Utilization of Deep Eutectic Solvents to Reduce the Release of Hazardous Gases to the Atmosphere: A Critical Review
Abstract
1. Introduction
- CO2 capture
- Capture of acidic gases
- Selectivity of DESs in capturing gases
- Desulfurization and denitrification of fuels
2. CO2 Capture
3. Capture of Acidic Gases
3.1. Oxide-Based Acid Gases
3.1.1. SO2 Capture
3.1.2. NOx Capture
3.2. NH3 Capture
3.3. H2S Capture
4. Selectivity of DESs in Capturing Gases
5. Desulfurization and Denitrification of Fuels
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, W.; Lin, L.C.; Peng, X.; Smit, B. Computational screening of porous metal-organic frameworks and zeolites for the removal of SO2 and NOx from flue gases. AIChE J. 2014, 60, 2314–2323. [Google Scholar] [CrossRef]
- Perera, F.P. Multiple Threats to Child Health from Fossil Fuel Combustion: Impacts of Air Pollution and Climate Change. Environ. Heal. Perspect. 2017, 125, 141–148. [Google Scholar] [CrossRef]
- Silas, K.; Ghani, W.A.W.A.K.; Choong, T.S.; Rashid, U. Carbonaceous materials modified catalysts for simultaneous SO2/NOx removal from flue gas: A review. Catal. Rev. 2019, 61, 134–161. [Google Scholar] [CrossRef]
- Lemaoui, T.; Benguerba, Y.; Darwish, A.S.; Abu Hatab, F.; Warrag, S.E.; Kroon, M.C.; Alnashef, I.M. Simultaneous dearomatization, desulfurization, and denitrogenation of diesel fuels using acidic deep eutectic solvents as extractive agents: A parametric study. Sep. Purif. Technol. 2021, 256, 117861. [Google Scholar] [CrossRef]
- Ladommatos, N.; Xiao, Z.; Zhao, H. Effects of fuels with a low aromatic content on diesel engine exhaust emissions. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2000, 214, 779–794. [Google Scholar] [CrossRef]
- Li, C.; Li, D.; Zou, S.; Li, Z.; Yin, J.; Wang, A.; Cui, Y.; Yao, Z.; Zhao, Q. Extraction desulfurization process of fuels with ammonium-based deep eutectic solvents. Green Chem. 2013, 15, 2793–2799. [Google Scholar] [CrossRef]
- Ali, M.C.; Yang, Q.; Fine, A.A.; Jin, W.; Zhang, Z.; Xing, H.; Ren, Q. Efficient removal of both basic and non-basic nitrogen compounds from fuels by deep eutectic solvents. Green Chem. 2016, 18, 157–164. [Google Scholar] [CrossRef]
- Alli, R.D.; Kroon, M.C. Extraction of benzothiazole and thiophene from their mixtures with n-heptane using tetrahexylammonium bromide-based deep eutectic solvents as extractive denitrogenation and desulfurization agents. Fluid Phase Equilibria 2018, 477, 1–11. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Environmental chemistry in the twenty-first century. Environ. Chem. Lett. 2017, 15, 329–346. [Google Scholar] [CrossRef]
- Krishnan, A.; Gopinath, K.; Vo, D.-V.N.; Malolan, R.; Nagarajan, V.M.; Arun, J. Ionic liquids, deep eutectic solvents and liquid polymers as green solvents in carbon capture technologies: A review. Environ. Chem. Lett. 2020, 1–24. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.; Simões, M. Green fuel production: Processes applied to microalgae. Environ. Chem. Lett. 2013, 11, 315–324. [Google Scholar] [CrossRef]
- Xu, H.-J.; Zhang, C.-F.; Zheng, Z.-S. Selective H2S Removal by Nonaqueous Methyldiethanolamine Solutions in an Experimental Apparatus. Ind. Eng. Chem. Res. 2002, 41, 2953–2956. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Ji, N.; Deng, S.; Zhao, J.; Li, Y.; Song, Y.; Li, H. Alternative pathways for efficient CO2 capture by hybrid processes—A review. Renew. Sustain. Energy Rev. 2018, 82, 215–231. [Google Scholar] [CrossRef]
- Haider, J.; Saeed, S.; Qyyum, M.A.; Kazmi, B.; Ahmad, R.; Muhammad, A.; Lee, M. Simultaneous capture of acid gases from natural gas adopting ionic liquids: Challenges, recent developments, and prospects. Renew. Sustain. Energy Rev. 2020, 123, 109771. [Google Scholar] [CrossRef]
- Lepaumier, H.; Picq, D.; Carrette, P.-L. New Amines for CO2Capture. I. Mechanisms of Amine Degradation in the Presence of CO2. Ind. Eng. Chem. Res. 2009, 48, 9061–9067. [Google Scholar] [CrossRef]
- Mirzaei, S.; Shamiri, A.; Aroua, M.K. A review of different solvents, mass transfer, and hydrodynamics for postcombustion CO2 capture. Rev. Chem. Eng. 2015, 31, 521–561. [Google Scholar] [CrossRef]
- Morgan, J.C.; Chinen, A.S.; Omell, B.; Bhattacharyya, D.; Tong, C.; Miller, D.C. Thermodynamic modeling and uncertainty quantification of CO2-loaded aqueous MEA solutions. Chem. Eng. Sci. 2017, 168, 309–324. [Google Scholar] [CrossRef]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3, 22739–22773. [Google Scholar] [CrossRef]
- Cents, A.; Brilman, D.W.F.; Versteeg, G. CO2 absorption in carbonate/bicarbonate solutions: The Danckwerts-criterion revisited. Chem. Eng. Sci. 2005, 60, 5830–5835. [Google Scholar] [CrossRef]
- Song, H.-J.; Park, S.; Kim, H.; Gaur, A.; Park, J.-W.; Lee, S.-J. Carbon dioxide absorption characteristics of aqueous amino acid salt solutions. Int. J. Greenh. Gas Control. 2012, 11, 64–72. [Google Scholar] [CrossRef]
- Bernardo, P.; Clarizia, G. 30 years of membrane technology for gas separation. Chem. Eng. Trans. 2013, 32, 1999–2004. [Google Scholar]
- Wazeer, I.; Hayyan, M.; Hadj-Kali, M.K. Deep eutectic solvents: Designer fluids for chemical processes. J. Chem. Technol. Biotechnol. 2018, 93, 945–958. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Li, C.-J.; Chan, T.-H. Comprehensive Organic Reactions in Aqueous Media; Wiley: Hoboken, NJ, USA, 2007; p. 191. [Google Scholar]
- Hyde, J.R.; Licence, P.; Carter, D.; Poliakoff, M. Continuous catalytic reactions in supercritical fluids. Appl. Catal. A Gen. 2001, 222, 119–131. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; Wiley: Hoboken, NJ, USA, 2010; p. 5. [Google Scholar]
- Kerton, F.M.; Marriott, R. Alternative Solvents for Green Chemistry; Royal Society of Chemistry (RSC): London, UK, 2013; pp. 11–16. [Google Scholar]
- Brennecke, J.F.; Maginn, E.J. Ionic liquids: Innovative fluids for chemical processing. AIChE J. 2001, 47, 2384–2389. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Radošević, K.; Redovniković, I.R.; Halambek, J.; Srček, V.G. A brief overview of the potential environmental hazards of ionic liquids. Ecotoxicol. Environ. Saf. 2014, 99, 1–12. [Google Scholar] [CrossRef]
- Pham, T.P.T.; Cho, C.-W.; Yun, Y.-S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef]
- Coutinho, J.A.; Pinho, S.P. Special Issue on Deep Eutectic Solvents: A foreword. Fluid Phase Equilibria 2017, 448, 1. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Šima, J. Use of Deep Eutectic Solvents in Polymer Chemistry–A Review. Molecules 2019, 24, 3978. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; Alnashef, I.M.; Mirghani, M.E.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; Al-Saadi, M.A.; Hayyan, A.; Alnashef, I.M.; Mirghani, M.E. Assessment of cytotoxicity and toxicity for phosphonium-based deep eutectic solvents. Chemosphere 2013, 93, 455–459. [Google Scholar] [CrossRef]
- Mao, S.; Li, K.; Hou, Y.; Liu, Y.; Ji, S.; Qin, H.; Lu, F. Synergistic effects of components in deep eutectic solvents relieve toxicity and improve the performance of steroid biotransformation catalyzed by Arthrobacter simplex. J. Chem. Technol. Biotechnol. 2018, 93, 2729–2736. [Google Scholar] [CrossRef]
- Wen, Q.; Chen, J.-X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef]
- Radošević, K.; Bubalo, M.C.; Srček, V.G.; Grgas, D.; Dragičević, T.L.; Redovniković, I.R. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, D.; Lu, Y.; Li, G.; Fu, L.; Mu, T. Volatility of Deep Eutectic Solvent Choline Chloride:N-Methylacetamide at Ambient Temperature and Pressure. Ind. Eng. Chem. Res. 2019, 58, 7308–7317. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q.; Liu, Z.; Li, Z.; Chen, W.; Zhou, L.; Qin, J.; Meng, Y.; Mu, T. Vaporization enthalpy, long-term evaporation and evaporation mechanism of polyethylene glycol-based deep eutectic solvents. New J. Chem. 2020, 44, 9493–9501. [Google Scholar] [CrossRef]
- Ali, E.; Hadj-Kali, M.K.; Mulyono, S.; Alnashef, I.M.; Fakeeha, A.; Mjalli, F.S.; Hayyan, A. Solubility of CO2 in deep eutectic solvents: Experiments and modelling using the Peng–Robinson equation of state. Chem. Eng. Res. Des. 2014, 92, 1898–1906. [Google Scholar] [CrossRef]
- Mulyono, S.; Hizaddin, H.F.; Alnashef, I.M.; Hashim, M.A.; Fakeeha, A.H.; Hadj-Kali, M.K. Separation of BTEX aromatics from n-octane using a (tetrabutylammonium bromide + sulfolane) deep eutectic solvent—Experiments and COSMO-RS prediction. RSC Adv. 2014, 4, 17597–17606. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Sarmad, S.; Mikkola, J.-P.; Ji, X. Carbon Dioxide Capture with Ionic Liquids and Deep Eutectic Solvents: A New Generation of Sorbents. ChemSusChem 2017, 10, 324–352. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, T.; Alnashef, I.M.; Qureshi, U.A.; Benguerba, Y. Potential applications of deep eutectic solvents in natural gas sweetening for CO2 capture. Rev. Chem. Eng. 2017, 33, 523–550. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Chen, Y.; Han, X.; Liu, Z.; Yu, D.; Guo, W.; Mu, T. Capture of Toxic Gases by Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2020, 8, 5410–5430. [Google Scholar] [CrossRef]
- Emami, S.; Shayanfar, A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm. Dev. Technol. 2020, 1–18. [Google Scholar] [CrossRef]
- Devries, N. CO2 Absorption into Concentrated Carbonate Solutions with Promoters at Elevated Temperatures; University of Illinois at Urbana-Champaign: Urbana, IL, USA, 2014. [Google Scholar]
- Wu, G.; Liu, Y.; Liu, G.; Pang, X. The CO2 Absorption in Flue Gas Using Mixed Ionic Liquids. Molecules 2020, 25, 1034. [Google Scholar] [CrossRef]
- Xie, Y. CO2 Separation with Ionic Liquids-From Properties to Process Simulation. Ph.D. Thesis, Luleå University of Technology, Luleå, Sweden, 2016. [Google Scholar]
- Li, X.; Hou, M.; Han, B.; Wang, X.; Zou, L. Solubility of CO2in a Choline Chloride + Urea Eutectic Mixture. J. Chem. Eng. Data 2008, 53, 548–550. [Google Scholar] [CrossRef]
- Akhmetshina, A.I.; Petukhov, A.N.; Mechergui, A.; Vorotyntsev, A.V.; Nyuchev, A.V.; Moskvichev, A.A.; Vorotyntsev, I. Evaluation of Methanesulfonate-Based Deep Eutectic Solvent for Ammonia Sorption. J. Chem. Eng. Data 2018, 63, 1896–1904. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Deng, D. Solubilities and Thermodynamic Properties of CO2 in Four Azole-Based Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63, 2091–2096. [Google Scholar] [CrossRef]
- Liu, X.; Gao, B.; Jiang, Y.; Ai, N.; Deng, D. Solubilities and Thermodynamic Properties of Carbon Dioxide in Guaiacol-Based Deep Eutectic Solvents. J. Chem. Eng. Data 2017, 62, 1448–1455. [Google Scholar] [CrossRef]
- Deng, D.; Jiang, Y.; Liu, X.; Zhang, Z.; Ai, N. Investigation of solubilities of carbon dioxide in five levulinic acid-based deep eutectic solvents and their thermodynamic properties. J. Chem. Thermodyn. 2016, 103, 212–217. [Google Scholar] [CrossRef]
- Altamash, T.; Nasser, M.S.; Elhamarnah, Y.; Magzoub, M.; Ullah, R.; Qiblawey, H.; Aparicio, S.; Atilhan, M. Gas solubility and rheological behavior study of betaine and alanine based natural deep eutectic solvents (NADES). J. Mol. Liq. 2018, 256, 286–295. [Google Scholar] [CrossRef]
- Ghaedi, H.; Ayoub, M.; Sufian, S.; Shariff, A.M.; Hailegiorgis, S.M.; Khan, S.N. CO2 capture with the help of Phosphonium-based deep eutectic solvents. J. Mol. Liq. 2017, 243, 564–571. [Google Scholar] [CrossRef]
- Sarmad, S.; Xie, Y.; Mikkola, J.-P.; Ji, X. Screening of deep eutectic solvents (DESs) as green CO2 sorbents: From solubility to viscosity. New J. Chem. 2017, 41, 290–301. [Google Scholar] [CrossRef]
- Zubeir, L.F.; Van Osch, D.J.G.P.; Rocha, M.A.A.; Banat, F.; Kroon, M.C. Carbon Dioxide Solubilities in Decanoic Acid-Based Hydrophobic Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63, 913–919. [Google Scholar] [CrossRef]
- Aldawsari, J.N.; Adeyemi, I.A.; Bessadok-Jemai, A.; Ali, E.; Alnashef, I.M.; Hadj-Kali, M.K. Polyethylene glycol-based deep eutectic solvents as a novel agent for natural gas sweetening. PLoS ONE 2020, 15, e0239493. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Gu, A.Z.; Brennecke, J.F. High-Pressure Phase Behavior of Ionic Liquid/CO2Systems. J. Phys. Chem. B 2001, 105, 2437–2444. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Li, C.; Cui, Y.; Li, S.; Yang, G.; Shen, Y. Absorption of Carbon Dioxide Using Ethanolamine-Based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 10403–10414. [Google Scholar] [CrossRef]
- Adeyemi, I.; Abu-Zahra, M.R.; Alnashef, I. Experimental Study of the Solubility of CO 2 in Novel Amine Based Deep Eutectic Solvents. Energy Procedia 2017, 105, 1394–1400. [Google Scholar] [CrossRef]
- Anthony, J.L.; Maginn, E.J.; Brennecke, J.F. Solubilities and Thermodynamic Properties of Gases in the Ionic Liquid 1-n-Butyl-3-methylimidazolium Hexafluorophosphate. J. Phys. Chem. B 2002, 106, 7315–7320. [Google Scholar] [CrossRef]
- Ren, H.; Lian, S.; Wang, X.; Zhang, Y.; Duan, E. Exploiting the hydrophilic role of natural deep eutectic solvents for greening CO2 capture. J. Clean. Prod. 2018, 193, 802–810. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, D.; Chen, W.; Fu, L.; Mu, T. Water absorption by deep eutectic solvents. Phys. Chem. Chem. Phys. 2019, 21, 2601–2610. [Google Scholar] [CrossRef]
- Ma, C.; Sarmad, S.; Mikkola, J.-P.; Ji, X. Development of Low-Cost Deep Eutectic Solvents for CO 2 Capture. Energy Procedia 2017, 142, 3320–3325. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, X.; Liu, W. Application of Deep Eutectic Solvents in Food Analysis: A Review. Molecules 2019, 24, 4594. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Lee, J.H.; Lee, H.J.; Jeong, Y.K.; Choi, J.W. Deep eutectic solvents as attractive media for CO2 capture. Green Chem. 2016, 18, 2834–2842. [Google Scholar] [CrossRef]
- Akopyan, A.V.; Eseva, E.; Polikarpova, P.; Kedalo, A.; Vutolkina, A.; Glotov, A.P. Deep Oxidative Desulfurization of Fuels in the Presence of Brönsted Acidic Polyoxometalate-Based Ionic Liquids. Molecules 2020, 25, 536. [Google Scholar] [CrossRef]
- Deng, D.; Liu, X.; Gao, B. Physicochemical Properties and Investigation of Azole-Based Deep Eutectic Solvents as Efficient and Reversible SO2 Absorbents. Ind. Eng. Chem. Res. 2017, 56, 13850–13856. [Google Scholar] [CrossRef]
- Deng, D.; Han, G.; Jiang, Y. Investigation of a deep eutectic solvent formed by levulinic acid with quaternary ammonium salt as an efficient SO2 absorbent. New J. Chem. 2015, 39, 8158–8164. [Google Scholar] [CrossRef]
- Zhang, K.; Ren, S.; Hou, Y.; Wu, W. Efficient absorption of SO2 with low-partial pressures by environmentally benign functional deep eutectic solvents. J. Hazard. Mater. 2017, 324, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liang, J.; Zhang, Y.; Wu, Y.; Hu, X. Unexpectedly efficient SO2 capture and conversion to sulfur in novel imidazole-based deep eutectic solvents. Chem. Commun. 2018, 54, 8964–8967. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, H.; Zhang, L.; Zhang, N.; Huang, Z.; Chen, Y.; Sun, Y.; Tantai, X. Novel Deep Eutectic Solvents for Highly Efficient and Reversible Absorption of SO2 by Preorganization Strategy. ACS Sustain. Chem. Eng. 2019, 7, 8347–8357. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, B.; Dou, H.; Zhang, L.; Tantai, X.; Sun, Y.; Zhang, H. Highly Efficient and Reversible Capture of Low Partial Pressure SO2 by Functional Deep Eutectic Solvents. Energy Fuels 2018, 32, 10737–10744. [Google Scholar] [CrossRef]
- Yang, D.; Hou, M.; Ning, H.; Zhang, J.; Ma, J.; Yang, G.; Han, B. Efficient SO2 absorption by renewable choline chloride–glycerol deep eutectic solvents. Green Chem. 2013, 15, 2261–2265. [Google Scholar] [CrossRef]
- Chen, C.-C.; Wang, C.-Y.; Huang, Y.-H. Reversible absorption of nitrogen dioxide by choline chloride-based deep eutectic solvents and their aqueous mixtures. Chem. Eng. J. 2021, 405, 126760. [Google Scholar] [CrossRef]
- Liu, X.; Gao, B.; Deng, D. SO2 absorption/desorption performance of renewable phenol-based deep eutectic solvents. Sep. Sci. Technol. 2018, 53, 2150–2158. [Google Scholar] [CrossRef]
- Sun, S.; Niu, Y.; Xu, Q.; Sun, Z.; Wei, X.-H. Efficient SO2 Absorptions by Four Kinds of Deep Eutectic Solvents Based on Choline Chloride. Ind. Eng. Chem. Res. 2015, 54, 8019–8024. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, H.; Wei, G.; Jiang, B.; Sun, Y.; Tantai, X.; Huang, Z.; Chen, Y. Efficient and Reversible Nitric Oxide Absorption by Low-Viscosity, Azole-Derived Deep Eutectic Solvents. J. Chem. Eng. Data 2019, 64, 3068–3077. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, J.; Wei, F. Characterization of caprolactam based eutectic ionic liquids and their application in SO2 absorption. J. Mol. Liq. 2013, 180, 19–25. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Conversion of CO2 to value-added products mediated by ionic liquids. Green Chem. 2019, 21, 2544–2574. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Y.; Li, Z.; Wu, Y.; Wang, J.; Liu, Z. Choline-based ionic liquids for CO2 capture and conversion. Sci. China Ser. B Chem. 2018, 62, 256–261. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Y.; Sun, X.; Zhang, Z.; Mu, T. Water sorption in ionic liquids: Kinetics, mechanisms and hydrophilicity. Phys. Chem. Chem. Phys. 2012, 14, 12252–12262. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Zhang, C.; Deng, X.; Gong, L. Efficient Absorption of Low Partial Pressure SO2 by 1-Ethyl-3-methylimidazolium Chloride Plus N-Formylmorpholine Deep Eutectic Solvents. Energy Fuels 2020, 34, 665–671. [Google Scholar] [CrossRef]
- Long, G.; Yang, C.; Yang, X.; Zhao, T.; Liu, F.; Cao, J. Bisazole-Based Deep Eutectic Solvents for Efficient SO2 Absorption and Conversion without Any Additives. ACS Sustain. Chem. Eng. 2020, 8, 2608–2613. [Google Scholar] [CrossRef]
- Sheng, K.; Kang, Y.; Li, J.; Xu, H.; Li, D. High-Efficiency Absorption of SO2 by a New Type of Deep Eutectic Solvents. Energy Fuels 2020, 34, 3440–3448. [Google Scholar] [CrossRef]
- Zhang, K.; Ren, S.; Yang, X.; Hou, Y.; Wu, W.; Bao, Y. Efficient absorption of low-concentration SO2 in simulated flue gas by functional deep eutectic solvents based on imidazole and its derivatives. Chem. Eng. J. 2017, 327, 128–134. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Wei, F.; Zhao, J. Characterization of amide–thiocyanates eutectic ionic liquids and their application in SO2 absorption. RSC Adv. 2013, 3, 2470–2476. [Google Scholar] [CrossRef]
- Cui, G.; Liu, J.; Lyu, S.; Wang, H.; Li, Z.; Wang, J. Efficient and Reversible SO2 Absorption by Environmentally Friendly Task-Specific Deep Eutectic Solvents of PPZBr + Gly. ACS Sustain. Chem. Eng. 2019, 7, 14236–14246. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, L.; Gong, R.; Li, M.; Ren, H.; Duan, E. Role of Hydrophilic Ammonium-Based Deep Eutectic Solvents in SO2 Absorption. Energy Fuels 2019, 34, 74–81. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, G.; Tantai, X.; Huang, Z.; Yang, H.; Zhang, L. Highly Efficient Nitric Oxide Absorption by Environmentally Friendly Deep Eutectic Solvents Based on 1,3-Dimethylthiourea. Energy Fuels 2017, 31, 12439–12445. [Google Scholar] [CrossRef]
- Duan, E.; Guo, B.; Zhang, D.; Shi, L.; Sun, H.; Wang, Y. Absorption of NO and NO2 in Caprolactam Tetrabutyl Ammonium Halide Ionic Liquids. J. Air Waste Manag. Assoc. 2011, 61, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, J.; Cui, G.; Luo, X.; Guo, Y.; Li, H. Highly efficient SO2capture through tuning the interaction between anion-functionalized ionic liquids and SO2. Chem. Commun. 2013, 49, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; He, H.; Gao, H.; Zhang, S.; Wang, J.; Huang, Y.; Zhang, S. Improving SO2 capture by tuning functional groups on the cation of pyridinium-based ionic liquids. RSC Adv. 2014, 5, 2470–2478. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, S.; Bai, L.; Gao, H.; Zhang, S.; Zhang, S. Novel Ether-Functionalized Pyridinium Chloride Ionic Liquids for Efficient SO2 Capture. Ind. Eng. Chem. Res. 2014, 53, 16832–16839. [Google Scholar] [CrossRef]
- Li, J.; Kang, Y.; Li, B.; Wang, X.; Li, D. PEG-Linked Functionalized Dicationic Ionic Liquids for Highly Efficient SO2 Capture through Physical Absorption. Energy Fuels 2018, 32, 12703–12710. [Google Scholar] [CrossRef]
- Yang, D.; Hou, M.; Ning, H.; Ma, J.; Kang, X.; Zhang, J.; Han, B. Reversible Capture of SO2through Functionalized Ionic Liquids. ChemSusChem 2013, 6, 1191–1195. [Google Scholar] [CrossRef]
- Zeng, S.; Gao, H.; Zhang, X.; Dong, H.; Zhang, S.; Zhang, S. Efficient and reversible capture of SO2 by pyridinium-based ionic liquids. Chem. Eng. J. 2014, 251, 248–256. [Google Scholar] [CrossRef]
- Yang, D.; Han, Y.; Qi, H.; Wang, Y.; Dai, S. Efficient absorption of SO2 by EmimCl-EG deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 6382–6386. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, S.; Jiang, D.-E.; Dai, S. SO2 absorption in EmimCl–TEG deep eutectic solvents. Phys. Chem. Chem. Phys. 2018, 20, 15168–15173. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, S.; Jiang, D.-E. Efficient Absorption of SO2 by Deep Eutectic Solvents Formed by Biobased Aprotic Organic Compound Succinonitrile and 1-Ethyl-3-methylimidazolium Chloride. ACS Sustain. Chem. Eng. 2019, 7, 9086–9091. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, M.; Ren, S.; Zhang, Q.; Hou, Y.; Wu, W. Highly Efficient Absorption of NO by Amine-Based Functional Deep Eutectic Solvents. Energy Fuels 2019, 34, 690–697. [Google Scholar] [CrossRef]
- Waite, S.L.; Li, H.; Page, A.J. NO2 Solvation Structure in Choline Chloride Deep Eutectic Solvents—The Role of the Hydrogen Bond Donor. J. Phys. Chem. B 2018, 122, 4336–4344. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Duan, X.; Gao, B.; Zhang, C.; Deng, X.; Gong, L. Efficient and reversible absorption of NH3 by functional azole–glycerol deep eutectic solvents. New J. Chem. 2019, 43, 11636–11642. [Google Scholar] [CrossRef]
- Deng, X.; Duan, X.; Gong, L.; Deng, D. Ammonia Solubility, Density, and Viscosity of Choline Chloride–Dihydric Alcohol Deep Eutectic Solvents. J. Chem. Eng. Data 2020, 65, 4845–4854. [Google Scholar] [CrossRef]
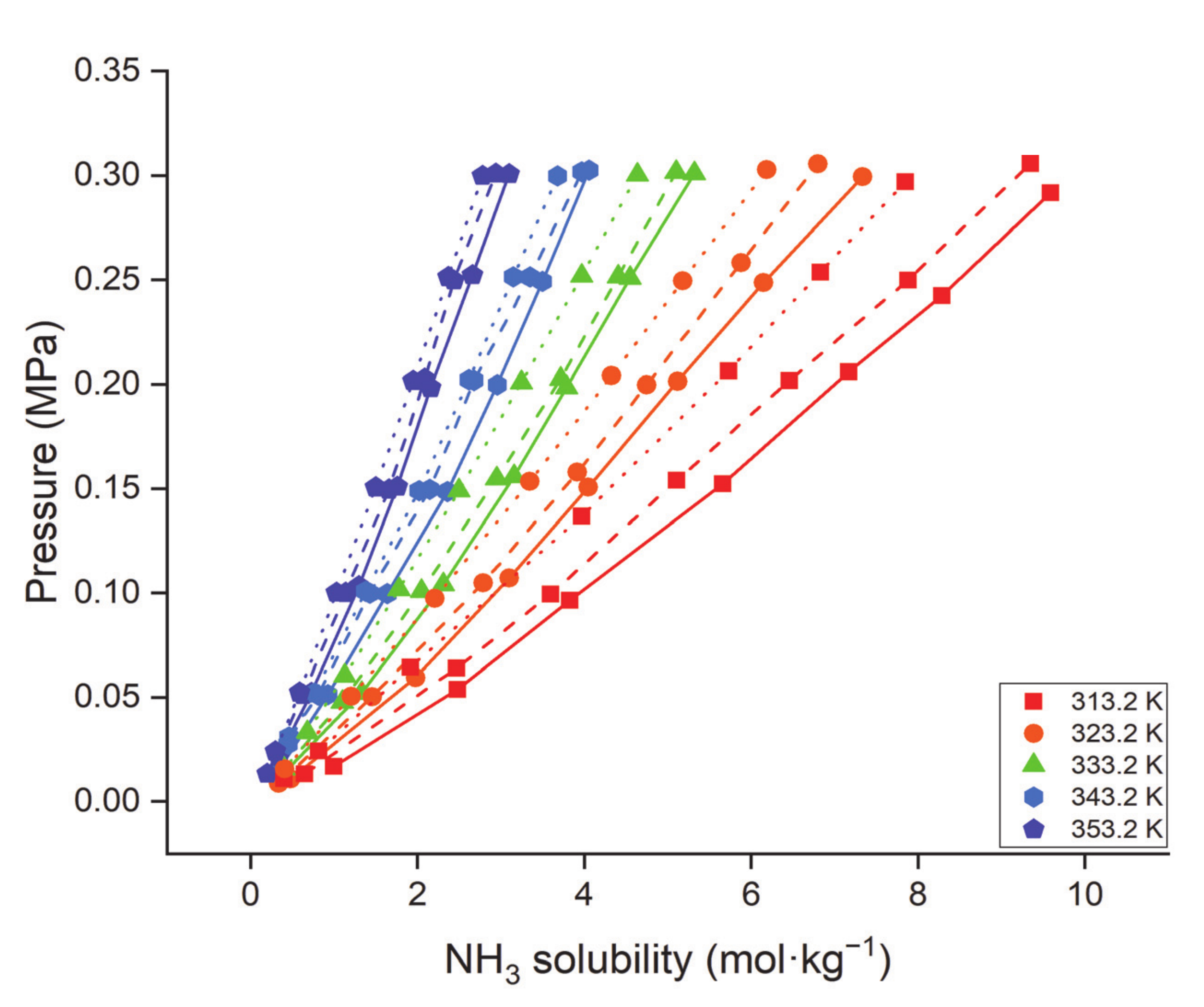
- Duan, X.; Gao, B.; Zhang, C.; Deng, D. Solubility and thermodynamic properties of NH3 in choline chloride-based deep eutectic solvents. J. Chem. Thermodyn. 2019, 133, 79–84. [Google Scholar] [CrossRef]
- Li, Z.-L.; Zhong, F.-Y.; Huang, J.-Y.; Peng, H.-L.; Huang, K. Sugar-based natural deep eutectic solvents as potential absorbents for NH3 capture at elevated temperatures and reduced pressures. J. Mol. Liq. 2020, 317, 113992. [Google Scholar] [CrossRef]
- Zhong, F.-Y.; Peng, H.-L.; Tao, D.-J.; Wu, P.-K.; Fan, J.-P.; Huang, K. Phenol-Based Ternary Deep Eutectic Solvents for Highly Efficient and Reversible Absorption of NH3. ACS Sustain. Chem. Eng. 2019, 7, 3258–3266. [Google Scholar] [CrossRef]
- Zhong, F.-Y.; Huang, K.; Peng, H.-L. Solubilities of ammonia in choline chloride plus urea at (298.2–353.2) K and (0–300) kPa. J. Chem. Thermodyn. 2019, 129, 5–11. [Google Scholar] [CrossRef]
- Li, Y.; Ali, M.C.; Yang, Q.; Zhang, Z.; Bao, Z.; Su, B.; Xing, H.; Ren, Q. Hybrid Deep Eutectic Solvents with Flexible Hydrogen-Bonded Supramolecular Networks for Highly Efficient Uptake of NH3. ChemSusChem 2017, 10, 3368–3377. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Zhong, F.-Y.; Liu, Y.; Huang, K. Effective and Reversible Capture of NH3 by Ethylamine Hydrochloride Plus Glycerol Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 10552–10560. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Huang, K. Densities and viscosities of, and NH3 solubilities in deep eutectic solvents composed of ethylamine hydrochloride and acetamide. J. Chem. Thermodyn. 2019, 139, 105883. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Zhong, F.-Y.; Zhou, L.-S.; Peng, H.-L.; Fan, J.-P.; Huang, K. Chemical dual-site capture of NH3 by unprecedentedly low-viscosity deep eutectic solvents. Chem. Commun. 2020, 56, 2399–2402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Kong, L.-Y.; Huang, K. NH3 Solubilities and Physical Properties of Ethylamine Hydrochloride Plus Urea Deep Eutectic Solvents. J. Chem. Eng. Data 2019, 64, 3821–3830. [Google Scholar] [CrossRef]
- Deng, D.; Deng, X.; Duan, X.; Gong, L. Protic guanidine isothiocyanate plus acetamide deep eutectic solvents with low viscosity for efficient NH3 capture and NH3/CO2 separation. J. Mol. Liq. 2020, 114719. [Google Scholar] [CrossRef]
- Deng, D.; Gao, B.; Zhang, C.; Duan, X.; Cui, Y.; Ning, J. Investigation of protic NH4SCN-based deep eutectic solvents as highly efficient and reversible NH3 absorbents. Chem. Eng. J. 2019, 358, 936–943. [Google Scholar] [CrossRef]
- Li, Z.-L.; Zhong, F.-Y.; Zhou, L.-S.; Tian, Z.; Huang, K. Deep Eutectic Solvents Formed by N-Methylacetamide and Heterocyclic Weak Acids for Highly Efficient and Reversible Chemical Absorption of Ammonia. Ind. Eng. Chem. Res. 2020, 59, 2060–2067. [Google Scholar] [CrossRef]
- Zhong, F.-Y.; Zhou, L.; Shen, J.; Liu, Y.; Fan, J.-P.; Huang, K. Rational Design of Azole-Based Deep Eutectic Solvents for Highly Efficient and Reversible Capture of Ammonia. ACS Sustain. Chem. Eng. 2019, 7, 14170–14179. [Google Scholar] [CrossRef]
- Liu, F.; Chen, W.; Mi, J.; Zhang, J.-Y.; Kan, X.; Zhong, F.-Y.; Huang, K.; Zheng, A.-M.; Jiang, L. Thermodynamic and molecular insights into the absorption of H 2 S, CO 2, and CH 4 in choline chloride plus urea mixtures. AIChE J. 2019, 65, e16574. [Google Scholar] [CrossRef]
- Wu, H.; Shen, M.; Chen, X.; Yu, G.; Abdeltawab, A.A.; Yakout, S.M. New absorbents for hydrogen sulfide: Deep eutectic solvents of tetrabutylammonium bromide/carboxylic acids and choline chloride/carboxylic acids. Sep. Purif. Technol. 2019, 224, 281–289. [Google Scholar] [CrossRef]
- Mao, J.; Ma, Y.; Zang, L.; Xue, R.; Xiao, C.; Ji, D. Efficient Adsorption of Hydrogen Sulfide at Room Temperature Using Fumed Silica-supported Deep Eutectic Solvents. Aerosol Air Qual. Res. 2020, 20, 203–2015. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Zhang, J.-B.; Zou, Y.-T.; Peng, H.-L.; Huang, K. Manufacturing Acidities of Hydrogen-Bond Donors in Deep Eutectic Solvents for Effective and Reversible NH3 Capture. ACS Sustain. Chem. Eng. 2020, 8, 13408–13417. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, X.; Zeng, S.; Liu, Y.; Dong, H.; Deng, C. Protic ionic liquid-based deep eutectic solvents with multiple hydrogen bonding sites for efficient absorption of NH3. AIChE J. 2020, 66, 16253. [Google Scholar] [CrossRef]
- Rogošić, M.; Kučan, K.Z. Deep eutectic solvents based on choline chloride and ethylene glycol as media for extractive denitrification/desulfurization/dearomatization of motor fuels. J. Ind. Eng. Chem. 2019, 72, 87–99. [Google Scholar] [CrossRef]
- Almashjary, K.H.; Khalid, M.; Dharaskar, S.; Jagadish, P.; Walvekar, R.; Gupta, T.C.S.M. Optimisation of extractive desulfurization using Choline Chloride-based deep eutectic solvents. Fuel 2018, 234, 1388–1400. [Google Scholar] [CrossRef]
- Makoś, P.; Boczkaj, G. Deep eutectic solvents based highly efficient extractive desulfurization of fuels—Eco-friendly approach. J. Mol. Liq. 2019, 296, 111916. [Google Scholar] [CrossRef]
- Lee, H.; Kang, S.; Jin, Y.; Jung, D.; Park, K.; Li, K.; Lee, J. Systematic investigation of the extractive desulfurization of fuel using deep eutectic solvents from multifarious aspects. Fuel 2020, 264, 116848. [Google Scholar] [CrossRef]
- Sudhir, N.; Yadav, P.; Nautiyal, B.; Singh, R.; Rastogi, H.; Chauhan, H. Extractive desulfurization of fuel with methyltriphenyl phosphonium bromide- tetraethylene glycol-based eutectic solvents. Sep. Sci. Technol. 2020, 55, 554–563. [Google Scholar] [CrossRef]
- Yin, J.; Wang, J.; Li, Z.; Li, D.; Yang, G.; Cui, Y.; Wang, A.; Li, C. Deep desulfurization of fuels based on an oxidation/extraction process with acidic deep eutectic solvents. Green Chem. 2015, 17, 4552–4559. [Google Scholar] [CrossRef]
- Li, J.-J.; Xiao, H.; Tang, X.-D.; Zhou, M. Green Carboxylic Acid-Based Deep Eutectic Solvents as Solvents for Extractive Desulfurization. Energy Fuels 2016, 30, 5411–5418. [Google Scholar] [CrossRef]
- Jiang, W.; Li, H.; Wang, C.; Liu, W.; Guo, T.; Liu, H.; Zhu, W.; Li, H. Synthesis of Ionic-Liquid-Based Deep Eutectic Solvents for Extractive Desulfurization of Fuel. Energy Fuels 2016, 30, 8164–8170. [Google Scholar] [CrossRef]
- Tang, X.-D.; Zhang, Y.-F.; Li, J.-J.; Zhu, Y.-Q.; Qing, D.-Y.; Deng, Y.-X. Deep Extractive Desulfurization with Arenium Ion Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2015, 54, 4625–4632. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, W.; Zhu, W.; Li, H.; Yin, S.; Chang, Y.; Li, H. A simple and cost-effective extractive desulfurization process with novel deep eutectic solvents. RSC Adv. 2016, 6, 30345–30352. [Google Scholar] [CrossRef]
- Warrag, S.E.E.; Darwish, A.S.; AbuHatab, F.O.S.; Adeyemi, I.A.; Kroon, M.C.; Alnashef, I.M. Combined Extractive Dearomatization, Desulfurization, and Denitrogenation of Oil Fuels Using Deep Eutectic Solvents: A Parametric Study. Ind. Eng. Chem. Res. 2020, 59, 11723–11733. [Google Scholar] [CrossRef]
- Jha, D.; Haider, M.B.; Kumar, R.; Sivagnanam, B.M. Extractive desulfurization of fuels using diglycol based deep eutectic solvents. J. Environ. Chem. Eng. 2020, 8, 104182. [Google Scholar] [CrossRef]
- Hizaddin, H.F.; Ramalingam, A.; Hashim, M.A.; Hadj-Kali, M.K. Evaluating the Performance of Deep Eutectic Solvents for Use in Extractive Denitrification of Liquid Fuels by the Conductor-like Screening Model for Real Solvents. J. Chem. Eng. Data 2014, 59, 3470–3487. [Google Scholar] [CrossRef]
- Hizaddin, H.F.; Hadj-Kali, M.K.; Ramalingam, A.; Hashim, M.A. Extractive denitrogenation of diesel fuel using ammonium- and phosphonium-based deep eutectic solvents. J. Chem. Thermodyn. 2016, 95, 164–173. [Google Scholar] [CrossRef]
- Hadj-Kali, M.K.; Mulyono, S.; Hizaddin, H.F.; Wazeer, I.; El-Blidi, L.; Ali, E.; Hashim, M.A.; Alnashef, I.M. Removal of Thiophene from Mixtures with n-Heptane by Selective Extraction Using Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2016, 55, 8415–8423. [Google Scholar] [CrossRef]
- Warrag, S.E.E.; Alli, R.D.; Kroon, M.C. Liquid–Liquid Equilibrium Measurements for the Extraction of Pyridine and Benzothiazole from n-Alkanes Using Deep Eutectic Solvents. J. Chem. Eng. Data 2019, 64, 4882–4890. [Google Scholar] [CrossRef]
- Warrag, S.E.; Darwish, A.S.; Adeyemi, I.A.; Hadj-Kali, M.K.; Kroon, M.C.; Alnashef, I. Extraction of pyridine from n-alkane mixtures using methyltriphenylphosphonium bromide-based deep eutectic solvents as extractive denitrogenation agents. Fluid Phase Equilibria 2020, 517, 112622. [Google Scholar] [CrossRef]
- Abu Hatab, F.; Darwish, A.S.; Lemaoui, T.; Warrag, S.E.E.; Benguerba, Y.; Kroon, M.C.; Alnashef, I.M. Extraction of Thiophene, Pyridine, and Toluene from n-Decane as a Diesel Model Using Betaine-Based Natural Deep Eutectic Solvents. J. Chem. Eng. Data 2020, 65, 5443–5457. [Google Scholar] [CrossRef]
- Kamarudin, A.F.; Hizaddin, H.F.; El-Blidi, L.; Ali, E.; Hashim, M.A.; Hadj-Kali, M.K. Performance of p-Toluenesulfonic Acid–Based Deep Eutectic Solvent in Denitrogenation: Computational Screening and Experimental Validation. Molecules 2020, 25, 5093. [Google Scholar] [CrossRef] [PubMed]
- Warrag, S.E.E.; Rodriguez, N.N.R.; Nashef, I.M.; Annaland, M.M.V.S.; Siepmann, J.I.; Kroon, M.C.; Peters, C.J. Separation of Thiophene from Aliphatic Hydrocarbons Using Tetrahexylammonium-Based Deep Eutectic Solvents as Extracting Agents. J. Chem. Eng. Data 2017, 62, 2911–2919. [Google Scholar] [CrossRef]
- Warrag, S.E.E.; Adeyemi, I.; Rodriguez, N.R.; Nashef, I.M.; Annaland, M.V.S.; Kroon, M.C.; Peters, C.C. Effect of the Type of Ammonium Salt on the Extractive Desulfurization of Fuels Using Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63, 1088–1095. [Google Scholar] [CrossRef]
- Warrag, S.E.E.; Pototzki, C.; Rodriguez, N.R.; Annaland, M.V.S.; Kroon, M.C.; Held, C.; Sadowski, G.; Peters, C.C. Oil desulfurization using deep eutectic solvents as sustainable and economical extractants via liquid-liquid extraction: Experimental and PC-SAFT predictions. Fluid Phase Equilibria 2018, 467, 33–44. [Google Scholar] [CrossRef]
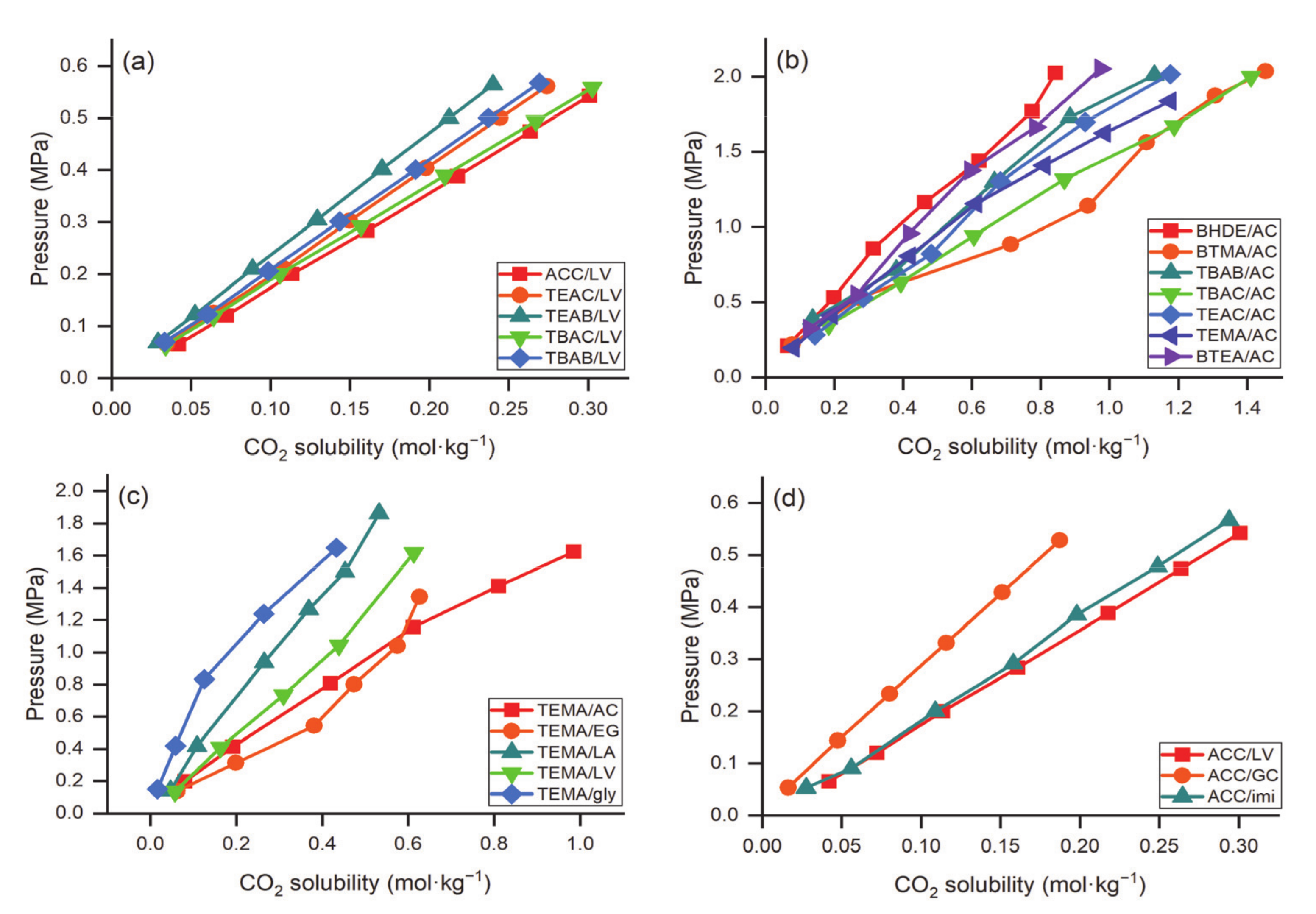
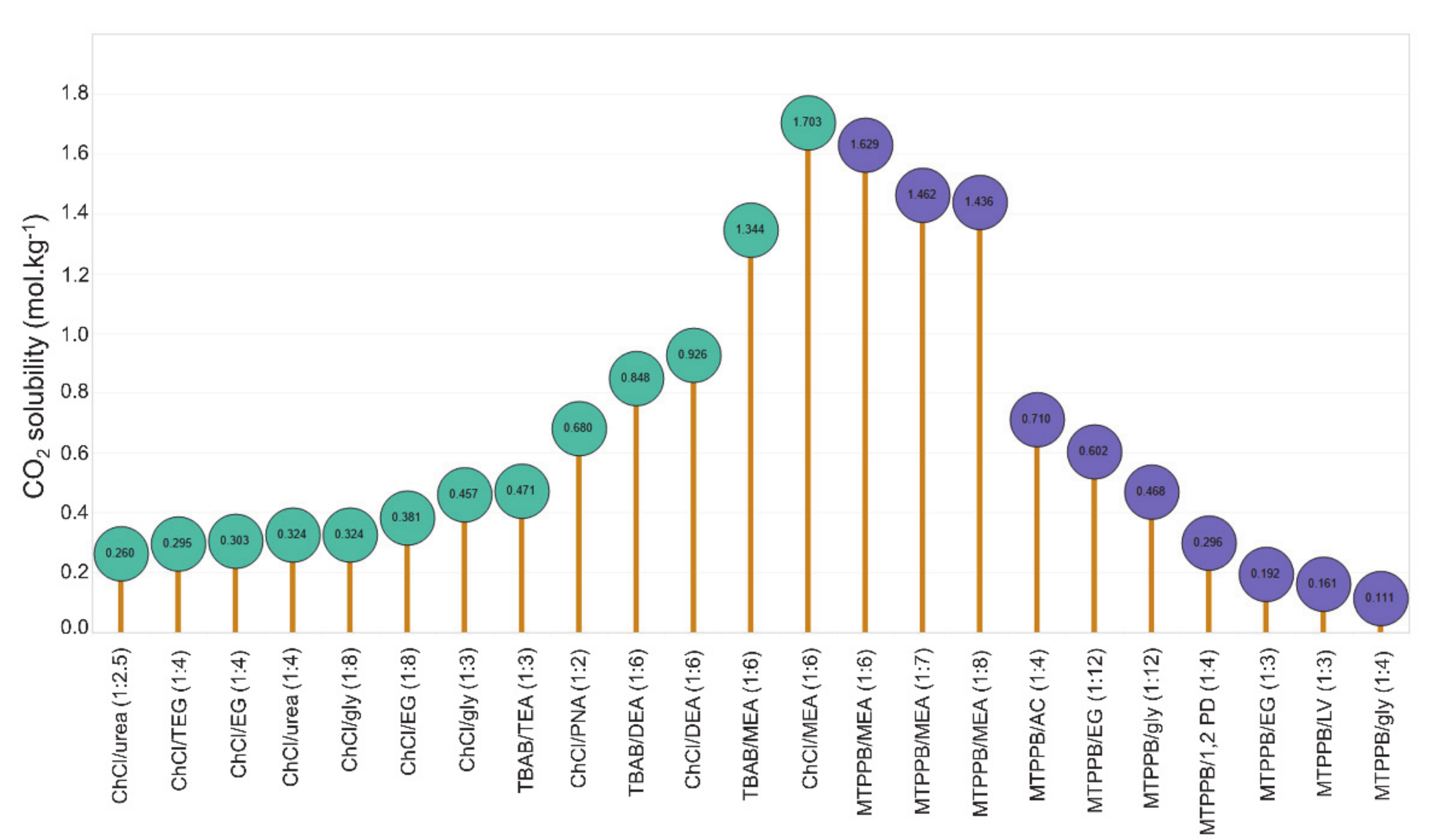
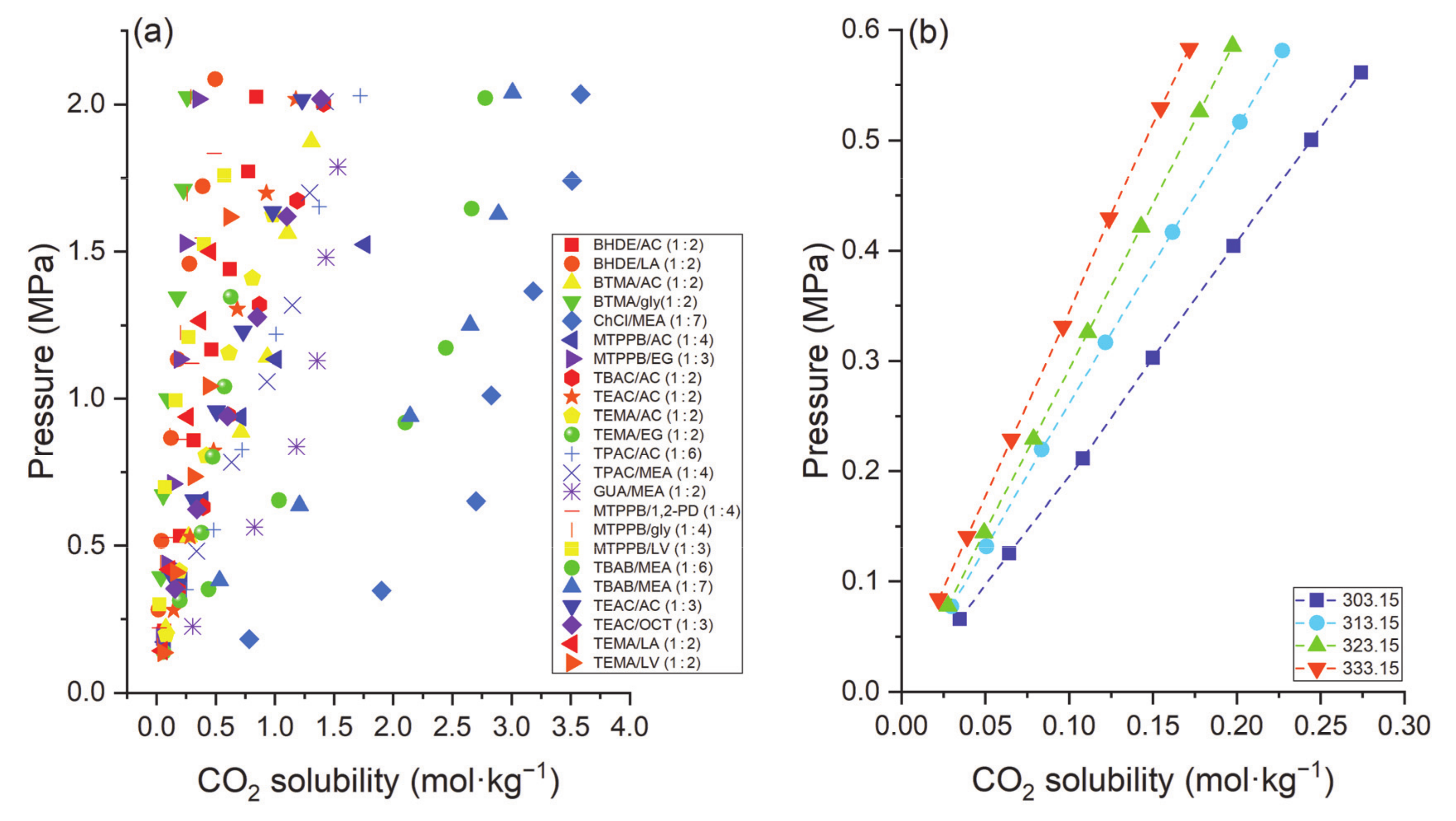


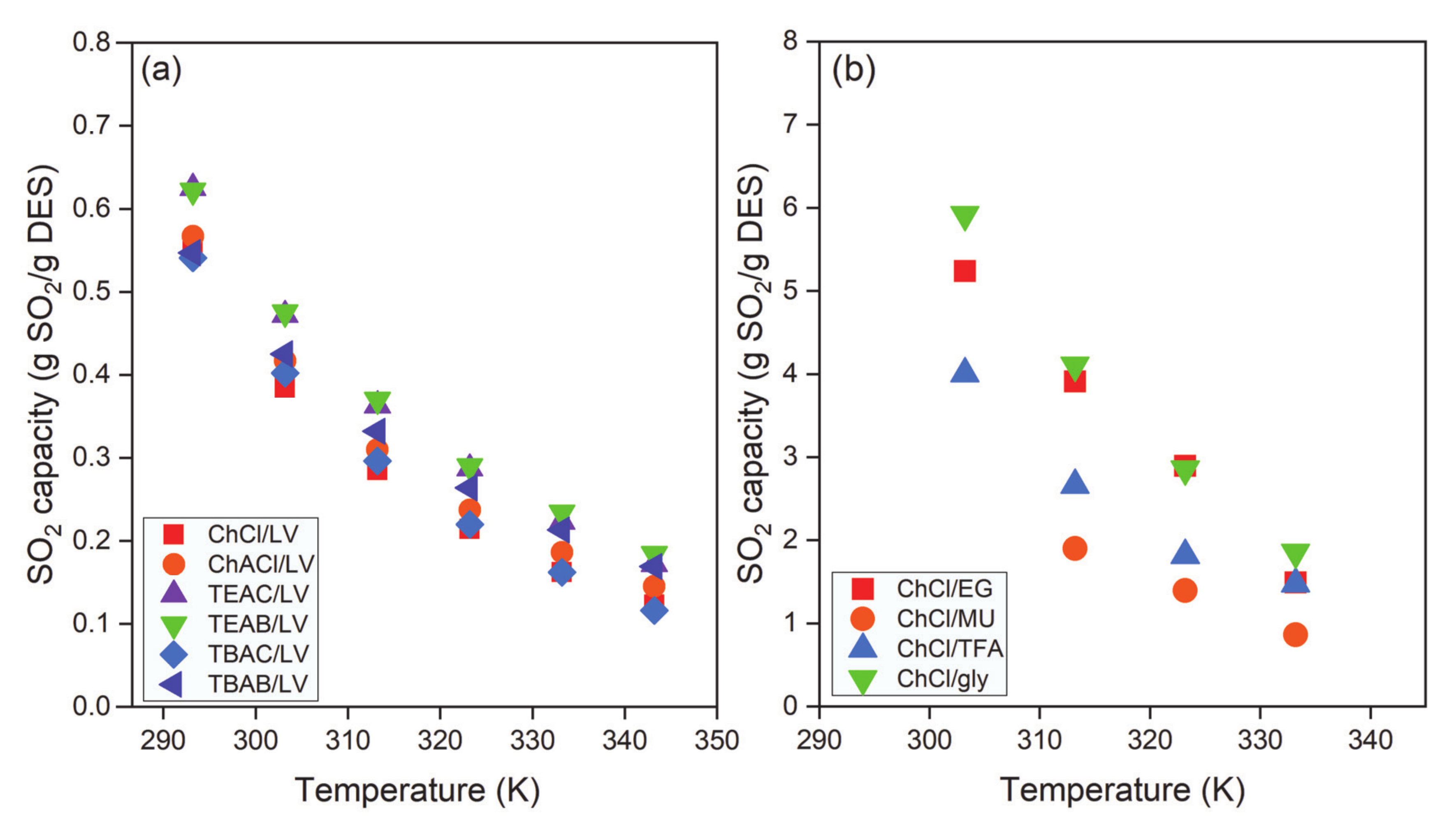
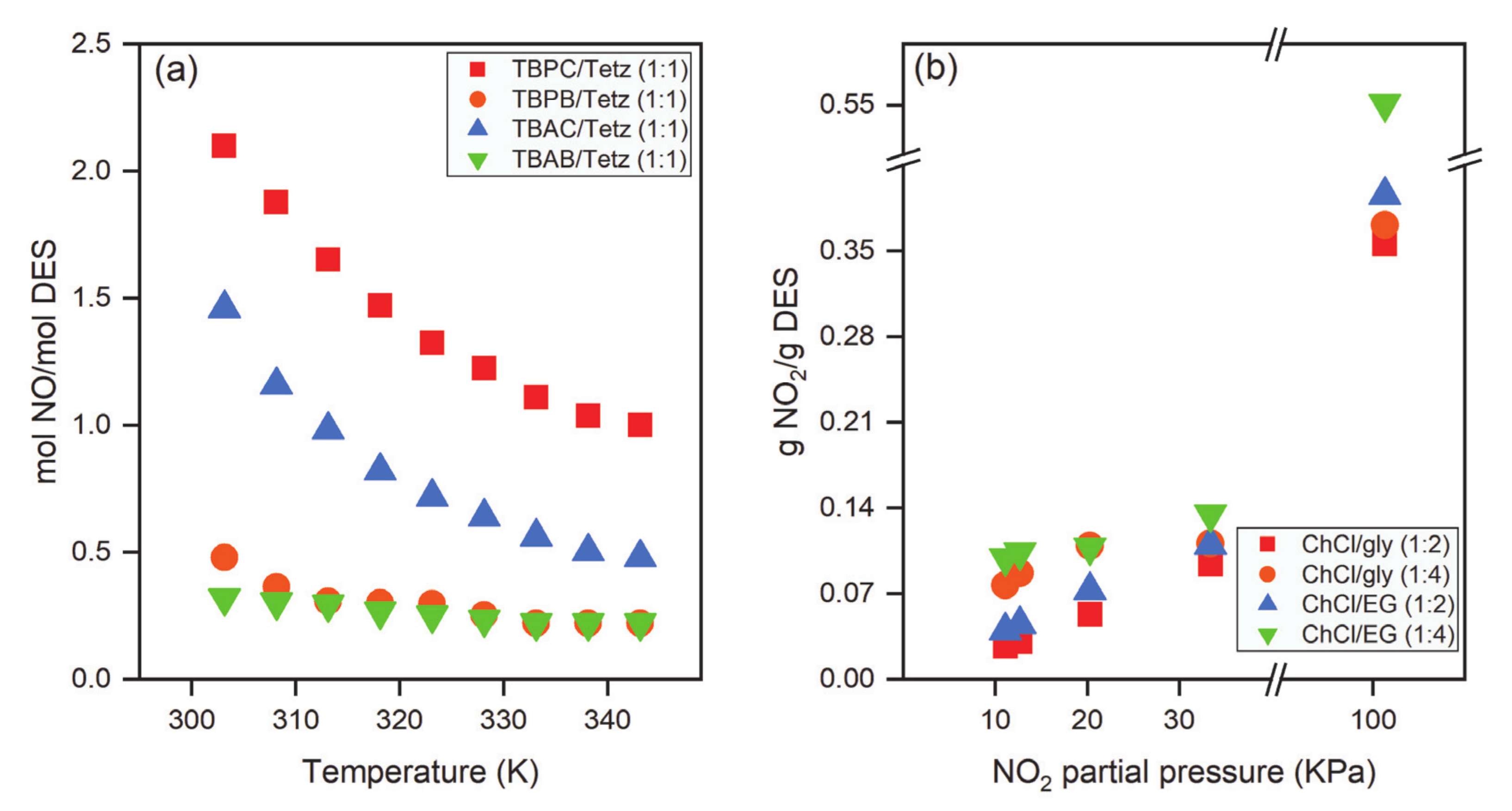
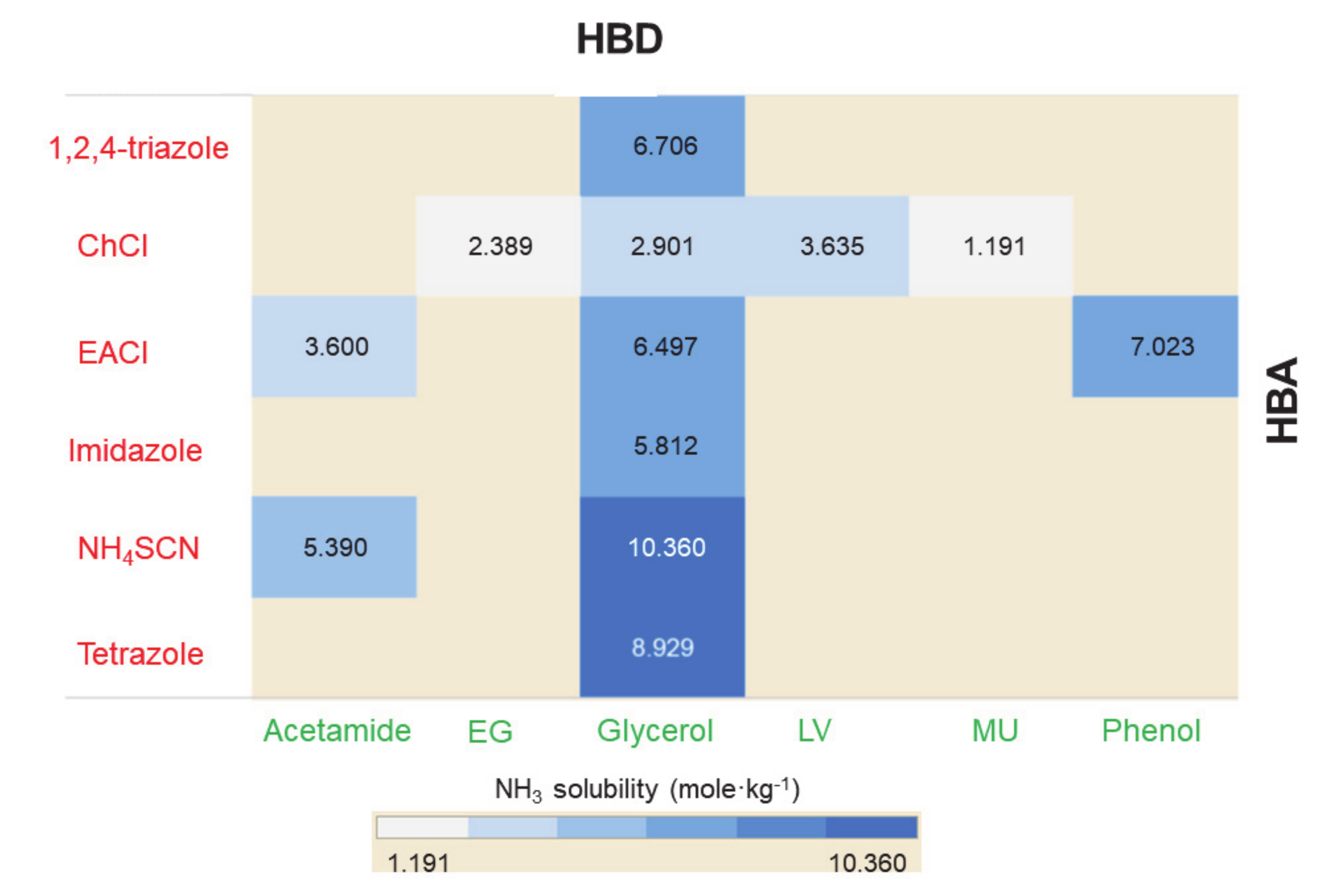
| DES | Molar Ratio | T, K | P, MPa | mCO2, mol·kg−1 | Refs. |
|---|---|---|---|---|---|
| [bmim][MeSO3] 1/urea | 1:1 | 303.15 | 0.423 | 0.245 | [55] |
| ACC 2/1,2,4-triazole | 1:1 | 303.15 | 0.497 | 0.186 | [56] |
| ACC/guaiacol | 1:3 | 303.15 | 0.432 | 0.127 | [57] |
| 1:4 | 303.15 | 0.432 | 0.133 | ||
| 1:5 | 303.15 | 0.428 | 0.140 | ||
| ACC/imidazole | 2:3 | 303.15 | 0.487 | 0.194 | [56] |
| 1:2 | 303.15 | 0.526 | 0.239 | ||
| 1:3 | 303.15 | 0.479 | 0.249 | ||
| ACC/LV 3 | 1:3 | 303.15 | 0.543 | 0.301 | [58] |
| Alanine/lactic acid | 1:1 | 308.15 | 0.494 | 0.279 | [59] |
| Alanine/ malic acid | 1:1 | 308.15 | 0.493 | 0.346 | |
| ATPPB 4/diethylene glycol | 1:4 | 303.15 | 0.739 | 0.174 | [60] |
| 1:10 | 303.15 | 0.734 | 0.145 | ||
| 1:16 | 303.15 | 0.742 | 0.122 | ||
| ATPPB/triethylene glycol | 1:4 | 303.15 | 0.718 | 0.193 | |
| 1:10 | 303.15 | 0.744 | 0.154 | ||
| 1:16 | 303.15 | 0.744 | 0.131 | ||
| Betaine/lactic acid | 1:1 | 308.15 | 0.493 | 0.623 | [59] |
| Betaine/malic acid | 1:1 | 318.15 | 0.493 | 0.287 | |
| BHDE 5/acetic acid | 1:2 | 298.15 | 0.533 | 0.199 | [61] |
| BHDE/lactic acid | 1:2 | 298.15 | 0.866 | 0.122 | |
| BTEA 6/acetic acid | 1:2 | 298.15 | 0.551 | 0.265 | |
| BTMA 7/acetic acid | 1:2 | 298.15 | 0.530 | 0.271 | |
| ChCl/MEA | 1:7 | 298.15 | 0.651 | 2.700 | |
| ChCl/guaiacol | 1:3 | 303.15 | 0.434 | 0.116 | [57] |
| 1:4 | 303.15 | 0.437 | 0.121 | ||
| 1:5 | 303.15 | 0.432 | 0.129 | ||
| ChCl/gly/acetic acid | 1:1:1 | 298.15 | 0.542 | 0.112 | [61] |
| DEH 8/guaiacol | 1:3 | 303.15 | 0.428 | 0.153 | [57] |
| 1:4 | 303.15 | 0.425 | 0.158 | ||
| 1:5 | 303.15 | 0.424 | 0.163 | ||
| GUA 9/MEA | 1:2 | 298.15 | 0.563 | 0.827 | [61] |
| MTOAB 10/decanoic acid | 1:2 | 298.15 | 0.490 | 0.285 | [62] |
| MTOAC 11/decanoic acid | 1:2 | 298.15 | 0.490 | 0.297 | |
| MTPPB 12/1,2-PD 13 | 1:4 | 298.15 | 0.861 | 0.228 | [61] |
| MTPPB/acetic acid | 1:4 | 298.15 | 0.652 | 0.390 | |
| MTPPB/ethylene glycol | 1:3 | 298.15 | 0.710 | 0.137 | |
| MTPPB/gly | 1:4 | 298.15 | 0.875 | 0.111 | |
| MTPPB/LV | 1:3 | 298.15 | 0.994 | 0.161 | |
| MTPPB/LV/acetic acid | 1:3:0.03 | 298.15 | 0.516 | 0.327 | [61] |
| TBAB 14/acetic acid | 1:2 | 298.15 | 0.715 | 0.380 | |
| TBAB/MEA | 1:6 | 298.15 | 0.654 | 1.036 | |
| 1:7 | 298.15 | 0.637 | 1.208 | ||
| TBAB/LV | 1:3 | 303.15 | 0.568 | 0.269 | [58] |
| TBAB/octanoic acid | 1:4 | 298.15 | 0.100 | 0.491 | [63] |
| TBAB/PEG-8 | 1:4 | 298.15 | 0.100 | 0.286 | |
| TBAC 15/acetic acid | 1:2 | 298.15 | 0.631 | 0.393 | [61] |
| TBAC/decanoic acid | 1:2 | 298.15 | 0.490 | 0.337 | [62] |
| TBAC/LV | 1:3 | 303.15 | 0.559 | 0.303 | [58] |
| TEAB 16/LV | 1:3 | 303.15 | 0.564 | 0.240 | |
| TEAC 17/acetic acid | 1:2 | 298.15 | 0.530 | 0.284 | [61] |
| 1:3 | 298.15 | 0.654 | 0.315 | ||
| TEAC/LV | 1:3 | 303.15 | 0.562 | 0.274 | [58] |
| TEAC/octanoic acid | 1:3 | 298.15 | 0.624 | 0.342 | [61] |
| TEMA 18/acetic acid | 1:2 | 298.15 | 0.413 | 0.192 | |
| TEMA/ethylene glycol | 1:2 | 298.15 | 0.314 | 0.199 | |
| TEMA/glycerol | 1:2 | 298.15 | 0.833 | 0.126 | |
| TEMA/lactic acid | 1:2 | 298.15 | 0.418 | 0.109 | |
| TEMA/LV | 1:2 | 298.15 | 0.409 | 0.163 | |
| TMAC 19/acetic acid | 1:4 | 298.15 | 0.519 | 0.296 | |
| TPAC 20/acetic acid | 1:6 | 298.15 | 0.554 | 0.481 | |
| TPAC/MEA | 1:4 | 298.15 | 0.481 | 0.338 | |
| 1:7 | 298.15 | 0.645 | 2.051 | ||
| TOAB 21/decanoic acid | 1:2 | 298.15 | 0.490 | 0.288 | [62] |
| TOAC 22/decanoic acid | 1:1.5 | 298.15 | 0.490 | 0.305 | |
| 1:2 | 298.15 | 0.490 | 0.307 |
| DES | Molar Ratio | mSO2, (T, K) | mNO, (T, K) | mNO2, (T, K) | Refs. |
|---|---|---|---|---|---|
| Deep eutectic solvents | |||||
| ACC/1,2,4-triazole | 1:1 | 0.227 (303.2) 1 | [74] | ||
| ACC/imidazole | 1:1.5 | 0.356 (303.2) 1 | |||
| ACC/LV | 1:3 | 0.567 (293) | [75] | ||
| ACC/imidazole | 1:2 | 0.989 (303.2) | [74] | ||
| 1:3 | 0.383 (303.2) 1 | ||||
| Betaine/EG | 1:3 | 0.366 (313.2) | [76] | ||
| BMIMB 2/acetamide | 1:1 | 1.00 (303.2) | [77] | ||
| BMIMB/DMU 3 | 1:1 | 0.920 (293.2) | [78] | ||
| BMIMC/ethyleneurea | 1:1 | 1.07 (293.2) | |||
| BMIMC/acetamide | 1:1 | 1.17 (303.2) | [77] | ||
| BMIMC 4/DMU | 1:2 | 0.950 (293.2) | [78] | ||
| 1:1 | 1.04 (293.2) | ||||
| 2:1 | 1.14 (293.2) | ||||
| BMIMC/Mim 5 | 1:1 | 1.31 (293.2) | [79] | ||
| 1:2 | 1.42 (293.2) | ||||
| BMIMC/imidazole | 2:1 | 1.32 (293.2) | |||
| 1:1 | 1.29 (293.2) | ||||
| 1:2 | 1.24 (293.2) | ||||
| ChCl/gly | 1:1 | 0.678 (293.2) | [80] | ||
| 1:2 | 0.482 (293.2) | 0.356 (298.2) | [80,81] | ||
| 1:3 | 0.380 (293.2) | [80] | |||
| 1:4 | 0.320 (293.2) | 0.371 (298.2) | [80,81] | ||
| ChCl/LV | 1:3 | 0.557 (293.2) | [75] | ||
| ChCl/GC | 1:3 | 0.528 (293.2) | [82] | ||
| 1:4 | 0.501 (293.2) | ||||
| 1:5 | 0.479 (293.2) | ||||
| ChCl/cardanol | 1:3 | 0.196 (293.2) | |||
| 1:4 | 0.170 (293.2) | ||||
| 1:5 | 0.149 (293.2) | ||||
| ChCl/EG | 1:2 | 0.700 (293.2) | 0.396 (298.2) | [81,83] | |
| 1:4 | 0.551 (298.2) | [81] | |||
| ChCl/malonic acid | 1:1 | 0.490 (293.2) | [83] | ||
| ChCl/urea | 1:2 | 0.350 (293.2) | |||
| ChCl/thiourea | 1:1 | 0.880 (293.2) | |||
| ChCl/tetrazolium | 1:1 | 0.860 (343.2) | [84] | ||
| ChCl/triazole | 1:1 | 0.670 (343.2) | |||
| ChCl/imid | 1:1 | 0.470 (343.2) | |||
| Caprolactam/imidazole | 1:1 | 1.66 (303.2) | [85] | ||
| Caprolactam/acetamide | 1:1 | 0.988 (303.2) | |||
| Carnitine/EG | 1:3 | 0.365 (313.2) | [76] | ||
| EMIMB/ethyleneurea | 1:1 | 0.910 (293.2) | [78] | ||
| EMIMC 6/DMU | 1:1 | 1.14 (293.2) | |||
| EMIMC/EG | 2:1 | 1.15 (293.2) | [86] | ||
| 1:1 | 1.03 (293.2) | ||||
| 1:2 | 0.820 (293.2) | ||||
| EMIMC/TEG | 1:1 | 0.910 (293.2) | [87] | ||
| 2:1 | 1.06 (293.2) | ||||
| 4:1 | 1.20 (293.2) | ||||
| 6:1 | 1.25 (293.2) | ||||
| EMIMC/succinonitrile | 1:1 | 1.13 (293.2) | [88] | ||
| 1:2 | 0.960 (293.2) | ||||
| 1:4 | 0.790 (293.2) | ||||
| EMIMC/FMP 7 | 1:1 | 0.220 (293.2) | [89] | ||
| 1:2 | 0.162 (303.2) | ||||
| 2:1 | 0.245 (303.2) | ||||
| EMIMC/acetamide | 1:1 | 1.25 (303.2) | [77] | ||
| 1:2 | 1.13 (303.2) | ||||
| 2:1 | 1.39 (303.2) | ||||
| EMIMC/imidazole | 2:1 | 1.40 (293.2) | [90] | ||
| EMIMC/1,2,4-triazole | 2:1 | 1.28 (293.2) | |||
| EMIMC/1,2,3-triazole | 2:1 | 1.18 (293.2) | |||
| EMIMC/tetrazole | 2:1 | 1.13 (293.2) | |||
| EMIMC/EPB 8 | 1:1 | 1.29 (293.2) | [91] | ||
| 2:1 | 1.34 (293.2) | ||||
| 3:1 | 1.39 (293.2) | ||||
| EMIMC/ethyleneurea | 1:1 | 1.14 (293.2) | [78] | ||
| HMIMC 9/acetamide | 1:1 | 1.02 (303.2) | [77] | ||
| Imidazole/gly | 1:2 | 0.163 (313.2) | 0.034 (313.2) | [92] | |
| KSCN 10/acetamide | 1:3 | 1.43 (293.2) | [93] | ||
| KSCN/caprolactam | 1:3 | 1.54 (293.2) | |||
| NH4SCN 11/acetamide | 1:3 | 1.37 (293.2) | |||
| NH4SCN/caprolactam | 1:3 | 1.47 (293.2) | |||
| PPZB 12/gly | 1:4 | 0.420 (293.2) | [94] | ||
| 1:5 | 0.380 (293.2) | ||||
| 1:6 | 0.350 (293.2) | ||||
| TBAB/caprolactam | 1:1 | 0.747 (293.2) | [95] | ||
| 1:2 | 0.764 (293.2) | ||||
| 1:3 | 0.719 (293.2) | ||||
| 1:4 | 0.696 (293.2) | ||||
| TBAB/LV | 1:3 | 0.547 (293.2) | [75] | ||
| TBAB/Tetz 13 | 1:1 | 0.320 (303.2) | [84] | ||
| TBAB/DMTU 14 | 1:1 | 1.00 (303.2) | [96] | ||
| TBAB/imidazole | 1:2 | 0.910 (293.2) | [79] | ||
| TBAB/caprolactam | 1:2 | 0.090 (343.2) | [97] | ||
| TBAC/Mim | 1:2 | 1.04 (293.2) | [79] | ||
| TBAC/imidazole | 1:2 | 0.960 (293.2) | |||
| TBAC/benzimidazole | 1:2 | 0.820 (293.2) | |||
| TBAC/pyrazole | 1:2 | 0.710 (293.2) | |||
| TBAC/tetrazole | 1:2 | 0.460 (293.2) | |||
| TBAC/ethyleneurea | 1:1 | 0.810 (293.2) | [78] | ||
| TBAC/LV | 1:3 | 0.541 (293.2) | [75] | ||
| TBAC/Tetz | 1:1 | 1.46 (303.2) | [84] | ||
| TBAC/DMU | 1:1 | 0.830 (293.2) | [78] | ||
| TBAC/DMTU | 1:1 | 0.830 (293.2) | 2.05 (303.2) | [96] | |
| TBAC/caprolactam | 1:2 | 0.130 (343.2) | [97] | ||
| TBAF 15/caprolactam | 1:2 | 0.160 (338.2) | |||
| TBPB 16/Tetz | 1:1 | 0.480 (303.2) | [84] | ||
| TBPB/DMTU | 1:1 | 1.13 (303.2) | [96] | ||
| TBPB/DMU | 1:1 | 0.660 (293.2) | |||
| 1:2 | 0.920 (293.2) | ||||
| 1:3 | 1.17 (293.2) | ||||
| TBPC 17/Mim | 1:2 | 1.04 (293.2) | [79] | ||
| TBPC/DMU | 1:1 | 0.830 (293.2) | [78] | ||
| TBPC/ethyleneurea | 1:1 | 0.810 (293.2) | |||
| TBPC/Tetz | 1:1 | 2.10 (303.2) | [84] | ||
| TBPC/Imid | 1:1 | 0.160 (303.2) | |||
| TBPC/triazole | 1:1 | 0.710 (303.2) | |||
| TBPC/DMTU | 1:1 | 2.13 (303.2) | [96] | ||
| 1:2 | 3.18 (303.2) | ||||
| 1:3 | 4.25 (303.2) | ||||
| TEAB/LV | 1:3 | 0.622 (293.2) | [84] | ||
| TEAC/LV | 1:3 | 0.625 (293.2) | |||
| Ionic Liquids | |||||
| [Emim][SCN] | 1.13 (293.2) | [98] | |||
| [NEt2C2Py][SCN] | 1.06 (293.2) | [99] | |||
| [E3Py][Cl] | 1.05 (293.2) | [100] | |||
| [E3Eim2][Cl]2 | 1.03 (293.2) | [101] | |||
| [Et2NEMim][Tetz] | 1.10 (293.2) | [102] | |||
| [C4Py][SCN] | 0.841 (293.2) | [103] | |||
| DES | Molar Ratio | Temperature | Pressure | mNH3 | Refs. |
|---|---|---|---|---|---|
| 1,2,4-triazole/gly | 1:3 | 313.15 | 0.10 | 6.706 | [109] |
| ChCl/1,4-BD 1 | 1:3 | 313.15 | 0.13 | 2.347 | [110] |
| 1:4 | 313.15 | 0.12 | 2.369 | ||
| ChCl/2,3-BD 2 | 1:3 | 313.15 | 0.13 | 2.073 | |
| 1:4 | 313.15 | 0.12 | 1.903 | ||
| ChCl/1,3-PD 3 | 1:3 | 313.15 | 0.13 | 2.513 | |
| 1:4 | 313.15 | 0.12 | 2.517 | ||
| ChCl/EG | 1:2 | 333.2 | 0.10 | 1.491 | [111] |
| ChCl/gly | 1:2 | 333.2 | 0.11 | 1.341 | |
| ChCl/MU 4 | 1:2 | 333.2 | 0.09 | 0.519 | |
| ChCl/xylose | 1:1 | 333.2 | 0.11 | 4.187 | [112] |
| 1.5:1 | 333.2 | 0.11 | 3.722 | ||
| 2:1 | 333.2 | 0.10 | 2.980 | ||
| ChCl/TFA 5 | 1:2 | 333.2 | 0.12 | 1.475 | [111] |
| ChCl/phenol/EG | 1:5:4 | 313.2 | 0.10 | 6.988 | [113] |
| 1:7:4 | 313.2 | 0.10 | 7.652 | ||
| ChCl/imidazole/EG | 3:7:14 | 313.2 | 0.10 | 4.909 | [106] |
| ChCl/triazole/EG | 3:7:14 | 313.2 | 0.10 | 6.495 | |
| ChCl/tetrazole/EG | 3:7:14 | 313.2 | 0.11 | 9.952 | |
| ChCl/urea | 1:1.5 | 313.2 | 0.11 | 1.436 | [114] |
| 1:2 | 313.2 | 0.11 | 1.599 | ||
| 1:2.5 | 313.2 | 0.10 | 1.355 | ||
| ChCl/PNA 6 | 1:2 | 313.2 | 0.10 | 2.445 | [115] |
| ChCl/LV | 1:2 | 313.2 | 0.10 | 4.631 | |
| 1:4 | 298.2 | 0.10 | 9.494 | [116] | |
| 1:5 | 298.2 | 0.10 | 9.443 | ||
| EACl/AA 7 | 1:1 | 313.2 | 0.10 | 3.830 | [117] |
| 1:2 | 313.2 | 0.10 | 3.600 | ||
| 1:3 | 323.2 | 0.10 | 2.210 | ||
| EaCl/phenol | 1:2 | 313.2 | 0.10 | 7.023 | [118] |
| 1:3 | 313.2 | 0.10 | 7.433 | ||
| 1:5 | 313.2 | 0.10 | 8.106 | ||
| 1:7 | 298.2 | 0.10 | 9.801 | ||
| EACl 8/gly | 1:2 | 298.2 | 0.11 | 9.631 | [116] |
| EACl/urea | 1:0.5 | 313.2 | 0.10 | 4.396 | [119] |
| 1:1 | 313.2 | 0.10 | 4.573 | ||
| 1:2 | 313.2 | 0.10 | 4.179 | ||
| GI 9/AA | 1:2 | 303.15 | 0.10 | 5.300 | [120] |
| 1:3 | 303.15 | 0.10 | 4.160 | ||
| 1:4 | 303.15 | 0.10 | 3.580 | ||
| Imidazole/gly | 1:3 | 313.15 | 0.10 | 5.812 | [109] |
| KSCN/gly | 2:3 | 313.15 | 0.10 | 5.970 | [121] |
| MAA 10/tetrazole | 2:1 | 313.2 | 0.10 | 8.000 | [122] |
| 2.5:1 | 313.2 | 0.10 | 6.650 | ||
| 3:1 | 313.2 | 0.10 | 5.940 | ||
| MAA/imidazole | 2:1 | 313.2 | 0.11 | 1.770 | |
| MAA/triazole | 2:1 | 313.2 | 0.10 | 3.650 | |
| NH4SCN/gly | 2:3 | 313.15 | 0.10 | 10.36 | [121] |
| NH4SCN/EG | 1:3 | 313.15 | 0.10 | 9.890 | |
| NH4SCN/urea | 2:3 | 313.15 | 0.10 | 8.590 | |
| NH4SCN/acetamide | 2:3 | 313.15 | 0.10 | 5.390 | |
| NH4SCN/caprolactam | 1:3 | 313.15 | 0.10 | 1.730 | |
| Tetrazole/gly | 1:3 | 33.15 | 0.10 | 8.929 | [109] |
| DES | Molar Ratio | Selectivity | Refs. | DES | Molar Ratio | Selectivity | Refs. |
|---|---|---|---|---|---|---|---|
| SO2/CO2 selectivity at 293.15 K, 0.1 MPa | NH3/CO2 selectivity at 313.15 K, 0.1 MPa | ||||||
| ACC/LV | 1:3 | 155 | [75] | 1,2,4-triazole/gly | 1:3 | 216 | [109] |
| ChCl/LV | 1:3 | 155 | [Im][NO3] 1/EG | 1:3 | 139 | [128] | |
| ChCl/GC | 1:3 | 258 | [82] | ChCl/Res/Gly | 1:3:5 | 142 | [115] |
| 1:4 | 237 | ChCl/Res | 1:3 | 64 | |||
| 1:5 | 214 | ChCl/phenol/Gly | 1:3:5 | 87 | |||
| ChCl/CD | 1:3 | 36.0 | ChCl/phenol | 1:3 | 54 | ||
| 1:4 | 30.0 | ChCl/Res/EG | 1:3:5 | 49 | |||
| 1:5 | 26.0 | ChCl/urea | 1:2 | 16.7 | [114] | ||
| TEAB/LV | 1:3 | 183 | [75] | ChCl/1,4-BD | 1:3 | 74.7 | [110] |
| TEAC/LV | 1:3 | 199 | 1:4 | 79.1 | |||
| TBAB/LV | 1:3 | 134 | ChCl/2,3-BD | 1:3 | 65.5 | ||
| TBAC/LV | 1:3 | 141 | 1:4 | 52.9 | |||
| PPZB/gly | 1:4 | 33.1 | [94] | GI/AA | 1:2 | 151 2 | [120] |
| 1:5 | 12.8 | 1:3 | 116 2 | ||||
| 1:6 | 9.50 | 1:4 | 972 2 | ||||
| Imidazole/gly | 1:3 | 37.3 | [109] | ||||
| NH4SCN/gly | 2:3 | 609 | |||||
| DES | Mixture | S Range | D Range | Refs. |
|---|---|---|---|---|
| MTPPB/EG (1:4) | Benzothiazole/n-hexane | 9.60–40.0 | 2.10–3.10 | [144] |
| MTPPB/EG (1:4) | Benzothiazole/n-heptane | 7.00–46.8 | 2.10–3.50 | |
| THAB/EG (1:2) | 7.00–27.50 | 2.36–3.76 | [8] | |
| THAB/gly (1:2) | 7.33–35.26 | 1.90–2.99 | ||
| TBPB/EG (1:2) | Indoline/n-hexadecane | 457–1,116 | 5.04–7.42 | [142] |
| TBAB/EG (1:2) | 833–2506 | 4.54–7.57 | ||
| MTPPB/gly (1:4) | Pyridine/n-hexane | 26.1–839.5 | 1.589–2.677 | [145] |
| MTPPB/EG (1:4) | 34.1–232.6 | 2.50–2.60 | [144] | |
| MTPPB/EG (1:4) | Pyridine/n-heptane | 30.1–276.9 | 2.60–3.40 | |
| TBAB/EG (1:2) | Pyridine/n-hexadecane | 727–1228 | 2.93–4.39 | [142] |
| TBPB/EG (1:2) | 157–437 | 3.24–4.60 | ||
| Betaine/LV (1:7) | Pyrene/n-decane | 255.3–48,500 | 6.127–97.00 | [146] |
| TBAB/EG (1:2) | Pyrrole/n-hexadecane | 6659–46,953 | 30.31–94.00 | [142] |
| TBPB/EG (1:2) | 1413–8159 | 27.37–98.00 | ||
| MTPPB/TEG (1:4) | Quinoline/n-heptane | 235.8–2327.9 | 4.120–9.574 | [139] |
| TBPB/PTSA (1:1) | 8644–10,866 | 363–467 | [147] | |
| TBPB/PTSA (1:1) | Quinoline/n-pentadecane | 3740–24,321 | 200–277 | |
| TBAB/EG (1:2) | Quinoline/n-hexadecane | 3229–4955 | 3.56–5.00 | [142] |
| TBPB/EG (1:2) | 141–594 | 3.71–7.80 | ||
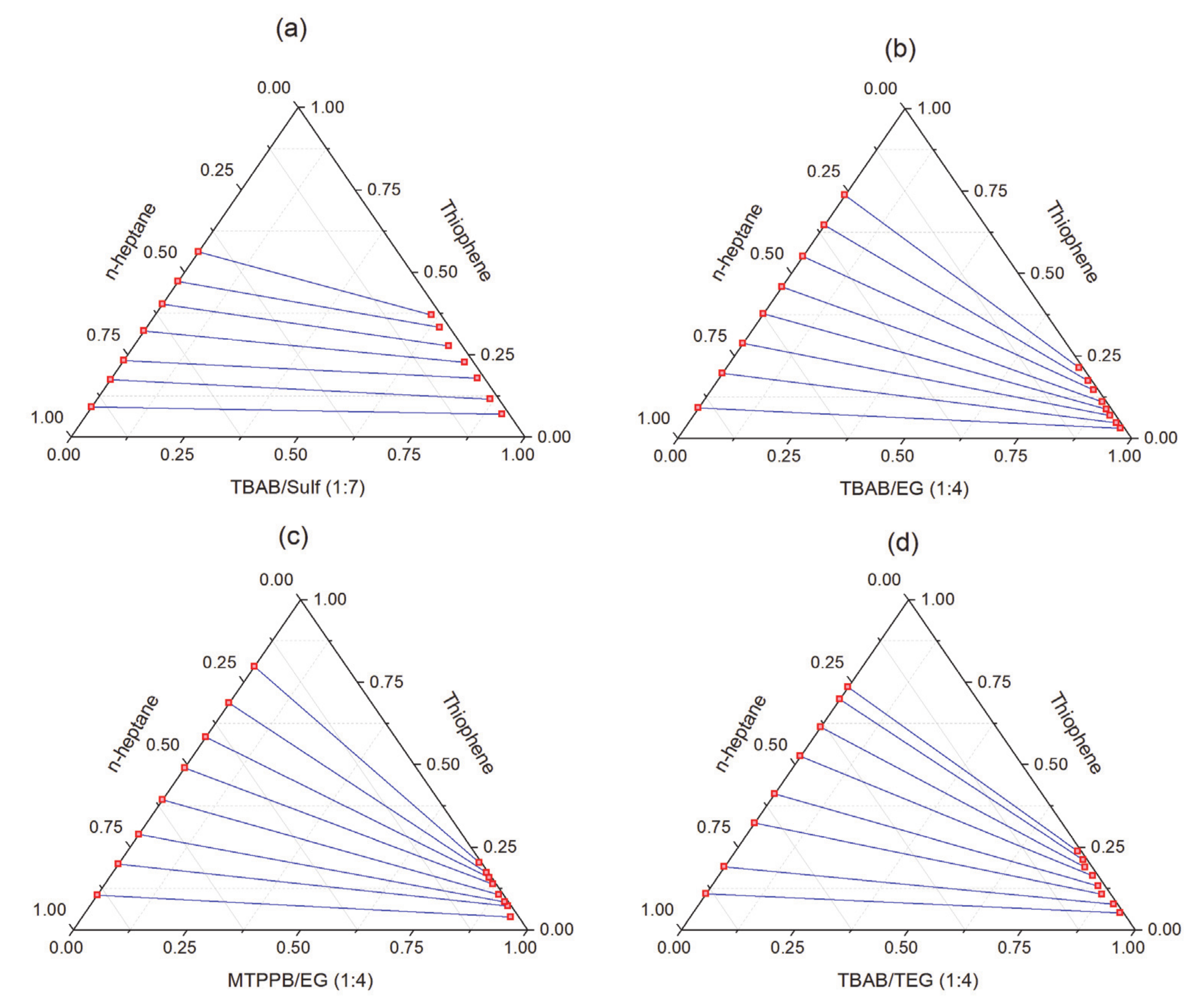
| TBAB/EG (1:4) | Thiophene/n-heptane | 8.79–30.22 | 0.233–0.325 | [143] |
| MTPPB/EG (1:4) | 10.12–20.19 | 0.251–0.373 | ||
| TBAB/TEG (1:4) | 10.70–51.95 | 0.302–0.464 | ||
| TBAB/Sulf (1:7) | 13.77–41.87 | 0.659–0.764 | ||
| MTPPB/TEG (1:4) | 20.80–161.40 | 0.402–0.647 | [139] | |
| THAB/EG (1:2) | 1.89–9.55 | 0.81–0.95 | [8] | |
| THAB/gly (1:2) | 3.21–14.8 | 0.66–0.79 | ||
| THAB/EG (1:2) | Thiophene/n-hexane | 1.73–8.05 | 0.810–0.935 | [148] |
| THAB/EG (1:2) | 2.08–11.71 | 0.797–0.981 | ||
| TEAC/EG (1:2) | 4.81–87.99 | 0.320–0.512 | [149] | |
| TEAC/gly (1:2) | 42.68–257.73 | 0.130–0.226 | ||
| MTPPB/EG (1:3) | 12.36–140.38 | 0.419–0.521 | ||
| MTPPB/gly (1:3) | 1.42–29.30 | 0.031–0.158 | ||
| THAB/gly (1:2) | Thiophene/n-octane | 1.40–12.99 | 0.662–0.789 | [148] |
| THAB/gly (1:2) | 1.66–17.30 | 0.665–0.794 | ||
| Betaine/LV (1:7) | 14.4–159.7 | 0.396–0.489 | [146] | |
| TEAC/EG (1:2) | 46.95–1009.11 | 0.378–0.701 | [150] | |
| TEAC/gly (1:2) | 137.09–794.21 | 0.205–0.360 | ||
| MTPPB/EG (1:3) | 26.44–465.49 | 0.409–0.550 | ||
| MTPPB/gly (1:3) | 107.89–673.64 | 0.129–0.245 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wazeer, I.; Hadj-Kali, M.K.; Al-Nashef, I.M. Utilization of Deep Eutectic Solvents to Reduce the Release of Hazardous Gases to the Atmosphere: A Critical Review. Molecules 2021, 26, 75. https://doi.org/10.3390/molecules26010075
Wazeer I, Hadj-Kali MK, Al-Nashef IM. Utilization of Deep Eutectic Solvents to Reduce the Release of Hazardous Gases to the Atmosphere: A Critical Review. Molecules. 2021; 26(1):75. https://doi.org/10.3390/molecules26010075
Chicago/Turabian StyleWazeer, Irfan, Mohamed K. Hadj-Kali, and Inas M. Al-Nashef. 2021. "Utilization of Deep Eutectic Solvents to Reduce the Release of Hazardous Gases to the Atmosphere: A Critical Review" Molecules 26, no. 1: 75. https://doi.org/10.3390/molecules26010075
APA StyleWazeer, I., Hadj-Kali, M. K., & Al-Nashef, I. M. (2021). Utilization of Deep Eutectic Solvents to Reduce the Release of Hazardous Gases to the Atmosphere: A Critical Review. Molecules, 26(1), 75. https://doi.org/10.3390/molecules26010075