Evaluation of Wound-Healing and Antioxidant Effects of Marantodes pumilum (Blume) Kuntze in an Excision Wound Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Extracts and Phytochemical Profiling
2.2. Topical Ointment Preparation
2.3. Experimental Animals
2.4. Excision Wound Model
2.5. Wound Healing Measurement
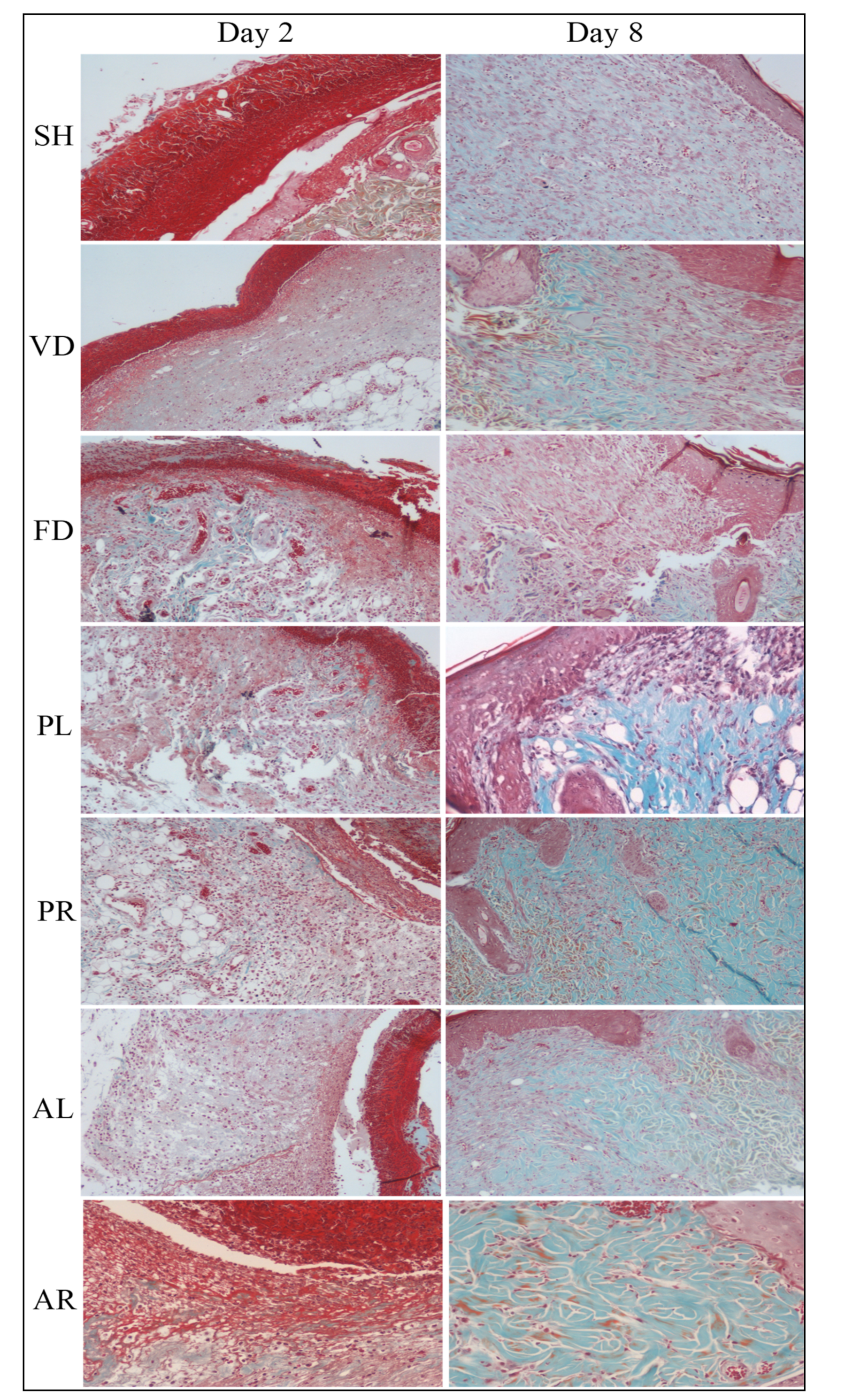
2.6. Histological Analysis
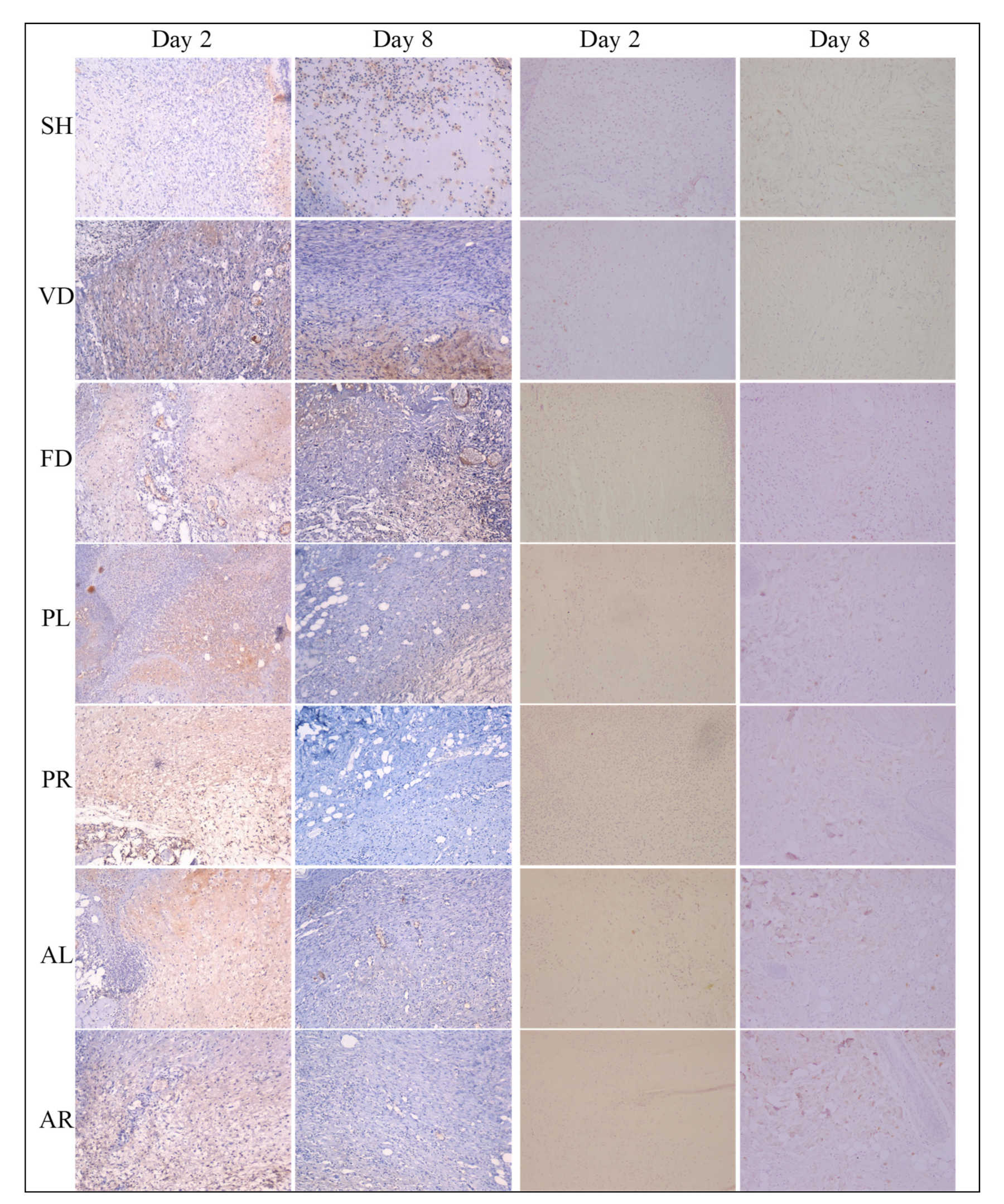
2.7. Immunohistochemistry Analysis
2.8. Biochemical Analysis
2.9. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
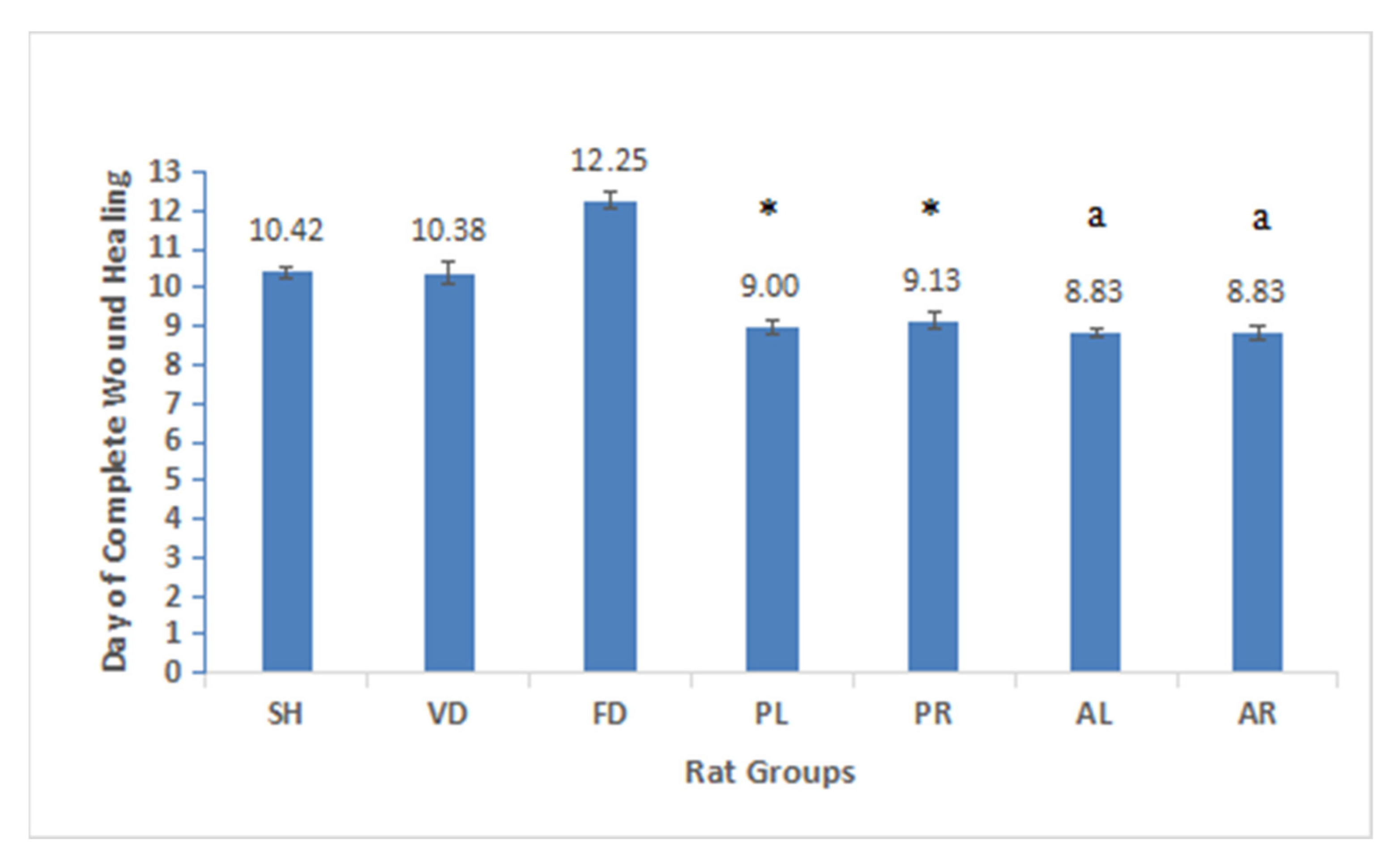
3.2. Determination of Wound Contraction
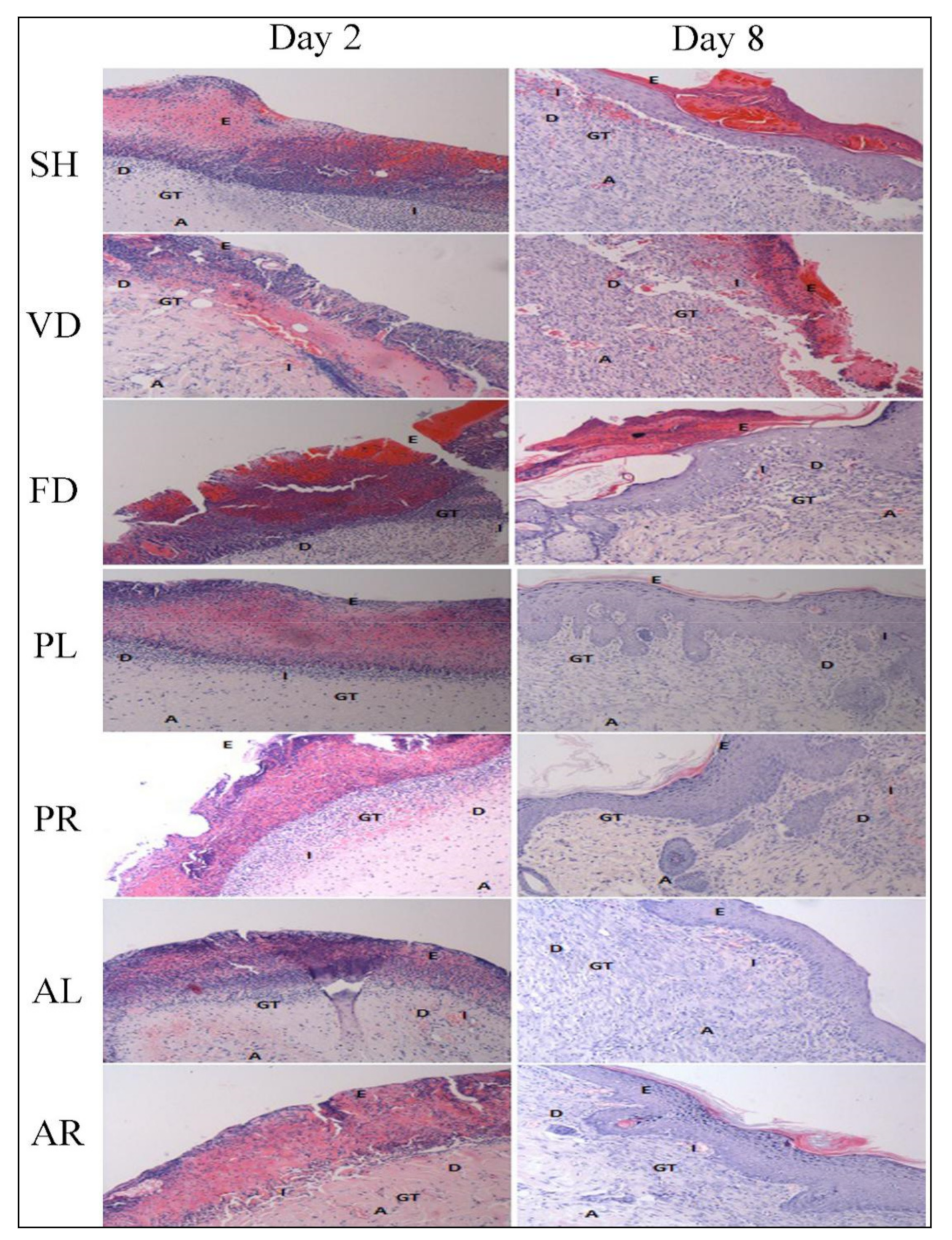
3.3. Histological Evaluation
3.4. Immunohistochemistry (IHC) Analysis
3.5. Superoxide Dismutase (SOD) Enzyme Activity
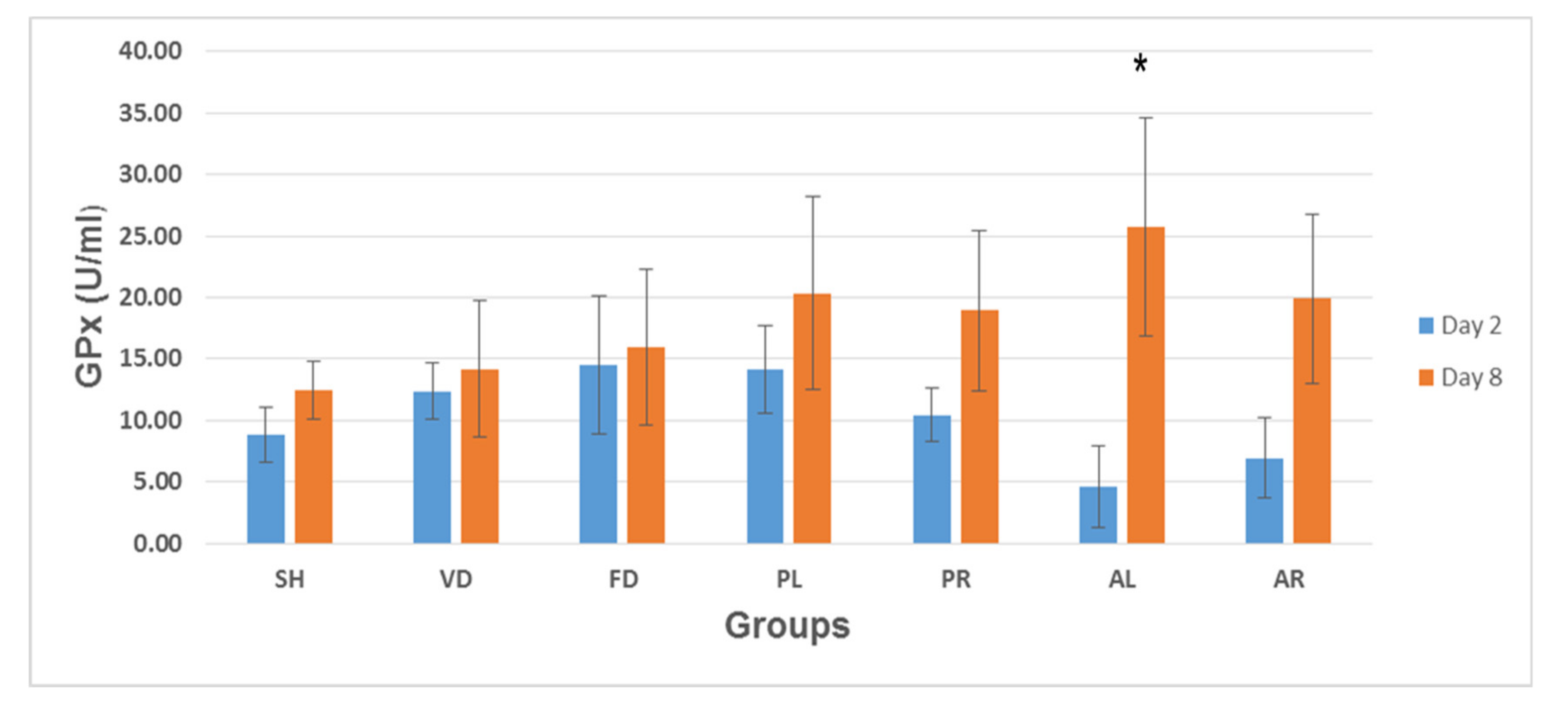
3.6. Glutathione Peroxidase (GPx) Enzyme Activity
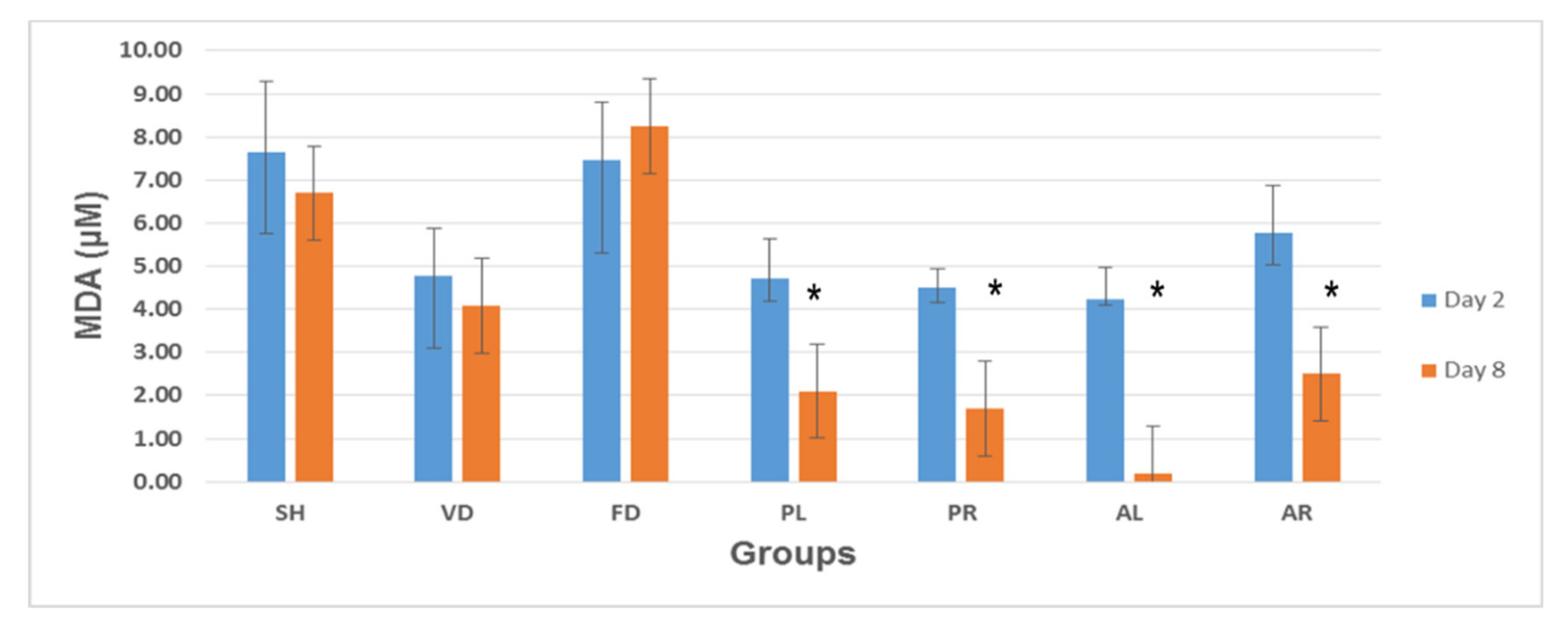
3.7. Lipid Peroxidation Analysis: Malondialdehyde (MDA) Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, R.G. Fundamentals of Cutaneous Surgery; CV Mosby: St Louis, MO, USA, 1988; pp. 75–99. [Google Scholar]
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Physiology and Healing Dynamics of Chronic Cutaneous Wounds. Am. J. Surg. 1998, 176, 26S–38S. [Google Scholar] [CrossRef]
- Rasche, H. Haemostasis and thrombosis: An overview. Eur. Heart J. Suppl. 2001, 3, Q3–Q7. [Google Scholar] [CrossRef]
- Shakespeare, P. Burn wound healing and skin substitutes. Burns 2001, 27, 517–522. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Heemskerk, J.W.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Williams, L.V.; Schwarzbauer, J.E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. [Google Scholar] [CrossRef]
- Chang, H.Y.; Sneddon, J.B.; Alizadeh, A.A.; Sood, R.; West, R.B.; Montgomery, K.; Chi, J.T.; van de Rijn, M.; Botstein, D.; Brown, P.O. Gene expression signature of fibroblast serum response predicts human cancer progression: Similarities between tumors and wounds. PLoS Biol. 2004, 2, e7. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The Role of Oxygen in Wound Healing: A Review of the Literature. Dermatol. Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef]
- The Plant List. Version 1.1. 2013. Available online: http://www.theplantlist.org/ (accessed on 1 January 2020).
- Stone, B.C. New and Noteworthy Malesian Myrsinaceae, III. On the genus Ardisia Sw. in Borneo. Proc. Acad. Nat. Sci. Phila. 1989, 141, 263–306. [Google Scholar]
- National Key Economic Area (NKEA). Available online: http://www.teraju.gov.my/nkea-info/?lang=en (accessed on 23 December 2020).
- Karimi, E.; Jaafar, H.Z. HPLC and GC-MS determination of bioactive compounds in microwave obtained extracts of three varieties of Labisia pumila Benth. Molecules 2011, 16, 6791–6805. [Google Scholar] [CrossRef]
- Hisham, D.M.N.; Lip, J.M.; Noh, J.M.; Normah, A.; Nabilah, M.N. Identification and isolation of methyl gallate as a polar chemical marker for Labisia pumila Benth. J. Trop. Agric. Food Sci. 2011, 39, 279–284. [Google Scholar]
- Avula, B.; Wang, Y.H.; Ali, Z.; Smillie, T.J.; Khan, I.A. Quantitative determination of triperpene saponins and alkenated-phenolics from Labisia pumila using an LC-UV/ELSD method and confirmation by LC-ESI-TOF. Planta Med. 2011, 77, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Al-Mekhlafi, N.A.; Shaari, K.; Abas, F.; Kneer, R.; Jeyaraj, E.J.; Stanslas, J.; Yamamoto, N.; Honda, T.; Lajis, N.H. Alkenylresorcinols and cytotoxic activity of the constituents isolated from Labisia pumila. Phytochemistry 2012, 80, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.U.; Azam, A.; Shuid, A.N.; Mohamed, I.N. Antioxidant and Anti-inflammatory Activities of Marantodes pumilum (Blume) Kuntze and Their Relationship with the Phytochemical Content. Rec. Nat. Prod. 2018, 12, 518–534. [Google Scholar] [CrossRef]
- Fazliana, M.; Gu, H.F.; Östenson, C.G.; Yusoff, M.M.; Nazaimoon, W.W. Labisia Pumila Extract Down-Regulates Hydroxysteroid (11-Beta) Dehydrogenase 1 Expression and Corticosterone Levels in Ovariectomized Rats. J. Nat. Med. 2012, 66, 257–264. [Google Scholar] [CrossRef]
- Shuid, A.N.; Ping, L.L.; Muhammad, N.; Mohamed, N.; Soelaiman, I.N. The Effects of Labisia pumila var. alata on Bone Markers and Bone Calcium in a Rat Model of Post-Menopausal Osteoporosis. J. Ethnopharmacol. 2011, 133, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, A.; Nazaimoon, W.W.; Norsyam, W.; Farihah, H.; Azian, A. Effect of Water Extract of Labisia Pumila Var Alata on Aorta of Ovariectomized Sprague Dawley Rats. Pak. J. Nutr. 2008, 7, 208–213. [Google Scholar] [CrossRef][Green Version]
- Ahmad, S.U.; Azam, A.; Shuid, A.N.; Mohamed, I.N. Phyto-estrogenic effects of Marantodes pumilum (Blume) Kuntze syn. Labisia pumila (Blume) Fern.-Vill. for the prevention and treatment of post-menopausal diseases. Indian J. Tradit. Knowl. 2017, 16, 208–215. [Google Scholar]
- Jamia, A.J.; Houghton, P.; Milligan, S.; Ibrahim, J. The Oestrogenic and Cytotoxic Effects of the Extracts of Labisia Pumila Var. Alata and Labisia Pumila Var. Pumila In Vitro. J. Sains Kesihat. 2003, 1, 53–60. [Google Scholar]
- Husniza, H. Estrogenic and Androgenic Activities of Kacip Fatimah (Labisia Pumila). In Abstracts of Research Projects; Institute for Medical Research, Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2002; p. 8. [Google Scholar]
- Ahmad, S.U.; Shuid, A.N.; Mohamed, I.N. Labisia pumila improves wound healing process in ovariectomized rat model. Bangladesh J. Pharmacol. 2018, 13, 106–113. [Google Scholar] [CrossRef]
- Karimi, E.; Jaafar, H.Z.; Ahmad, S. Phytochemical Analysis and Antimicrobial Activities of Methanolic Extracts of Leaf, Stem and Root from Different Varieties of Labisa Pumila Benth. Molecules 2011, 16, 4438–4450. [Google Scholar] [CrossRef]
- Norhaiza, M.; Maziah, M.; Hakiman, M. Antioxidative Properties of Leaf Extracts of a Popular Malaysian Herb, Labisia Pumila. J. Med. Plant Res. 2009, 3, 217–223. [Google Scholar]
- Sanusi, R.; Ab-Shukor, N.A.; Sulaiman, M.R. Anti-Inflammatory Effects of Labisia pumila (Blume) F. Vill-Naves. Aqueous Extract. Sains Malays. 2013, 42, 1511–1516. [Google Scholar]
- Dharmani, M.; Kamarulzaman, K.; Giribabu, N.; Choy, K.W.; Zuhaida, M.Z.; Aladdin, N.A.; Jamal, A.J.; Mustafa, M.R. Effect of Marantodes pumilum Blume (Kuntze) var.alata on β-cell function and insulin signaling in ovariectomised diabetic rats. Phytomedicine 2019, 65, 153101. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.U.; Azam, A.; Shuid, A.N.; Mohamed, I.N. A Dose Optimization Study of Marantodes pumilum (Blume) Kuntze Extracts for Wound Healing Effect in Animal Model. Bangladesh J. Med. Sci. (Under review).
- Ibrahim, N.I.; Khamis, M.F.; Yunoh, M.F.M.; Abdullah, S.; Mohamed, N.; Shuid, A.N. Targeted Delivery of Lovastatin and Tocotrienol to Fracture Site Promotes Fracture Healing in Osteoporosis Model: Micro-Computed Tomography and Biomechanical Evaluation. PLoS ONE 2014, 9, e115595. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.A.; Zaki, M.Z.M.; Leng, T.M.; Rahman, N.H.A.; Arshad, S.A.; Hamid, A. Alocasia Denudata Engler Treatment Enhance Open Wound Healing Activities in Wistar Rat’s Skin. J. Ethnopharmacol. 2015, 176, 258–267. [Google Scholar] [CrossRef]
- Fernandes, C.P.M.; Vaz, T.L.; Capella, S.D.O.; Garcia, E.F.; Tillmann, M.T.; Félix, S.R.; Nobre, M.D.O. Comparing open wound measuring methods popularly used in experimental studies. Braz. J. Vet. Res. Anim. Sci. 2015, 52, 106–111. [Google Scholar] [CrossRef][Green Version]
- Lynch, M.J.; Raphael, S.S.; Mellor, L.D.; Spare, P.D.; Inwood, M.J.H. Medical Laboratory Technology and Clinical Pathology, 2nd ed.; W. B. Saunders Co.: Toronto, ON, Canada, 1969. [Google Scholar]
- Abramov, Y.; Golden, B.; Sullivan, M.; Botros, S.M.; Miller, J.J.; Alshahrour, A.; Goldberg, R.P.; Sand, P.K. Histologic Characterization of Vaginal vs. Abdominal Surgical Wound Healing in a Rabbit Model. Wound Repair Regen. 2007, 15, 80–86. [Google Scholar] [CrossRef]
- Rizzi, S.C.; Upton, Z.; Bott, K.; Dargaville, T.R. Recent Advances in Dermal Wound Healing: Biomedical Device Approaches. Expert Rev. Med. Devices 2010, 7, 143–154. [Google Scholar] [CrossRef]
- Houghton, P.J.; Hylands, P.J.; Mensah, A.Y.; Hensel, A.; Deters, A.M. In vitro tests and ethnopharmacological investigations: Wound healing as an example. J. Ethnopharmacol. 2005, 100, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.I.; Brunicardi, F.C. Schwartz’s Principles of Surgery, 9th ed.; McGraw-Hill Education, Medical Pub. Division: New York, NY, USA, 2010. [Google Scholar]
- Henry, G.; Garner, W.L. Inflammatory Mediators in Wound Healing. Surg. Clin. N. Am. 2003, 83, 483–507. [Google Scholar] [CrossRef]
- Ohta, H.; Yatomi, Y.; Sweeney, E.A.; Hakomori, S.I.; Igarashi, Y. A possible role of sphingosine in induction of apoptosis by tumor necrosis factor-α, in human neutrophils. FEBS Lett. 1994, 355, 267–270. [Google Scholar] [CrossRef]
- Kuwahara, R.T.; Rasberry, R. Chemical Peels. Archived 25 October 2008. Available online: https://emedicine.medscape.com (accessed on 15 September 2019).
- Forrest, L. Current Concepts in Soft Connective Tissue Wound Healing. Br. J. Surg. 1983, 70, 133–140. [Google Scholar] [CrossRef]
- Song, G.; Nguyen, D.T.; Pietramaggiori, G.; Scherer, S.; Chen, B.; Zhan, Q.; Ogawa, R.; Yannas, I.; Wagers, A.J.; Orgill, D.P. Use of the Parabiotic Model in Studies of Cutaneous Wound Healing to Define the Participation of Circulating Cells. Wound Repair Regen. 2010, 18, 426–432. [Google Scholar] [CrossRef]
- Simon, P.E.; Al Moutran, H.; Romo, T. Skin Wound Healing. Available online: https://emedicine.medscape.com/article/884594-overview (accessed on 1 January 2020).
- Grinnell, F.; Billingham, R.E.; Burgess, L. Distribution of Fibronectin during Wound Healing In Vivo. J. Investig. Dermatol. 1981, 76, 181–189. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Broughton, G., II; Janis, J.E.; Attinger, C.E. The Basic Science of Wound Healing. Plast. Reconstr. Surg. 2006, 117 (Suppl. 7), 12S–34S. [Google Scholar] [CrossRef]
- Nazzal, M.; Osman, M.; Albeshri, H.; Abbas, D.; Angel, C. Wound Healing: Schwartz’s Principles of Surgery, 11th ed.; McGraw-Hill Education, Medical Pub. Division: New York, NY, USA, 2019. [Google Scholar]
- Madri, J.A.; Marx, M. Matrix Composition, Organization and Soluble Factors: Modulators of Microvascular Cell Differentiation In Vitro. Kidney Int. 1992, 41, 560–565. [Google Scholar] [CrossRef]
- Montesano, R.; Orci, L.; Vassalli, P. In Vitro Rapid Organization of Endothelial Cells into Capillary-Like Networks Is Promoted by Collagen Matrices. J. Cell Biol. 1983, 97, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Adelmann-Grill, B. Collagenolytic Cleavage Products of Collagen Type I as Chemoattractants for Human Dermal Fibroblasts. Eur. J. Cell Biol. 1985, 36, 104–107. [Google Scholar] [PubMed]
- Hynes, R.O. Integrins: Versatility, Modulation, and Signaling in Cell Adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Mercandetti, M.; Cohen, A.J. Wound Healing and Repair. Available online: https://emedicine.medscape.com/article/1298129-overview (accessed on 20 January 2008).
- Esimone, C.; Nworu, C.; Jackson, C. Cutaneous Wound Healing Activity of a Herbal Ointment Containing the Leaf Extract of Jatropha Curcas L. (Euphorbiaceae). Int. J. Appl. Res. Nat. Prod. 2008, 1, 1–4. [Google Scholar]
- Sanwal, R.; Chaudhary, A.K. Wound Healing and Antimicrobial Potential of Carissa Spinarum Linn in Albino Mice. J. Ethnopharmacol. 2011, 135, 792–796. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, Oxidative Stress and the Biology of Ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Clark, L.; Moon, R. Hyperbaric oxygen in the treatment of life-threatening soft-tissue infections. Respir Care Clin. N. Am. 1999, 5, 203–219. [Google Scholar]
- Siwik, D.A.; Pagano, P.J.; Colucci, W.S. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am. J. Physiol. Cell Physiol. 2001, 280, 53–60. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.; Grootveld, M. Methods for the measurements of hydroxyl radicals in biomedical systems: Deoxyribose degradation and aromatic hydroxylation. Methods Biochem. Anal. 1988, 33, 59–90. [Google Scholar]
- Altavilla, D.; Squadrito, F.; Polito, F.; Irrera, N.; Calò, M.; Cascio, P.L.; Galeano, M.; Cava, L.L.; Minutoli, L.; Marini, H.; et al. Activation of adenosine A2A receptors restores the altered cell-cycle machinery during impaired wound healing in genetically diabetic mice. Surgery 2011, 149, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tang, W.; Li, Y.; Yang, F.; Dowd, D.R.; Macdonald, P.N. Osteoblast-Specific Transcription Factor Osterix Increases Vitamin D Receptor Gene Expression in Osteoblasts. PLoS ONE 2011, 6, e26504. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, K.; Vijayakumar, R.; Vanitha, P.; Natarajan, S.; Manjula, C.; Rajaguru, P.; Sivasubramanian, S.; Gunasekaran, P. Protective effect of gallic acid on alloxan-induced oxidative stress and osmotic fragility in rats. Hum. Exp. Toxicol. 2014, 33, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Omur, A.D.; Coyan, K. Protective effects of the antioxidants curcumin, ellagic acid and methionine on motility, mitochondrial transmembrane potential, plasma membrane and acrosome integrity in freeze-thawed Merino ram sperm. Vet. Med. 2016, 61, 10–16. [Google Scholar] [CrossRef]
- Yousef, A.I.; El-Masry, O.S.; Abdel Mohsen, M.A. Impact of Cellular Genetic Make-up on Colorectal Cancer Cell Lines Response to Ellagic Acid: Implications of small interfering RNA. Asian Pac. J. Cancer Prev. 2016, 17, 743–748. [Google Scholar] [CrossRef]
- Padma, V.V.; Sowmya, P.; Felix, T.A.; Baskaran, R.; Poornima, P. Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem. Toxicol. 2011, 49, 991–998. [Google Scholar] [CrossRef]
- Panda, S.K. Assay Guided Comparison for Enzymatic and Non-Enzymatic Antioxidant Activities with Special Reference to Medicinal Plants. In Antioxidant Enzyme; El-Missiry, M.A., Ed.; InTech (Online): London, UK, 2012; pp. 38–400. [Google Scholar]



| Extracts | Retention Time (min) | [M − H]− m/z, (Da) | [M + H]+ m/z, (Da) | Fragment Ions | Detected Chemical Constituents |
|---|---|---|---|---|---|
| MPvp leaves | 1.43 | 165 | 147, 105, 75 | Hydroxylated cinnamic acid | |
| 1.76 | 331 | 303, 211, 192, 169, 124 | Monogalloyl glucose isomer | ||
| 1.88 | 191 | 173, 129, 110, 87 | Quinic acid | ||
| 2.13 | 169 | 124, 95, 68 | Gallic acid | ||
| 2.72 | 483 | 331, 169, 124 | Digalloyl glucose | ||
| 2.85 | 179 | 135, 107 | Caffeic acid | ||
| 3.11 | 301 | 257, 229, 185 | Ellagic acid | ||
| 3.72 | 470 | 452, 318, 300, 256, 169 | Gallic acid derivative | ||
| 3.77 | 136 | 108 | p-hydroxybenzoic acid | ||
| 3.99 | 451 | 433, 361, 289, 165 | Catechin-7-O-glucoside | ||
| 4.28 | 147 | 118, 102 | Cinnamic acid | ||
| 4.39 | 289 | 245, 201, 181 | Catechin | ||
| 4.95 | 463 | 373, 316, 300, 271, 179 | Myricetin derivative | ||
| 5.23 | 331 | 287, 271, 211, 179, 150 | Monogalloyl glucose | ||
| 6.58 | 327 | 309, 201, 171 | Methyl-2-[cyclohex-2-en-1-yl(hydroxy)methyl]-3-hydroxy-4-(2-hydroxyethyl)-3-methyl-5-oxoprolinate | ||
| MPvp roots | 2.12 | 169 | 124, 95, 68 | Gallic acid | |
| 2.37 | 315 | 195, 163, 152, 123 | Protocatechuic acid hexoside | ||
| 2.84 | 179 | 135, 107 | Caffeic acid | ||
| 2.98 | 153 | 123, 108 | Protocatechuic Acid | ||
| 3.12 | 301 | 257, 229, 185 | Ellagic acid | ||
| 5.00 | 483 | 313, 169, 139 | Digalloyl glucose | ||
| 6.18 | 329 | 311, 201, 171 | 3,30-di-O-methyl ellagic acid | ||
| MPva leaves | 2.12 | 169 | 124, 95, 68 | Gallic acid | |
| 2.94 | 179 | 135, 107 | Caffeic acid | ||
| 3.05 | 153 | 123, 108, 66 | Protocatechuic acid | ||
| 3.13 | 301 | 257, 229, 185 | Ellagic acid | ||
| 4.00 | 636 | 484, 465, 313, 169 | Gallic acid derivative | ||
| 4.79 | 136 | 92, 64 | p-hydroxybenzoic acid | ||
| 5.24 | 331 | 287, 271, 179, 151 | Monogalloyl glucose | ||
| 5.69 | 327 | 309, 201, 171, 137 | Methyl-2-[cyclohex-2-en-1-yl(hydroxy)methyl]-3-hydroxy-4-(2-hydroxyethyl)-3-methyl-5-oxoprolinate | ||
| 5.91 | 329 | 311, 211, 171, 139 | 3,30-di-O-methyl ellagic acid | ||
| 6.64 | 327 | 291, 211, 171 | Methyl-2-[cyclohex-2-en-1-yl(hydroxy)methyl]-3-hydroxy-4-(2-hydroxyethyl)-3-methyl-5-oxoprolinate isomer | ||
| MPva roots | 1.37 | 377 | 341, 215, 179 | Caffeic acid derivative | |
| 1.49 | 191 | 172, 111, 84 | Quinic acid | ||
| 2.13 | 169 | 124, 95, 68 | Gallic acid | ||
| 2.65 | 153 | 123, 108, 64 | Protocatechuic acid | ||
| 2.84 | 179 | 135, 107 | Caffeic acid | ||
| 3.11 | 301 | 257, 229, 185 | Ellagic acid | ||
| 4.16 | 289 | 245, 225, 209, 137 | Catechin |
| Extracts | Content (mg/g) | ||
|---|---|---|---|
| Gallic Acid | Caffeic Acid | Ellagic Acid | |
| MPvp leaves | 6.810 | <0 | 0.0585 |
| MPvp roots | 4.080 | 0.00052 | 0.335 |
| MPva leaves | 5.540 | <0 | 0.0701 |
| MPva roots | 2.240 | <0 | 0.115 |
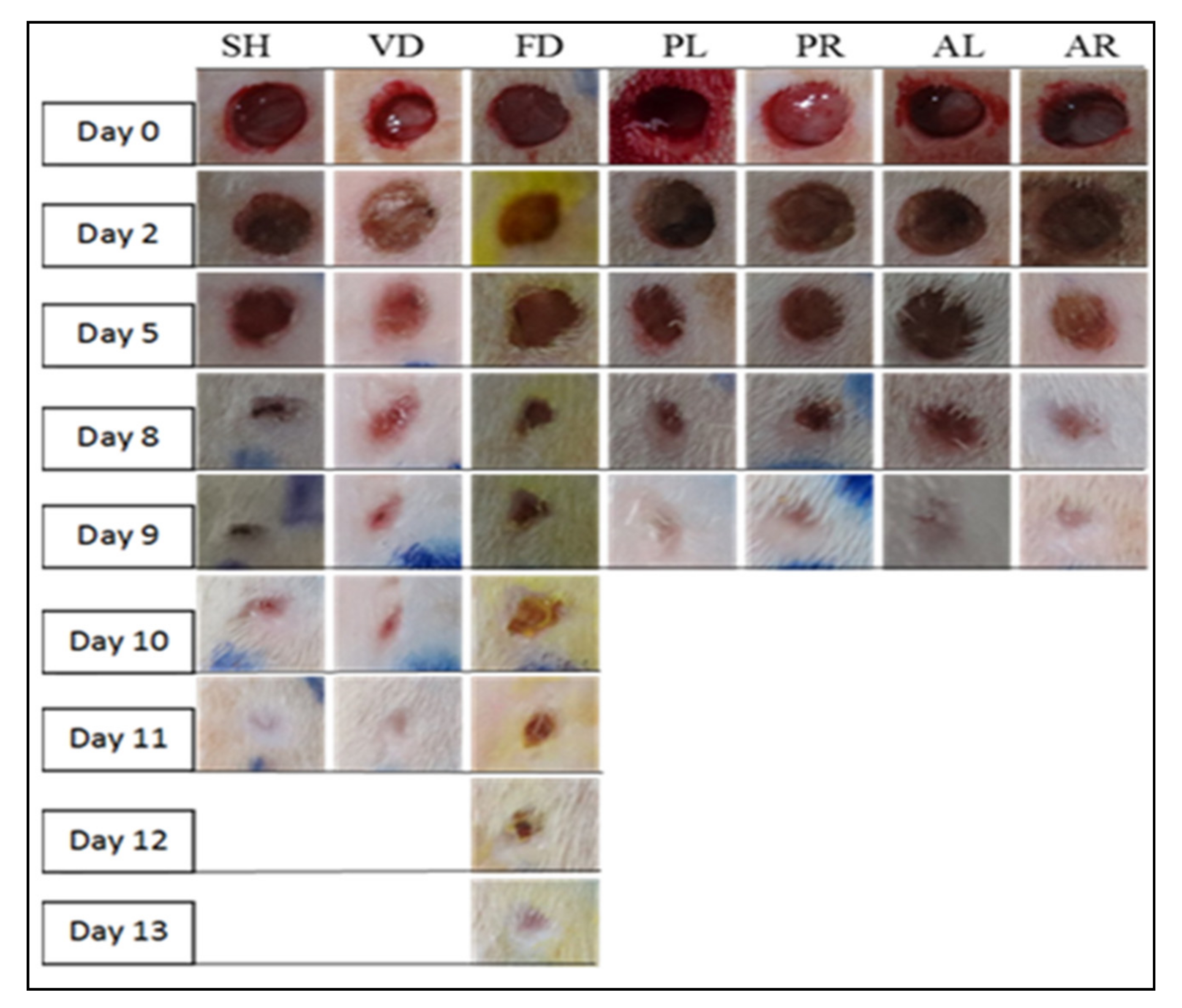
| Macroscopic Observations | Day | ||||||
|---|---|---|---|---|---|---|---|
| SH | VD | FD | PL | PR | AL | AR | |
| Excision | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Secreting fluid | 1–3 | 1–3 | 1–3 | 1–2 | 1–2 | 1–2 | 1–2 |
| Scab | 4–7 | 4–7 | 4–9 | 3–5 | 3–5 | 3–5 | 3–5 |
| Granulation tissue | 8–9 | 8–9 | 10–11 | 6–8 | 6–8 | 6–7 | 6–7 |
| Fibrous | 10–11 | 10–11 | 12–13 | 9 | 9 | 8–9 | 8–9 |
| Groups | Re-Epithelialization | Inflammatory Cell Infiltration | Fibroblast Cell Proliferation | Neo-Vascularization | Granulation Tissue Formation | Collagen Deposition | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 2 | Day 8 | Day 2 | Day 8 | Day 2 | Day 8 | Day 2 | Day 8 | Day 2 | Day 8 | Day 2 | Day 8 | |
| SH | 0.33 ± 0.21 | 2.17 ± 0.11 | 2.00 ± 0.26 | 1.50 ± 0.22 | 1.77 ± 0.17 | 2.17 ± 0.17 | 0.50 ± 0.22 | 1.83 ± 0.31 | 1.67 ± 0.21 | 2.67 ± 0.21 | 1.00 ± 0.00 | 1.58 ± 0.15 |
| VD | 0.33 ± 0.21 | 2.17 ± 0.31 | 2.00 ± 0.26 | 1.67 ± 0.21 | 1.33 ± 0.21 | 2.33 ± 0.21 | 0.83 ± 0.17 | 2.00 ± 0.26 | 1.67 ± 0.21 | 2.17 ± 0.17 | 0.67 ± 0.21 | 1.67 ± 0.17 |
| FD | 0.33 ± 0.21 | 2.08 ± 0.15 | 2.00 ± 0.26 | 1.83 ± 0.17 | 1.67 ± 0.33 | 2.33 ± 0.21 | 0.67 ± 0.21 | 1.67 ± 0.21 | 1.67 ± 0.21 | 2.00 ± 0.00 | 0.67± 0.21 | 1.42 ± 0.20 |
| PL | 0.33 ± 0.21 | 2.83 ± 0.11 * | 1.83 ± 0.17 | 1.00 ± 0.22 | 1.17 ± 0.17 | 2.67 ± 0.21 | 0.92 ± 0.08 | 2.75 ± 0.17 a | 2.00 ± 0.26 | 2.33 ± 0.21 | 0.83± 0.11 | 2.67 ± 0.17 * |
| PR | 0.50 ± 0.22 | 2.92 ± 0.08 * | 1.83 ± 0.31 | 0.92 ± 0.08 a | 1.33 ± 0.21 | 2.67 ± 0.21 | 1.17 ± 0.17 | 2.58 ± 0.20 | 2.33 ± 0.21 | 2.50 ± 0.22 | 1.00± 0.00 | 2.58 ± 0.20 * |
| AL | 0.67 ± 0.33 | 3.00 ± 0.00 * | 1.83 ± 0.17 | 0.67 ± 0.17 a | 1.17 ± 0.17 | 3.00 ± 0.00 * | 1.17 ± 0.17 | 2.75 ± 0.17 a | 2.00 ± 0.00 | 2.83 ± 0.17 a | 1.17 ± 0.17 | 2.58 ± 0.20 * |
| AR | 0.17 ± 0.17 | 2.92 ± 0.08 * | 1.33 ± 0.21 | 0.67 ± 0.21 a | 1.83 ± 0.17 | 2.67 ± 0.21 | 0.92 ± 0.08 | 2.42 ± 0.20 | 2.00 ± 0.00 | 2.83 ± 0.17 a | 1.00 ± 0.00 | 2.58 ± 0.20 * |
| Score of Proteins Expression | Day | SH | VD | FD | PL | PR | AL | AR |
|---|---|---|---|---|---|---|---|---|
| Fibronectin | Day 2 | 1.58 ± 0.15 | 1.17 ± 0.17 | 0.92 ± 0.17 | 2.92 ± 0.08 | 2.83 ± 0.11 | 3.00 ± 0.00 | 2.58 ± 0.20 |
| Day 8 | 1.20 ± 0.11 | 1.17 ± 0.21 | 1.63 ± 0.17 | 0.40 ± 0.15 * | 0.50 ± 0.17 * | 0.50 ± 0.17 * | 0.83 ± 0.11 * | |
| Fibroblast | Day 2 | 1.17 ± 0.11 | 1.33 ± 0.21 | 1.17 ± 0.11 | 2.17 ± 0.17 | 2.67 ± 0.21 | 2.50 ± 0.22 | 2.33 ± 0.21 |
| Day 8 | 2.17 ± 0.17 | 2.42 ± 0.20 | 2.00 ± 0.00 | 3.00 ± 0.00 | 2.92 ± 0.08 | 3.00 ± 0.00 | 2.83 ± 0.11 | |
| Collagen-III | Day 2 | 2.20 ± 0.15 | 2.10 ± 0.17 | 2.58 ± 0.20 | 2.67 ± 0.17 | 2.70 ± 0.20 | 2.72 ± 0.20 | 2.58 ± 0.20 |
| Day 8 | 1.20 ± 0.11 | 1.50 ± 0.21 | 1.57 ± 0.21 | 0.63 ± 0.17 * | 0.40 ± 0.20 * | 0.50 ± 0.22 * | 0.50 ± 0.17 * | |
| Collagen-I | Day 2 | 0.67 ± 0.21 | 0.50 ± 0.22 | 0.50 ± 0.22 | 0.83 ± 0.11 | 1.25 ± 0.17 | 1.25 ± 0.17 | 1.33 ± 0.21 |
| Day 8 | 1.17 ± 0.25 | 1.17 ± 0.17 | 1.33 ± 0.17 | 1.50 ± 0.18 | 2.00 ± 0.17 | 2.10 ± 0.18 | 2.00 ± 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.U.; Binti Aladdin, N.-A.; Jamal, J.A.; Shuid, A.N.; Mohamed, I.N. Evaluation of Wound-Healing and Antioxidant Effects of Marantodes pumilum (Blume) Kuntze in an Excision Wound Model. Molecules 2021, 26, 228. https://doi.org/10.3390/molecules26010228
Ahmad SU, Binti Aladdin N-A, Jamal JA, Shuid AN, Mohamed IN. Evaluation of Wound-Healing and Antioxidant Effects of Marantodes pumilum (Blume) Kuntze in an Excision Wound Model. Molecules. 2021; 26(1):228. https://doi.org/10.3390/molecules26010228
Chicago/Turabian StyleAhmad, Shihab Uddin, Nor-Ashila Binti Aladdin, Jamia Azdina Jamal, Ahmad Nazrun Shuid, and Isa Naina Mohamed. 2021. "Evaluation of Wound-Healing and Antioxidant Effects of Marantodes pumilum (Blume) Kuntze in an Excision Wound Model" Molecules 26, no. 1: 228. https://doi.org/10.3390/molecules26010228
APA StyleAhmad, S. U., Binti Aladdin, N.-A., Jamal, J. A., Shuid, A. N., & Mohamed, I. N. (2021). Evaluation of Wound-Healing and Antioxidant Effects of Marantodes pumilum (Blume) Kuntze in an Excision Wound Model. Molecules, 26(1), 228. https://doi.org/10.3390/molecules26010228