ESI-MS Analysis of Thiol-yne Click Reaction in Petroleum Medium
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Procedures
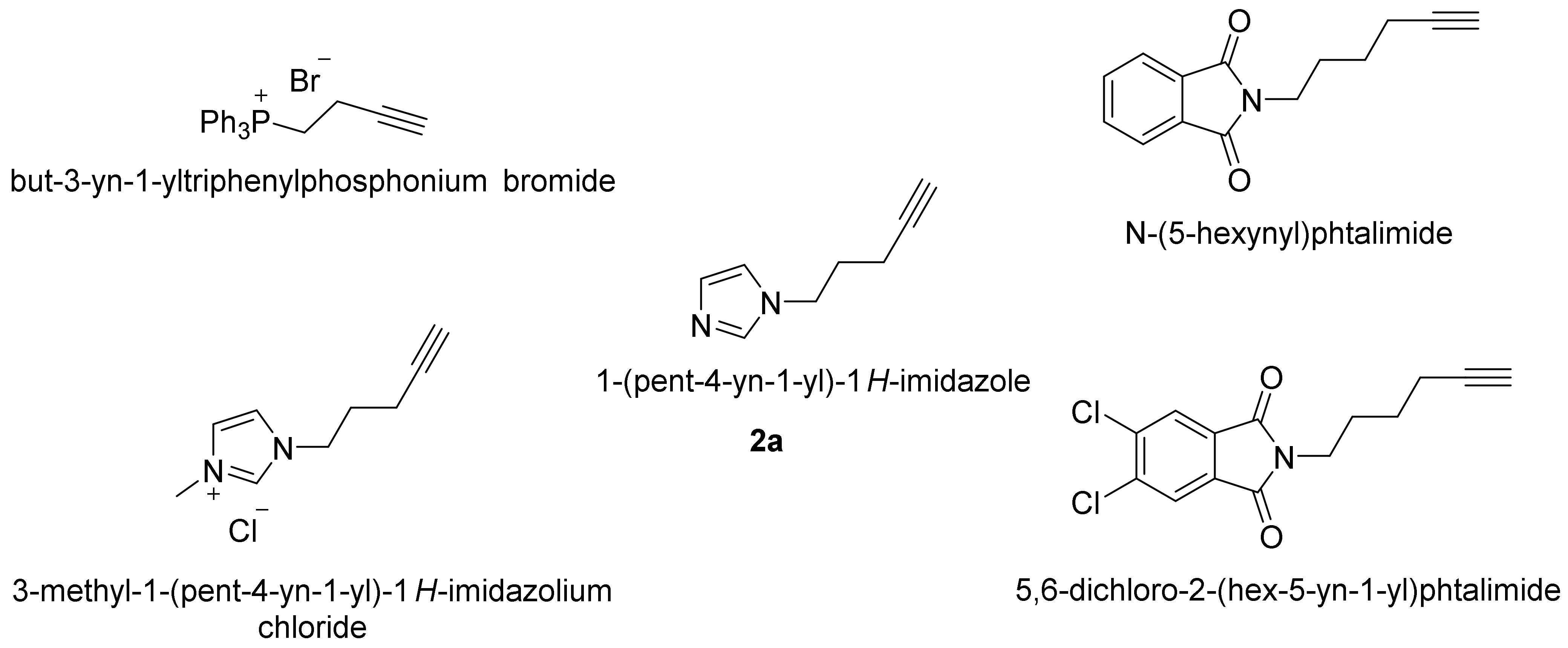
4.2. Synthesis of 1-(pentyn-4-yn-1-yl)-1H-imidazole (2a)
4.3. Model Reaction of Thiol 1a with Alkyne 2a in Petroleum Medium
4.4. Hydrothiolation of Alkyne 2a in Petroleum Medium
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tissot, B.P.; Welte, D.H. Composition of Crude Oils. In Petroleum Formation and Occurrence; Springer: Berlin/Heidelberg, Germany, 1984; pp. 375–414. [Google Scholar]
- Behbehani, H.; Al-Qallaf, M.A.; EL-Dusouqui, O.M.E. Comparison Study for the Distribution of Organo-Sulfur Containing Compounds of Two Kuwaiti Crude Oil Distillates. Pet. Sci. Technol. 2005, 23, 219–233. [Google Scholar] [CrossRef]
- Orr, W.L.; Sinninghe Damsté, J.S. Geochemistry of Sulfur in Petroleum Systems. In Geochemistry of Sulfur in Fossil Fuels; American Chemical Society: Washington, DC, USA, 1990; pp. 2–29. [Google Scholar]
- Lowe, A.B. Thiol-yne ‘click’/coupling chemistry and recent applications in polymer and materials synthesis and modification. Polymer 2014, 55, 5517–5549. [Google Scholar] [CrossRef]
- Kultys, A. Sulfur-Containing Polymers. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; ISBN 9780471440260. [Google Scholar]
- Boyd, D.A. Sulfur and Its Role In Modern Materials Science. Angew. Chem. Int. Ed. 2016, 55, 15486–15502. [Google Scholar] [CrossRef] [PubMed]
- Godoi, M.; Leitemberger, A.; Böhs, L.M.C.; Silveira, M.V.; Rafique, J.; D’Oca, M.G.M. Rice straw ash extract, an efficient solvent for regioselective hydrothiolation of alkynes. Environ. Chem. Lett. 2019, 17, 1441–1446. [Google Scholar] [CrossRef]
- Degtyareva, E.S.; Borkovskaya, E.V.; Ananikov, V.P. Applying Green Metrics to Eco-Friendly Synthesis of Sulfur-Substituted Conjugated Dienes Based on Atom-Economic Hydrothiolation. ACS Sustain. Chem. Eng. 2019, 7, 9680–9689. [Google Scholar] [CrossRef]
- Wei, C.; He, Y.; Wang, J.; Ye, X.; Wojtas, L.; Shi, X. Hexafluoroisopropanol-Promoted Disulfidation and Diselenation of Alkyne, Alkene, and Allene. Org. Lett. 2020, 22, 5462–5465. [Google Scholar] [CrossRef]
- Dass, C. Basics of Mass Spectrometry. In Fundamentals of Contemporary Mass Spectrometry; Dass, C., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 1–14. [Google Scholar]
- Cho, Y.; Ahmed, A.; Islam, A.; Kim, S. Developments in FT-ICR MS instrumentation, ionization techniques, and data interpretation methods for petroleomics. Mass Spectrom. Rev. 2015, 34, 248–263. [Google Scholar] [CrossRef]
- Vetere, A.; Schrader, W. Mass Spectrometric Coverage of Complex Mixtures: Exploring the Carbon Space of Crude Oil. ChemistrySelect 2017, 2, 849–853. [Google Scholar] [CrossRef]
- Borisov, R.S.; Kulikova, L.N.; Zaikin, V.G. Mass Spectrometry in Petroleum Chemistry (Petroleomics). Pet. Chem. 2019, 59, 1055–1076. [Google Scholar] [CrossRef]
- Kondyli, A.; Schrader, W. Evaluation of the combination of different atmospheric pressure ionization sources for the analysis of extremely complex mixtures. Rapid Commun. Mass Spectrom. 2020, 34, e8676. [Google Scholar] [CrossRef]
- Fenn, J.; Mann, M.; Meng, C.; Wong, S.; Whitehouse, C. Electrospray ionization for mass spectrometry of large biomolecules. Science 1989, 246, 64–71. [Google Scholar] [CrossRef]
- Zhan, D.; Fenn, J.B. Electrospray mass spectrometry of fossil fuels. Int. J. Mass Spectrom. 2000, 194, 197–208. [Google Scholar] [CrossRef]
- Palacio Lozano, D.C.; Gavard, R.; Arenas-Diaz, J.P.; Thomas, M.J.; Stranz, D.D.; Mejía-Ospino, E.; Guzman, A.; Spencer, S.E.F.; Rossell, D.; Barrow, M.P. Pushing the analytical limits: New insights into complex mixtures using mass spectra segments of constant ultrahigh resolving power. Chem. Sci. 2019, 10, 6966–6978. [Google Scholar] [CrossRef]
- Panda, S.K.; Andersson, J.T.; Schrader, W. Mass-spectrometric analysis of complex volatile and nonvolatile crude oil components: A challenge. Anal. Bioanal. Chem. 2007, 389, 1329–1339. [Google Scholar] [CrossRef]
- Marshall, A.G.; Rodgers, R.P. Petroleomics: The Next Grand Challenge for Chemical Analysis. Acc. Chem. Res. 2004, 37, 53–59. [Google Scholar] [CrossRef]
- Quirke, J.M.E.; Adams, C.L.; Van Berkel, G.J. Chemical Derivatization for Electrospray Ionization Mass Spectrometry. 1. Alkyl Halides, Alcohols, Phenols, Thiols, and Amines. Anal. Chem. 1994, 66, 1302–1315. [Google Scholar] [CrossRef]
- Liu, P.; Shi, Q.; Chung, K.H.; Zhang, Y.; Pan, N.; Zhao, S.; Xu, C. Molecular characterization of sulfur compounds in venezuela crude oil and its SARA fractions by electrospray ionization fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2010, 24, 5089–5096. [Google Scholar] [CrossRef]
- Janusson, E.; McGarvey, G.B.; Islam, F.; Rowan, C.; McIndoe, J.S. Selective mass spectrometric analysis of thiols using charge-tagged disulfides. Analyst 2016, 141, 5520–5526. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Ananikov, V.P. Transition-Metal-Catalyzed C−S, C−Se, and C−Te Bond Formation via Cross-Coupling and Atom-Economic Addition Reactions. Chem. Rev. 2011, 111, 1596–1636. [Google Scholar] [CrossRef]
- Kalck, P. Sulfur in Catalysis. In Applied Homogeneous Catalysis with Organometallic Compounds; Wiley: Weinheim, Germany, 2017; pp. 1557–1578. ISBN 9783527651733. [Google Scholar]
- Burykina, J.V.; Shlapakov, N.S.; Gordeev, E.G.; König, B.; Ananikov, V.P. Selectivity control in thiol–yne click reactions via visible light induced associative electron upconversion. Chem. Sci. 2020, 11, 10061–10070. [Google Scholar] [CrossRef]
- Dondoni, A.; Marra, A. Metal-Catalyzed and Metal-Free Alkyne Hydrothiolation: Synthetic Aspects and Application Trends. Eur. J. Org. Chem. 2014, 2014, 3955–3969. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Yamamoto, Y.; Ogawa, A. Catalytic synthesis of sulfur and phosphorus compounds via atom-economic reactions. Mendeleev Commun. 2020, 30, 129–138. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Nájera, C.; Yus, M. Catalysis and regioselectivity in hydrofunctionalization reactions of unsaturated carbon bonds. Part III. Russ. Chem. Rev. 2021, 90, 70–93. [Google Scholar] [CrossRef]
- Desnoyer, A.N.; Love, J.A. Recent advances in well-defined, late transition metal complexes that make and/or break C–N, C–O and C–S bonds. Chem. Soc. Rev. 2017, 46, 197–238. [Google Scholar] [CrossRef]
- Ogawa, A. Transition-Metal-Catalyzed S–H and Se–H Bonds Addition to Unsaturated Molecules. In Hydrofunctionalization; Ananikov, V.P., Tanaka, M., Eds.; Topics in Organometallic Chemistry; Springer: Berlin/Heidelberg, Germany, 2013; Volume 43, pp. 325–360. ISBN 978-3-642-33734-5. [Google Scholar]
- Li, J.; Yang, S.; Wu, W.; Jiang, H. Recent developments in palladium-catalyzed C–S bond formation. Org. Chem. Front. 2020, 7, 1395–1417. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Li, H.; Sun, Q.; Xiong, L.; Yin, G. Highly regio- and stereoselective synthesis of bis-sulfanyl substituted conjugated dienes by copper–palladium cooperative catalysis. Org. Chem. Front. 2021, 8, 628–634. [Google Scholar] [CrossRef]
- Lee, C.-F.; Basha, R.S.; Badsara, S.S. Engineered C–S Bond Construction. Top. Curr. Chem. 2018, 376, 25. [Google Scholar] [CrossRef] [PubMed]
- Sundaravelu, N.; Sangeetha, S.; Sekar, G. Metal-catalyzed C-S bond formation using sulfur surrogates. Org. Biomol. Chem. 2021, 19, 1459–1482. [Google Scholar] [CrossRef] [PubMed]
- Degtyareva, E.S.; Burykina, J.V.; Fakhrutdinov, A.N.; Gordeev, E.G.; Khrustalev, V.N.; Ananikov, V.P. Pd-NHC Catalytic System for the Efficient Atom-Economic Synthesis of Vinyl Sulfides from Tertiary, Secondary, or Primary Thiols. ACS Catal. 2015, 5. [Google Scholar] [CrossRef]
- Annesley, T.M. Ion Suppression in Mass Spectrometry. Clin. Chem. 2003, 49, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Orlov, N.V.; Chistyakov, I.V.; Khemchyan, L.L.; Ananikov, V.P.; Beletskaya, I.P.; Starikova, Z.A. Exclusive Selectivity in the One-Pot Formation of C–C and C–Se Bonds Involving Ni-Catalyzed Alkyne Hydroselenation: Optimization of the Synthetic Procedure and a Mechanistic Study. J. Org. Chem. 2014, 79, 12111–12121. [Google Scholar] [CrossRef]
- Marion, N.; de Frémont, P.; Puijk, I.M.; Ecarnot, E.C.; Amoroso, D.; Bell, A.; Nolan, S.P. N-Heterocyclic Carbene–Palladium Complexes [(NHC)Pd(acac)Cl]: Improved Synthesis and Catalytic Activity in Large-Scale Cross-Coupling Reactions. Adv. Synth. Catal. 2007, 349, 2380–2384. [Google Scholar] [CrossRef]
- Perl, N.R.; Ide, N.D.; Prajapati, S.; Perfect, H.H.; Durón, S.G.; Gin, D.Y. Annulation of Thioimidates and Vinyl Carbodiimides to Prepare 2-Aminopyrimidines, Competent Nucleophiles for Intramolecular Alkyne Hydroamination. Synthesis of (−)-Crambidine. J. Am. Chem. Soc. 2010, 132, 1802–1803. [Google Scholar] [CrossRef]
- Yu, T.-B.; Bai, J.Z.; Guan, Z. Cycloaddition-Promoted Self-Assembly of a Polymer into Well-Defined β Sheets and Hierarchical Nanofibrils. Angew. Chem. Int. Ed. 2009, 48, 1097–1101. [Google Scholar] [CrossRef]


| Thiol 1a Concentration, M | Thiol 1a Concentration, % | Observed Intensity of Alkyne 2a Signal in ESI-HRMS | Observed Intensity of Product 3a Signal in ESI-HRMS |
|---|---|---|---|
| 1 | 8 | 1.25 × 106 | 1.25 × 106 |
| 0.1 | 1 | 1.25 × 106 | 1.25 × 106 |
| 0.05 | 0.2 | 1.2 × 106 | 1.25 × 106 |
| 0.01 | 0.1 | 1.2 × 106 | 6 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degtyareva, E.S.; Burykina, J.V.; Ananikov, V.P. ESI-MS Analysis of Thiol-yne Click Reaction in Petroleum Medium. Molecules 2021, 26, 2896. https://doi.org/10.3390/molecules26102896
Degtyareva ES, Burykina JV, Ananikov VP. ESI-MS Analysis of Thiol-yne Click Reaction in Petroleum Medium. Molecules. 2021; 26(10):2896. https://doi.org/10.3390/molecules26102896
Chicago/Turabian StyleDegtyareva, Evgeniya S., Julia V. Burykina, and Valentine P. Ananikov. 2021. "ESI-MS Analysis of Thiol-yne Click Reaction in Petroleum Medium" Molecules 26, no. 10: 2896. https://doi.org/10.3390/molecules26102896
APA StyleDegtyareva, E. S., Burykina, J. V., & Ananikov, V. P. (2021). ESI-MS Analysis of Thiol-yne Click Reaction in Petroleum Medium. Molecules, 26(10), 2896. https://doi.org/10.3390/molecules26102896







