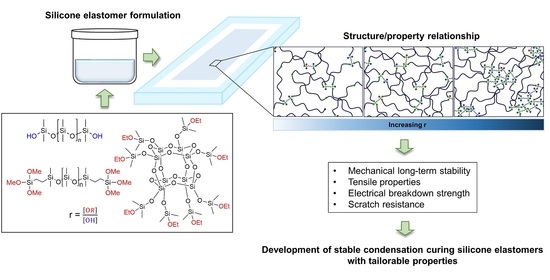
Reliable Condensation Curing Silicone Elastomers with Tailorable Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Condensation Curing Silicone Elastomer Formulations
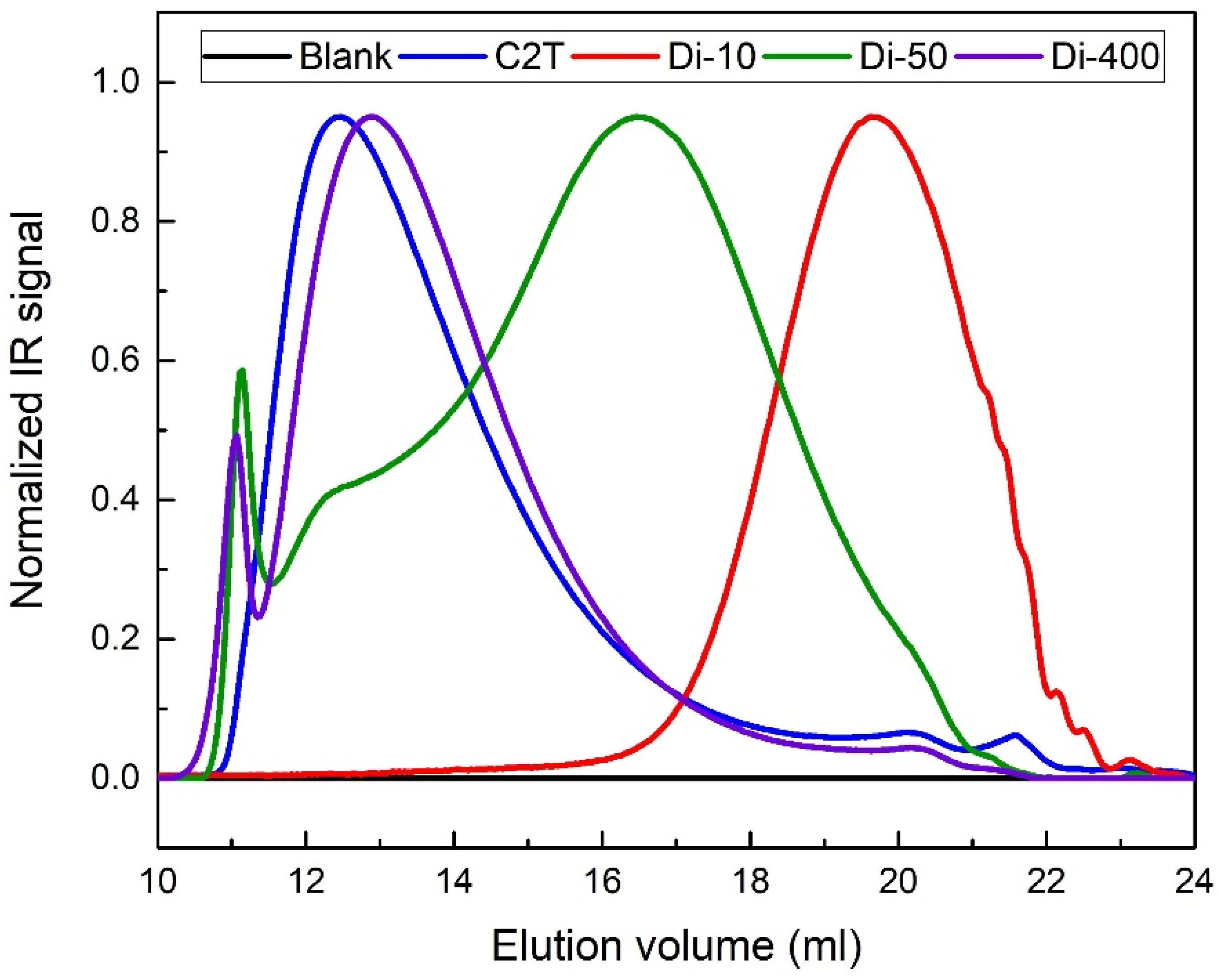
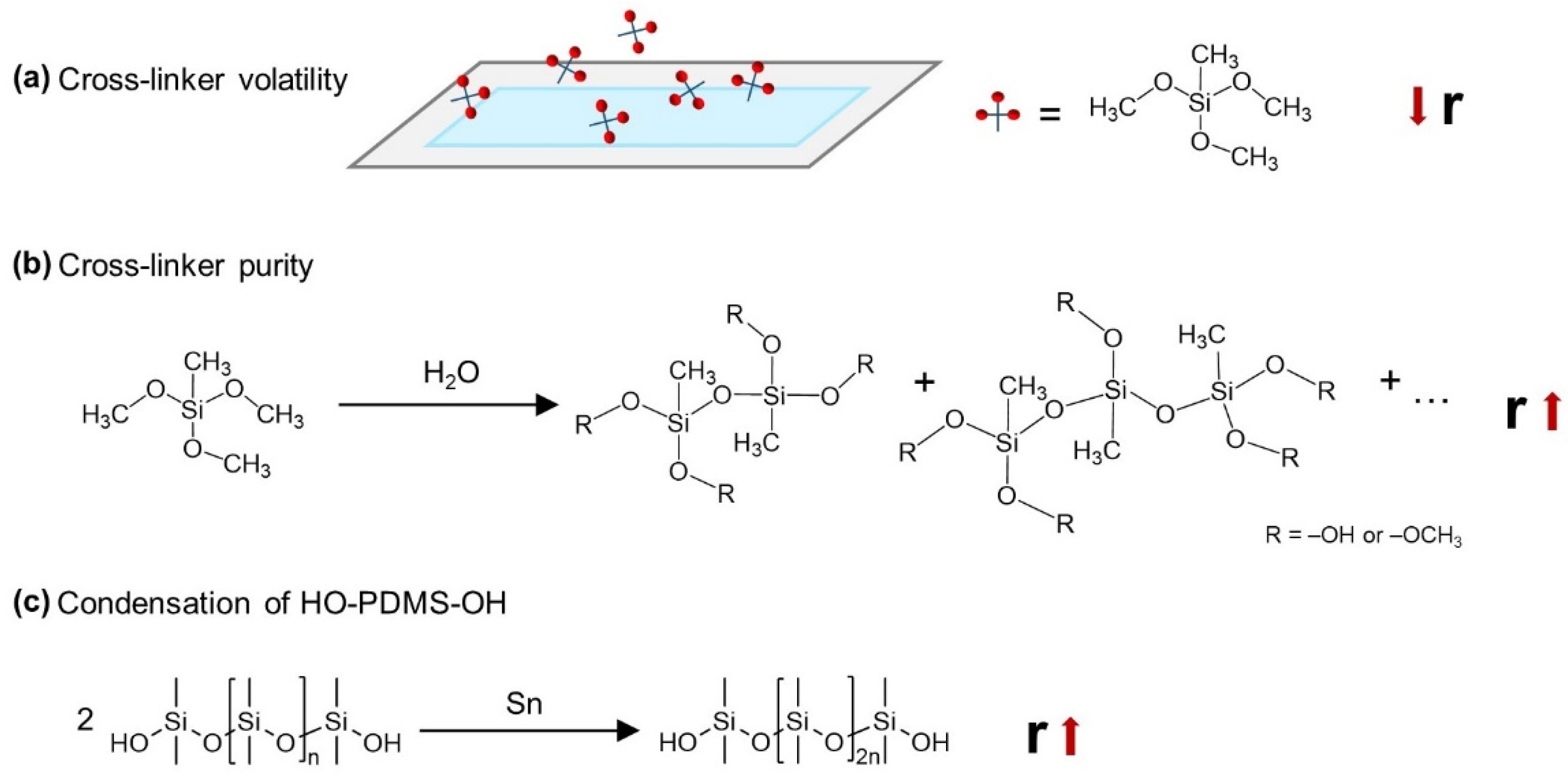
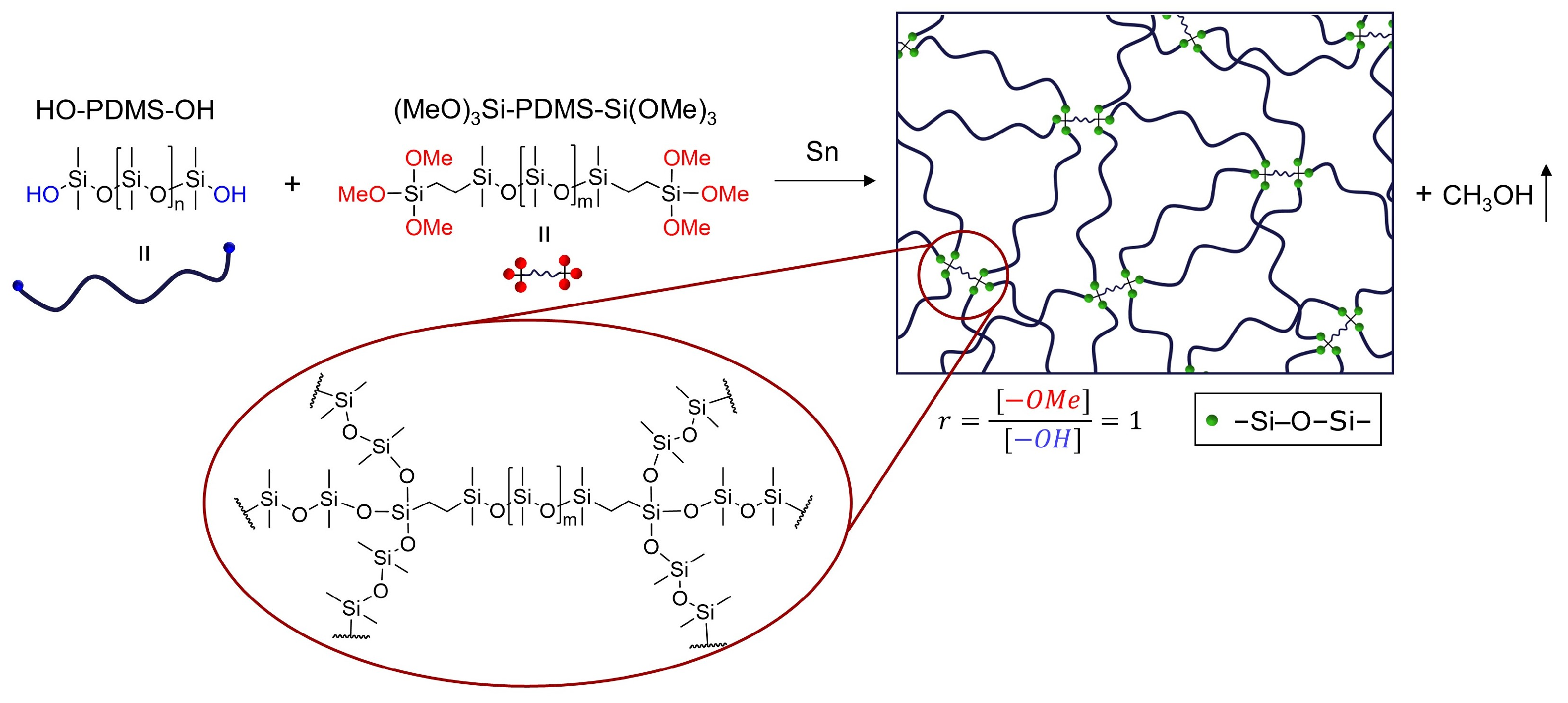
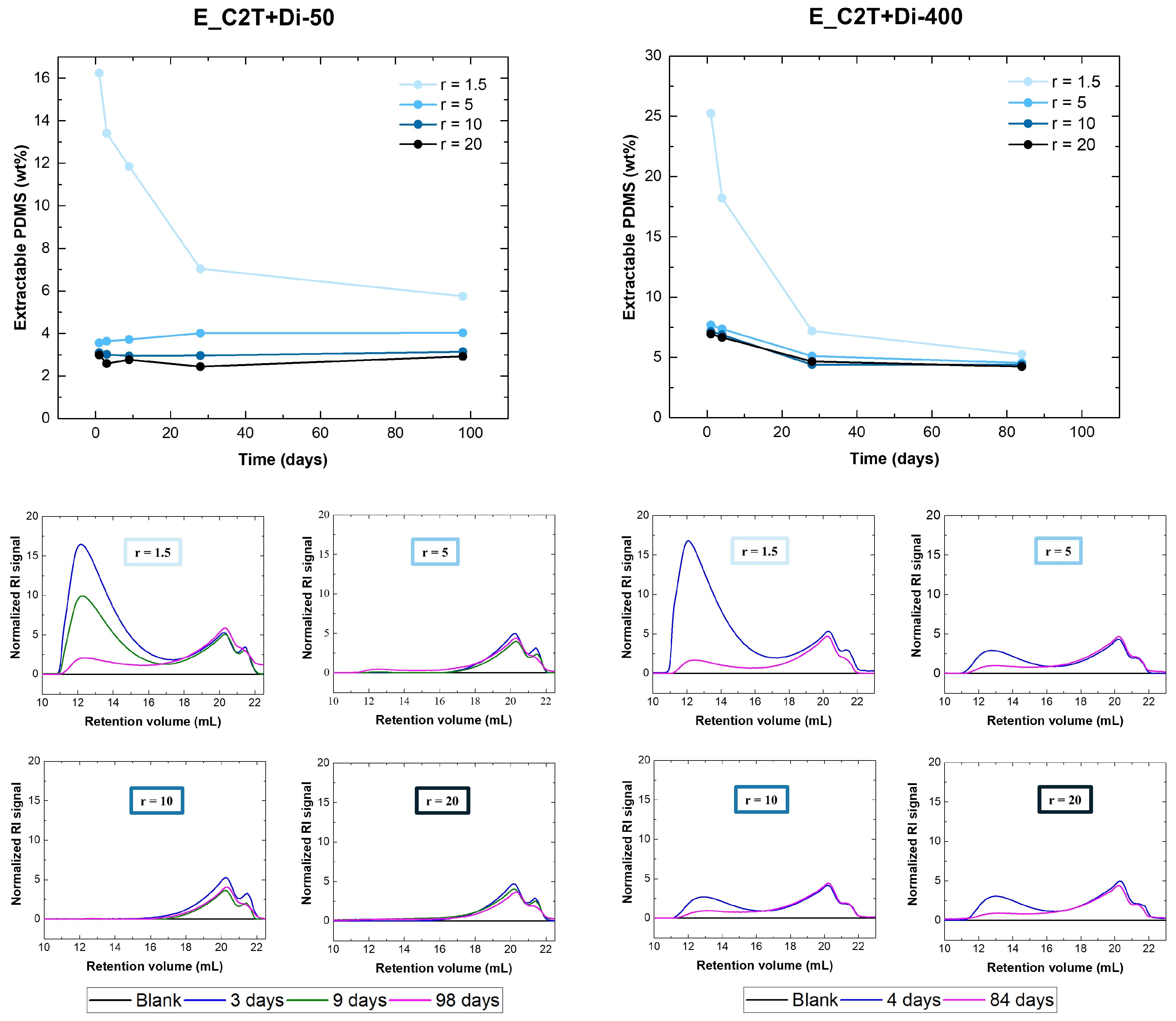
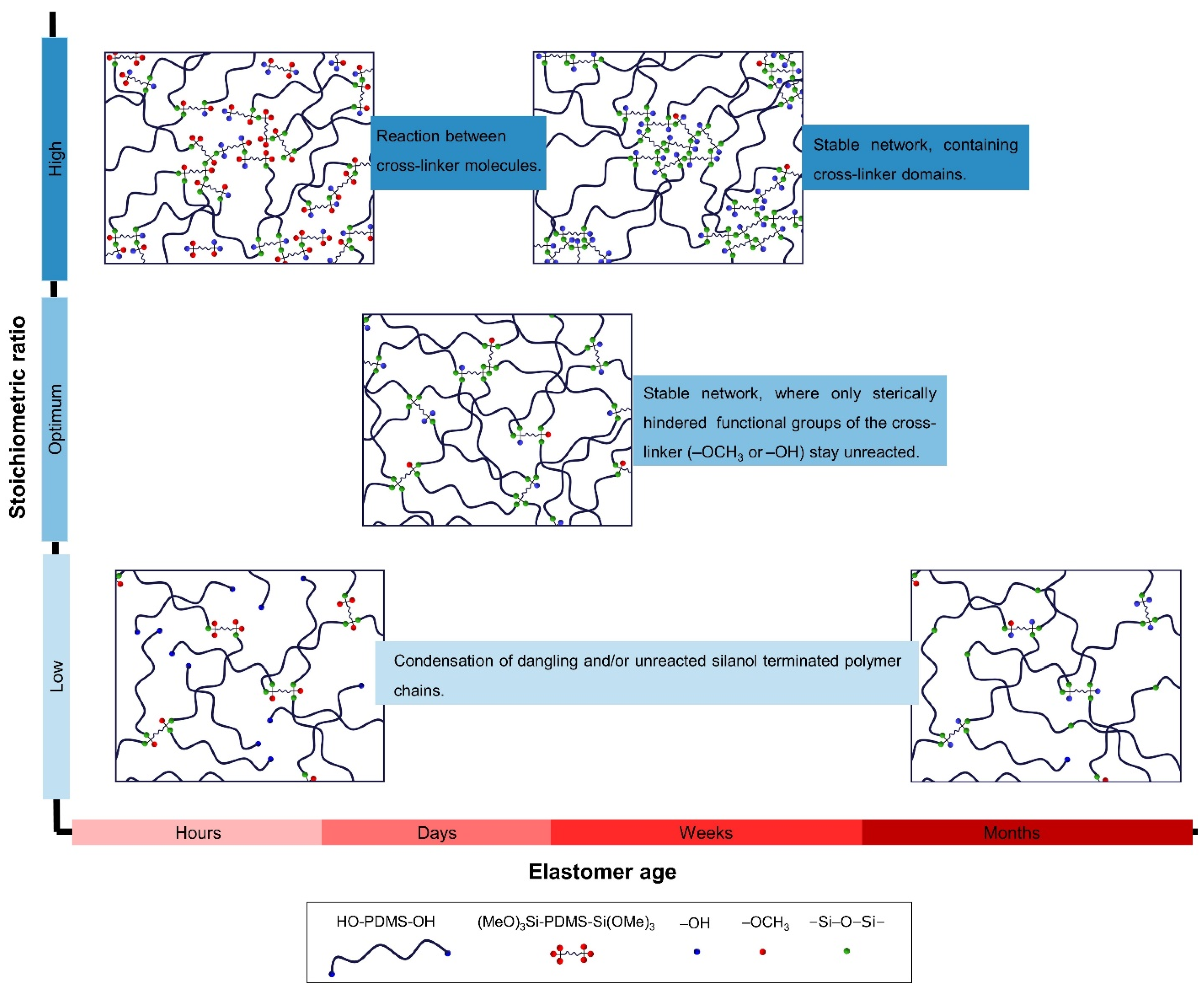
2.1.1. Trimethoxysilane-Terminated Polysiloxane Cross-Linkers
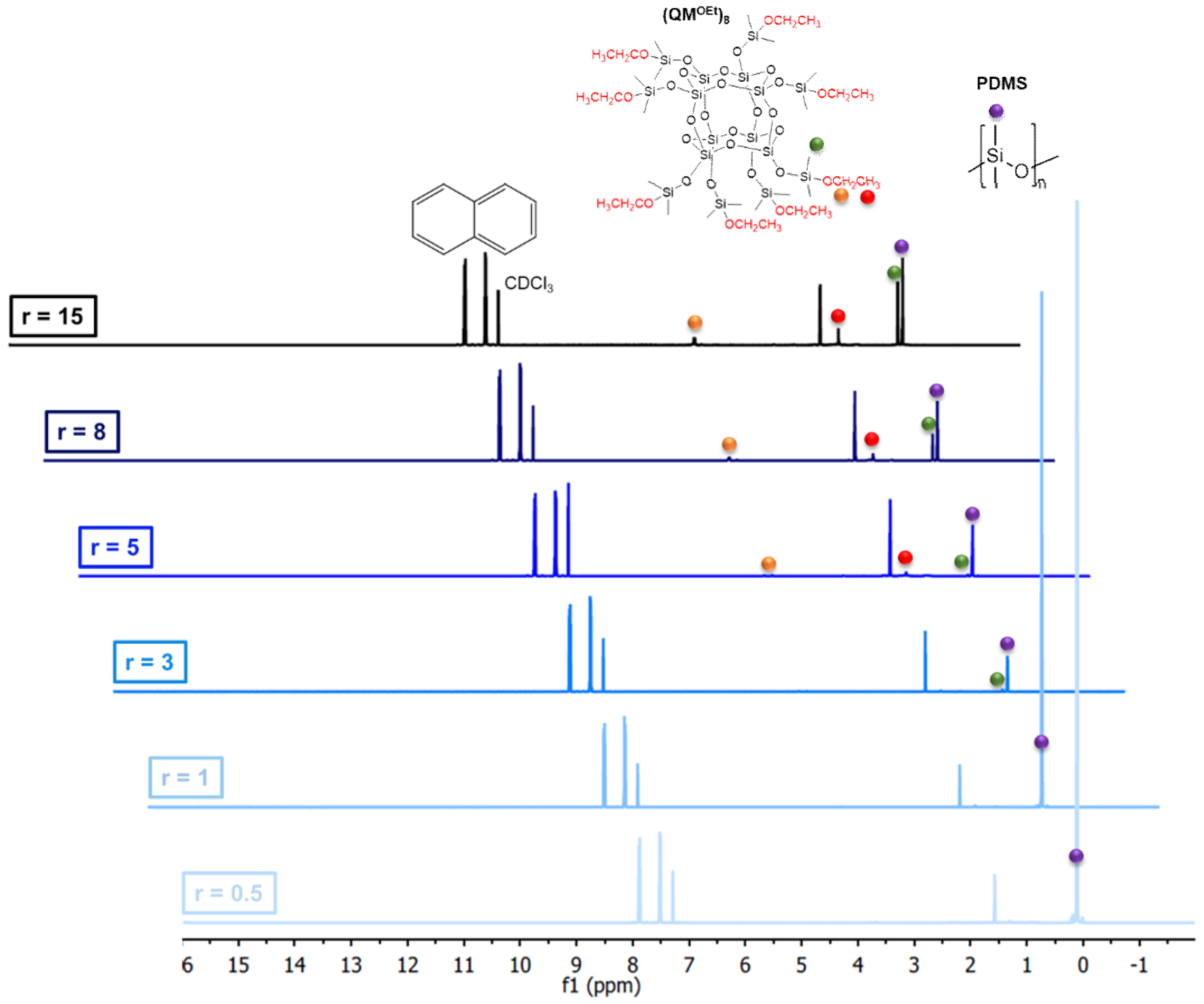
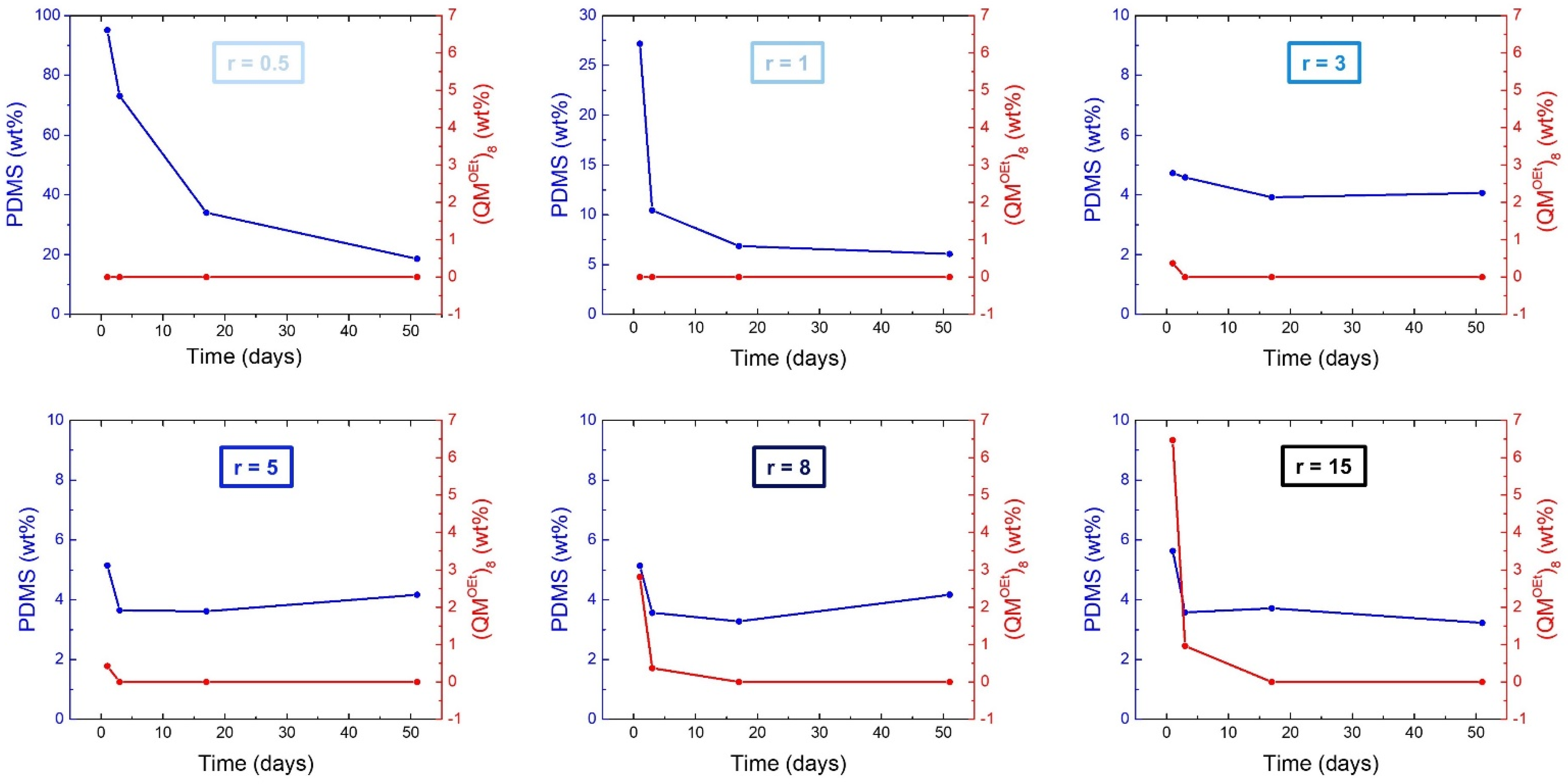
2.1.2. Silsesquioxane Cross-Linker
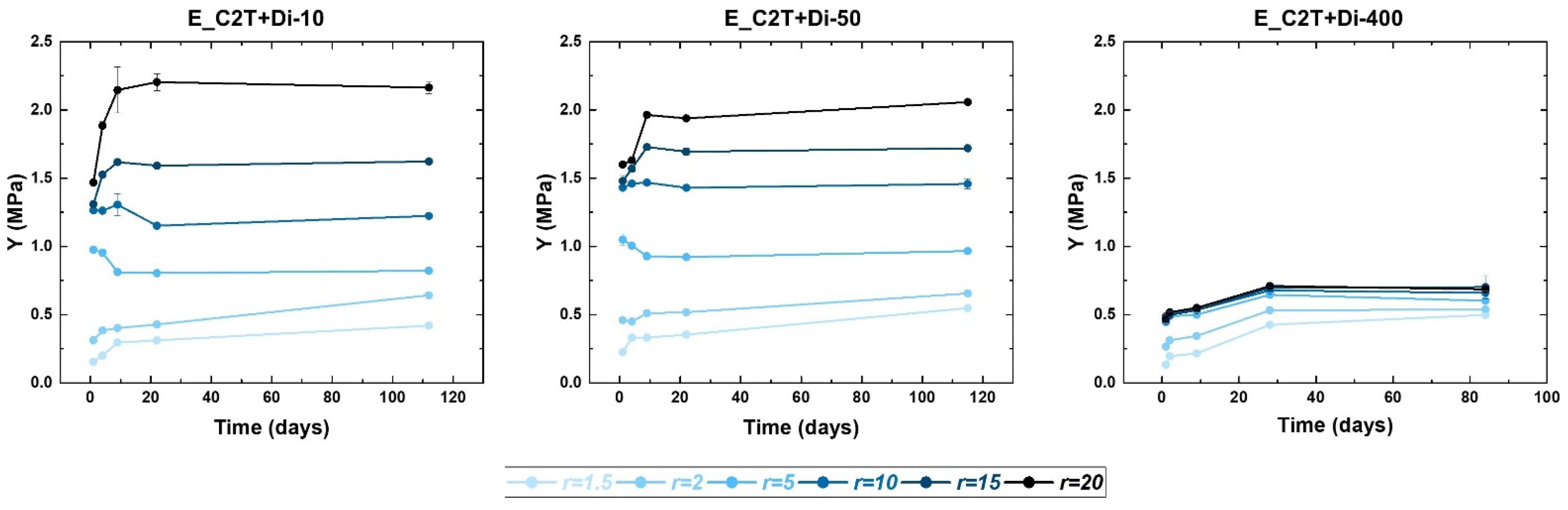
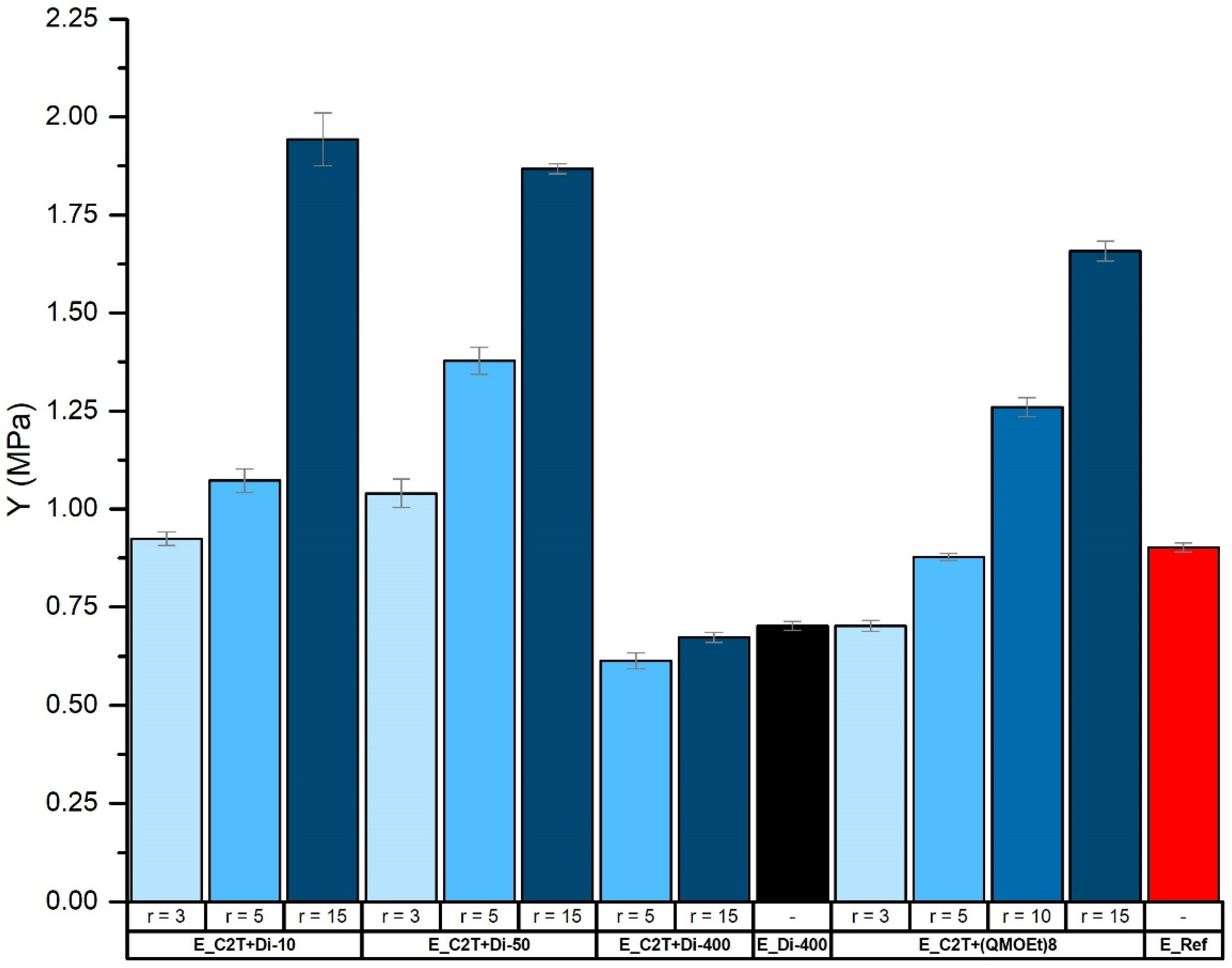
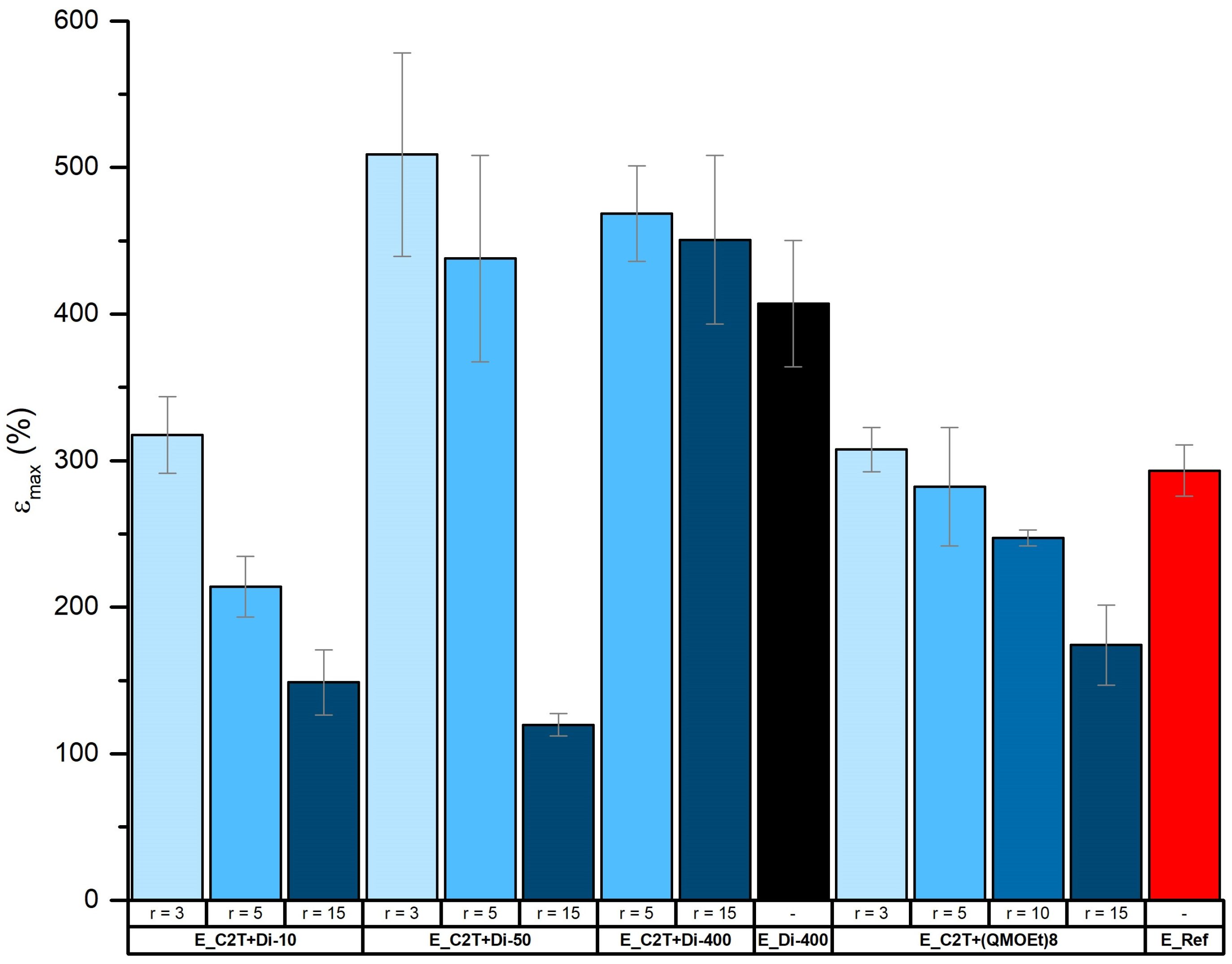
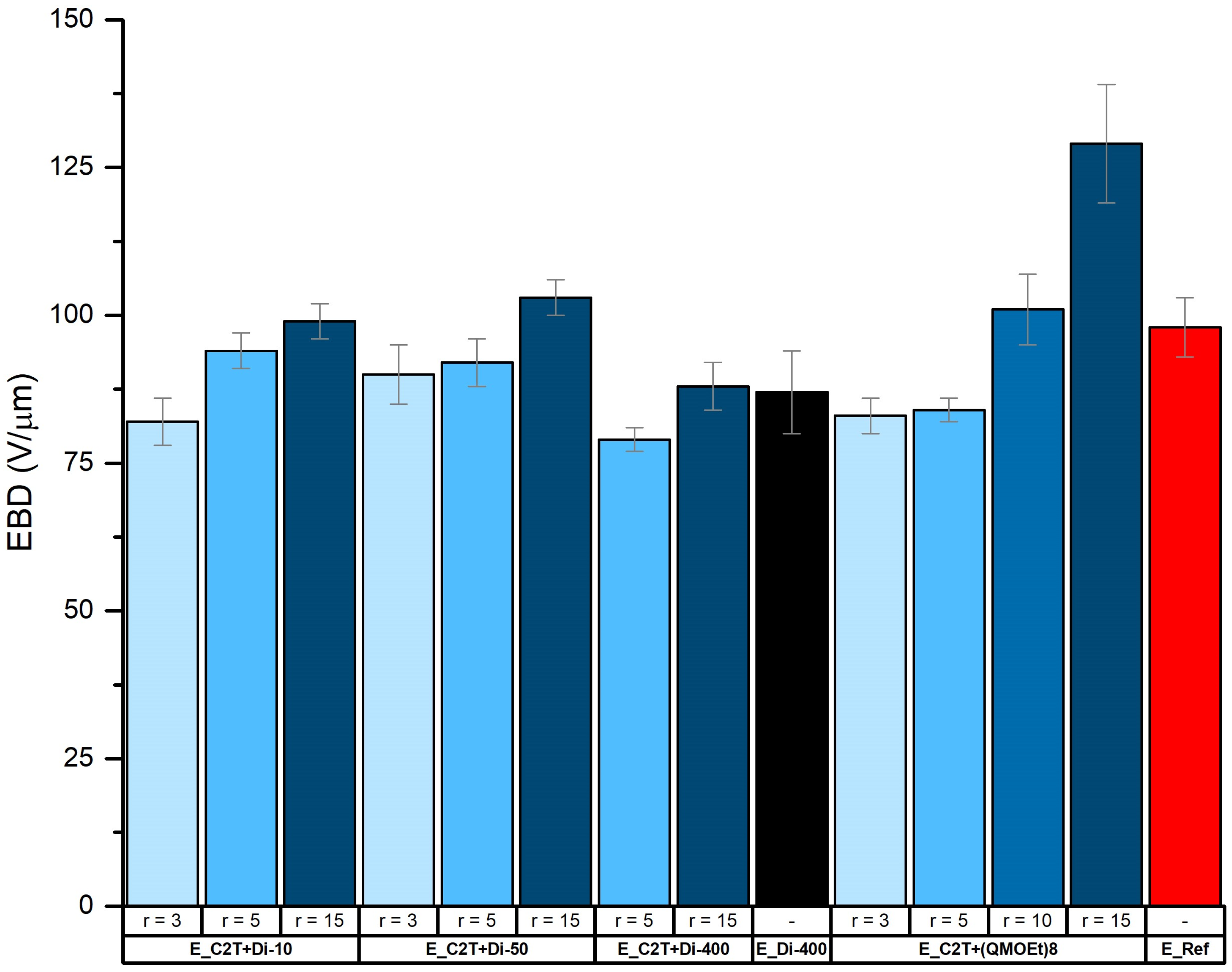
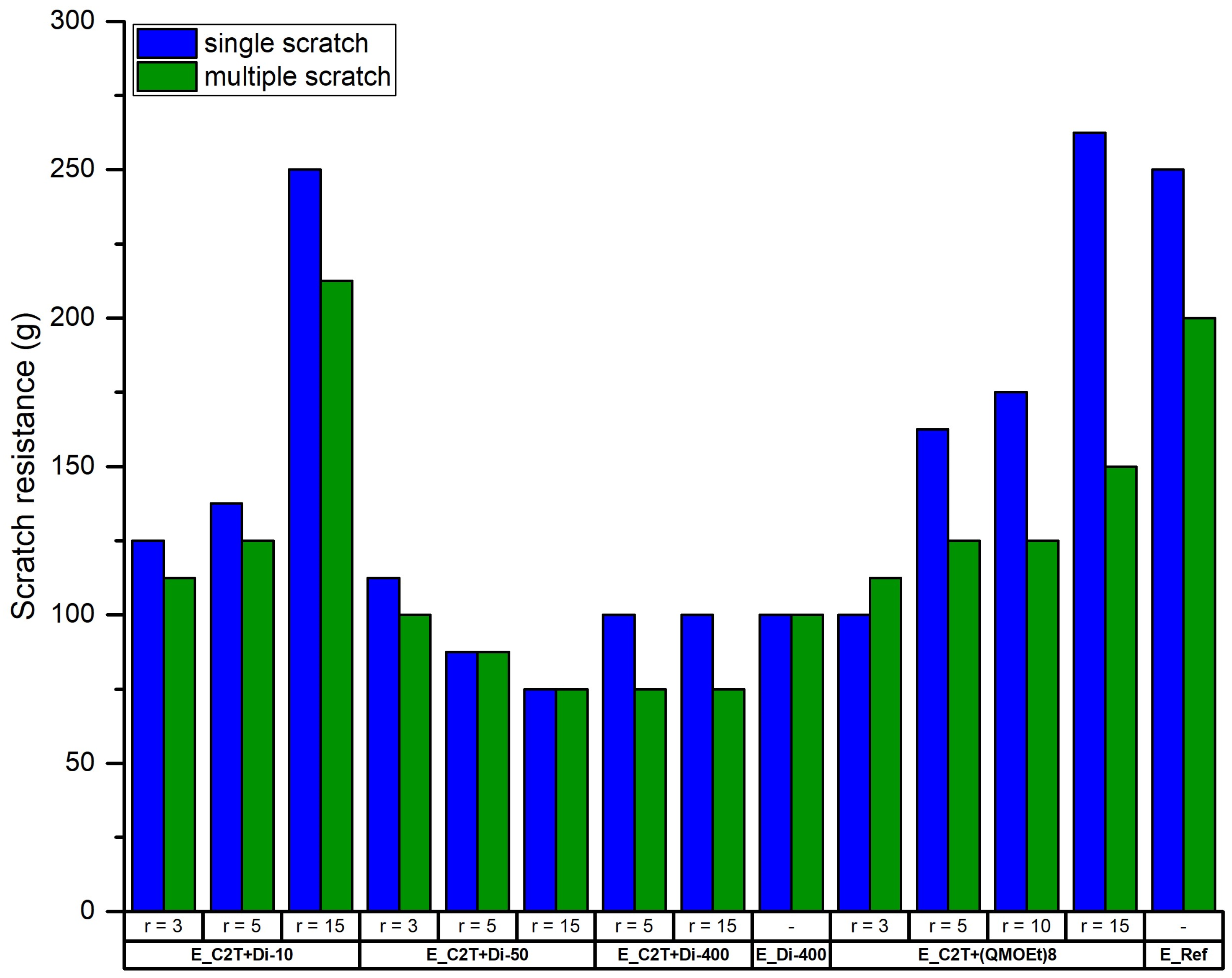
2.2. Mechanically Stable Silicone Elastomer Films and Their Properties
3. Materials and Methods
3.1. Materials
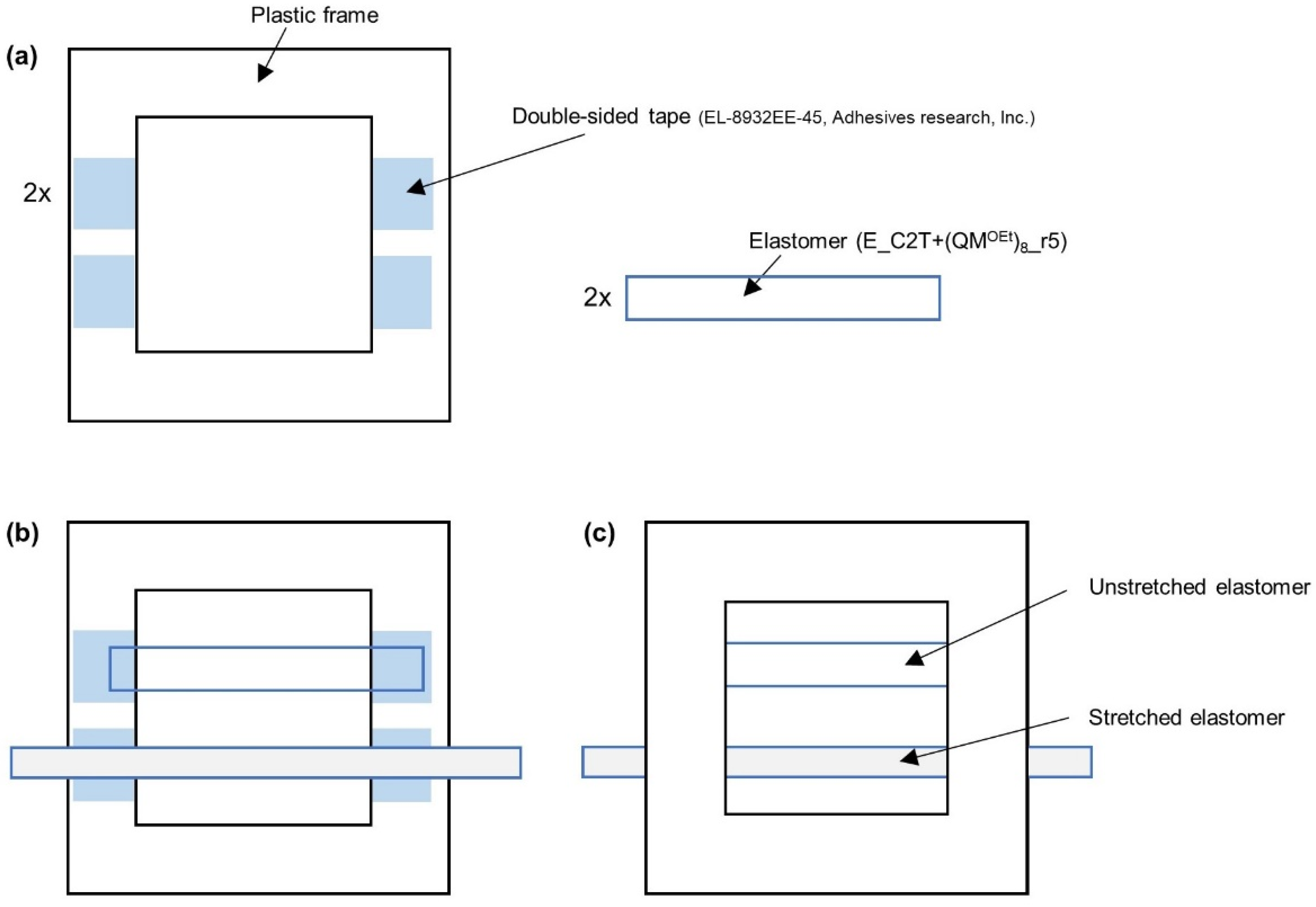
3.2. Elastomer Film Preparation
3.3. Evaluation of the Elastomer Film Stability
3.4. Evaluation of the Silicone Elastomer Films Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
| Compound | SEC | 1H NMR Mn (g/mol) | |
|---|---|---|---|
| Mn (g/mol) | PDI | ||
| Di-10 | 1200 | 1.49 | 1300 |
| Di-50 | 5500 | 4.06 | 4600 |
| Di-400 | 20,300 | 2.25 | 23,000 |
| C2T | 20,200 | 2.18 | - |
| C2T (~20,200 g/mol) + Di-10 (~1200 g/mol) * | |||||||
| Name | r | C2T (g) | Di-10 | Sn_DL | |||
| wt% | g | wt% | g | µL | |||
| E_C2T+Di-10_r1.5 | 1.5 | 3.88 | 3 | 0.13 | 0.5 | 0.02 | 19 |
| E_C2T+Di-10_r2 | 2 | 3.85 | 4 | 0.15 | |||
| E_C2T+Di-10_r5 | 5 | 3.64 | 9 | 0.36 | |||
| E_C2T+Di-10_r10 | 10 | 3.34 | 17 | 0.66 | |||
| E_C2T+Di-10_r15 | 15 | 3.08 | 23 | 0.92 | |||
| E_C2T+Di-10_r20 | 20 | 2.87 | 28 | 1.13 | |||
| C2T (~20,200 g/mol) + Di-50 (~5500 g/mol) * | |||||||
| Name | r | C2T (g) | Di-50 | Sn_DL | |||
| wt% | g | wt% | g | µL | |||
| E_C2T+Di-50_r1.5 | 1.5 | 3.52 | 12 | 0.48 | 0.5 | 0.02 | 19 |
| E_C2T+Di-50_r2 | 2 | 3.39 | 15 | 0.61 | |||
| E_C2T+Di-50_r5 | 5 | 2.75 | 31 | 1.25 | |||
| E_C2T+Di-50_r10 | 10 | 2.10 | 48 | 1.90 | |||
| E_C2T+Di-50_r15 | 15 | 1.69 | 58 | 2.31 | |||
| E_C2T+Di-50_r20 | 20 | 1.42 | 65 | 2.58 | |||
| C2T (~20,200 g/mol) + Di-400 (~20,300 g/mol) * | |||||||
| Name | r | C2T (g) | Di-400 | Sn_DL | |||
| wt% | g | wt% | g | µL | |||
| E_C2T+Di-400_r1.5 | 1.5 | 2.66 | 34 | 1.34 | 0.5 | 0.02 | 19 |
| E_C2T+Di-400_r2 | 2 | 2.40 | 40 | 1.6 | |||
| E_C2T+Di-400_r5 | 5 | 1.50 | 63 | 2.50 | |||
| E_C2T+Di-400_r10 | 10 | 0.92 | 77 | 3.08 | |||
| E_C2T+Di-400_r15 | 15 | 0.66 | 84 | 3.34 | |||
| E_C2T+Di-400_r20 | 20 | 0.52 | 87 | 3.48 | |||
| C2T (~20,200 g/mol) + (QMOEt)8 (1370.404 g/mol) ** | |||||||
| Name | r | C2T (g) | (QMOEt)8 | Sn_DL | |||
| wt% | µL *** | wt% | g | µL | |||
| E_C2T+(QMOEt)8_r0.5 | 0.5 | 0.5 | 1 | 5 | 0.5 | 0.02 | 19 |
| E_C2T+(QMOEt)8_r1 | 1 | 0.49 | 2 | 10 | |||
| E_C2T+(QMOEt)8_r3 | 3 | 0.48 | 5 | 30 | |||
| E_C2T+(QMOEt)8_r5 | 5 | 0.46 | 8 | 48 | |||
| E_C2T+(QMOEt)8_r8 | 8 | 0.44 | 12 | 73 | |||
| E_C2T+(QMOEt)8_r15 | 15 | 0.40 | 20 | 125 | |||
| C2T (~20,200 g/mol) + Di-10 (~1200 g/mol) * | |||||||
| Name | r | C2T (g) | Di-10 | Sn_DL | |||
| wt% | g | wt% | g | µL | |||
| E_C2T+Di-10_r3 | 3 | 5.66 | 6 | 0.34 | 0.5 | 0.03 | 28 |
| E_C2T+Di-10_r5 | 5 | 5.46 | 9 | 0.54 | |||
| E_C2T+Di-10_r15 | 15 | 4.63 | 23 | 1.37 | |||
| C2T (~20,200 g/mol) + Di-50 (~5500 g/mol) * | |||||||
| Name | r | C2T (g) | Di-50 | Sn_DL | |||
| wt% | g | wt% | g | µL | |||
| E_C2T+Di-50_r3 | 3 | 4.72 | 21 | 1.28 | 0.5 | 0.03 | 28 |
| E_C2T+Di-50_r5 | 5 | 4.13 | 31 | 1.87 | |||
| E_C2T+Di-50_r15 | 15 | 2.54 | 58 | 3.46 | |||
| C2T (~20,200 g/mol) + Di-400 (~20,300 g/mol) ** | |||||||
| Name | r | C2T (g) | Di-400 | Sn_DL | |||
| wt% | g | wt% | g | µL | |||
| E_C2T+Di-400_r5 | 5 | 1.50 | 63 | 2.50 | 0.5 | 0.03 | 28 |
| E_C2T+Di-400_r15 | 15 | 0.66 | 83 | 3.34 | |||
| E_Di-400 | - | - | 100 | 4 | |||
| C2T (~20,200 g/mol) + (QMOEt)8 (1370.404 g/mol) ** | |||||||
| Name | r | C2T (g) | (QMOEt)8 | Sn_DL | |||
| wt% | µL *** | wt% | g | µL | |||
| E_C2T+(QMOEt)8_r3 | 3 | 3.81 | 5 | 255 | 0.5 | 0.02 | 19 |
| E_C2T+(QMOEt)8_r5 | 5 | 3.69 | 8 | 411 | |||
| E_C2T+(QMOEt)8_r10 | 10 | 3.42 | 15 | 763 | |||
| E_C2T+(QMOEt)8_r15 | 15 | 3.19 | 20 | 1067 | |||
| E_Ref | Reference coating = commercially used silicone elastomer coating prepared via condensation curing chemistry | ||||||
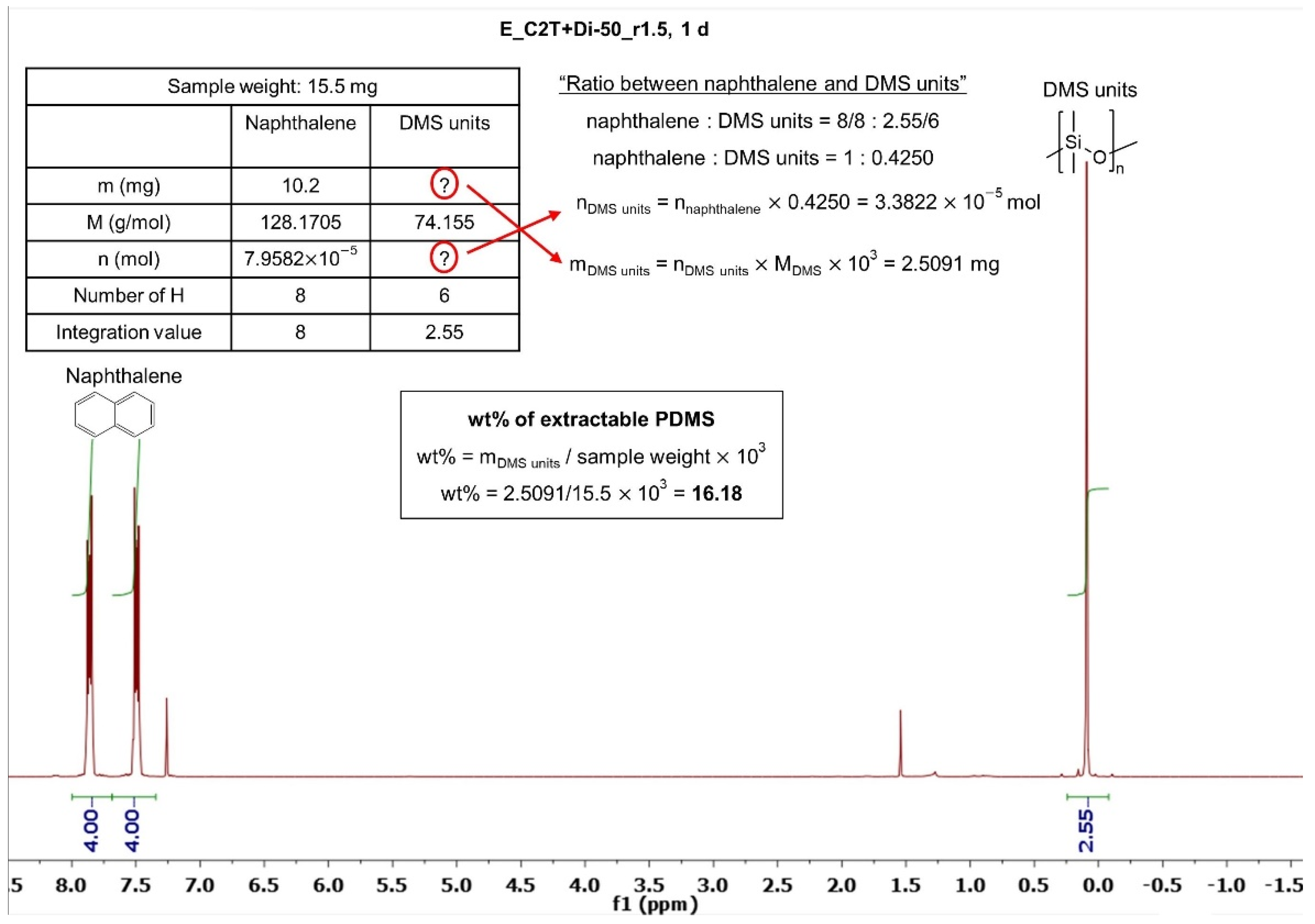
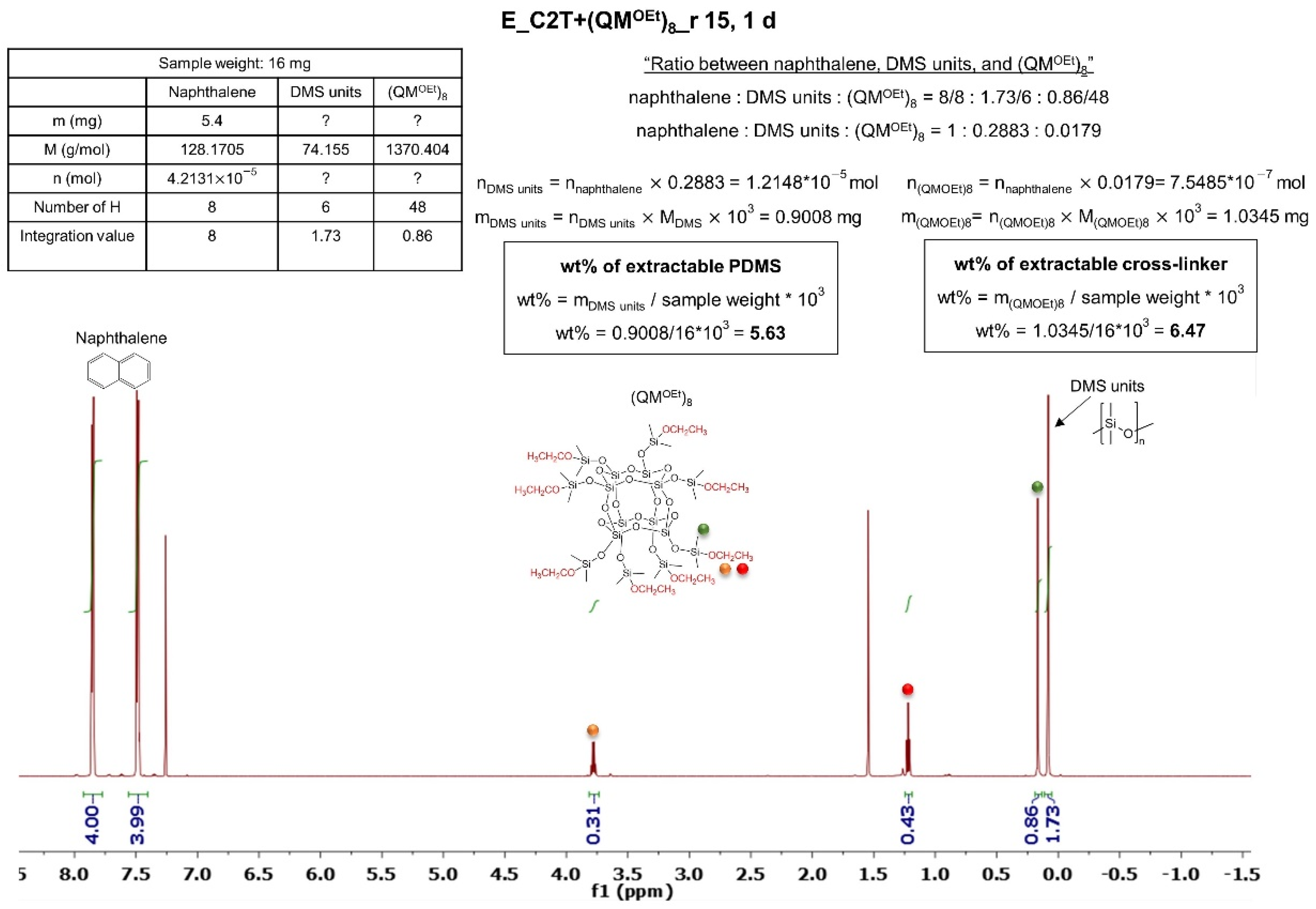
| Elastomer | Extractable PDMS (wt%) | Sol Fraction (wt%) |
|---|---|---|
| E_C2T+Di-10_r3 | 4.1 | 4.2 |
| E_C2T+Di-10_r5 | 4.1 | 3.8 |
| E_C2T+Di-10_r15 | 3.8 | 3.1 |
| E_C2T+Di-50_r3 | 4.2 | 3.3 |
| E_C2T+Di-50_r5 | 3.5 | 3.5 |
| E_C2T+Di-50_r15 | 3.5 | 3.6 |
| E_C2T+Di-400_r5 | 4.6 | 4.7 |
| E_C2T+Di-400_r15 | 4.6 | 3.8 |
| E_Di-400 | 4.3 | 2.5 |
| E_C2T+(QMOEt)8_r3 | 4.3 | 5.0 |
| E_C2T+(QMOEt)8_r5 | 4.2 | 3.7 |
| E_C2T+(QMOEt)8_r10 | 3.9 | 2.7 |
| E_C2T+(QMOEt)8_r15 | 3.6 | 5.0 |
| E_Ref | 7.0 | 6.3 |

References
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- De Buyl, F. Silicone sealants and structural adhesives. Int. J. Adhes. Adhes. 2001, 21, 411–422. [Google Scholar] [CrossRef]
- Gutowski, W.S.; Wolf, A.; Deng, P.; Zhang, Y.; Yin, Y. Application of polydimethylsiloxane (PDMS) polymers as structural adhesives, sealants, and high-performance functional coatings. J. Mach. Constr. Maint. 2019, 3, 7–17. [Google Scholar]
- Beers, M.D.; Thompson, J.E. Oil Resistant Low Modulus Silicone Sealant Composition. U.S. Patent 4,514,529, 30 April 1985. [Google Scholar]
- Stein, J.; Burnell, T.B. Sprayable, Condensation Curable Silicone Foul Release Coatings and Articles Coated Therewith. U.S. Patent 5,904,988, 18 May 1999. [Google Scholar]
- Kimura, T.; Ikeno, M. Room Temperature Fast Curable Compositions. U.S. Patent 6,306,998 B1, 23 October 2001. [Google Scholar]
- Lucas, G.M. Process for Producing Alkoxy-Terminated Polysiloxanes. U.S. Patent 4,599,394, 8 July 1986. [Google Scholar]
- Ogliani, E.; Yu, L.; Skov, A.L.; Skov, A.L. Designing reliable silicone elastomers for high-temperature applications. Polym. Degrad. Stab. 2018, 157, 175–180. [Google Scholar] [CrossRef]
- Skov, A.L.; Vudayagiri, S.; Skov, A.L. How to tailor flexible silicone elastomers with mechanical integrity: A tutorial review. Chem. Soc. Rev. 2019, 48, 1448–1464. [Google Scholar] [CrossRef]
- Jurásková, A.; Dam-Johansen, K.; Olsen, S.M.; Skov, A.L. Factors influencing mechanical long-term stability of condensation curing silicone elastomers. J. Polym. Res. 2020, 27, 1–14. [Google Scholar] [CrossRef]
- Tang, M.Y.; Mark, J.E. Effect of composition and cross-link functionality on the elastomeric properties of bimodal networks. Macromolecules 1984, 17, 2616–2619. [Google Scholar] [CrossRef]
- Sprung, M.M.; Guenther, F.O. The Partial Hydrolysis of Methyltrimethoxysilane. J. Am. Chem. Soc. 2005, 77, 4173–4175. [Google Scholar] [CrossRef]
- Smith, K.A. Polycondensation of methyltrimethoxysilane. Macromolecules 1987, 20, 2514–2520. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef]
- Clarson, S.J.; Wang, Z.; Mark, J.E. Effect of stannous 2-ethylhexanoate on the network formation and chain extension reactions of α,ω-dihydroxy terminated poly(dimethylsiloxane). Eur. Polym. J. 1990, 26, 621–622. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, J. Preparation and characterization of incompletely condensed POSS and its application in RTV composites. J. Elastomers Plast. 2016, 49, 157–172. [Google Scholar] [CrossRef]
- Ji, J.-Y.; Ge, X.; Pang, X.; Liu, R.; Wen, S.; Sun, J.; Liang, W.; Ge, J.; Chen, X. Synthesis and Characterization of Room Temperature Vulcanized Silicone Rubber Using Methoxyl-Capped MQ Silicone Resin as Self-Reinforced Cross-Linker. Polymers 2019, 11, 1142. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Gao, X.; Zhang, D.; Liu, Y.; Huang, G. Synthesis and thermal properties of modified room temperature vulcanized (RTV) silicone rubber using polyhedral oligomeric silsesquioxane (POSS) as a cross linking agent. RSC Adv. 2014, 4, 41453–41460. [Google Scholar] [CrossRef]
- Sun, J.; Kong, J.; He, C. Liquid polyoctahedral silsesquioxanes as an effective and facile reinforcement for liquid silicone rubber. J. Appl. Polym. Sci. 2019, 136, 46996. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, G.; Shi, Y.; Zhang, G.; Liu, Y. Polyhedral oligomeric silsesquioxane/silica/polydimethylsiloxane rubber composites with enhanced mechanical and thermal properties. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Mohammad, S.A.; Wee, A.G.; Rumsey, D.J.; Schricker, S.R. Maxillofacial Materials Reinforced with Various Concentrations of Polyhedral Silsesquioxanes. J. Dent. Biomech. 2010, 1, 701845. [Google Scholar] [CrossRef]
- Silau, H.; Stabell, N.B.; Petersen, F.R.; Pham, M.; Yu, L.; Skov, A.L. Weibull Analysis of Electrical Breakdown Strength as an Effective Means of Evaluating Elastomer Thin Film Quality. Adv. Eng. Mater. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Dissado, L.A.; Fothergill, J.C. Electrical Degradation and Breakdown in Polymers. Polymer 1993, 34, 3966. [Google Scholar] [CrossRef]
- Yu, L.; Skov, A.L. Molecular Strategies for Improved Dielectric Elastomer Electrical Breakdown Strengths. Macromol. Rapid Commun. 2018, 39, 1–6. [Google Scholar] [CrossRef]
- Vaicekauskaite, J.; Skov, A.L.; Vudayagiri, S.; Skov, A.L. Mapping the mechanical and electrical properties of commercial silicone elastomer formulations for stretchable transducers. J. Mater. Chem. C 2020, 8, 1273–1279. [Google Scholar] [CrossRef]
- Chalker, P.; Bull, S.; Rickerby, D. A review of the methods for the evaluation of coating-substrate adhesion. Mater. Sci. Eng. A 1991, 140, 583–592. [Google Scholar] [CrossRef]
- Li, J.; Beres, W. Scratch Test for Coating/Substrate Systems—A Literature Review. Can. Met. Q. 2007, 46, 155–173. [Google Scholar] [CrossRef]
- Sangermano, M.; Messori, M. Scratch Resistance Enhancement of Polymer Coatings. Macromol. Mater. Eng. 2010, 295, 603–612. [Google Scholar] [CrossRef]
- Madsen, F.B.; Daugaard, A.E.; Hvilsted, S.; Skov, A.L. The Current State of Silicone-Based Dielectric Elastomer Transducers. Macromol. Rapid Commun. 2016, 37, 378–413. [Google Scholar] [CrossRef] [PubMed]
- Jurásková, A.; Skov, A.L.; Brook, M.A. A Mild Route to Convert SiH Compounds to Their Alkoxy Analogues. Ind. Eng. Chem. Res. 2020, 59, 18412–18418. [Google Scholar] [CrossRef]
- Zakaria, S.; Madsen, F.B.; Skov, A.L. Post Curing as an Effective Means of Ensuring the Long-term Reliability of PDMS Thin Films for Dielectric Elastomer Applications. Polym. Technol. Eng. 2017, 56, 83–95. [Google Scholar] [CrossRef]
- Gale, C.B.; Brook, M.A.; Skov, A.L. Compatibilization of porphyrins for use as high permittivity fillers in low voltage actuating silicone dielectric elastomers. RSC Adv. 2020, 10, 18477–18486. [Google Scholar] [CrossRef]

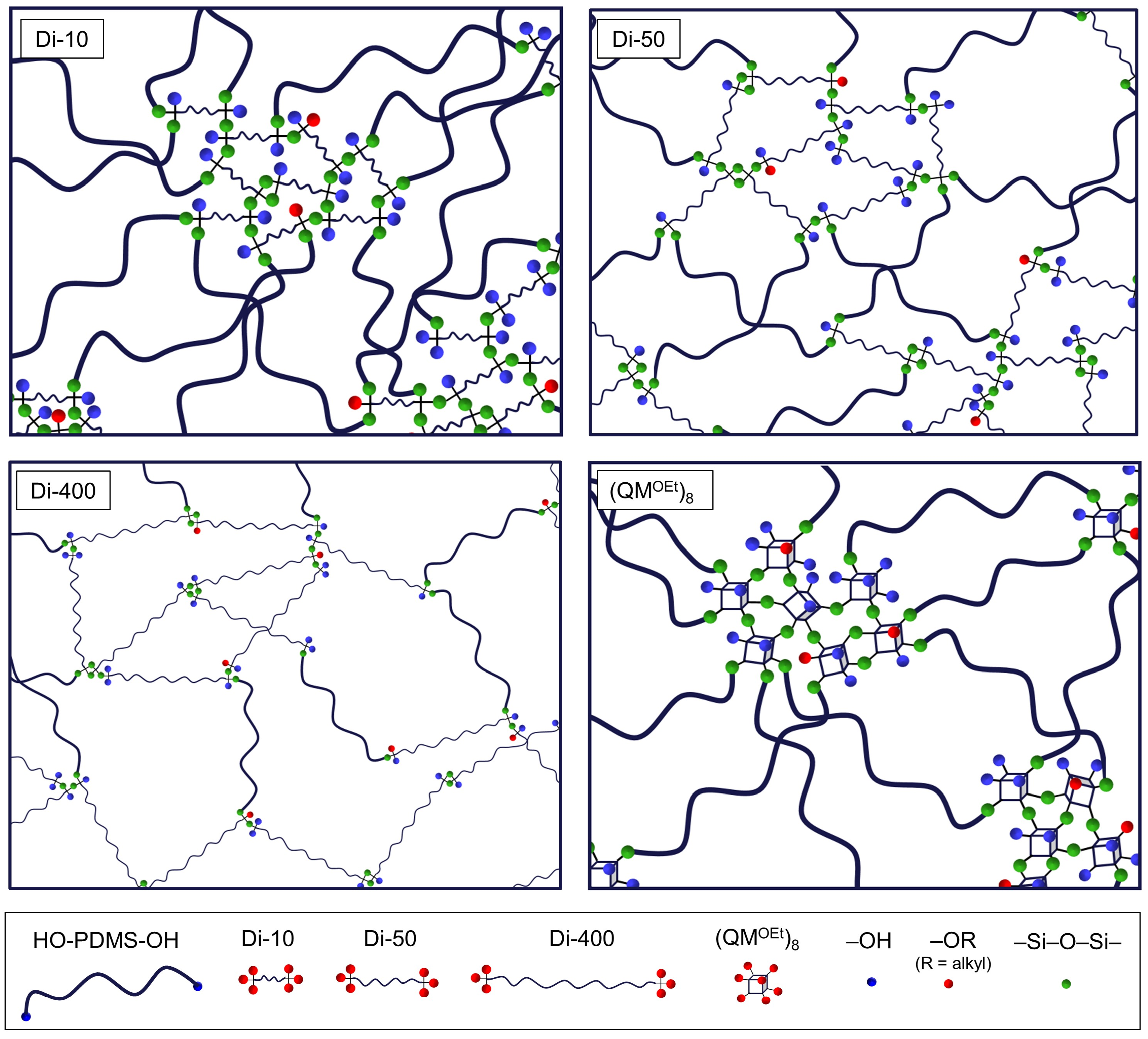

| Compound | Abbreviation | Molecular Weight (g/mol) | Chemical Structure |
|---|---|---|---|
| HO-PDMS-OH | C2T | 20,200 * |  |
| (MeO)3Si-PDMS-Si(OMe)3 | Di-10 | 1200 * | 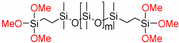 |
| Di-50 | 5500 * | ||
| Di-400 | 20,300 * | ||
| (QMOEt)8 | (QMOEt)8 | 1370.4 ** | 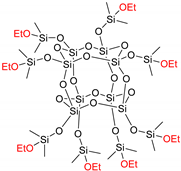 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurásková, A.; Møller Olsen, S.; Dam-Johansen, K.; Brook, M.A.; Skov, A.L. Reliable Condensation Curing Silicone Elastomers with Tailorable Properties. Molecules 2021, 26, 82. https://doi.org/10.3390/molecules26010082
Jurásková A, Møller Olsen S, Dam-Johansen K, Brook MA, Skov AL. Reliable Condensation Curing Silicone Elastomers with Tailorable Properties. Molecules. 2021; 26(1):82. https://doi.org/10.3390/molecules26010082
Chicago/Turabian StyleJurásková, Alena, Stefan Møller Olsen, Kim Dam-Johansen, Michael A. Brook, and Anne Ladegaard Skov. 2021. "Reliable Condensation Curing Silicone Elastomers with Tailorable Properties" Molecules 26, no. 1: 82. https://doi.org/10.3390/molecules26010082
APA StyleJurásková, A., Møller Olsen, S., Dam-Johansen, K., Brook, M. A., & Skov, A. L. (2021). Reliable Condensation Curing Silicone Elastomers with Tailorable Properties. Molecules, 26(1), 82. https://doi.org/10.3390/molecules26010082