Chemical Composition and Antibacterial and Antioxidant Activity of a Citrus Essential Oil and Its Fractions
Abstract
1. Introduction
2. Results
2.1. The Vacuum Fractionation
2.2. Chemical Composition of the Fractions
2.3. Antibacterial Activity and Antioxidant Capacity
2.4. Relationship between Chemical Composition and Biological Activities
3. Discussion
4. Materials and Methods
4.1. Essential Oils Supply
4.2. Fractionation of Citrus Essential Oil
4.3. Chemical Composition of Essential Oils
4.4. Antibacterial Activity
4.4.1. Bacterial Strains
4.4.2. Determination of Minimal Inhibitory Concentration (MIC)
4.4.3. Minimal Bactericidal Concentration (MBC)
4.5. Antioxidant Capacity
4.5.1. Reagents
4.5.2. ORAC Assay
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dosoky, N.S.; Setzer, W.N. Biological activities and safety of citrus spp. Essential oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [PubMed]
- Tranchida, P.Q.; Bonaccorsi, I.; Dugo, P.; Mondello, L.; Dugo, G. Analysis of Citrus essential oils: State of the art and future perspectives. A review. Flavour Fragr. J. 2012, 27, 98–123. [Google Scholar] [CrossRef]
- Sawamura, M. Introduction and Overview. In Citrus Essential Oils: Flavor and Fragance; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. General Safety Guidelines. In Essential Oil Safety; Elsevier: Amsterdam, The Netherlands, 2014; pp. 649–654. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.; Cho, M.H. Citrus essential oils: Extraction, authentication and application in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Espina, L.; Gelaw, T.K.; de Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. Mechanism of Bacterial Inactivation by (+)-Limonene and Its Potential Use in Food Preservation Combined Processes. PLoS ONE 2013, 8, e56769. [Google Scholar] [CrossRef] [PubMed]
- Erasto, P.; Viljoen, A.M. Limonene—A review: Biosynthetic, ecological and pharmacological relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef] [PubMed]
- EU Regulation (EC), No.1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union 2003, 268, 29–43.
- Food Safety Commission of Japan Antimicrobial-resistant Bacteria Arising from the Use of Colistin Sulfate in the Livestock (Antimicrobial-resistant Bacteria). Food Saf. 2017, 5, 24–28. [CrossRef] [PubMed]
- Johnson, R. Potential Trade Implications of Restrictions on Antimicrobial Use in Animal Production-CRS Report; DIANE Publishing: Darby, PA, USA, 2010. [Google Scholar]
- Liu, Y.; Liu, J.-H. Monitoring Colistin Resistance in Food Animals, An Urgent Threat. Expert Rev. Anti. Infect. Ther. 2018, 16, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Wu, Y. China bans colistin as a feed additive for animals. Lancet Infect. Dis. 2016, 16, 1102–1103. [Google Scholar] [CrossRef]
- Brüssow, H. Adjuncts and alternatives in the time of antibiotic resistance and in-feed antibiotic bans. Microb. Biotechnol. 2017, 10, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, C.M.S.; Ikeda, N.Y.; Miano, A.C.; Saldaña, E.; Moreno, A.M.; Stashenko, E.; Contreras-Castillo, C.J.; Da Gloria, E.M. Unraveling the selective antibacterial activity and chemical composition of citrus essential oils. Sci. Rep. 2019, 9, 17719. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, C.M.S.; de Alencar, S.M.; de Sousa, R.L.M.; Moreno, A.M.; Da Gloria, E.M. Antimicrobial activity of several essential oils on pathogenic and beneficial bacteria. Ind. Crops Prod. 2017, 97, 128–136. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Tiihonen, K.; Kettunen, H.; Peuranen, S.; Schulze, H.; Rautonen, N. In vitro effects of essential oils on potential pathogens and beneficial members of the normal microbiota. Vet. Med. (Praha) 2010, 55, 71–78. [Google Scholar] [CrossRef]
- Si, W.; Gong, J.; Tsao, R.; Zhou, T.; Yu, H.; Poppe, C.; Johnson, R.; Du, Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006, 100, 296–305. [Google Scholar] [CrossRef]
- Simitzis, P.E. Enrichment of Animal Diets with Essential Oils—A Great Perspective on Improving Animal Performance and Quality Characteristics of the Derived Products. Medicines 2017, 4, 35. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Agostini, F.; Muniz, L.A.R.; Pauletti, G.F. Fractionating of green Mandarin (Citrus deliciosa Tenore) essential oil by vacuum fractional distillation. J. Food Eng. 2016, 178, 90–94. [Google Scholar] [CrossRef]
- Falcão, M.A.; Fianco, A.L.B.; Lucas, A.M.; Pereira, M.A.A.; Torres, F.C.; Vargas, R.M.F.; Cassel, E. Determination of antibacterial activity of vacuum distillation fractions of lemongrass essential oil. Phytochem. Rev. 2012, 11, 405–412. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Alvim, I.D.; Contreras Castillo, C.J.; Da Gloria, E.M. Microencapsulation Enhances the in vitro Antibacterial Activity of a Citrus Essential Oil. J. Essent. Oil-Bear. Plants 2020, 23, 985–997. [Google Scholar] [CrossRef]
- Beneti, S.C.; Rosset, E.; Corazza, M.L.; Frizzo, C.D.; Di Luccio, M.; Oliveira, J.V. Fractionation of citronella (Cymbopogon winterianus) essential oil and concentrated orange oil phase by batch vacuum distillation. J. Food Eng. 2011, 102, 348–354. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.L.; Zara, S.; Sanna, M.L.; Addis, R.; Maldini, M.; Foddai, M.; Rourke, J.P.; Chessa, M.; Pintore, G. Chemical characterization, antioxidant capacity and antimicrobial activity against food related microorganisms of Citrus limon var. pompia leaf essential oil. LWT Food Sci. Technol. 2016, 69, 579–585. [Google Scholar] [CrossRef]
- Aggarwal, K.K.; Khanuja, S.P.S.; Ahmad, A.; Santha Kumar, T.R.; Gupta, V.K.; Kumar, S. Antimicrobial activity profiles of the two enantiomers of limonene and carvone isolated from the oils ofMentha spicata andAnethum sowa. Flavour Fragr. J. 2002, 17, 59–63. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Settanni, L.; Palazzolo, E.; Guarrasi, V.; Aleo, A.; Mammina, C.; Moschetti, G.; Germanà, M.A. Inhibition of foodborne pathogen bacteria by essential oils extracted from citrus fruits cultivated in Sicily. Food Control 2012, 26, 326–330. [Google Scholar] [CrossRef]
- Espina, L.; Somolinos, M.; Lorán, S.; Conchello, P.; García, D.; Pagán, R. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control 2011, 22, 896–902. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef]
- Oumzil, H.; Ghoulami, S.; Rhajaoui, M.; Ilidrissi, A.; Fkih-Tetouani, S.; Faid, M.; Benjouad, A. Antibacterial and antifungal activity of essential oils of Mentha suaveolens. Phyther. Res. 2002, 16, 727–731. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Eller, G.; Ngassoum, M.B.; Maponmetsem, P.M. Composition and antimicrobial activity of cymbopogon giganteus (Hochst.) chiov. Essential flower, leaf and stem oils from cameroon. J. Essent. Oil Res. 2007, 19, 485–489. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Lamien-Meda, A.; Bayala, B.; Obame, L.C.; Ilboudo, A.J.; Franz, C.; Novak, J.; Nebié, R.C.; Dicko, M.H. Chemical composition and antimicrobial activity of Cymbopogon citratus and Cymbopogon giganteus essential oils alone and in combination. Phytomedicine 2011, 18, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Marshall, M.R.; Wei, C. Antibacterial activity of some essential oil components against five foodborne pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Contreras-Castillo, C.J.; Da Gloria, E.M. In vitro mechanism of antibacterial action of a citrus essential oil on an enterotoxigenic Escherichia coli and Lactobacillus rhamnosus. J. Appl. Microbiol. 2020, 129, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Rostro-Alanis, M.d.J.; Báez-González, J.; Torres-Alvarez, C.; Parra-Saldívar, R.; Rodriguez-Rodriguez, J.; Castillo, S. Chemical Composition and Biological Activities of Oregano Essential Oil and Its Fractions Obtained by Vacuum Distillation. Molecules 2019, 24, 1904. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Yi, F.; Jin, R.; Sun, J.; Ma, B.; Bao, X. Evaluation of mechanical-pressed essential oil from Nanfeng mandarin (Citrus reticulata Blanco cv. Kinokuni) as a food preservative based on antimicrobial and antioxidant activities. LWT 2018, 95, 346–353. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, N.; Peng, Y.; Zhu, C.; Pan, S. Peel oils from three Citrus species: Volatile constituents, antioxidant activities and related contributions of individual components. J. Food Sci. Technol. 2019, 56, 4492–4502. [Google Scholar] [CrossRef]
- Giannenas, I.; Bonos, E.; Christaki, E.; Florou-paneri, P. Essential Oils and their Application in Animal Nutrition. Med Aromat Plants 2013, 2, 1–12. [Google Scholar] [CrossRef]
- Li, P.; Piao, X.; Ru, Y.; Han, X.; Xue, L.; Zhang, H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian Australas. J. Anim. Sci. 2012, 25, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.T.; Liu, L.; Long, S.F.; Pan, L.; Piao, X.S. Effect of organic acids and essential oils on performance, intestinal health and digestive enzyme activities of weaned pigs. Anim. Feed Sci. Technol. 2018, 235, 110–119. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, X.; Zhang, Q.; Li, P.; Zhao, P.; Li, Q.; Liu, J.; Piao, X. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim. Sci. J. 2015, 86, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Kratz, D.P. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oils by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publ.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
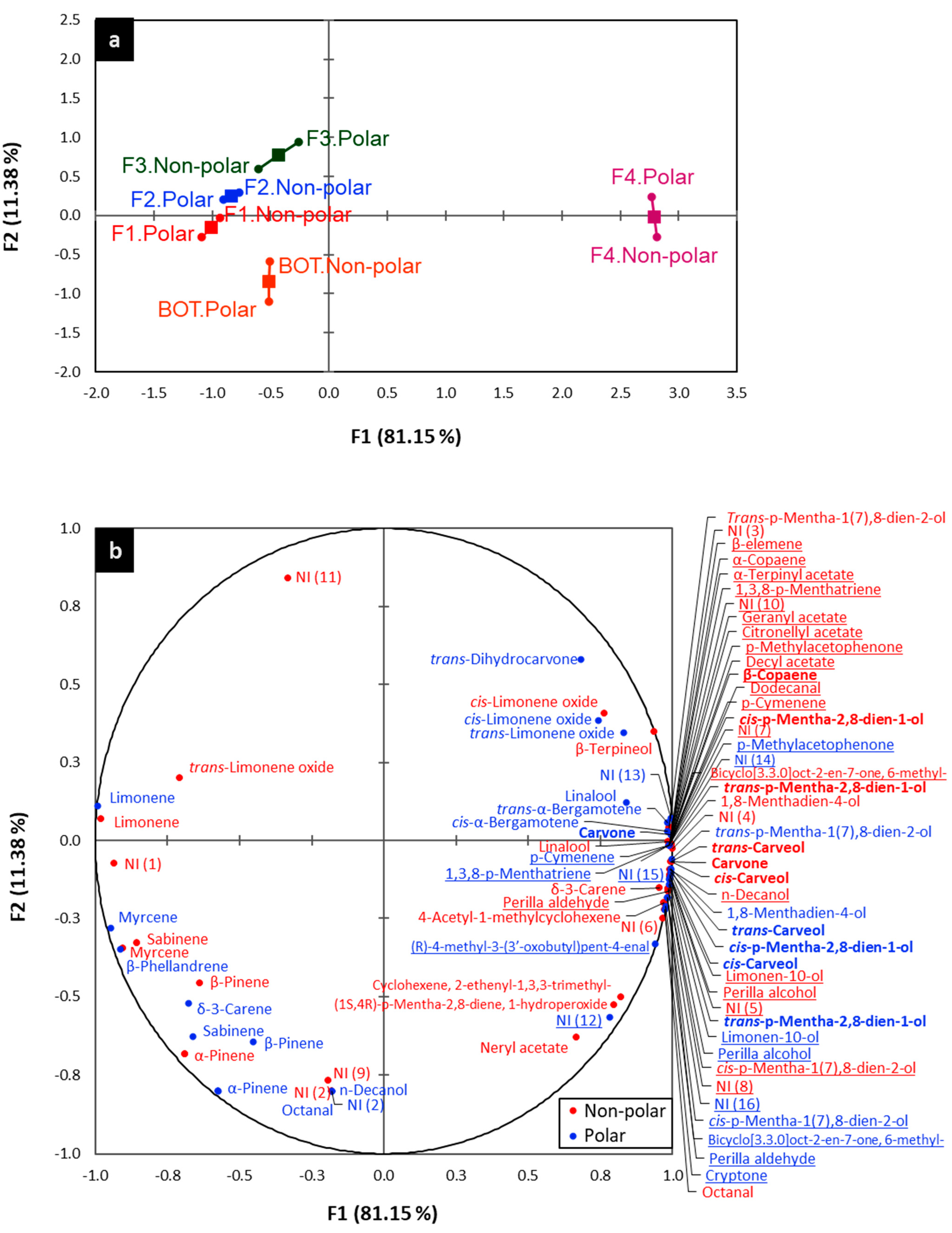
| Compounds 1 | LRIc 2 | LRIL 3 | BOT 6,* | F1 6 | F2 6 | F3 6 | F4 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NP 4 | P 5 | NP | P | NP | P | NP | P | NP | P | NP | P | NP | P | |
| α-Pinene | 936 | 1019 | - | 1025.4 | 0.06 | 0.13 | 0.06 | 0.11 | 0.03 | 0.05 | 0.01 | - | - | - |
| Sabinene | 975 | 1118 | 976 | 1122 | 0.04 | 0.08 | 0.06 | 0.10 | 0.04 | 0.06 | 0.02 | - | - | - |
| β-Pinene | 981 | 1105 | 980 | 1100 | 0.01 | 0.02 | 0.02 | 0.03 | 0.01 | - | - | - | - | - |
| Myrcene | 989 | 1158 | 991 | 1160.2 | 0.12 | 0.24 | 0.14 | 0.28 | 0.12 | 0.23 | 0.06 | 0.15 | - | - |
| Octanal | 1003 | - | 1002.8 | - | 0.03 | 0.002 | 0.01 | - | 0.01 | - | 0.01 | - | 0.09 | - |
| δ-3-Carene | 1015 | 1146 | 1011 | 1146.8 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.01 | - | 0.07 | - |
| β-Phellandrene | - | 1208 | - | 1209.3 | - | 0.02 | - | 0.02 | - | 0.02 | - | 0.01 | - | - |
| NI (1) | 1028 | - | - | - | 0.04 | - | 0.05 | - | 0.03 | - | 0.04 | - | - | - |
| Limonene | 1037 | 1201 | 1031 | 1198.2 | 10.96 | 13.73 | 11.06 | 16.41 | 10.86 | 15.80 | 11.81 | 15.66 | 0.13 | 0.08 |
| p-Cymenene | 1092 | 1430 | 1087.9 | 1437.5 | - | - | - | - | - | - | - | - | 0.11 | 0.08 |
| Linalool | 1099 | 1538 | 1098 | 1543.3 | 0.04 | 0.06 | 0.02 | 0.03 | 0.03 | 0.04 | 0.04 | 0.07 | 0.09 | 0.09 |
| 1,3,8-p-Menthatriene | 1116 | 1390 | 1111 | 1411 | - | - | - | - | - | - | - | - | 0.06 | 0.03 |
| trans-p-Mentha-2,8-dien-1-ol | 1125 | 1622 | 1123 | 1639 | 0.12 | 0.36 | 0.06 | 0.09 | 0.07 | 0.09 | 0.13 | 0.23 | 1.12 | 1.31 |
| 4-Acetyl-1-methylcyclohexene | 1133 | - | 1137 | - | 0.02 | - | - | - | 0.01 | - | - | - | 0.08 | - |
| cis-Limonene oxide | 1137 | 1446 | 1134 | 1450.5 | 0.27 | 0.33 | 0.19 | 0.23 | 0.22 | 0.26 | 0.44 | 0.50 | 0.48 | 0.53 |
| cis-p-Mentha-2,8-dien-1-ol | 1141 | 1664 | 1138 | 1652.1 | - | 0.24 | - | 0.07 | - | 0.06 | - | 0.15 | 1.41 | 1.15 |
| trans-Limonene oxide | 1141 | 1458 | 1139 | 1461.6 | 0.23 | 0.16 | 0.13 | 0.11 | 0.15 | 0.12 | 0.30 | 0.27 | - | 0.33 |
| β-Terpineol | 1153 | - | 1157 | - | - | - | - | - | 0.01 | - | 0.02 | - | 0.05 | - |
| Bicyclo [3.3.0]oct-2-en-7-one, 6-methyl- | 1172 | 1694 | - | - | - | 0.02 | - | - | - | - | - | - | 0.16 | 0.10 |
| Cryptone | - | 1673 | - | 1674.8 | - | 0.02 | - | - | - | - | - | - | - | 0.08 |
| 1,8-Menthadien-4-ol | 1183 | 1680 | 1189 | 1681 | 0.02 | 0.08 | 0.01 | - | 0.01 | - | 0.02 | 0.05 | 0.27 | 0.40 |
| p-Methylacetophenone | 1189 | 1765 | 1182.7 | 1765 | - | - | - | - | - | - | - | - | 0.07 | 0.05 |
| trans-p-Mentha-1(7),8-dien-2-ol | 1191 | 1789 | 1180.5 | 1791 | - | 0.05 | 0.01 | - | 0.01 | - | 0.02 | 0.04 | 0.28 | 0.28 |
| NI (2) | 1192 | 1732 | - | - | 0.03 | 0.48 | - | - | - | - | - | - | - | - |
| NI (3) | 1201 | - | - | - | 0.06 | - | 0.02 | - | 0.03 | - | 0.08 | - | 0.28 | - |
| NI (4) | 1204 | - | 1205.4 | - | 0.09 | - | 0.03 | - | 0.03 | - | 0.09 | - | 0.53 | - |
| trans-Carveol | 1222 | 1828 | 1217 | 1836.3 | 0.25 | 0.40 | 0.05 | 0.06 | 0.04 | 0.05 | 0.13 | 0.20 | 2.09 | 2.20 |
| cis-p-Mentha-1(7),8-dien-2-ol | 1233 | 1880 | 1233 | 1894.9 | 0.02 | 0.03 | - | - | - | - | - | - | 0.12 | 0.15 |
| cis-Carveol | 1236 | 1858 | 1229 | 1854.4 | 0.11 | 0.18 | 0.03 | 0.03 | 0.02 | 0.02 | 0.07 | 0.09 | 0.76 | 0.96 |
| Carvone | 1249 | 1731 | 1243 | 1733.6 | 0.23 | 0.11 | 0.04 | 0.06 | 0.03 | 0.04 | 0.13 | 0.22 | 1.77 | 2.12 |
| n-Decanol | 1274 | 1746 | 1272.1 | - | 0.02 | 0.07 | - | - | - | - | - | - | 0.21 | - |
| Perilla aldehyde | 1281 | 1781 | 1273.4 | 1793.9 | 0.03 | 0.04 | - | - | - | - | - | - | 0.17 | 0.17 |
| NI (5) | 1289 | 1947 | - | - | 0.06 | - | - | - | - | - | - | - | 0.49 | - |
| Limonen-10-ol | 1294 | 1985 | 1239 | 1979 | 0.02 | 0.03 | - | - | - | - | - | - | 0.17 | 0.23 |
| Perilla alcohol | 1303 | 1994 | 1296.3 | 2006.6 | 0.04 | 0.03 | - | - | - | - | - | - | 0.35 | 0.20 |
| Cyclohexene, 2-ethenyl-1,3,3-trimethyl- | 1308 | - | - | - | 0.21 | - | 0.01 | - | - | - | 0.03 | - | 0.30 | - |
| (1S,4R)-p-Mentha-2,8-diene, 1-hydroperoxide | 1322 | - | - | - | 0.20 | - | - | - | - | - | 0.02 | - | 0.27 | - |
| NI (6) | 1335 | - | - | - | 0.13 | - | - | - | - | - | 0.02 | - | 0.40 | - |
| NI (7) | 1344 | - | - | - | - | - | - | - | - | - | - | - | 0.12 | - |
| Citronellyl acetate | 1349 | - | 1352.4 | - | - | - | - | - | - | - | - | - | 0.25 | - |
| α-Terpinyl acetate | 1357 | - | 1347 | - | - | - | - | - | - | - | - | - | 0.14 | - |
| Neryl acetate | 1360 | - | 1362.9 | - | 0.21 | - | - | - | - | - | 0.02 | - | 0.21 | - |
| NI (8) | 1367 | - | - | - | 0.04 | - | - | - | - | - | - | - | 0.24 | - |
| Geranyl acetate | 1375 | - | 1381 | - | - | - | - | - | - | - | - | - | 0.24 | - |
| NI (9) | 1377 | - | - | - | 0.16 | - | - | - | - | - | 0.01 | - | - | - |
| α-Copaene | 1386 | - | 1377 | - | - | - | - | - | - | - | - | - | 0.13 | - |
| NI (10) | 1393 | - | - | - | - | - | - | - | - | - | - | - | 0.16 | - |
| β-elemene | 1396 | - | 1390.4 | - | - | - | - | - | - | - | - | - | 0.13 | - |
| Dodecanal | 1409 | - | 1408.1 | - | - | - | - | - | - | - | - | - | 0.19 | - |
| Decyl acetate | 1416 | - | 1407.1 | - | - | - | - | - | - | - | - | - | 0.11 | - |
| cis-α-Bergamotene | 1420 | 1552 | 1414.5 | 1559.1 | - | 0.11 | - | 0.03 | - | 0.05 | - | 0.13 | - | 0.34 |
| trans-α-Bergamotene | 1440 | 1559 | 1434.5 | 1575.7 | - | 0.06 | - | 0.06 | - | 0.03 | - | 0.09 | - | 0.26 |
| β-Copaene | 1447 | - | 1433.1 | - | - | - | - | - | - | - | - | - | 1.10 | - |
| NI (11) | 1701 | - | - | - | - | - | - | - | 0.02 | - | 0.03 | - | - | - |
| trans-Dihydrocarvone | - | 1609 | - | 1623.1 | - | - | - | - | - | - | - | 0.03 | - | 0.03 |
| NI (12) | - | 1633 | - | - | - | 0.03 | - | - | - | - | - | - | - | 0.04 |
| NI (13) | - | 1746 | - | - | - | - | - | - | - | - | - | 0.05 | - | 0.43 |
| NI (14) | - | 1839 | - | - | - | - | - | - | - | - | - | - | - | 0.34 |
| NI (15) | - | 1919 | - | - | - | - | - | - | - | - | - | - | - | 0.10 |
| (R)-4-methyl-3-(3′-oxobutyl)pent-4-enal | - | 1946 | - | - | - | 0.10 | - | - | - | - | - | - | - | 0.26 |
| NI (16) | - | 1978 | - | - | - | 0.01 | - | - | - | - | - | - | - | 0.05 |
| EO/Fractions | Antibacterial Parameter (mg/mL) | Bacterial Strain | |
|---|---|---|---|
| E. coli U21 | L. rhamnosus ATCC 7469 | ||
| F1 | MIC | - | - |
| MBC | - | - | |
| F2 | MIC | - | - |
| MBC | - | - | |
| F3 | MIC | - | - |
| MBC | - | - | |
| F4 | MIC | 3.70 | 14.80 |
| MBC | 3.70 | 14.80 | |
| BOT * | MIC | 1.85 | 3.70 |
| MBC | 1.85 | 7.40 | |
| EO/Fractions | Antioxidant Capacity µmol Trolox/g of Pure Sample |
|---|---|
| F1 | 477.64 ± 35.45 b |
| F2 | 343.24 ± 26.57 c |
| F3 | 211.69 ± 14.73 d |
| F4 | 850.67 ± 16.04 a |
| BOT | 785.14 ± 79.35 a |
| α-Tocoferol | 339.21 ± 14.55 c |
| BHT | 312.16 ± 11.02 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosio, C.M.S.; Diaz-Arenas, G.L.; Agudelo, L.P.A.; Stashenko, E.; Contreras-Castillo, C.J.; da Gloria, E.M. Chemical Composition and Antibacterial and Antioxidant Activity of a Citrus Essential Oil and Its Fractions. Molecules 2021, 26, 2888. https://doi.org/10.3390/molecules26102888
Ambrosio CMS, Diaz-Arenas GL, Agudelo LPA, Stashenko E, Contreras-Castillo CJ, da Gloria EM. Chemical Composition and Antibacterial and Antioxidant Activity of a Citrus Essential Oil and Its Fractions. Molecules. 2021; 26(10):2888. https://doi.org/10.3390/molecules26102888
Chicago/Turabian StyleAmbrosio, Carmen M. S., Gloria L. Diaz-Arenas, Leidy P. A. Agudelo, Elena Stashenko, Carmen J. Contreras-Castillo, and Eduardo M. da Gloria. 2021. "Chemical Composition and Antibacterial and Antioxidant Activity of a Citrus Essential Oil and Its Fractions" Molecules 26, no. 10: 2888. https://doi.org/10.3390/molecules26102888
APA StyleAmbrosio, C. M. S., Diaz-Arenas, G. L., Agudelo, L. P. A., Stashenko, E., Contreras-Castillo, C. J., & da Gloria, E. M. (2021). Chemical Composition and Antibacterial and Antioxidant Activity of a Citrus Essential Oil and Its Fractions. Molecules, 26(10), 2888. https://doi.org/10.3390/molecules26102888