Detection of Paratuberculosis in Dairy Herds by Analyzing the Scent of Feces, Alveolar Gas, and Stable Air
Abstract
1. Introduction
2. Results
2.1. Visualization of VOC Datasets from Feces, Alveolar Gas, and Stable Air
2.2. Identification and Reproducibility of Significant VOCs Present in Fecal Headspaces and Alveolar Gas
2.3. Classification of MAP Status from VOCs Present above Feces or in Alveolar Gas
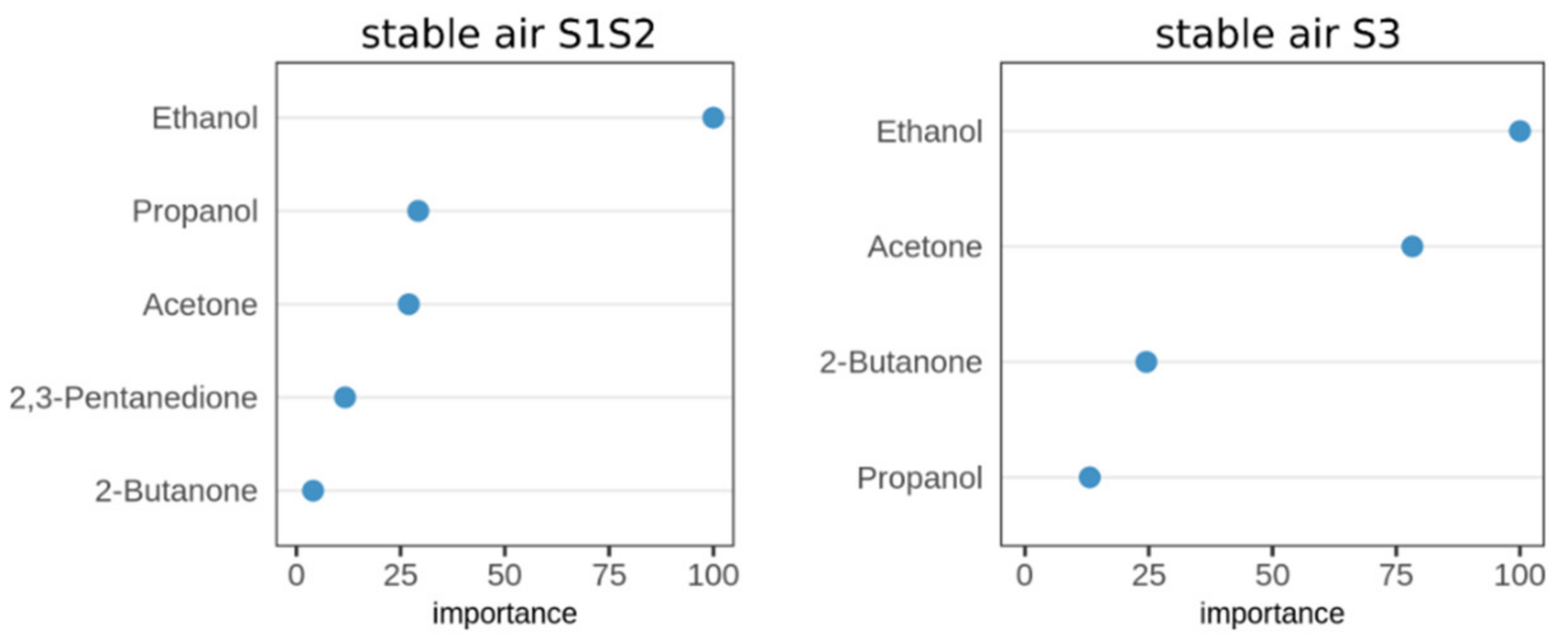
2.4. Identification of Significant VOCs Classifying for Paratuberculosis in Stable Air
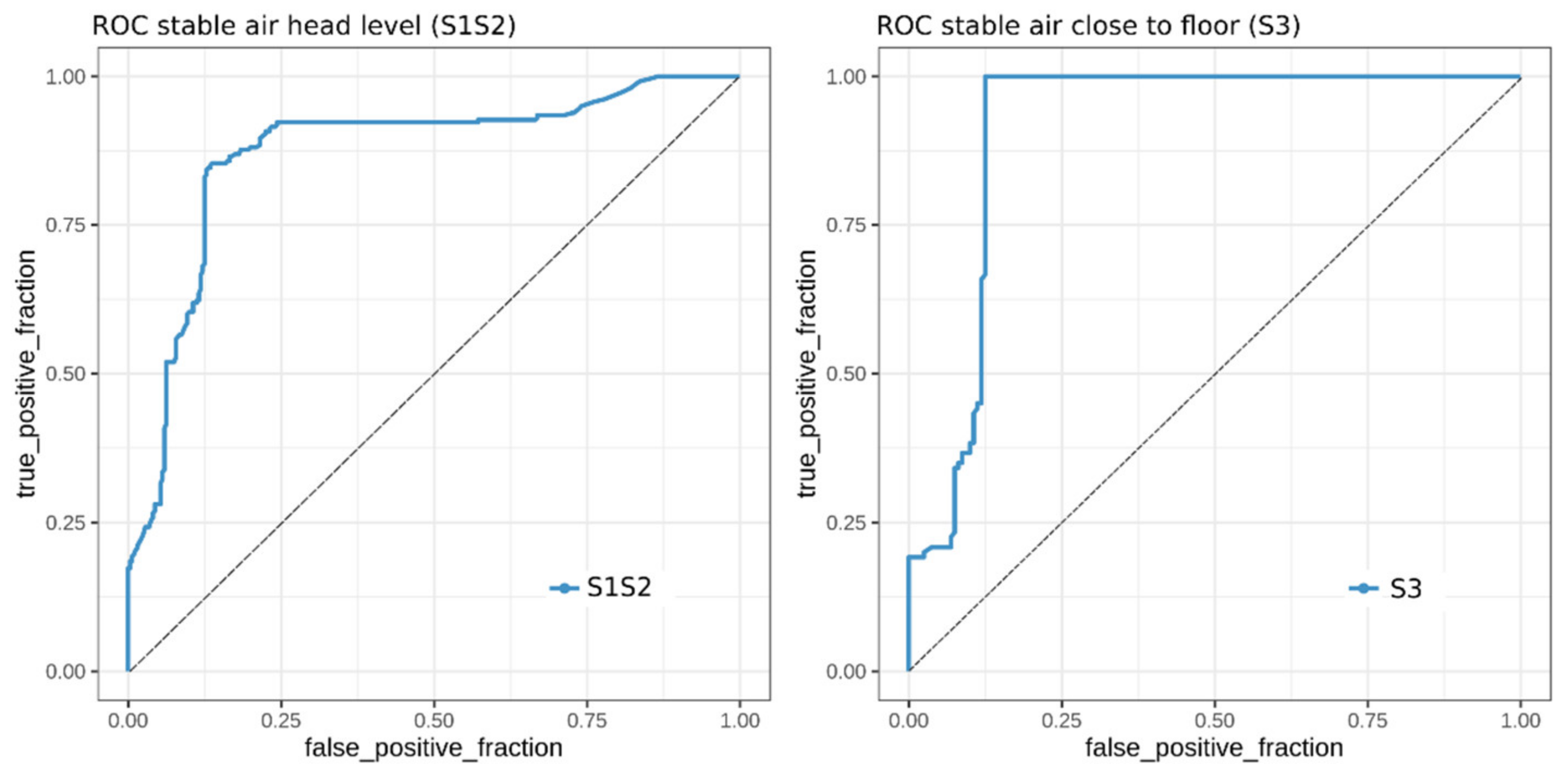
2.5. Classification of MAP Status from VOCs Present in Stable Air
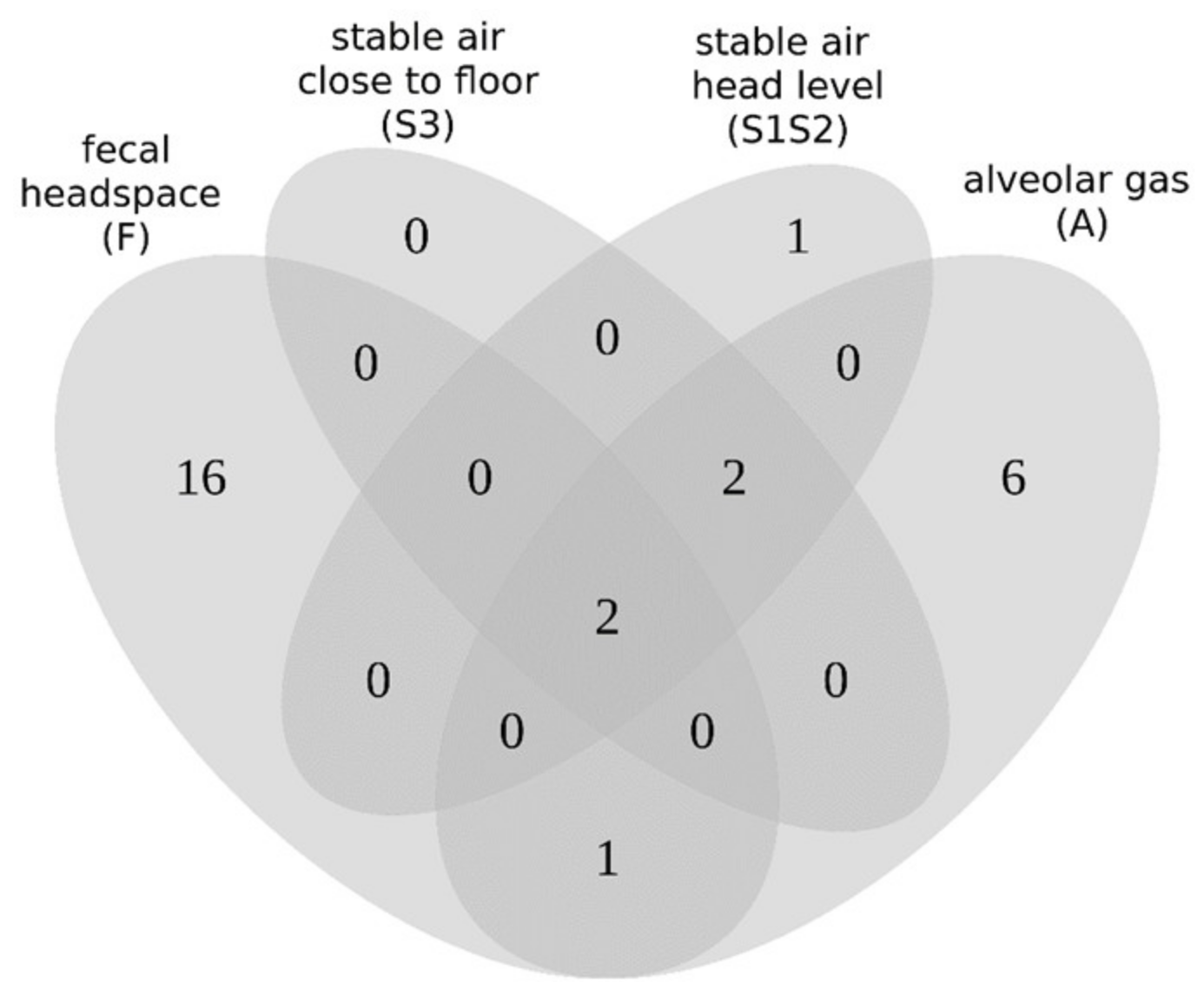
2.6. Comparison of Resulting VOC Sets
3. Discussion
3.1. Biological Aspects
3.1.1. Sources of Variability in VOC Profiles
3.1.2. VOCs Indicative of MAP Infection
3.2. Methodological Aspects
4. Animals, Materials, and Methods
4.1. Legislation and Ethical Approval
4.2. Characteristics of Herds and Animals Used for Sample Collection
4.3. Sampling and Preconcentration of Headspace above Feces, Alveolar Gas, and Stable Air
- S1: at the level of the cow’s head, without the presence of any facemask;
- S2: in front of the cow’s head while wearing the facemask (used for exhaled breath sampling);
- S3: in the area close to the floor of the stable, i.e., close to emissions from feces, manure, or slurry;
- S4: in the area above the animals, far away from slurry or wastes, and partially floated naturally by fresh air.
4.4. Analysis and Biochemical Identification of VOCs
4.5. Data Preprocessing
4.6. Feature Selection
4.7. Classification Model Building
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, A.; Vanleeuwen, J.A.; Dohoo, I.R.; Keefe, G.P.; Haddad, J.P.; Tremblay, R.; Scott, H.M.; Whiting, T. Production effects of pathogens causing bovine leukosis, bovine viral diarrhea, paratuberculosis, and neosporosis. J. Dairy Sci. 2007, 90, 659–669. [Google Scholar] [CrossRef]
- Sweeney, R.W. Pathogenesis of Paratuberculosis. Vet. Clin. N. Am. Food A 2011, 27, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Brüning, A.; Bellamy, K.; Talbot, D.; Anderson, J. A rapid chromatographic strip test for the pen-side diagnosis of rinderpest virus. J. Virol. Methods 1999, 81, 143–154. [Google Scholar] [CrossRef]
- Ferris, N.P.; Clavijo, A.; Yang, M.; Velazquez-Salinas, L.; Nordengrahn, A.; Hutchings, G.H.; Kristersson, T.; Merza, M. Development and laboratory evaluation of two lateral flow devices for the detection of vesicular stomatitis virus in clinical samples. J. Virol. Methods 2012, 180, 96–100. [Google Scholar] [CrossRef]
- Reid, S.M.; Ferris, N.P.; Bruning, A.; Hutchings, G.H.; Kowalska, Z.; Akerblom, L. Development of a rapid chromatographic strip test for the pen-side detection of foot-and-mouth disease virus antigen. J. Virol. Methods 2001, 96, 189–202. [Google Scholar] [CrossRef]
- Fleming, J.R.; Sastry, L.; Wall, S.J.; Sullivan, L.; Ferguson, M.A. Proteomic Identification of Immunodiagnostic Antigens for Trypanosoma vivax Infections in Cattle and Generation of a Proof-of-Concept Lateral Flow Test Diagnostic Device. PLoS Negl. Trop. Dis. 2016, 10, e0004977. [Google Scholar] [CrossRef]
- Hanon, J.B.; Vandenberge, V.; Deruelle, M.; De Leeuw, I.; De Clercq, K.; Van Borm, S.; Koenen, F.; Liu, L.; Hoffmann, B.; Batten, C.A.; et al. Inter-laboratory evaluation of the performance parameters of a Lateral Flow Test device for the detection of Bluetongue virus-specific antibodies. J. Virol. Methods 2016, 228, 140–150. [Google Scholar] [CrossRef]
- Bergmann, A.; Trefz, P.; Fischer, S.; Klepik, K.; Walter, G.; Steffens, M.; Ziller, M.; Schubert, J.K.; Reinhold, P.; Köhler, H.; et al. In Vivo Volatile Organic Compound Signatures of Mycobacterium avium subsp. paratuberculosis. PLoS ONE 2015, 10, e0123980. [Google Scholar] [CrossRef]
- Ellis, C.K.; Rice, S.; Maurer, D.; Stahl, R.; Waters, W.R.; Palmer, M.V.; Nol, P.; Rhyan, J.C.; VerCauteren, K.C.; Koziel, J.A. Use of fecal volatile organic compound analysis to discriminate between nonvaccinated and BCG-Vaccinated cattle prior to and after Mycobacterium bovis challenge. PLoS ONE 2017, 12, e0179914. [Google Scholar] [CrossRef]
- Ellis, C.K.; Stahl, R.S.; Nol, P.; Waters, W.R.; Palmer, M.V.; Rhyan, J.C.; VerCauteren, K.C.; McCollum, M.; Salman, M.D. A Pilot Study Exploring the Use of Breath Analysis to Differentiate Healthy Cattle from Cattle Experimentally Infected with Mycobacterium bovis. PLoS ONE 2014, 9, e89280. [Google Scholar]
- Peled, N.; Ionescu, R.; Nol, P.; Barash, O.; McCollum, M.; VerCauteren, K.; Koslow, M.; Stahl, R.; Rhyan, J.; Haick, H. Detection of volatile organic compounds in cattle naturally infected with Mycobacterium bovis. Sensors Actuators B Chem. 2012, 171, 588–594. [Google Scholar] [CrossRef]
- Reinhold, P.E.; Gierschner, P.; Küntzel, A.; Schubert, J.K.; Miekisch, W.; Köhler, H.U. Chapter 27: Ruminants. In Breathborne Biomarkers and the Human Volatilome, 2nd ed.; Beauchamp, J., Davis, C., Pleil, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 441–460. [Google Scholar]
- Bos, L.D.; Sterk, P.J.; Schultz, M.J. Volatile metabolites of pathogens: A systematic review. PLoS Pathog. 2013, 9, e1003311. [Google Scholar] [CrossRef]
- Kasbohm, E.; Fischer, S.; Küntzel, A.; Oertel, P.; Bergmann, A.; Trefz, P.; Miekisch, W.; Schubert, J.K.; Reinhold, P.; Ziller, M.; et al. Strategies for the identification of disease-related patterns of volatile organic compounds: Prediction of paratuberculosis in an animal model using random forests. J. Breath Res. 2017, 11, 047105. [Google Scholar] [CrossRef]
- Sundell, J. Reflections on the history of indoor air science, focusing on the last 50 years. Indoor Air 2017, 27, 708–724. [Google Scholar] [CrossRef]
- Krüger, C.; Köhler, H.; Liebler-Tenorio, E.M. Sequential Development of Lesions 3, 6, 9, and 12 Months After Experimental Infection of Goat Kids with Mycobacterium avium subsp. paratuberculosis. Vet. Pathol. 2015, 52, 276–290. [Google Scholar] [CrossRef]
- Crossley, B.M.; Zagmutt-Vergara, F.J.; Fyock, T.L.; Whitlock, R.H.; Gardner, I.A. Fecal shedding of Mycobacterium avium subsp paratuberculosis by dairy cows. Vet. Microbiol. 2005, 107, 257–263. [Google Scholar] [CrossRef]
- Manning, E.J.B.; Collins, M.T. Mycobacterium avium subsp paratuberculosis: Pathogen, pathogenesis and diagnosis. Rev. Sci. Tech. OIE 2001, 20, 133–150. [Google Scholar] [CrossRef]
- Küntzel, A.; Weber, M.; Gierschner, P.; Trefz, P.; Miekisch, W.; Schubert, J.K.; Reinhold, P.; Köhler, H. Core profile of volatile organic compounds related to growth of Mycobacterium avium subspecies paratuberculosis—A comparative extract of three independent studies. PLoS ONE 2019, 14, e0221031. [Google Scholar] [CrossRef]
- Vitense, P.; Kasbohm, E.; Klassen, A.; Gierschner, P.; Trefz, P.; Weber, M.; Miekisch, W.; Schubert, J.K.; Möbius, P.; Reinhold, P.; et al. Detection of Mycobacterium avium ssp. paratuberculosis in Cultures from Fecal and Tissue Samples Using VOC Analysis and Machine Learning Tools. Front. Vet. Sci. 2021, 8, 620327. [Google Scholar] [CrossRef]
- Van Malderen, K.; De Man, J.; De Winter, B.Y.; De Schepper, H.U.; Lamote, K. Volatomics in Inflammatory Bowel Disease and Irritable Bowel Syndrome: Present and Future. Gastroenterology 2020, 158, S884. [Google Scholar] [CrossRef]
- Ahmed, I.; Fayyaz, F.; Nasir, M.; Niaz, Z.; Furnari, M.; Perry, L. Extending landscape of volatile metabolites as novel diagnostic biomarkers of inflammatory bowel disease—A review. Scand. J. Gastroenterol. 2016, 51, 385–392. [Google Scholar]
- Lukins, H.B.; Foster, J.W. Methyl Ketone Metabolism in Hydrocarbon-Utilizing Mycobacteria. J. Bacteriol. 1963, 85, 1074–1087. [Google Scholar] [CrossRef]
- Anderson, J.C. Measuring Breath Acetone for Monitoring Fat Loss: Review. Obesity 2015, 23, 2327–2334. [Google Scholar] [CrossRef]
- Gross, J.J.; Bruckmaier, R.M. Review: Metabolic challenges in lactating dairy cows and their assessment via established and novel indicators in milk. Animal 2019, 13, S75–S81. [Google Scholar] [CrossRef]
- Eisenreich, W.; Bacher, A.; Arigoni, D.; Rohdich, F. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell. Mol. Life Sci. 2004, 61, 1401–1426. [Google Scholar] [CrossRef]
- Eisenreich, W.; Schwarz, M.; Cartayrade, A.; Arigoni, D.; Zenk, M.H.; Bacher, A. The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chem. Biol. 1998, 5, R221–R233. [Google Scholar] [CrossRef]
- Salerno-Kennedy, R.; Cashman, K.D. Potential applications of breath isoprene as a biomarker in modern medicine: A concise overview. Wien. Klin. Wochenschr. 2005, 117, 180–186. [Google Scholar] [CrossRef]
- Kurada, S.; Alkhouri, N.; Fiocchi, C.; Dweik, R.; Rieder, F. Review article: Breath analysis in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2015, 41, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Koyama, Y.; Shin, W.; Akamatsu, T.; Tsuruta, A.; Masuda, Y.; Uchiyama, K. Selective Detection of Target Volatile Organic Compounds in Contaminated Air Using Sensor Array with Machine Learning: Aging Notes and Mold Smells in Simulated Automobile Interior Contaminant Gases. Sensors 2020, 20, 2687. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, D.M.; Runyon, J.B.; Richardson, B.A. Signals of speciation: Volatile organic compounds resolve closely related sagebrush taxa, suggesting their importance in evolution. New Phytol. 2016, 211, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.L.B.; Keefe, G.P.; Barkema, H.W.; Sockett, D.C. Evaluation of three ELISAs for Mycobacterium avium subsp paratuberculosis using tissue and fecal culture as comparison standards. Vet. Microbiol. 2005, 110, 105–111. [Google Scholar] [CrossRef]
- Köhler, H.; Burkert, B.; Pavlik, I.; Diller, R.; Geue, L.; Conraths, F.J.; Martin, G. Evaluation of five ELISA test kits for the measurement of antibodies against Mycobacterium avium subspecies paratuberculosis in bovine serum. Berl. Münch. Tierärztl. Wochenschr. 2008, 121, 203–210. [Google Scholar]
- Blanco Vazquez, C.; Alonso-Hearn, M.; Juste, R.A.; Canive, M.; Iglesias, T.; Iglesias, N.; Amado, J.; Vicente, F.; Balseiro, A.; Casais, R. Detection of latent forms of Mycobacterium avium subsp. paratuberculosis infection using host biomarker-based ELISAs greatly improves paratuberculosis diagnostic sensitivity. PLoS ONE 2020, 15, e0236336. [Google Scholar] [CrossRef]
- Paratuberkulose. Amtliche Methode und Falldefinition. 2016. Available online: https://www.openagrar.de/receive/openagrar_mods_00058039 (accessed on 15 April 2016).
- Fischer, S.; Trefz, P.; Bergmann, A.; Steffens, M.; Ziller, M.; Miekisch, W.; Schubert, J.S.; Köhler, H.; Reinhold, P. Physiological variability in volatile organic compounds (VOCs) in exhaled breath and released from faeces due to nutrition and somatic growth in a standardized caprine animal model. J. Breath Res. 2015, 9, 027108. [Google Scholar] [CrossRef]
- Küntzel, A.; Oertel, P.; Trefz, P.; Miekisch, W.; Schubert, J.K.; Köhler, H.; Reinhold, P. Animal science meets agricultural practice: Preliminary results of an innovative technical approach for exhaled breath analysis in cattle under field conditions. Berl. Münch. Tierärztl. Wochenschr. 2018, 131, 444–452. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2019. Available online: https://www.r-project.org/ (accessed on 31 March 2021).
- Kolde, R. Pheatmap: Pretty Heatmaps. R Package Version 1.0.8. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 28 March 2021).
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Oertel, P.; Küntzel, A.; Reinhold, P.; Köhler, H.; Schubert, J.K.; Kolb, J.; Miekisch, W. Continuous real-time breath analysis in ruminants: Effect of eructation on exhaled VOC profiles. J. Breath Res. 2018, 12, 036014. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Carter, J.V.; Pan, J.; Rai, S.N.; Galandiuk, S. ROC-ing along: Evaluation and interpretation of receiver operating characteristic curves. Surgery 2016, 159, 1638–1645. [Google Scholar] [CrossRef]



| Biological Specimen | Herd Status: MAP-Negative Herds/Animals/Samples | Herd Status: MAP-Positive Herds/Animals/Samples |
|---|---|---|
| alveolar gas | 4/46/85 | 4/30/49 |
| headspace above feces | 4/47/93 | 4/30/58 |
| Locations of Stable Air Sampling | Herd Status: MAP-Negative Herds/Samples | Herd Status: MAP-Positive Herds/Samples |
|---|---|---|
| S1: head level, without face mask | 4/8 | 4/7 |
| S2: head level, through face mask | 4/8 | 4/6 |
| S3: close to floor contaminated with manure or feces | 4/8 | 4/8 |
| S4: distant from animals, slurry, or wastes, partially floated by fresh air | 4/8 | 4/7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, M.; Gierschner, P.; Klassen, A.; Kasbohm, E.; Schubert, J.K.; Miekisch, W.; Reinhold, P.; Köhler, H. Detection of Paratuberculosis in Dairy Herds by Analyzing the Scent of Feces, Alveolar Gas, and Stable Air. Molecules 2021, 26, 2854. https://doi.org/10.3390/molecules26102854
Weber M, Gierschner P, Klassen A, Kasbohm E, Schubert JK, Miekisch W, Reinhold P, Köhler H. Detection of Paratuberculosis in Dairy Herds by Analyzing the Scent of Feces, Alveolar Gas, and Stable Air. Molecules. 2021; 26(10):2854. https://doi.org/10.3390/molecules26102854
Chicago/Turabian StyleWeber, Michael, Peter Gierschner, Anne Klassen, Elisa Kasbohm, Jochen K. Schubert, Wolfram Miekisch, Petra Reinhold, and Heike Köhler. 2021. "Detection of Paratuberculosis in Dairy Herds by Analyzing the Scent of Feces, Alveolar Gas, and Stable Air" Molecules 26, no. 10: 2854. https://doi.org/10.3390/molecules26102854
APA StyleWeber, M., Gierschner, P., Klassen, A., Kasbohm, E., Schubert, J. K., Miekisch, W., Reinhold, P., & Köhler, H. (2021). Detection of Paratuberculosis in Dairy Herds by Analyzing the Scent of Feces, Alveolar Gas, and Stable Air. Molecules, 26(10), 2854. https://doi.org/10.3390/molecules26102854






