Evaluation of Ionic Liquids and Ionic Liquids Active Pharmaceutical Ingredients Inhibition in Elastase Enzyme Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of Ionic Liquids Inhibition in Elastase Enzyme Activity
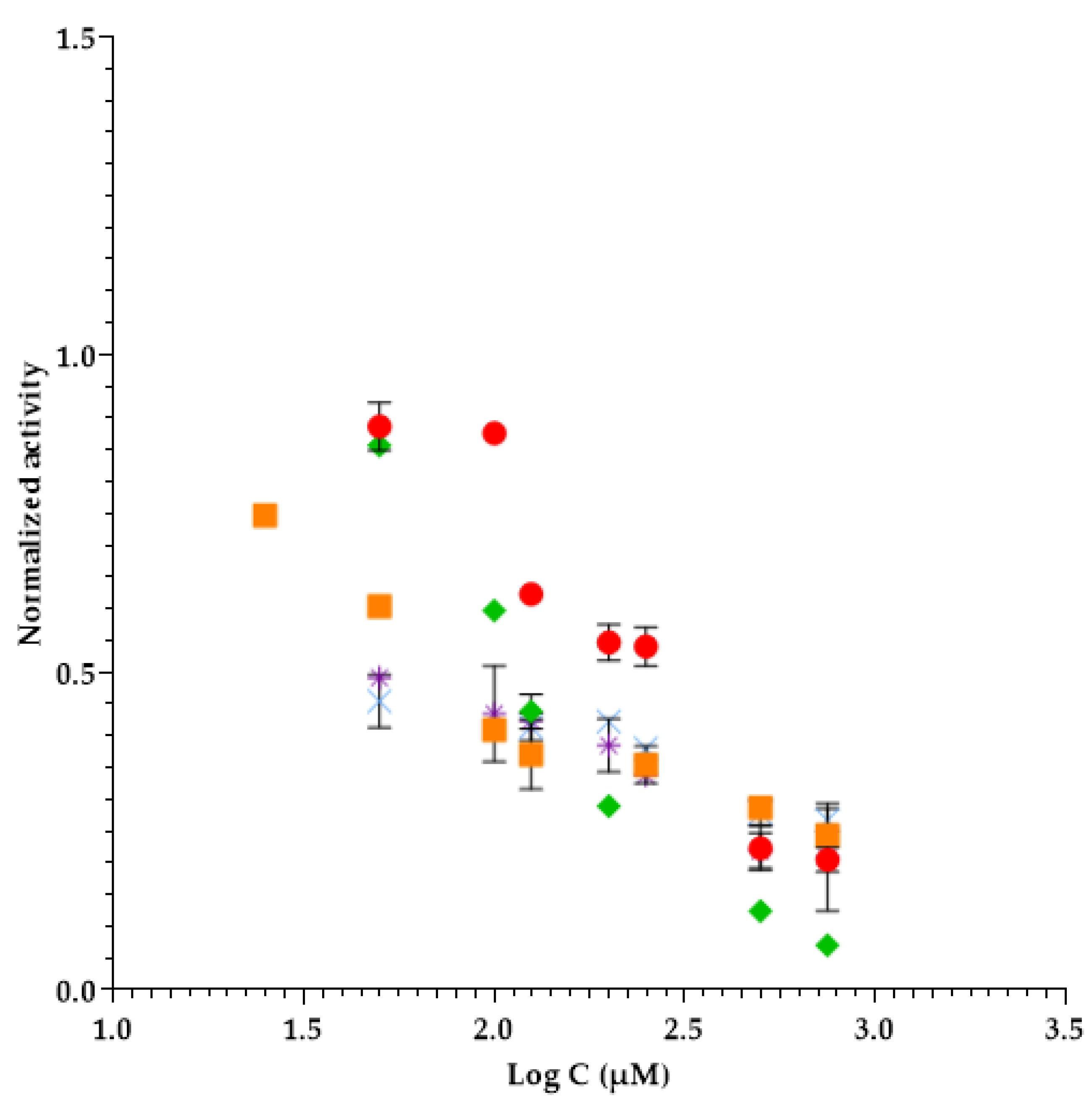
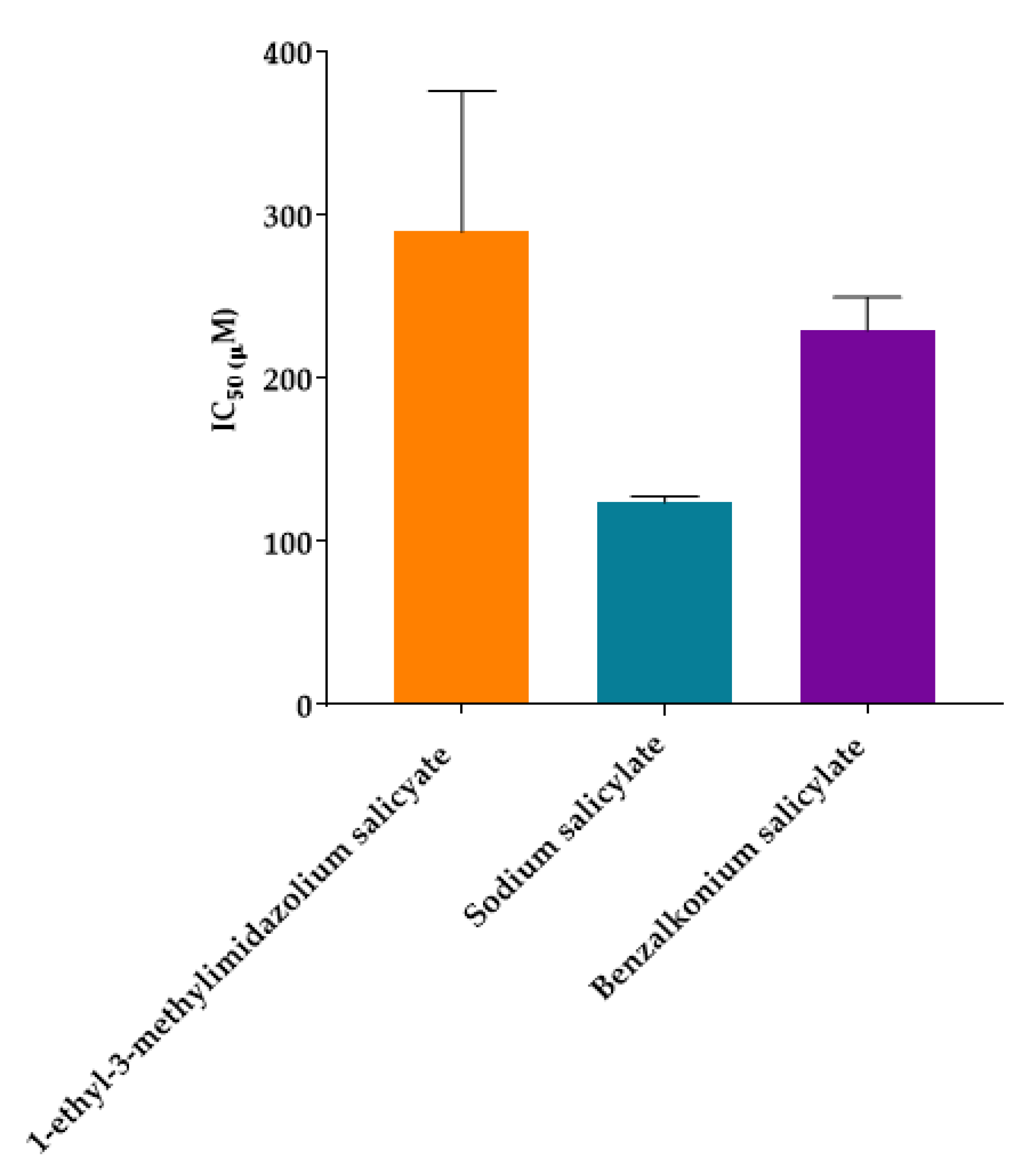
2.2. Evaluation of Ionic Liquids Active Pharmaceutical Ingredients (IL-APIs) Inhibition in Elastase Enzyme Activity
3. Materials and Methods
3.1. Reagents
3.2. Apparatus
3.3. Optimization of Elastase Porcine Reaction
3.4. Optimized Batch Procedure
3.5. Experimental Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hasmann, A.; Gewessler, U.; Hulla, E.; Schneider, K.P.; Binder, B.; Francesko, A.; Tzanov, T.; Schintler, M.; Van der Palen, J.; Guebitz, G.M.; et al. Sensor materials for the detection of human neutrophil elastase and cathepsin G activity in wound fluid. Exp. Dermatol. 2011, 20, 508–513. [Google Scholar] [CrossRef] [PubMed]
- von Nussbaum, F.; Li, V. Neutrophil elastase inhibitors for the treatment of (cardio)pulmonary diseases: Into clinical testing with pre-adaptive pharmacophores. Bioorganic. Med. Chem. Lett. 2015, 25, 4370–4381. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharm. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.D.; Costa, E.; Guedes, R.C.; Moreira, R. Targeting COPD: Advances on low-molecular-weight inhibitors of human neutrophil elastase. Med. Res. Rev. 2013, 33, E73–E101. [Google Scholar] [CrossRef]
- Kafienah, W.; Buttle, D.J.; Burnett, D.; Hollander, A.P. Cleavage of native type I collagen by human neutrophil elastase. Biochem. J. 1998, 330 Pt 2, 897–902. [Google Scholar] [CrossRef]
- Tsaka, T.; Herkner, K.R. Polymorphonuclear elastase in neonatal sepsis. Clin. Chim. Acta 1990, 193, 103–111. [Google Scholar] [CrossRef]
- Peters, K.M.; Koberg, K.; Rosendahl, T.; Haubeck, H.D. PMN elastase in bone and joint infections. Int. Orthop. 1994, 18, 352–355. [Google Scholar] [CrossRef]
- Diegelmann, R.; Evans, M. Wound Healing: An Overview of Acute, Fibrotic and Delayed Healing. Front. Biosci. J. Virtual Libr. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Ferreira, A.V.; Perelshtein, I.; Perkas, N.; Gedanken, A.; Cunha, J.; Cavaco-Paulo, A. Detection of human neutrophil elastase (HNE) on wound dressings as marker of inflammation. Appl. Microbiol. Biotechnol. 2017, 101, 1443–1454. [Google Scholar] [CrossRef]
- Lv, X.; Chen, K.; Shi, G.; Lin, W.; Bai, H.; Li, H.; Tang, G.; Wang, C. Design and tuning of ionic liquid–based HNO donor through intramolecular hydrogen bond for efficient inhibition of tumor growth. Sci. Adv. 2020, 6, eabb7788. [Google Scholar] [CrossRef]
- Zhao, H. Methods for stabilizing and activating enzymes in ionic liquids—A review. J. Chem. Technol. Biotechnol. 2010, 85, 891–907. [Google Scholar] [CrossRef]
- Hough, W.L.; Smiglak, M.; Rodríguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The third evolution of ionic liquids: Active pharmaceutical ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Kelley, S.P.; Gurau, G.; Rogers, R.D. Chemistry: Develop ionic liquid drugs. Nature 2015, 528, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Bica, K.; Rogers, R.D. Confused ionic liquid ions—a “liquification” and dosage strategy for pharmaceutically active salts. Chem. Commun. 2010, 46, 1215–1217. [Google Scholar] [CrossRef]
- Stoimenovski, J.; MacFarlane, D.R.; Bica, K.; Rogers, R.D. Crystalline vs. Ionic Liquid Salt Forms of Active Pharmaceutical Ingredients: A Position Paper. Pharm. Res. 2010, 27, 521–526. [Google Scholar] [CrossRef]
- Ferraz, R.; Branco, L.C.; Marrucho, I.M.; Araújo, J.M.M.; Rebelo, L.P.N.; da Ponte, M.N.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž. Development of novel ionic liquids based on ampicillin. MedChemComm 2012, 3, 494–497. [Google Scholar] [CrossRef]
- Bica, K.; Rijksen, C.; Nieuwenhuyzen, M.; Rogers, R.D. In search of pure liquid salt forms of aspirin: Ionic liquid approaches with acetylsalicylic acid and salicylic acid. Phys. Chem. Chem. Phys. 2010, 12, 2011–2017. [Google Scholar] [CrossRef]
- Bode, W.; Meyer, E., Jr.; Powers, J.C. Human leukocyte and porcine pancreatic elastase: X-ray crystal structures, mechanism, substrate specificity, and mechanism-based inhibitors. Biochemistry 1989, 28, 1951–1963. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Toxicity of Ionic Liquids: Eco(cyto)activity as Complicated, but Unavoidable Parameter for Task-Specific Optimization. ChemSusChem 2014, 7, 336–360. [Google Scholar] [CrossRef]
- Dong, X.; Fan, Y.; Zhang, H.; Zhong, Y.; Yang, Y.; Miao, J.; Hua, S. Inhibitory effects of ionic liquids on the lactic dehydrogenase activity. Int. J. Biol. Macromol. 2016, 86, 155–161. [Google Scholar] [CrossRef]
- Fan, Y.; Dong, X.; Li, X.; Zhong, Y.; Kong, J.; Hua, S.; Miao, J.; Li, Y. Spectroscopic studies on the inhibitory effects of ionic liquids on lipase activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 159, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko Bubalo, M.; Radošević, K.; Gaurina Srček, V.; Das, R.N.; Popelier, P.; Roy, K. Cytotoxicity towards CCO cells of imidazolium ionic liquids with functionalized side chains: Preliminary QSTR modeling using regression and classification based approaches. Ecotoxicol. Environ. Saf. 2015, 112, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Naushad, M.; Alothman, Z.A.; Khan, A.B.; Ali, M. Effect of ionic liquid on activity, stability, and structure of enzymes: A review. Int. J. Biol. Macromol. 2012, 51, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.M.O.; Pereira, S.A.P.; Passos, M.L.C.; Costa, S.P.F.; Pinto, P.C.A.G.; Araujo, A.R.T.S.; Saraiva, M.L.M.F.S. Assessment of ionic liquids’ toxicity through the inhibition of acylase I activity on a microflow system. Chemosphere 2017, 173, 351–358. [Google Scholar] [CrossRef]
- Costa, S.P.F.; Martins, B.S.F.; Pinto, P.C.A.G.; Saraiva, M.L.M.F.S. Automated cytochrome c oxidase bioassay developed for ionic liquids’ toxicity assessment. J. Hazard. Mater. 2016, 309, 165–172. [Google Scholar] [CrossRef]
- Gao, W.-W.; Zhang, F.-X.; Zhang, G.-X.; Zhou, C.-H. Key factors affecting the activity and stability of enzymes in ionic liquids and novel applications in biocatalysis. Biochem. Eng. J. 2015, 99, 67–84. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Gonçalves, A.M.M.; Sintra, T.; Pereira, J.L.; Gonçalves, F.; Coutinho, J.A.P. Designing ionic liquids: The chemical structure role in the toxicity. Ecotoxicology 2013, 22, 1–12. [Google Scholar] [CrossRef]
- Arning, J.; Stolte, S.; Böschen, A.; Stock, F.; Pitner, W.R.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Qualitative and quantitative structure activity relationships for the inhibitory effects of cationic head groups, functionalised side chains and anions of ionic liquids on acetylcholinesterase. Green Chem. 2008, 10, 47–58. [Google Scholar] [CrossRef]
- Stock, F.; Hoffmann, J.; Ranke, J.; Stoermann, R.; Ondruschka, B.; Jastorff, B. Effects of Ionic Liquids on the Acetylcholinesterase —A Structure-Activity Relationship Consideration. Green Chem. 2004, 6, 286–290. [Google Scholar] [CrossRef]
- Docherty, K.M.; Kulpa, J.C.F. Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem. 2005, 7, 185–189. [Google Scholar] [CrossRef]
- Paul, T.; Anastas, S.; Peter, W.; Stark, A. Green Solvents: Ionic Liquids; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 6. [Google Scholar]
- Luis, P.; Garea, A.; Irabien, A. Quantitative structure–activity relationships (QSARs) to estimate ionic liquids ecotoxicity EC50 (Vibrio fischeri). J. Mol. Liq. 2010, 152, 28–33. [Google Scholar] [CrossRef]
- Biczak, R. Quaternary ammonium salts with tetrafluoroborate anion: Phytotoxicity and oxidative stress in terrestrial plants. J. Hazard. Mater. 2016, 304, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Frade, R.F.M.; Rosatella, A.A.; Marques, C.S.; Branco, L.C.; Kulkarni, P.S.; Mateus, N.M.M.; Afonso, C.A.M.; Duarte, C.M.M. Toxicological evaluation on human colon carcinoma cell line (CaCo-2) of ionic liquids based on imidazolium, guanidinium, ammonium, phosphonium, pyridinium and pyrrolidinium cations. Green Chem. 2009, 11, 1660–1665. [Google Scholar] [CrossRef]
- Pinto, P.C.A.G.; Ribeiro, D.M.G.P.; Azevedo, A.M.O.; Dela Justina, V.; Cunha, E.; Bica, K.; Vasiloiu, M.; Reis, S.; Saraiva, M.L.M.F.S. Active pharmaceutical ingredients based on salicylate ionic liquids: Insights into the evaluation of pharmaceutical profiles. New J. Chem. 2013, 37, 4095–4102. [Google Scholar] [CrossRef]
- Costa, S.P.F.; Justina, V.D.; Bica, K.; Vasiloiu, M.; Pinto, P.C.A.G.; Saraiva, M.L.M.F.S. Automated evaluation of pharmaceutically active ionic liquids’ (eco)toxicity through the inhibition of human carboxylesterase and Vibrio fischeri. J. Hazard. Mater. 2014, 265, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Fincke, A.C.; Tikhomirov, A.S.; Braune, A.; Girbl, T.; Gilberg, E.; Bajorath, J.; Blaut, M.; Nourshargh, S.; Gütschow, M. Design of an Activity-Based Probe for Human Neutrophil Elastase: Implementation of the Lossen Rearrangement To Induce Förster Resonance Energy Transfers. Biochemistry 2018, 57, 742–752. [Google Scholar] [CrossRef]
- Peterson, M.E.; Daniel, R.M.; Danson, M.J.; Eisenthal, R. The dependence of enzyme activity on temperature: Determination and validation of parameters. Biochem. J. 2007, 402, 331–337. [Google Scholar] [CrossRef]
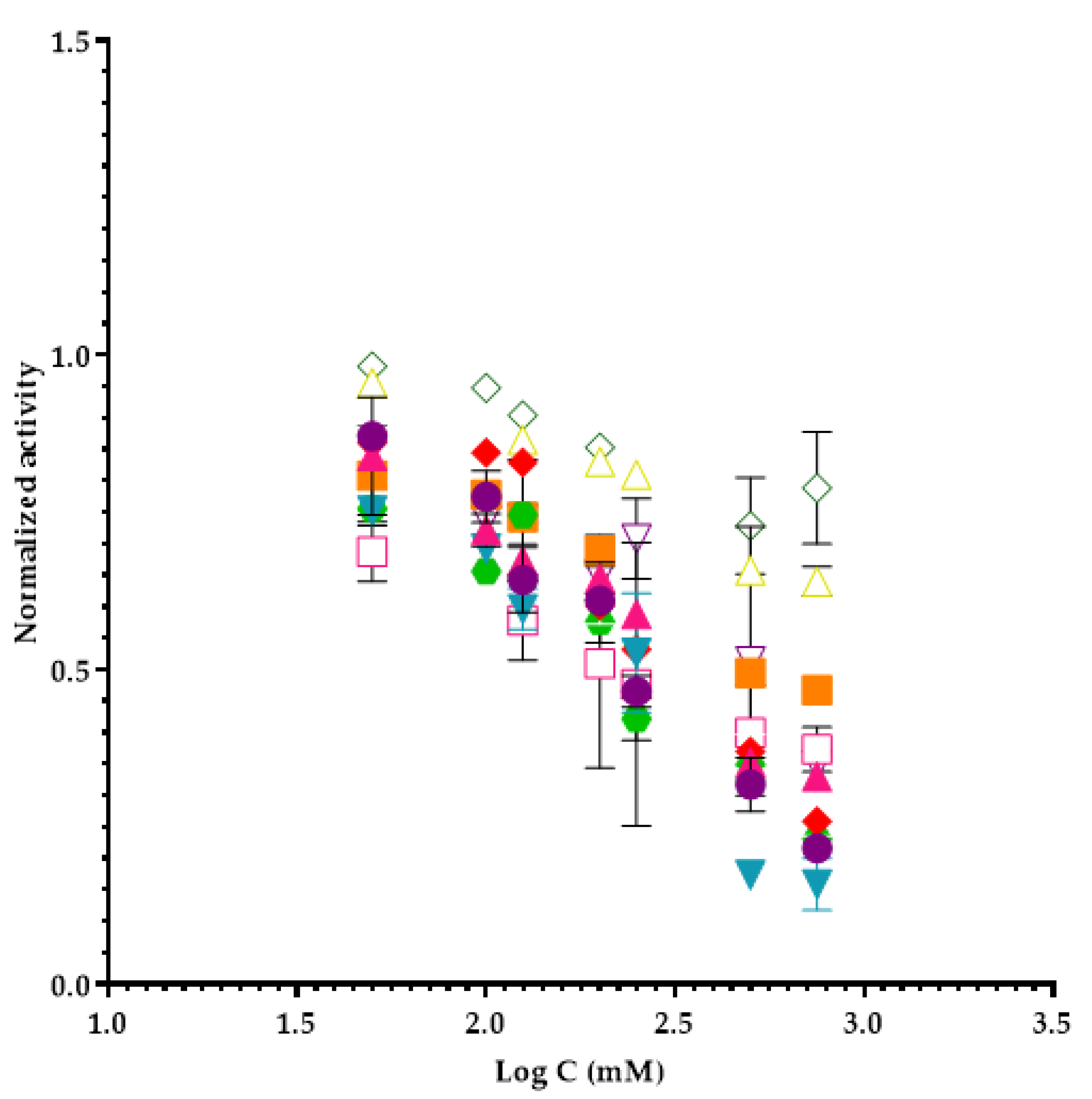
| Ionic Liquid (IL) | IL Abbreviation | Molecular Weight (g mol−1) | EC50 (mM) ± SD (1) | Confidence Interval |
|---|---|---|---|---|
| MeOSuc-Ala-Ala-Pro-Val-chloromethylketone | - | 503.0 | 0.47 ± 0.05 | ±0.47 |
| 1-Butyl-3-methylimidazolium tetrafluoroborate | [bmim] BF4 | 226.0 | 208 ± 8 | ±70 |
| 1-Ethyl-3-methylimidazolium tetrafluoroborate | [emim] BF4 | 197.9 | 201 ± 51 | ±457 |
| 1-Butyl-3-methylimidazolium chloride | [bmim] Cl | 174.7 | 248 ± 49 | ±441 |
| 1-Ethyl-3-methylimidazolium trifluoromethanesulfonate | [emim] Tfms | 260.2 | 239 ± 5 | ±46 |
| 1-Butyl-1-methylpyrrolidinium chloride | [bmpyrr] Cl | 177.7 | 194 ± 1 | ±12 |
| 1-Butyl-4-methylpyridinium tetrafluoroborate | [bmpy] BF4 | 237.0 | 239 ± 3 | ±30 |
| Tetrabutylammonium acetate | [tba] Ac | 301.5 | 176 ± 6 | ±55 |
| Tetrabutylammonium chloride | [tba] Cl | 277.9 | 233 ± 24 | ±216 |
| Tetrabutylphosphonium methanesulfonate | [Tbph] Ms | 354.5 | 229.9 ± 0.6 | ±5 |
| 1-Butyl-3-methylimidazolium acetate | [bmim] Ac | 198.3 | 289 ± 22 | ±200 |
| Ionic Liquid Active Pharmaceutical Ingredients (IL-APIs) | Molecular Weight (g mol−1) | EC50 (µM) ± SD (1) | Confidence Intervals |
|---|---|---|---|
| Lithium bistriflimide | 287.1 | 209 ± 12 | ±110 |
| 1-ethyl-3-methylimidazolium salicylate | 248.3 | 290 ± 86 | ±774 |
| Benzethonium chloride | 448.1 | 166 ± 4 | ±40 |
| Sodium salicylate * | 160.1 | 124 ± 4 | ±33 |
| Benzalkonium salicylate | 452.8 | 230 ± 20 | ±182 |
| Trihexyltetradecylphosphonium docusate | 905.4 | >700 | - |
| Benzethonium salicylate | 549.7 | >500 | - |
| Benzethonium docusate | 834.2 | >200 | - |
| Benzethonium bistriflimide | 692.8 | >1000 | - |
| Tributylmethylphosphonium salicylate | 621.0 | >200 | - |
| Trihexyltetradecyphosphonium chloride | 519.3 | >100 | - |
| Parameter Tested | Range | Selected Values |
|---|---|---|
| Temperature | 25 °C and 37 °C | 37 °C |
| Reaction time | 10 min, 15 min and 20 min | 10 min |
| Substrate concentration | 50 µM, 60 µM, 75 µM, 100 µM and 150 µM | 100 µM |
| Enzyme volume | 12.5 µL, 20 µL, 30 µL and 50 µL | 30 µL |
| Enzyme concentration | 0.0874 U; 0.0139 U; 0.0209 U and 0.0349 U | 0.0209 U |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, F.A.R.; Pereira, S.A.P.; Araujo, A.R.T.S.; Saraiva, M.L.M.F.S. Evaluation of Ionic Liquids and Ionic Liquids Active Pharmaceutical Ingredients Inhibition in Elastase Enzyme Activity. Molecules 2021, 26, 200. https://doi.org/10.3390/molecules26010200
Mota FAR, Pereira SAP, Araujo ARTS, Saraiva MLMFS. Evaluation of Ionic Liquids and Ionic Liquids Active Pharmaceutical Ingredients Inhibition in Elastase Enzyme Activity. Molecules. 2021; 26(1):200. https://doi.org/10.3390/molecules26010200
Chicago/Turabian StyleMota, Fátima A. R., Sarah A. P. Pereira, André R. T. S. Araujo, and M. Lúcia M. F. S. Saraiva. 2021. "Evaluation of Ionic Liquids and Ionic Liquids Active Pharmaceutical Ingredients Inhibition in Elastase Enzyme Activity" Molecules 26, no. 1: 200. https://doi.org/10.3390/molecules26010200
APA StyleMota, F. A. R., Pereira, S. A. P., Araujo, A. R. T. S., & Saraiva, M. L. M. F. S. (2021). Evaluation of Ionic Liquids and Ionic Liquids Active Pharmaceutical Ingredients Inhibition in Elastase Enzyme Activity. Molecules, 26(1), 200. https://doi.org/10.3390/molecules26010200








