The Proton Dissociation of Bio-Protic Ionic Liquids: [AAE]X Amino Acid Ionic Liquids
Abstract
1. Introduction
2. Results
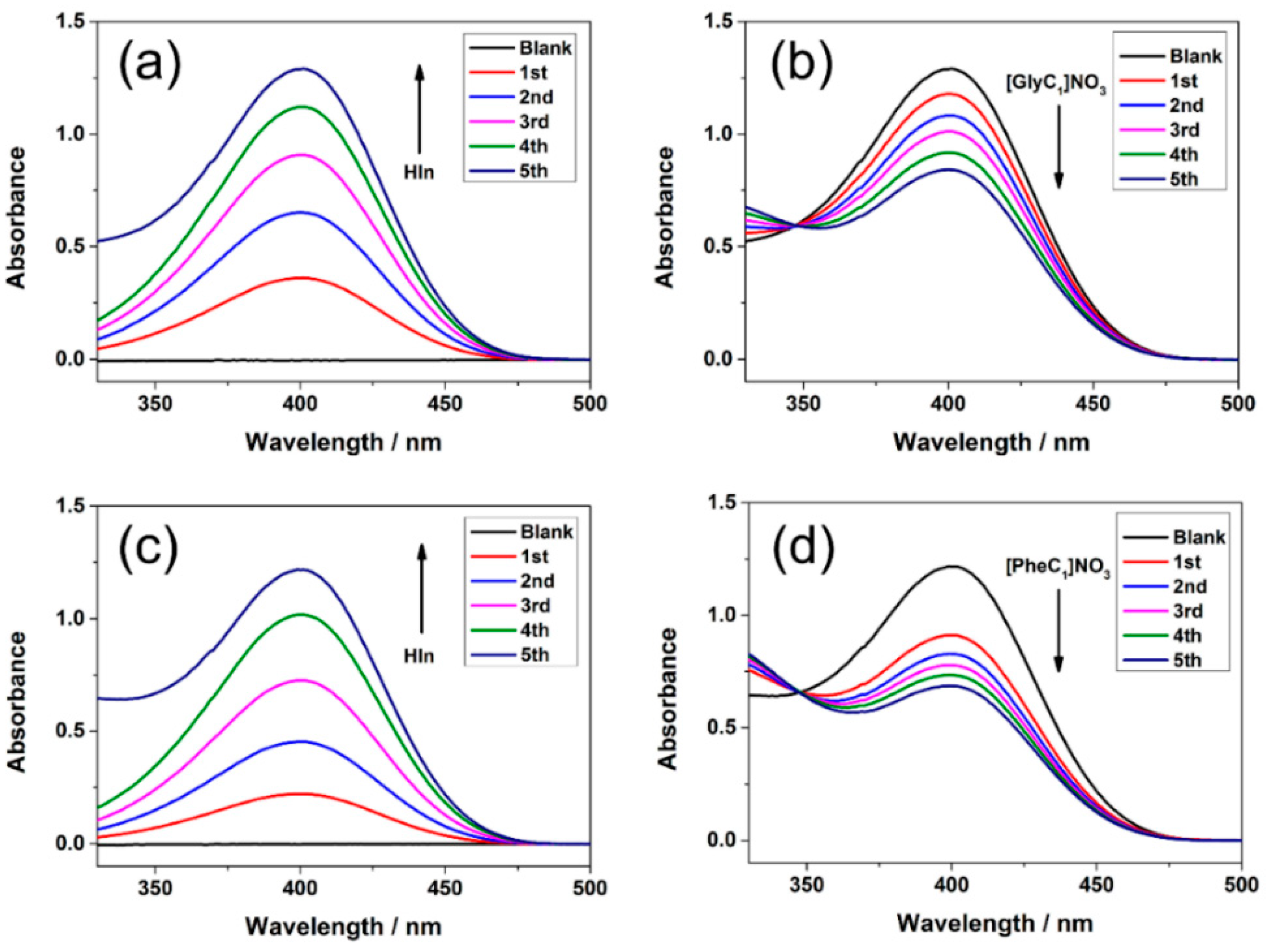
2.1. Overlapping Indicator Method (OIM)
2.2. Potential Titration Method (PTM)
2.3. pKa Values Measured by the OIM in Ethanol
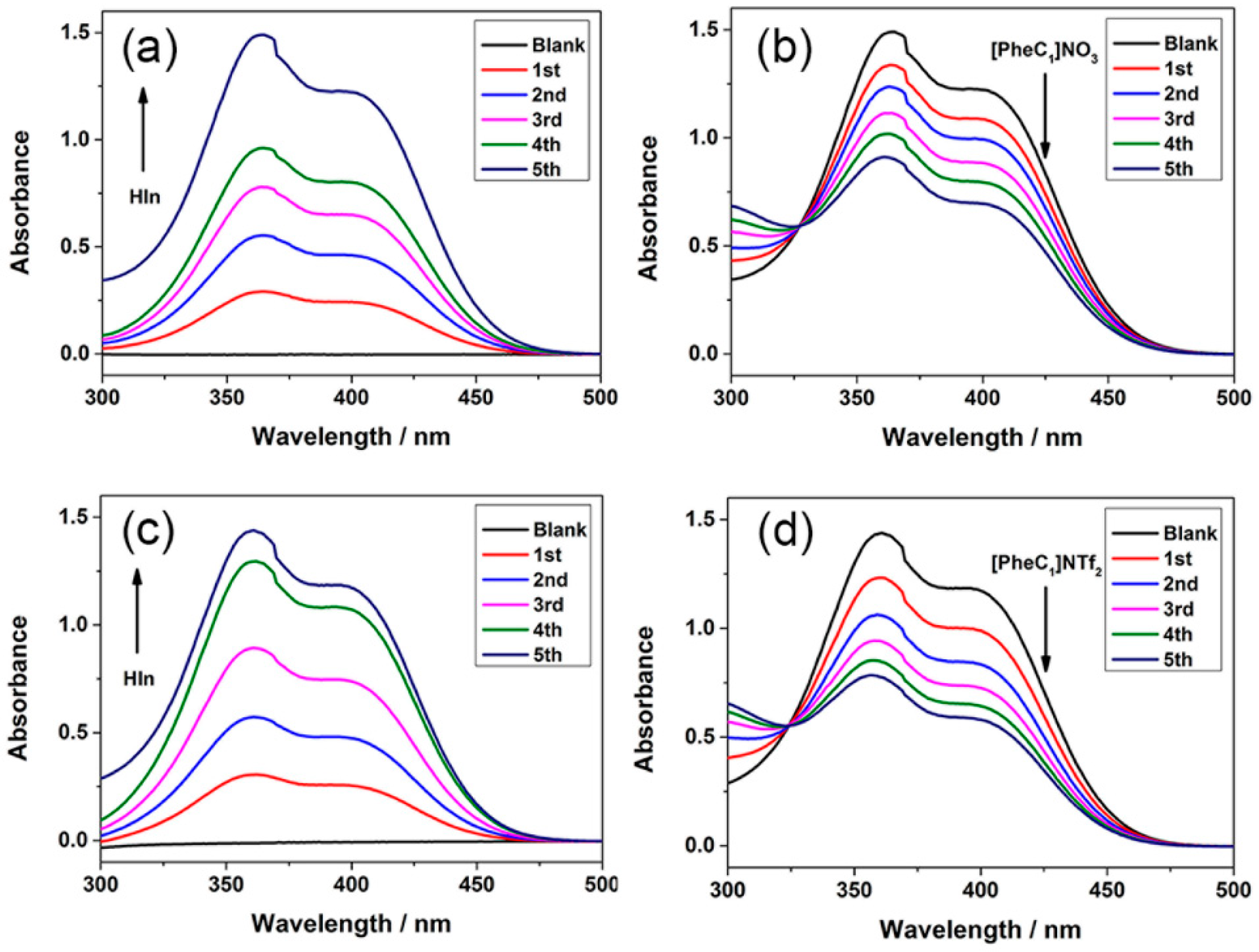
2.4. Hammett Acidity
2.5. pKa Values Calculated by DFT
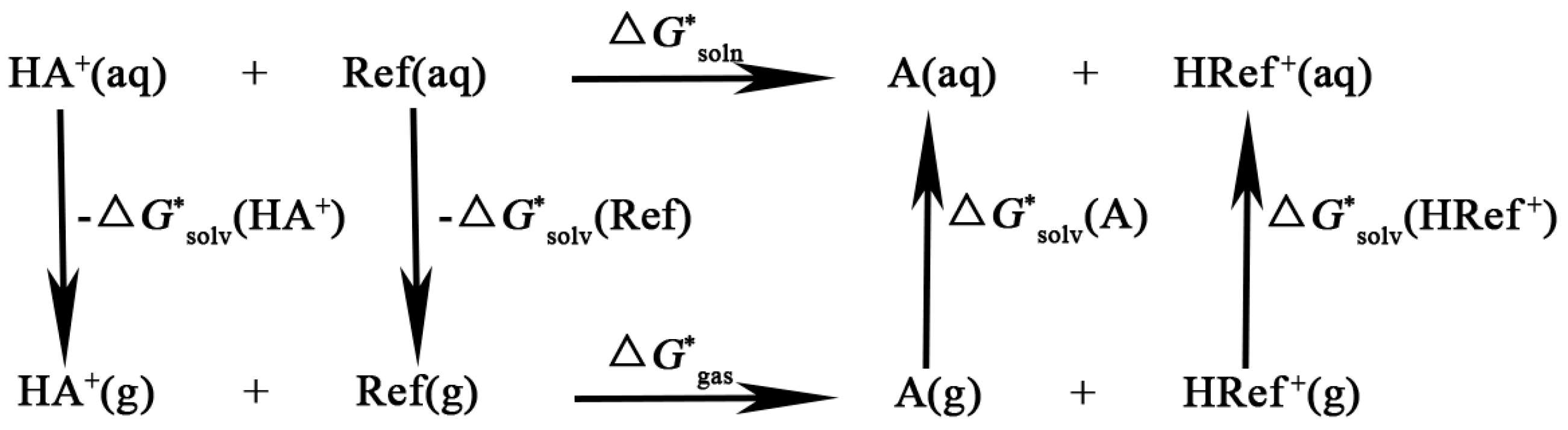
3. Discussion
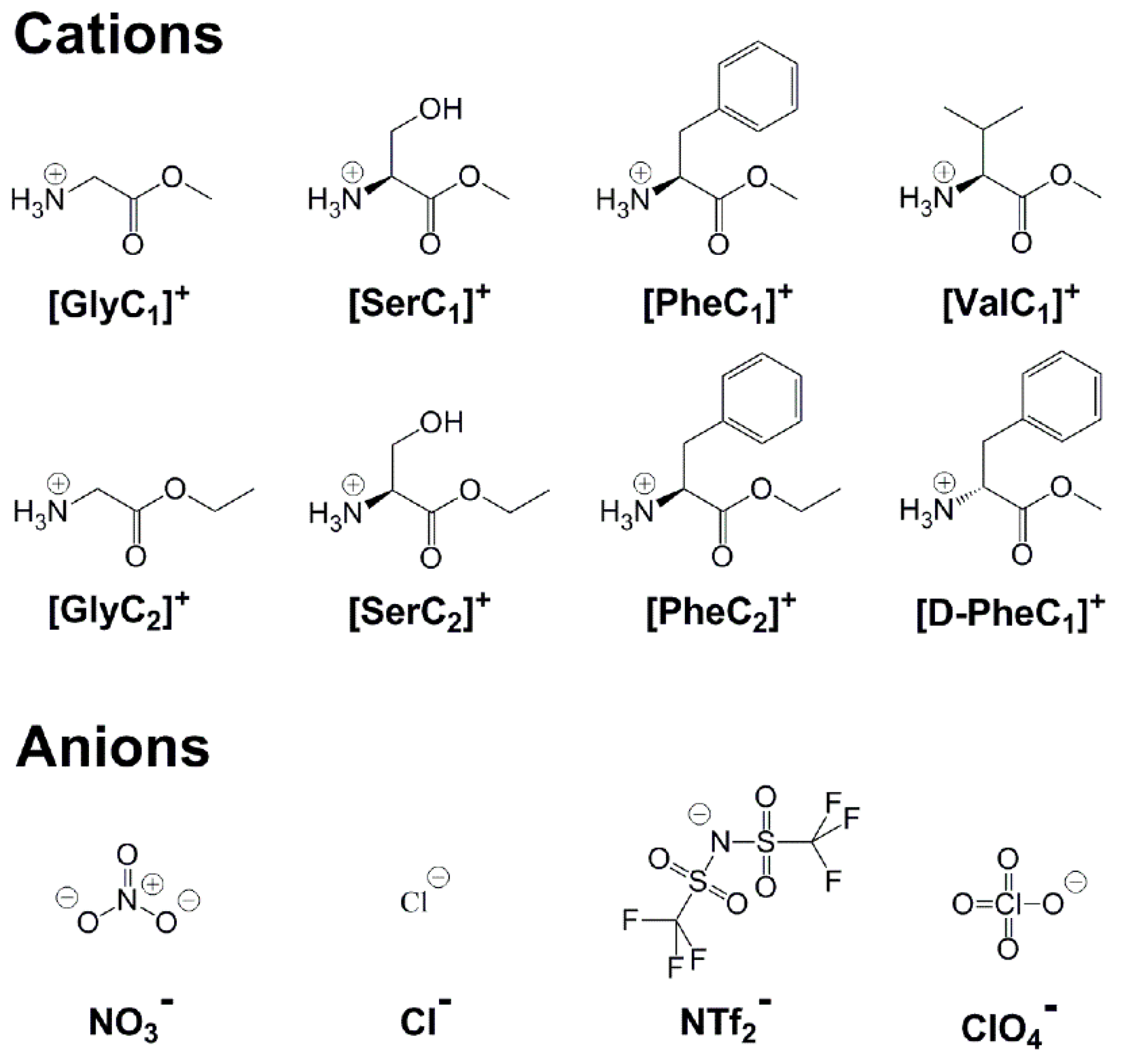
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 1, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Evolving structure–property relationships and expanding applications. Chem. Rev. 2015, 20, 11379–11448. [Google Scholar] [CrossRef] [PubMed]
- Fernicola, A.; Panero, S.; Scrosati, B.; Tamada, M.; Ohno, H. New types of brönsted acid–base ionic liquids-based membranes for applications in PEMFCs. Chem. Phys. Chem. 2007, 8, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Weingärtner, H. Protic ionic liquids with unusually high dielectric permittivities. Chem. Phys. Chem. 2008, 9, 2172–2173. [Google Scholar] [CrossRef]
- Billard, I.; Ouadi, A.; Gaillard, C. Liquid–liquid extraction of actinides, lanthanides, and fission products by use of ionic liquids: From discovery to understanding. Anal. Bioanal. Chem. 2011, 400, 1555–1566. [Google Scholar] [CrossRef]
- Pollet, P.; Davey, E.A.; Urena-Benavides, E.E.; Eckert, C.A.; Liotta, C.L. Solvents for sustainable chemical processes. Green Chem. 2014, 16, 1034–1055. [Google Scholar] [CrossRef]
- Sjöblom, M.; Antonopoulou, I.; Jiménez, I.G.; Maciel, A.O.; Khokarale, S.G.; Mikkola, J.P.; Rova, U.; ChristakopouloS, P. Enzyme-Assisted CO2 Absorption in aqueous amino acid ionic liquid amine blends. ACS Sustain. Chem. Eng. 2020, 36, 13672–13682. [Google Scholar] [CrossRef]
- Amarasekara, A.S. Acidic ionic liquids. Chem. Rev. 2016, 10, 6133–6183. [Google Scholar] [CrossRef]
- He, L.; Qin, S.; Chang, T.; Sun, Y.; Gao, X. Biodiesel synthesis from the esterification of free fatty acids and alcohol catalyzed by long-chain Brønsted acid ionic liquid. Catal. Sci. Technol. 2013, 3, 1102–1107. [Google Scholar] [CrossRef]
- Kotadia, D.A.; Soni, S.S. Symmetrical and unsymmetrical Brønsted acidic ionic liquids for the effective conversion of fructose to 5-hydroxymethyl furfural. Catal. Sci. Technol. 2013, 3, 469–474. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Zhu, Q.; Zhang, Z.; Wu, C.; Han, B. Transformation of Atmospheric CO2 Catalyzed by Protic Ionic Liquids: Efficient Synthesis of 2-Oxazolidinones. Angew. Chem. Int. Ed. 2015, 54, 5399–5403. [Google Scholar] [CrossRef] [PubMed]
- Manna, A.; Kumar, A. Invoking pairwise interactions in water-promoted diels–alder reactions by using ionic liquids as cosolvents. Chem. Phys. Chem. 2014, 15, 3067–3077. [Google Scholar] [CrossRef] [PubMed]
- Hulsbosch, J.; De Vos, D.E.; Binnemans, K.; Ameloot, R. Biobased ionic liquids: Solvents for a green processing industry? ACS Sustain. Chem. Eng. 2016, 4, 2917–2931. [Google Scholar] [CrossRef]
- Dong, L.; He, L.; Tao, G.; Huang, M.; Hu, C. Theoretical enthalpies of formation of [AA]X and [AAE]X type amino acid ionic liquids. J. Chem. Eng. Data 2013, 58, 1176–1185. [Google Scholar] [CrossRef]
- Tao, G.; He, L.; Sun, N.; Kou, Y. New generation ionic liquids: Cations derived from amino acids. Chem. Commun. 2005, 28, 3562–3564. [Google Scholar] [CrossRef]
- Ohno, H.; Fukumoto, K. Amino Acid Ionic Liquids. Acc. Chem. Res. 2007, 40, 1122–1129. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, K.; Tang, F.; Yao, L.; Yang, F.; Nie, Z.; Yao, S. Amino acid ionic liquids as chiral ligands in ligand-exchange chiral separations. Chem. Eur. J. 2009, 15, 9889–9896. [Google Scholar] [CrossRef]
- Gurkan, B.E.; de la Fuente, J.C.; Mindrup, E.M.; Ficke, L.E.; Goodrich, B.F.; Price, E.A.; Schneider, W.F.; Brennecke, J.F. Equimolar CO2 absorption by anion-functionalized ionic liquids. J. Am. Chem. Soc. 2010, 132, 2116–2117. [Google Scholar] [CrossRef]
- Dong, L.; He, L.; Tao, G.; Hu, C. High yield of ethyl valerate from the esterification of renewable valeric acid catalyzed by amino acid ionic liquid. RSC Adv. 2013, 3, 4806–4813. [Google Scholar] [CrossRef]
- Roshan, K.R.; Jose, T.; Kim, D.; Cherian, K.A.; Park, D.W. Microwave-assisted one pot-synthesis of amino acid ionic liquids in water: Simple catalysts for styrene carbonate synthesis under atmospheric pressure of CO2. Catal. Sci. Technol. 2014, 4, 963–970. [Google Scholar] [CrossRef]
- Shiri, M. Prolinium Triflate: A protic ionic liquid which acts as water-tolerant catalyst in the alkylation of indoles. J. Iran. Chem. Soc. 2013, 10, 1019–1023. [Google Scholar] [CrossRef]
- He, L.; Tao, G.; Parrish, D.A.; Shreeve, J.M. Slightly viscous amino acid ionic liquids: Synthesis, properties, and calculations. J. Phys. Chem. B 2009, 113, 15162–15169. [Google Scholar] [CrossRef] [PubMed]
- Capello, C.; Fischer, U.; Hungerbuhler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Gui, J.; Cong, X.; Liu, D.; Zhang, X.; Hu, Z.; Sun, Z. Novel Brønsted acidic ionic liquid as efficient and reusable catalyst system for esterification. Catal. Commun. 2004, 5, 473–477. [Google Scholar] [CrossRef]
- Manabe, K.; Iimura, S.; Sun, X.; Kobayashi, S. Dehydration reactions in water. Brønsted acid−surfactant-combined catalyst for ester, ether, thioether, and dithioacetal formation in water. J. Am. Chem. Soc. 2002, 124, 11971–11978. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Synergistic conversion of glucose into 5-hydroxymethylfurfural in ionic liquid–water mixtures. Bioresour. Technol. 2012, 109, 224–228. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, J.; Ye, L.; Lin, L.; Yuan, Y. Effects of acidity and immiscibility of lactam-based Brønsted-acidic ionic liquids on their catalytic performance for esterification. Green Chem. 2010, 12, 661–665. [Google Scholar] [CrossRef]
- Akiyama, T.; Itoh, J.; Yokota, K.; Fuchibe, K. Enantioselective mannich-type reaction catalyzed by a chiral brønsted acid. Angew. Chem. Int. Ed. 2004, 43, 1566–1568. [Google Scholar] [CrossRef]
- Chiappe, C.; Rajamani, S.; D’Andrea, F. A dramatic effect of the ionic liquid structure in esterification reactions in protic ionic media. Green Chem. 2013, 15, 137–143. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Campelo, J.M.; Francavilla, M.; Romero, A.A.; Luque, R.; Menendez-Vazquez, C.; Garcia, A.B.; Garcia-Suarez, E.J. Efficient microwave-assisted production of furfural from C5 sugars in aqueous media catalysed by Brönsted acidic ionic liquids. Catal. Sci. Technol. 2012, 2, 1828–1832. [Google Scholar] [CrossRef]
- Barhdadi, R.; Troupel, M.; Comminges, C.; Laurent, M.; Doherty, A. Electrochemical Determination of pKa of N-Bases in Ionic Liquid Media. J. Phys. Chem. B 2012, 116, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Crespo, G.A.; Afshar, M.G.; Bakker, E. Direct Detection of Acidity, Alkalinity, and pH with Membrane Electrodes. Anal. Chem. 2012, 84, 10165–10169. [Google Scholar] [CrossRef] [PubMed]
- Millan, D.; Rojas, M.; Santos, J.G.; Morales, J.; Isaacs, M.; Diaz, C.; Pavez, P. Toward a pKa Scale of N-base Amines in Ionic Liquids. J. Phys. Chem. B 2014, 118, 4412–4418. [Google Scholar] [CrossRef] [PubMed]
- Deive, F.J.; Rodriguez, A.; Pereiro, A.B.; Araujo, J.M.M.; Longo, M.A.; Coelho, M.A.Z.; Lopes, J.N.C.; Esperanca, J.; Rebelo, L.P.N.; Marrucho, I.M. Ionic liquid-based aqueous biphasic system for lipase extraction. Green Chem. 2011, 13, 390–396. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Paniagua, M.; Melero, J.A.; Riisager, A. Efficient Isomerization of Glucose to Fructose over Zeolites in Consecutive Reactions in Alcohol and Aqueous Media. J. Am. Chem. Soc. 2013, 135, 5246–5249. [Google Scholar] [CrossRef]
- Mihichuk, L.M.; Driver, G.W.; Johnson, K.E. Brønsted Acidity and the Medium: Fundamentals with a Focus on Ionic Liquids. Chem. Phys. Chem. 2011, 12, 1622–1632. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Yuan, W.; Zhao, N.; Zhu, Q.; He, L.; Tao, G. Hydrogen-Bonding-Driven Ion-Pair Formation in Protic Ionic Liquid Aqueous Solution. Chem. Phys. Chem. 2020, 255, 117650. [Google Scholar] [CrossRef]
- Matthews, W.S.; Bares, J.E.; Bartmess, J.E.; Bordwell, F.G.; Cornforth, F.J.; Drucker, G.E.; Margolin, Z.; McCallum, R.J.; McCollum, G.J.; Vanier, N.R. Equilibrium acidities of carbon acids. VI. Establishment of an absolute scale of acidities in dimethyl sulfoxide solution. J. Am. Chem. Soc. 1975, 97, 7006–7014. [Google Scholar] [CrossRef]
- Chu, Y.; Deng, H.; Cheng, J. An Acidity Scale of 1,3-Dialkylimidazolium Salts in Dimethyl Sulfoxide Solution. J. Org. Chem. 2007, 72, 7790–7793. [Google Scholar] [CrossRef]
- Fickling, M.; Fischer, A.; Mann, B.R.; Packer, J.; Vaughan, J. Hammett Substituent Constants for Electron-withdrawing Substituents: Dissociation of Phenols, Anilinium Ions and Dimethylanilinium Ions. J. Am. Chem. Soc. 1959, 81, 4226–4230. [Google Scholar] [CrossRef]
- Mchedlov-Petrosyan, N.O. Ionization and Tautomerism of Hydroxyxanthenes and Some Other Dyes in Ethanol. Russ. J. Gen. Chem. 2003, 73, 267–274. [Google Scholar] [CrossRef]
- Danovich, D.K.; Turchaninov, V.K. Basicity of azoles. 1. Investigation of total energy of pyrazole and imidazole derivatives by the partitioning method. Bull. Pol. Acad. Sci. Tech. 1989, 38, 1182–1187. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Zhang, L.; He, L.; Hong, C.; Qin, S.; Tao, G. Brønsted acidity of bio-protic ionic liquids: The acidic scale of [AA]X amino acid ionic liquids. Green Chem. 2015, 17, 5154–5163. [Google Scholar] [CrossRef]
- Espinosa, S.; Bosch, E.; Rosés, M. Retention of ionizable compounds in high-performance liquid chromatography: 14. Acid–base pK values in acetonitrile–water mobile phases. J. Chromatogr. A 2002, 964, 55–66. [Google Scholar] [CrossRef]
- Albert, A.; Serjeant, E.P. The Determination of Ionization Constants, 3rd ed.; Chapman and Hall Press: New York, NY, USA, 1984. [Google Scholar]
- Zevatskii, Y.E.; Samoilov, D.V. Empirical method for description of solvent effect on the ionization constants of NH acids. Russ. J. Org. Chem. 2008, 44, 1737–1744. [Google Scholar] [CrossRef]
- Gibson, G.T.T.; Mohamed, M.F.; Neverov, A.A.; Brown, R.S. Potentiometric Titration of Metal Ions in Ethanol. Inorg. Chem. 2006, 45, 7891–7902. [Google Scholar] [CrossRef] [PubMed]
- Thomazeau, C.; Olivier-Bourbigou, H.; Magna, L.; Luts, S.; Gilbert, B. Determination of an Acidic Scale in Room Temperature Ionic Liquids. J. Am. Chem. Soc. 2003, 125, 5264–5265. [Google Scholar] [CrossRef]
- Glasoe, P.K. Dissociation Constants for Some Nitrophenols and Salicylic Acid in Deuterium Oxide. J. Phys. Chem. 1965, 69, 4416–4417. [Google Scholar] [CrossRef]
- Choudhary, V.; Mushrif, S.H.; Ho, H.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Ho, J.; Coote, M.L. A universal approach for continuum solvent pK a calculations: Are we there yet? Theor. Chem. Acc. 2010, 125, 3–21. [Google Scholar] [CrossRef]
- Pliego, J.R.; Riveros, J.M. Theoretical Calculation of pKa Using the Cluster−Continuum Model. J. Phys. Chem. A 2002, 106, 7434–7439. [Google Scholar] [CrossRef]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of Density Functionals by Combining the Method of Constraint Satisfaction with Parametrization for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
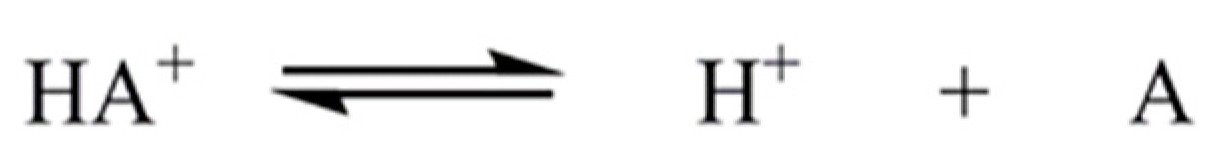


| Compound | pKa | SD |
|---|---|---|
| [GlyC1]NO3 | 7.67 | 0.02 |
| [GlyC2]NO3 | 7.73 | 0.02 |
| [ValC1]NO3 | 7.54 | 0.03 |
| [SerC1]NO3 | 7.23 | 0.04 |
| [SerC2]NO3 | 7.10 | 0.02 |
| [PheC1]NO3 | 7.20 | 0.01 |
| [PheC2]NO3 | 7.26 | 0.04 |
| [MIM]+ | 7.13 (a) | / |
| Gly | 9.78 (b) | / |
| Phe | 9.31 (b) | / |
| EAN | 10.43 (c) | / |
| [Et2N]NO3 | 10.68 (c) | / |
| [Et3N]NO3 | 10.55 (c) | / |
| [Pyri]+ | 5.17 (d) | / |
| Compound | pKa | Average pKa | SD | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| [PheC1]Cl | 7.25 | 7.26 | 7.26 | 7.25 | 7.24 | 7.25 | 0.01 |
| [PheC1]NO3 | 7.21 | 7.20 | 7.21 | 7.21 | 7.20 | 7.20 | 0.01 |
| [PheC1]NTf2 | 7.23 | 7.25 | 7.24 | 7.24 | 7.23 | 7.24 | 0.01 |
| [PheC1]ClO4 | 7.21 | 7.20 | 7.16 | 7.20 | 7.21 | 7.20 | 0.02 |
| [PheC1]Cl | 7.25 | 7.26 | 7.26 | 7.25 | 7.24 | 7.25 | 0.01 |
| [D-PheC1]Cl | 7.17 | 7.16 | 7.18 | 7.16 | 7.18 | 7.17 | 0.01 |
| [PheC1]NO3 | 7.21 | 7.20 | 7.21 | 7.21 | 7.20 | 7.20 | 0.01 |
| [D-PheC1]NO3 | 7.16 | 7.15 | 7.18 | 7.20 | 7.18 | 7.17 | 0.02 |
| Compound | pKa | SD | |
|---|---|---|---|
| OIM | PTM | ||
| [GlyC1]NO3 | 7.67 | 7.67 | 0.00 |
| [GlyC2]NO3 | 7.73 | 7.82 | 0.09 |
| [PheC1]NO3 | 7.20 | 7.19 | 0.01 |
| [PheC2]NO3 | 7.26 | 7.21 | 0.05 |
| [PheC1]Cl | 7.25 | 7.19 | 0.06 |
| [SerC1]NO3 | 7.23 | 7.24 | 0.01 |
| [PheC1]NTf2 | 7.24 | 7.12 | 0.12 |
| Compound | pKa | SD |
|---|---|---|
| [GlyC1]NO3 | 9.05 | 0.02 |
| [ValC1]NO3 | 8.86 | 0.04 |
| [SerC1]NO3 | 8.88 | 0.03 |
| [PheC1]NO3 | 8.54 | 0.01 |
| [PheC2]NO3 | 8.61 | 0.03 |
| [PheC1]NTf2 | 8.61 | 0.03 |
| [D-PheC1]NO3 | 8.59 | 0.01 |
| [EtNH3]+ | 12.0 (a) | / |
| [Et2NH2]+ | 10.7 (a) | / |
| [Et3NH]+ | 10.22 (b) | / |
| [MIM]+ | 7.50 (b) | / |
| [Pyri]+ | 4.30 (a) | / |
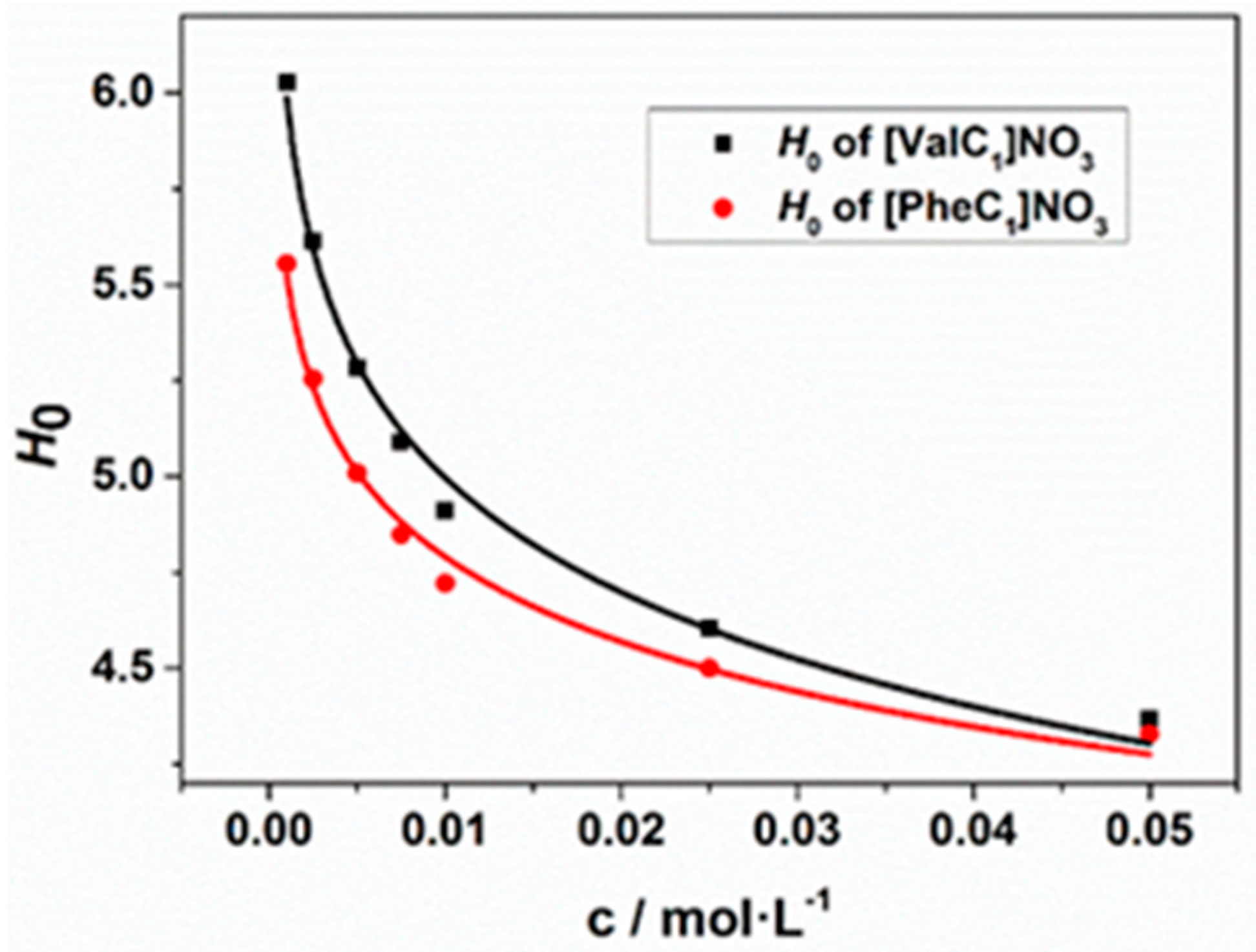
| Compound | H0 | pKa (a) |
|---|---|---|
| [ValC1]NO3 | 4.37 | 7.54 |
| [PheC1]NO3 | 4.33 | 7.20 |
| Phe | 6.44 | 9.31 |
| [MIM]Cl | 4.35 | 7.13 (c) |
| EAN | u.d. (b) | 10.43 |
| [Et2N]NO3 | u.d. (b) | 10.68 |
| [Et3N]NO3 | u.d. (b) | 10.55 |
| [AAE]+ | pKa(Cal) (a) | pKa(Exp) (b) | pKa(Cal) (c) | pKa(Exp) (d) |
|---|---|---|---|---|
| [GlyC1]+ | 7.11 | 7.67 | 8.54 | 9.08 |
| [GlyC2]+ | 7.15 | 7.73 | 8.60 | / |
| [ValC1]+ | 7.83 | 7.54 | 9.34 | 8.85 |
| [SerC1]+ | 7.40 | 7.23 | 8.96 | 8.88 |
| [SerC2]+ | 7.69 | 7.10 | 9.27 | / |
| [PheC1]+ | 7.24 | 7.20 | 8.78 | 8.50 |
| [PheC2]+ | 7.46 | 7.26 | 9.05 | 8.61 |
| [D-PheC1]+ | 7.24 | 7.17 | 8.78 | 8.58 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.; Hong, C.-B.; Jiao, P.-C.; Xiang, H.; Zhang, Y.; Cai, H.-Q.; Wang, S.-L.; Tao, G.-H. The Proton Dissociation of Bio-Protic Ionic Liquids: [AAE]X Amino Acid Ionic Liquids. Molecules 2021, 26, 62. https://doi.org/10.3390/molecules26010062
He T, Hong C-B, Jiao P-C, Xiang H, Zhang Y, Cai H-Q, Wang S-L, Tao G-H. The Proton Dissociation of Bio-Protic Ionic Liquids: [AAE]X Amino Acid Ionic Liquids. Molecules. 2021; 26(1):62. https://doi.org/10.3390/molecules26010062
Chicago/Turabian StyleHe, Ting, Cheng-Bin Hong, Peng-Chong Jiao, Heng Xiang, Yan Zhang, Hua-Qiang Cai, Shuang-Long Wang, and Guo-Hong Tao. 2021. "The Proton Dissociation of Bio-Protic Ionic Liquids: [AAE]X Amino Acid Ionic Liquids" Molecules 26, no. 1: 62. https://doi.org/10.3390/molecules26010062
APA StyleHe, T., Hong, C.-B., Jiao, P.-C., Xiang, H., Zhang, Y., Cai, H.-Q., Wang, S.-L., & Tao, G.-H. (2021). The Proton Dissociation of Bio-Protic Ionic Liquids: [AAE]X Amino Acid Ionic Liquids. Molecules, 26(1), 62. https://doi.org/10.3390/molecules26010062





