nejad, M.; Bardania, H.; Aliakbari, F.; Baradaran-Rafii, A.; Elahi, E.; Monti, D.; Morshedi, D. Formulation of nanoliposome-encapsulated bevacizumab (Avastin): Statistical optimization for enhanced drug encapsulation and properties evaluation. Int. J. Pharm. 2020, 590, 119895. [Google Scholar] [CrossRef] [PubMed]
- Gorjian, H.; Amiri, Z.R.; Milani, J.M.; Khaligh, N.G. Preparation and characterization of the encapsulated myrtle extract nanoliposome and nanoniosome without using cholesterol and toxic organic solvents: A comparative study. Food Chem. 2021, 342, 128342. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Res. Lett. 2018, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, H.; Cheraghi, N.; Khanjari, A.; Rezaeigolestani, M.; Basti, A.A.; Kamkar, A.; Aghaee, E.M. Incorporation of nanoencapsulated garlic essential oil into edible films: A novel approach for extending shelf life of vacuum-packed sausages. Meat Sci. 2020, 166, 108135. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules. 2017, 22, 989. [Google Scholar] [CrossRef]
- Risaliti, L.; Kehagia, A.; Daoultzi, E.; Lazari, D.; Bergonzi, M.C.; Vergkizi-Nikolakaki, S.; Bilia, A.R. Liposomes loaded with Salvia triloba and Rosmarinus officinalis essential oils: In vitro assessment of antioxidant, antiinflammatory and antibacterial activities. J. Drug Deliv. Sci. Technol. 2019, 51, 493–498. [Google Scholar] [CrossRef]
- Jebali, A.; Karimabad, M.N.; Ahmadi, Z.; Khorramdel, H.; Kaeidi, A.; Mirzaei, M.; Hassanshahi, G. Attenuation of inflammatory response in the EAE model by PEGlated nanoliposome of pistachio oils. J. Neuroimmunol. 2020, 347, 577352. [Google Scholar] [CrossRef] [PubMed]
- Salari, S.; Salari, R. Nanoliposomal system of rosemary essential oil made by specific human cell phospholipids and evaluation of its anti-cancer properties. Appl. Nanosci. 2019, 9, 2085–2089. [Google Scholar] [CrossRef]
- Arabi, M.H.; Mirzapour, A.; Chabok, H.; Shafiee Ardestani, M.; Saffari, M. Preparation methods of nanoliposomes containing Zataria multiflora essential oil: A comparative study. Biosci. Biotechnol. Res. Comm. 2017, 10, 151–160. [Google Scholar] [CrossRef]
- Bo, R.; Dai, X.; Huang, J.; Wei, S.; Liu, M.; Li, J. Evaluation of optimum conditions for decoquinate nanoliposomes and their anticoccidial efficacy against diclazuril-resistant Eimeria tenella infections in broilers. Vet. Parasitol. 2020, 283, 109186. [Google Scholar] [CrossRef] [PubMed]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of nanomaterials: Tools and challenges. Nanomater. Food Appl. 2019, 313–353. [Google Scholar] [CrossRef]
- Danaei, M.; Kalantari, M.; Raji, M.; Fekri, H.S.; Saber, R.; Asnani, G.P.; Taheriazam, A. Probing nanoliposomes using single particle analytical techniques: Effect of excipients, solvents, phase transition and zeta potential. Heliyon 2018, 4, 01088. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.R.; Noda, I.; Jung, Y.M. What is the origin of positional fluctuation of spectral features: True frequency shift or relative intensity changes of two overlapped bands? Appl. Spectrosc. 2010, 64, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Chibowski, E.; Szcześ, A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption 2016, 22, 755–765. [Google Scholar] [CrossRef]
- Makino, K.; Yamada, T.; Kimura, M.; Oka, T.; Ohshima, H.; Kondo, T. Temperature-and ionic strength-induced conformational changes in the lipid head group region of liposomes as suggested by zeta potential data. Bio. Chem. 1991, 41, 175–183. [Google Scholar] [CrossRef]
- Kistiakowsky, G.B.; Romeyn Jr, H.; Ruhoff, J.R.; Smith, H.A.; Vaughan, W.E. Heats of organic reactions. I. The apparatus and the heat of hydrogenation of ethylene. J. Am. Chem. Soc. 1935, 57, 65–75. [Google Scholar] [CrossRef]
- Cugia, F.; Monduzzi, M.; Ninham, B.W.; Salis, A. Interplay of ion specificity, pH and buffers: Insights from electrophoretic mobility and pH measurements of lysozyme solutions. RSC. Adv. 2013, 3, 5882–5888. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-based nanoparticles for drug delivery systems. Charact. Biol. Nanomater. Drug Deliv. 2019, 47–76. [Google Scholar] [CrossRef]
- Tai, K.; Liu, F.; He, X.; Ma, P.; Mao, L.; Gao, Y.; Yuan, F. The effect of sterol derivatives on properties of soybean and egg yolk lecithin liposomes: Stability, structure and membrane characteristics. Food Res. Int. 2018, 109, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Maji, R.; Roychowdhury, S.; Ghosh, S. Toxicological concerns of engineered nanosize drug delivery systems. Am. J. Ther. 2016, 23, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Damari, S.P.; Shamrakov, D.; Varenik, M.; Koren, E.; Nativ-Roth, E.; Barenholz, Y.; Regev, O. Practical aspects in size and morphology characterization of drug-loaded nano-liposomes. Int. J. Pharm. 2018, 547, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Sarabandi, K.; Jafari, S.M.; Mohammadi, M.; Akbarbaglu, Z.; Pezeshki, A.; Heshmati, M.K. Production of reconstitutable nanoliposomes loaded with flaxseed protein hydrolysates: Stability and characterization. Food Hydrocoll. 2019, 96, 442–450. [Google Scholar] [CrossRef]
- Mazloomi, S.N.; Mahoonak, A.S.; Ghorbani, M.; Houshmand, G. Physicochemical properties of chitosan-coated nanoliposome loaded with orange seed protein hydrolysate. J. Food Eng. 2020, 280, 109976. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Reque, P.M.; Brandelli, A. Effect of oleic acid, cholesterol, and octadecylamine on membrane stability of freeze-dried liposomes encapsulating natural antimicrobials. Food Bioprocess Technol. 2020, 13, 599–610. [Google Scholar] [CrossRef]
- Hammoud, Z.; Gharib, R.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. New findings on the incorporation of essential oil components into liposomes composed of lipoid S100 and cholesterol. Int. J. Pharm. 2019, 561, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Mun’delanji, C.V. Structure-dependent interactions of polyphenols with a biomimetic membrane system. Biophys. Acta. Biomembr. 2014, 1838, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
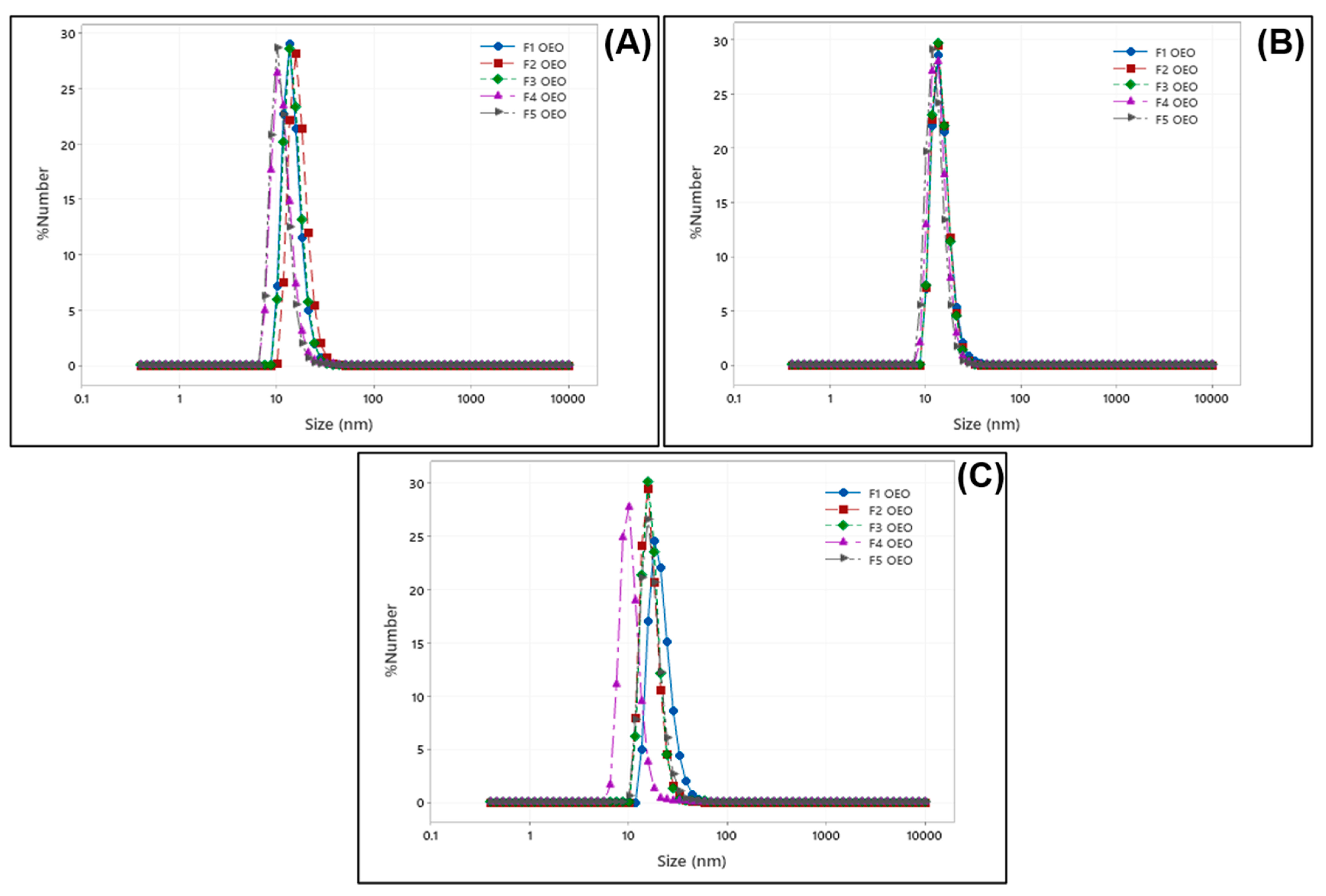
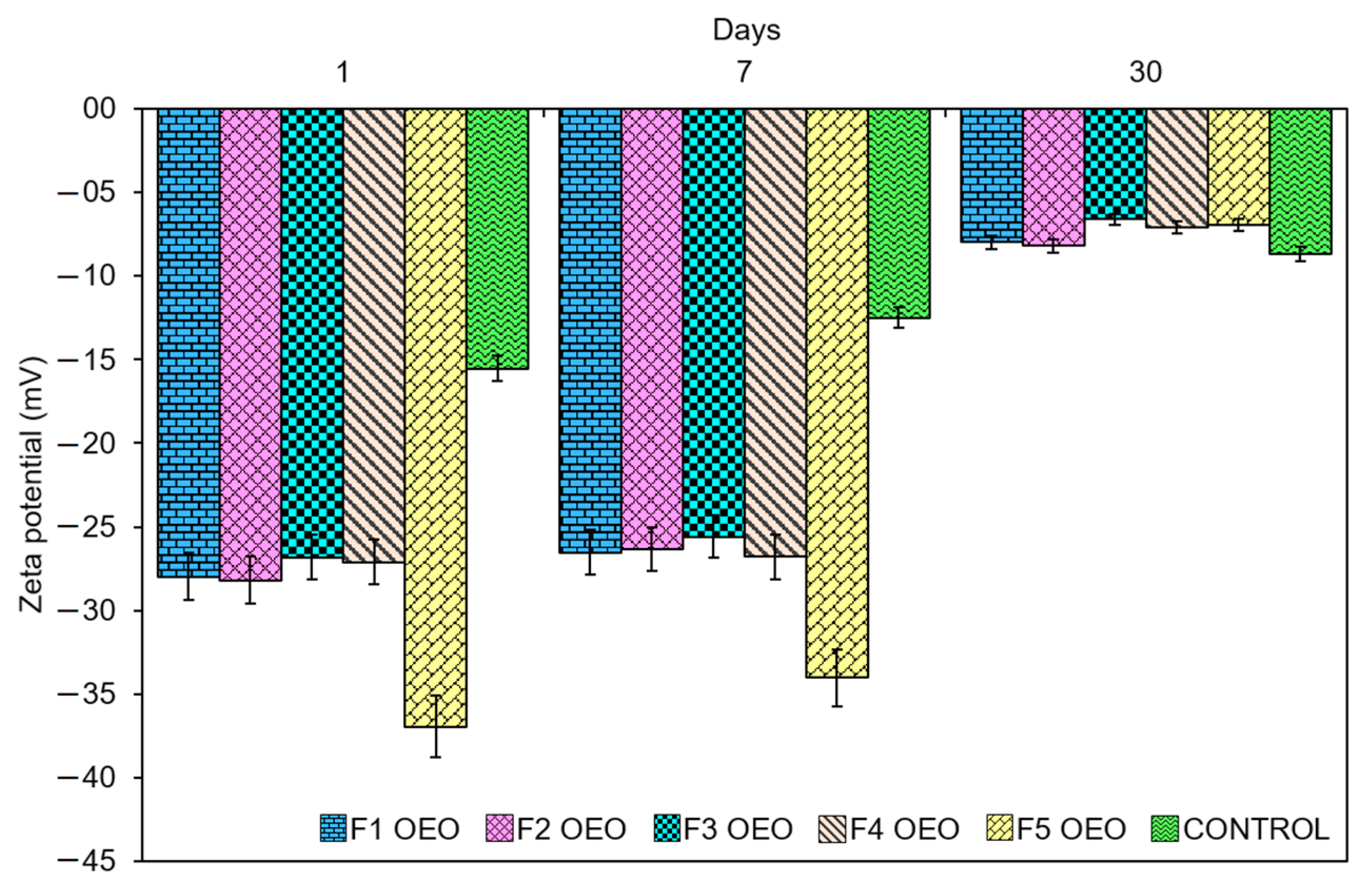
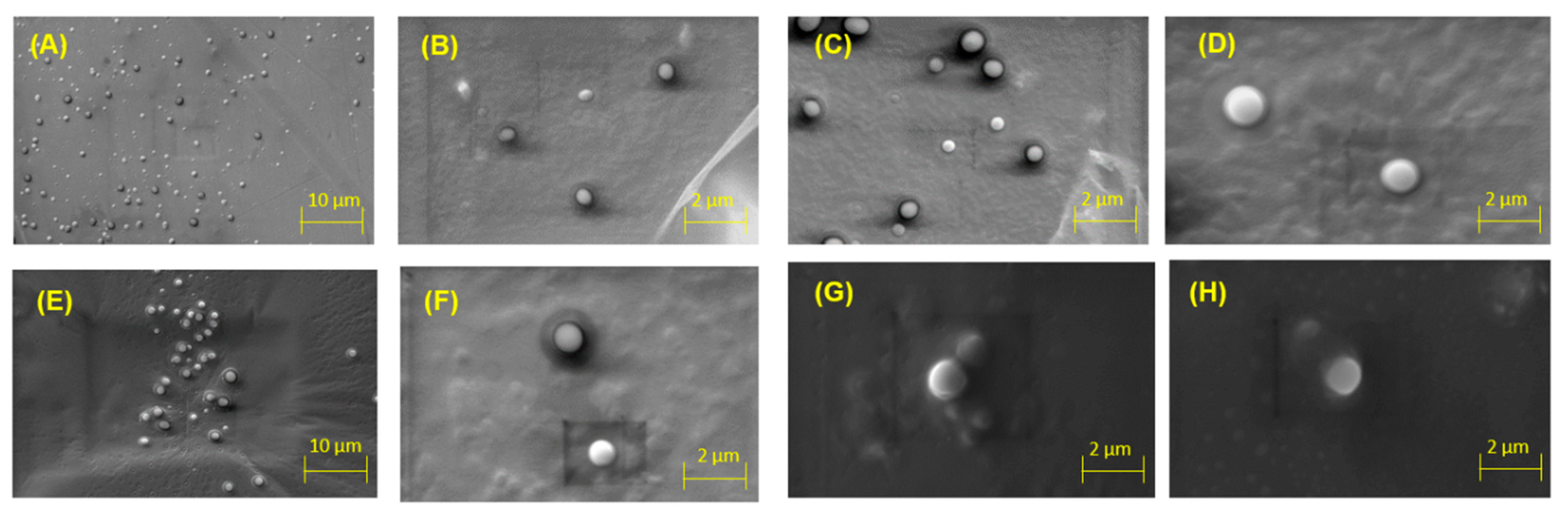
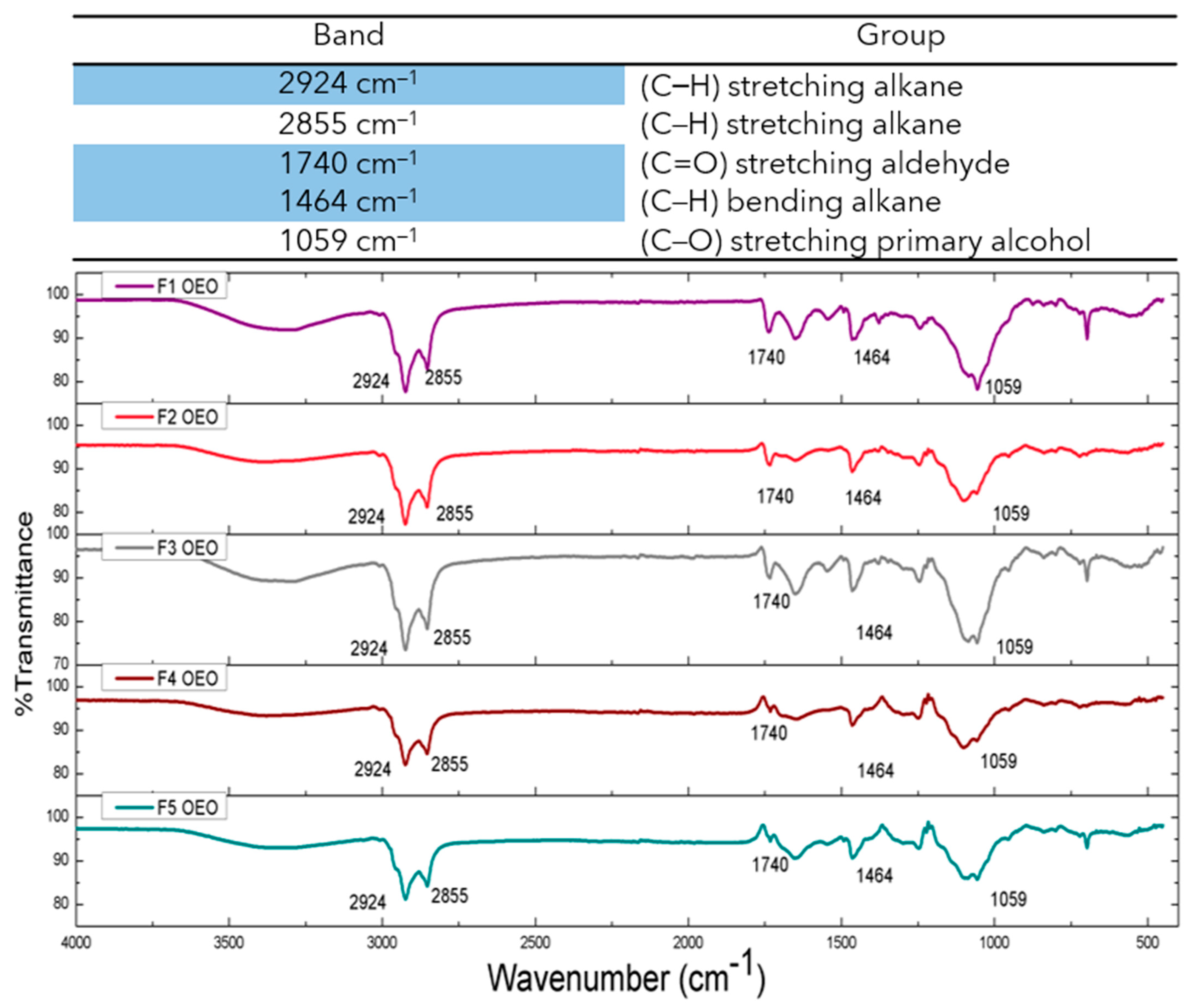
| Sample Name | OEO Concentration (mM) | Size 1st Day (nm) | PdI 1st Day | Size 1st Week (nm) | PdI 1st Week | Size 1st Month (nm) | PdI 1st Month |
|---|---|---|---|---|---|---|---|
| F1 OEO | 6.2 | 79.63 ± 0.92 | 0.413 ± 0.015 | 77.46 ± 0.66 | 0.396 ± 0.003 | 110.4 ± 0.98 | 0.479 ± 0.049 |
| F2 OEO | 4.9 | 87.30 ± 0.60 | 0.311 ± 0.008 | 88.48 ± 1.95 | 0.338 ± 0.041 | 92.27 ± 1.58 | 0.461 ± 0.004 |
| F3 OEO | 3.7 | 81.36 ± 0.75 | 0.302 ± 0.010 | 78.30 ± 0.64 | 0.419 ± 0.005 | 100.19 ± 0.56 | 0.490 ± 0.003 |
| F4 OEO | 2.5 | 83.14 ± 2.38 | 0.402 ± 0.071 | 81.09 ± 0.55 | 0.434 ± 0.003 | 98.51 ± 0.45 | 0.504 ± 0.007 |
| F5 OEO | 1.2 | 81.83 ± 1.06 | 0.434 ± 0.930 | 90.33 ± 0.51 | 0.350 ± 0.043 | 101.06 ± 0.76 | 0.484 ± 0.007 |
| Control | - | 32.43 ± 1.47 | 0.250 ± 0.002 | 105.10 ± 1.65 | 0.292 ± 0.007 | 110.83 ± 0.83 | 0.464 ± 0.003 |
| Sample Name | OEO Concentration (mM) | Zeta Potential 1st Day (mV) | Zeta Potential 1st Week (mV) | Zeta Potential 1st Month (mV) |
|---|---|---|---|---|
| F1 OEO | 6.2 | −27.99 ± 1.15 | −26.54 ± 0.36 | −7.98 ± 0.93 |
| F2 OEO | 4.9 | −28.20 ± 0.78 | −26.32 ± 0.47 | −8.22 ± 1.79 |
| F3 OEO | 3.7 | −26.82 ± 0.37 | −25.59 ± 0.53 | −6.62 ± 0.75 |
| F4 OEO | 2.5 | −27.11 ± 0.52 | −26.80 ± 0.44 | −7.13 ± 1.07 |
| F5 OEO | 1.2 | −36.94 ± 0.36 | −34.03 ± 0.20 | −6.97 ± 0.61 |
| Control | - | −27.54 ± 1.49 | −20.5 ± 0.7 | −8.66 ± 1.43 |
| Sample Name | OEO Concentration (mM) | Day 1 | Day 30 |
|---|---|---|---|
| F1 OEO | 6.2 | 79.55 ± 6.9 | 78.7 ± 5.4 |
| F2 OEO | 4.9 | 79.10 ± 5.3 | 75.3 ± 3.9 |
| F3 OEO | 3.7 | 77.60 ± 4.3 | 76.1 ± 2.7 |
| F4 OEO | 2.5 | 77.29 ± 2.4 | 68.09 ± 1.9 |
| F5 OEO | 1.2 | 51.50 ± 1.3 | 43.2 ± 2.4 |
| OEO Concentration (µL/mL) | MGI (%) OEO Nanoliposomes | MGI (%) Control (OEO) |
|---|---|---|
| 0.25 | 28.13 ± 1.72 | 20.9 ± 1.51 |
| 0.5 | 31.93 ± 1.55 | 32.73 ± 2.28 |
| 1 | 44.26 ± 3.95 | 35.13 ± 3.41 |
| 1.5 | 81.66 ± 0.86 | 40.1 ± 2.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Pérez, K.M.; Medina, D.I.; Narayanan, J.; Parra-Saldívar, R.; Iqbal, H.M.N. Synthesis and Nano-Sized Characterization of Bioactive Oregano Essential Oil Molecule-Loaded Small Unilamellar Nanoliposomes with Antifungal Potentialities. Molecules 2021, 26, 2880. https://doi.org/10.3390/molecules26102880
Aguilar-Pérez KM, Medina DI, Narayanan J, Parra-Saldívar R, Iqbal HMN. Synthesis and Nano-Sized Characterization of Bioactive Oregano Essential Oil Molecule-Loaded Small Unilamellar Nanoliposomes with Antifungal Potentialities. Molecules. 2021; 26(10):2880. https://doi.org/10.3390/molecules26102880
Chicago/Turabian StyleAguilar-Pérez, Katya M., Dora I. Medina, Jayanthi Narayanan, Roberto Parra-Saldívar, and Hafiz M. N. Iqbal. 2021. "Synthesis and Nano-Sized Characterization of Bioactive Oregano Essential Oil Molecule-Loaded Small Unilamellar Nanoliposomes with Antifungal Potentialities" Molecules 26, no. 10: 2880. https://doi.org/10.3390/molecules26102880
APA StyleAguilar-Pérez, K. M., Medina, D. I., Narayanan, J., Parra-Saldívar, R., & Iqbal, H. M. N. (2021). Synthesis and Nano-Sized Characterization of Bioactive Oregano Essential Oil Molecule-Loaded Small Unilamellar Nanoliposomes with Antifungal Potentialities. Molecules, 26(10), 2880. https://doi.org/10.3390/molecules26102880








