Abstract
Peptoids, or poly-N-substituted glycines, are characterised by broad structural diversity. Compared to peptides, they are less restricted in rotation and lack backbone-derived H bonding. Nevertheless, certain side chains force the peptoid backbone into distinct conformations. Designable secondary structures like helices or nanosheets arise from this knowledge. Herein, we report the copper-catalysed alkyne-azide cycloaddition (CuAAC) of macrocycles to form innovative tube-like tricyclic peptoids, giving access to host–guest chemistry or storage applications. Different linker systems make the single tubes tuneable in size and enable modifications within the gap. An azobenzene linker, which is reversibly switchable in conformation, was successfully incorporated and allowed for light-triggered changes of the entire tricyclic structure.
1. Introduction
Fundamental knowledge of the relationship between structure and function is a principal topic in both biological and material science. Rational design of conformationally stable macromolecules gives access to various applications regarding catalysis, host–guest chemistry, and cargo transport. Peptoids, or poly-N-substituted glycines, have been shown to form stable secondary structures, such as macrocycles [1,2,3], helices [4,5,6], or even nanosheets [7,8,9,10]. As bioinspired oligomers, peptoids possess antimicrobial characteristics [11,12,13,14,15,16,17,18,19,20,21,22,23] and hold promise for effective pharmaceuticals [20,24,25,26,27]. Attached to catalysts, they can induce enantioselectivity by structure [28,29]. In the form of nanosheets, peptoids act as molecular sensors or artificial membranes [7,8,30,31,32,33,34].
The submonomer method, firstly described by Zuckermann et al. [35], gives access to an infinite number of structurally diverse oligopeptoids, as various amines are easily introduced as sidechains. This remarkable diversity provides the basis for rationally designed functional foldamers that render peptoids a promising substance class for various applications.
Although the peptoid backbone is highly flexible due to its rather free rotating methylene unit, it can be forced into distinct conformations by a selection of certain sidechains. Thereby, aniline derivatives favour trans-conformations, while some aliphatic amines cis-conformations (Scheme 1) [36,37,38,39,40].
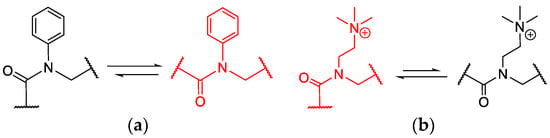
Scheme 1.
Conformational equilibrium of a peptoid backbone. (a) N-aryl sidechain enforcing trans-conformation [36] and (b) positively charged N-alkyl sidechain favouring cis-amide conformation [40].
Secondary structures can thus be induced via sequence control [41,42], but the scope of conformational permutations and the influence of certain side chains still have not been fully investigated. To constrain secondary structures, peptoid macrocycles of different sizes have been synthesised [1,2,37,38,43,44,45,46,47,48]. Among them, octameric macrocycles whose spatial structures were determined via X-ray crystallography and nuclear magnetic resonance (NMR) techniques [3,37,38,43,44,48,49]. Cyclic octamers were capable of performing host–guest interactions within their cavity [38,44,49] and could be further modified using copper-catalysed alkyne-azide [3 + 2] cycloaddition (CuAAC) [37,50,51]. CuAAC is a high-yielding, bio-orthogonal method that enables fast and facile modifications [49,50]. Kirshenbaum et al. reported the use of this kind of “click” chemistry to form cyclic peptoids [2,51]. By CuAAC of distinct side chains, the group was, furthermore, able to build up bi- and tricyclic structures [37].
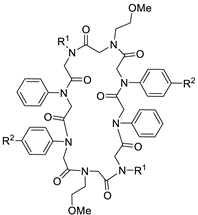
To date, only one of these tube-like tricyclic structures is known (Figure 1). It was built up by CuAAC of two cyclic octamers with one alkyne and one azide moiety each [37]. Herein, we report the design of further rigidified tubular structures with modified linkage structures to enlarge the applicability of these unique peptoid macrocycles.
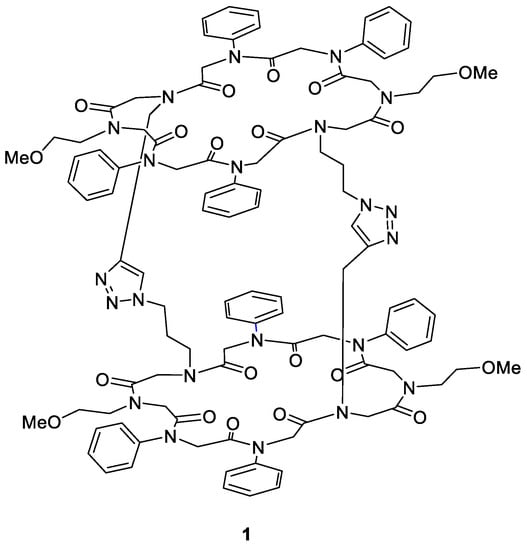
Figure 1.
Tricyclic peptoid reported by the group of Kirshenbaum in 2012 [37].
2. Results and Discussion
2.1. Design and Synthesis of Cyclic Peptoids
The stepwise synthesis of peptoid macrocycles was carried out using the published submonomer method on solid support [35]. Acetylation and substitution steps were alternated until the desired chain length was achieved. After cleavage from the resin, linear precursors were converted in the solution without further purification. Cyclisation was carried out according to a protocol by Kirshenbaum et al. [1] without exceeding the peptoid concentration of 4.80 mM to circumvent dimerisation (Scheme 2).
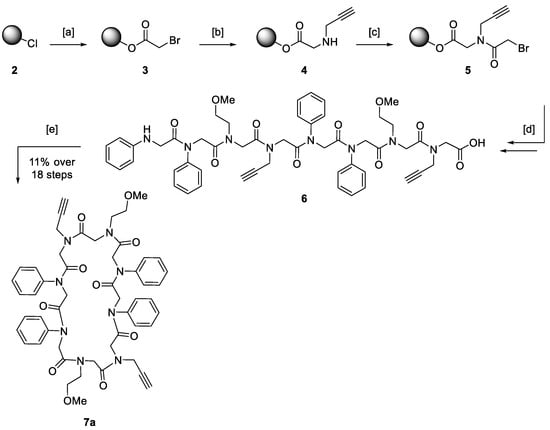
Scheme 2.
A general approach for the stepwise synthesis of cyclic peptoids as derivatives of model structure 7a, including solid-phase synthesis on a chlorotrityl chloride resin (2) and cyclisation of the linear precursor in solution. (a) Bromoacetic acid, N,N’-diisopropylethylamine, methylene chloride (DCM), 21 °C, 1 h; (b) propargylamine, N,N’-dimethylformamide (DMF), 21 °C, 1 h; (c) bromoacetic acid, N,N’-diisopropylcarbodiimide, DMF, 21 °C, 30 min; (d) (1). Alternating coupling of a desired amine following (b) and bromoacetic acid following (c); (2). hexafluoroisopropanol, DCM, 21 °C, 30 min; (e) PyBOP, DIPEA, dry DCM, 21 °C, 16 h.
By combining both solid- and liquid-phase techniques, plenty of peptoid macrocycles were readily accessible. Alkyne and azide moieties were incorporated to give access to post-synthetic modifications via CuAAC [50].
Peptoids were designed with the objective of the final backbone topology c-c-t-t-c-c-t-t, where “c” indicates cis-conformation and “t” indicates trans-conformation of the single-peptoid subunits. In our previous study, structural data of a macrocyclic octamer similar to peptoid 7a that showed the desired backbone conformation were obtained [38]. Thereby, incorporation of N-aryl amines stabilised the trans-amide structure elements. Different N-alkyl amines gave access to cis-conformations, ensuring the spatial arrangement required for cyclisation. Based on these former results, peptoid 7a was contrived to represent the model structure of every macrocycle synthesised.
Considering further cycloaddition reactions, each peptoid was functionalised with either two alkyne or azide moieties. This design should enable the combination of two different macrocycles to form a tricyclic structure via intermolecular CuAAC of their sidechains. Functional amines capable of the click reaction were both aliphatic and aromatic. Four different cyclic peptoids (7a–7d, Table 1) were synthesised according to the general approach described in Scheme 2. The cyclic octamers consist of four aryl and alkyl building blocks each. The respective monomers were arranged pairwise to ensure the desired backbone topology. Macrocycles 7a and 7b contain two adjacent anilines, as well as aliphatic functionalities as capable moieties for cycloaddition next to a methoxyethyl residue. Peptoids 7c and 7d are decorated with para-substituted anilines and two adjacent methoxyethyl sidechains.

Table 1.
Synthesised eight-membered cyclic peptoids 7a–7d, capable of copper-catalysed alkyne-azide cycloaddition (CuAAC).
Purification was carried out via preparative reverse-phase high performance liquid chromatography (HPLC) solely after the cyclisation step. Product formation was confirmed via matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) measurements. Macrocycles 7a–7d were obtained in moderate yields (8.0% to 25%) over 18 steps each. Purity was determined by integration of analytical reverse-phase HPLC traces and turned out to account for 87% to 99%.
2.2. Topology of Cyclic Peptoids
A precondition for successful CuAAC of two macrocycles is a defined spatial arrangement of the alkyne and azide moieties. Both structural elements have to point upward or downward on the backbone, but necessarily in the same direction. Only one 3D structure of macrocyclic octamers resembling the synthesised ones is known from the literature [38]. Vollrath et al. published crystallographic data of a peptoid similar to macrocycle 7a in 2013. The building blocks of the published octamer comply with the ones of structure 7a, but the positions of both the two aliphatic alkyne side chains and the methoxyethyl residues are interchanged. The backbone of this similar macrocycle showed the desired topology c-c-t-t-c-c-t-t, with sidechains pointing alternatingly up and down and both alkyne moieties located on the same side of the ring level.
It was assumed that peptoid 7a would be arranged in the same topology due to structural analogy. Unfortunately, no measurable crystals of 7a were obtained. However, macrocycle 7b, equipped with two aliphatic azides instead of the alkyne moieties, was successfully crystallised. X-ray diffraction experiments revealed its stabilised structure (Figure 2).
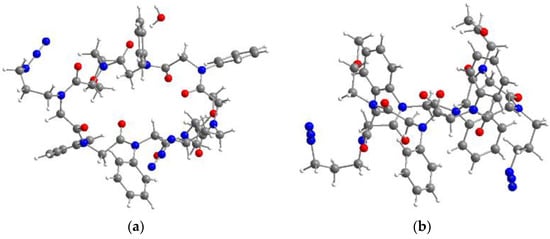
Figure 2.
Molecular structure of cyclic peptoid 7b revealing the backbone topology c-c-t-t-c-c-t-t with both aliphatic azides located on the same side of the ring level. (a) Top view; (b) side view.
Comparison of the structural data of the previously published octamer and peptoid 7b disclosed an equal backbone topology, including identical angles [38]. The sidechains are alternatingly located above and below the ring level, with both azide moieties pointing in the same direction. In contrast to propargylamine, the functional group of azidopropylamine is separated by three methylene units. The enlargement of conformational freedom by rotation around carbon single bonds makes the latter more flexible.
For cyclic octamers with aromatic alkyne or azide moieties, like peptoids 7c and 7d, neither conformational data nor information about the orientation of both functionalisable groups have been found so far. Measurable crystals of peptoid 7c were obtained after multiple attempts, and their structure was elucidated via X-ray diffraction (Figure 3).
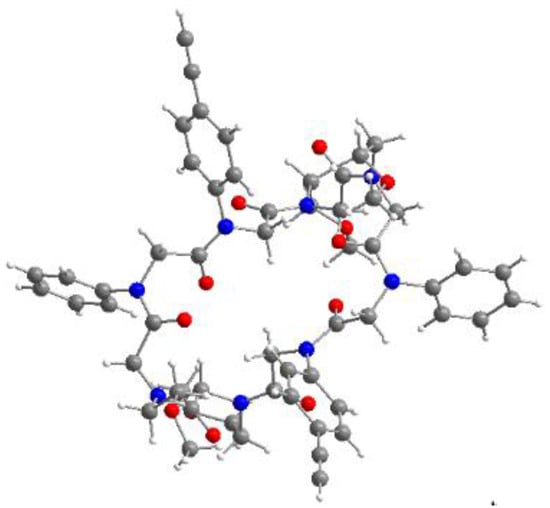
Figure 3.
Molecular structure of one of the two independent molecules of the cyclic peptoid 7c decorated with two aromatic alkynes. The structural data coincide with those obtained for macrocycle 7b.
The backbone topology equals the one known from other eight-membered macrocycles [1,38]. Again, the side chains are alternatingly located above and below the ring level, resulting in both alkyne moieties pointing into the same direction. These data coincide with those of the cyclic octamers 7a and 7b [38]. Due to the aromatic systems introduced, the functionalisable groups were rigidified compared to derivatives decorated with aliphatic moieties.
Although no measurable crystals of macrocycle 7d could be obtained, a similar conformation was expected due to the strong structural similarities to the remaining three macrocycles 7a–7c.
Every crystallised compound revealed the desired backbone topology c-c-t-t-c-c-t-t, as proven for model structure 7a [38]. Aliphatic and aromatic azides, as well as alkynes, were successfully incorporated, and the design allowed for a location on the same side of the ring level. The latter constitutes a prerequisite for further modifications via CuAAC to design tricyclic tube-like structures.
2.3. CuAAC of Two Cyclic Peptoids
Copper-catalysed alkyne-azide cycloaddition is an effective method for crosslinking different structures. The four macrocycles mentioned were conjugated pairwise via CuAAC following a modified protocol by Jagasia et al. [52]. Catalysis was performed with tetrakis(acetonitrile)copper(I) hexafluorophosphate (Cu(CH3CN)4PF6) and 2,6-lutidine. The reaction was carried out in dry methylene chloride under inert conditions. Monitoring of the product formation via analytical reverse-phase HPLC revealed a reaction time of three days for every starting material to be converted. Subsequent purification was carried out via preparative reverse-phase HPLC.
CuAAC of peptoids 7a and 7b, decorated with aliphatic functional groups, yielded the tricyclic compound 8 (Scheme 3).
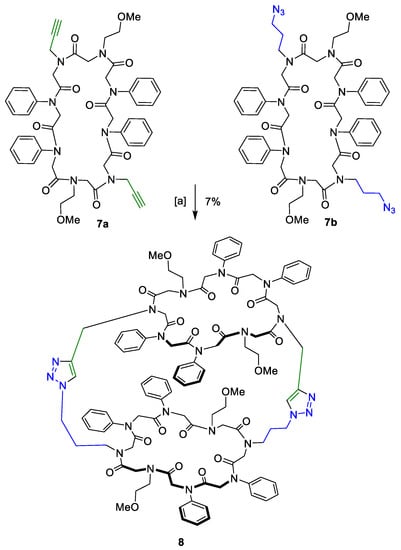
Scheme 3.
CuAAC of peptoids 7a and 7b decorated with aliphatic functional groups. (a) 2,6-Lutidine, Cu(CH3CN)4PF6, dry DCM, 21 °C, 3 days.
The tube-like structure was isolated in 7% yield. Analysis via MALDI-TOF and analytical HPLC revealed product formation with a purity of more than 99%. To validate conversion, IR measurements of both the azide-functionalised starting material and the final tricycle were taken. The characteristic azide band visible in the IR trace of peptoid 7b was omitted in the product spectrum. This measurement verified the formation of the tricyclic structure with both azide moieties converted into a triazole.
The CuAAC of the two macrocycles decorated with aromatic alkynes and azides (7c and 7d) was carried out following the procedure mentioned above. Product 8 was isolated in 6% yield and 98% purity (Scheme 4).
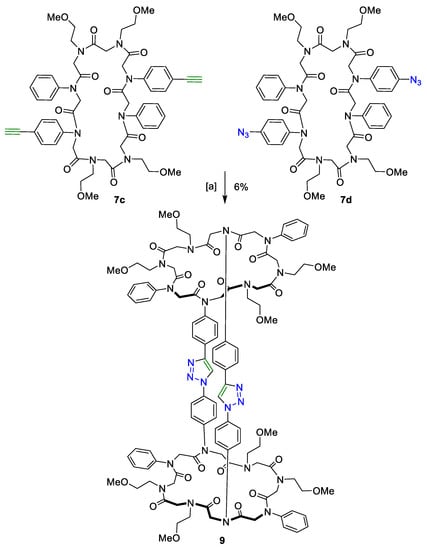
Scheme 4.
Tricycle 9 as a product of the CuAAC of peptoids 7c and 7d decorated with aromatic functional groups. (a) 2,6-Lutidine, Cu(CH3CN)4PF6, dry DCM, 21 °C, 3 days.
The yields for the CuAAC of two aliphatic and of two aromatic residues are similar. Therefore, it is reasonable that neither flexibility nor rigidity is advantageous for this kind of reaction.
2.4. CuAAC of Cyclic Peptoids and Small Molecules
To increase the structural diversity of the tube-like tricyclic peptoids, small molecules were incorporated by tuning the size of the tube and allowing access to further functionalisation. For size enlargement of the cavity in between the macrocycles, 1,4-diethynylbenzene (10) was attached to peptoid 7b (Scheme 5).
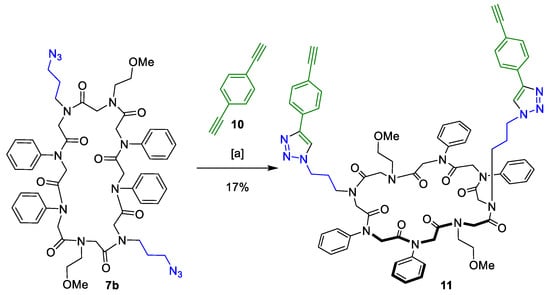
Scheme 5.
CuAAC of the macrocyclic peptoid 7b and 1,4-diethynylbenzene (10). (a) 2,6-Lutidine, Cu(CH3CN)4PF6, dry DCM, 21 °C, 3 days.
The bifunctionalised linker 10 was added in excess to enable full conversion and to circumvent intramolecular crosslinking. The reaction conditions complied with the ones mentioned for the CuAAC of the two peptoid macrocycles. Compound 11 was isolated in 17% yield after purification. Product formation was verified via MALDI-TOF and purity was determined to be 99%. The CuAAC of linker conjugate 11 and another azide-functionalised peptoid macrocycle failed.
To enable further structural diversity that was adjustable via light, an azobenzene linker system was synthesised according to a published procedure [53]. Azobenzenes are known for their ability to switch their conformation from cis to trans and vice versa after irradiation with light [54,55]. They have been used as submonomers in solid-phase synthesis and have been shown to affect the structure of the resulting peptoid derivatives due to triggerable cis-trans-isomerisation [56].
The effectivity of the conformational change after UV radiation was monitored via NMR for the synthesised linker (see Supplementary Materials). The potent azobenzene should then be incorporated as a spacer between two macrocycles. So far, examples for only linear peptoids with an azobenzene switch have been found [56]. Herein, we report the first peptoid macrocycle attached to an azobenzene. Conjugate 13 (Scheme 6) was synthesised via CuAAC according to the conditions mentioned above. Product 13 was obtained in 19% yield and 98% purity, similarly to the yield obtained for the CuAAC of the simplified linker 10 (Scheme 5).
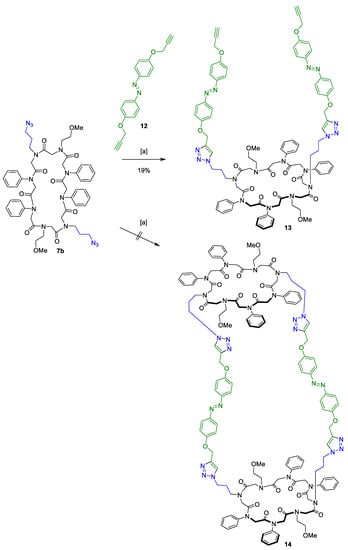
Scheme 6.
CuAAC of the cyclic peptoid 7b and the azobenzene linker 12 revealed conjugate 13, but did not form the tricyclic structure 14. (a) 2,6-Lutidine, Cu(CH3CN)4PF6, dry DCM, 21 °C, 3 days.
The subsequent switch experiments should confirm the conformational change of the entire system 13. NMR spectra of product 13 both before and after irradiation with UV light were measured (Figure 4).
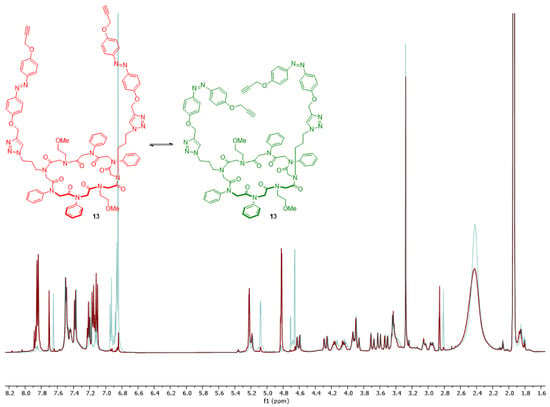
Figure 4.
Switch experiment of conjugate 13, whose conformation change from trans (red) to cis (green) after irradiation with UV light was monitored via nuclear magnetic resonance (NMR).
Significant changes in the aromatic area were observed. Additionally, the signals of the terminal alkyne and methylene groups of the azobenzene linkers shifted. UV-Vis measurements verified these results (see Supplementary Materials). Therefore, the azobenzene of conjugate 13 can switch efficaciously from trans- to cis-conformation after irradiation with UV light.
Unfortunately, the desired tricyclic structure 14 with one peptoid macrocycle attached to each alkyne moiety of the azobenzene linker could not be isolated (Scheme 6). It is conceivable that the chosen linker system is unable to close the tricyclic tube via CuAAC due to its high degree of flexibility. Compound 13 polymerised to an insoluble solid instead.
To circumvent polymerisation, a rigidified azobenzene linker system was synthesised according to a procedure known from the literature [57]. Switch experiments revealed conformational changes with effectivities of 97% for trans- and 95% cis-conformers (see Supplementary Materials).
The CuAAC of the rigidified linker and peptoid 7b was carried out as described before, using an excess of the azobenzene linker to circumvent intramolecular crosslinking. After three days, macrocycle 15 and the tube-like structure 16 were isolated (Figure 5).
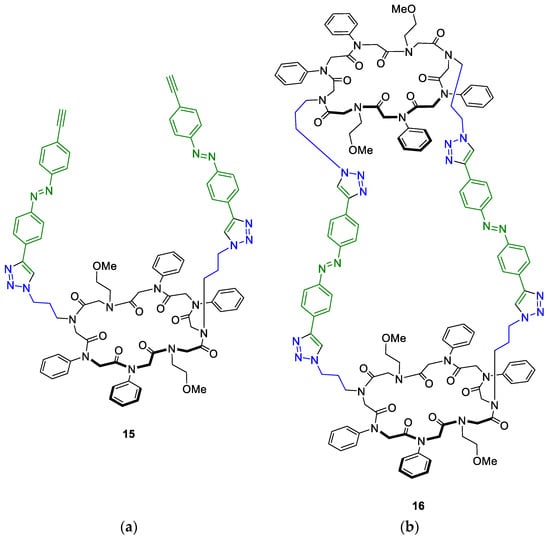
Figure 5.
Products of the CuAAC of peptoid 7b and a rigidified azobenzene linker (green). (a) Conjugate 15 displays the linker with one peptoid macrocycle attached to one alkyne moiety each. (b) CuAAC of both alkyne moieties led to the tube-like structure 16.
Structure 15 with peptoid conjugation to one alkyne moiety of the azobenzene linker was obtained in 3% yield after purification. Interestingly, the yield is significantly less compared to the CuAAC of the more flexible azobenzene linker 12 described above. In return, the desired tube-like tricyclic peptoid structure 16 was additionally obtained in a similar yield of 2%. Masses of both products were confirmed via MALDI-TOF and electrospray ionization (ESI-TOF). NMR measurements verified the receipt of the single structures (see Supplementary Materials).
Switch experiments of both molecules were monitored via NMR after irradiation with UV light (365 nm) for 30 min. The conformational change from trans to cis was verified by significant shifts in the aromatic region (Figure 6). In the case of product 15, an additional signal shift of the terminal alkyne proton was observable after irradiation (see Supplementary Materials).
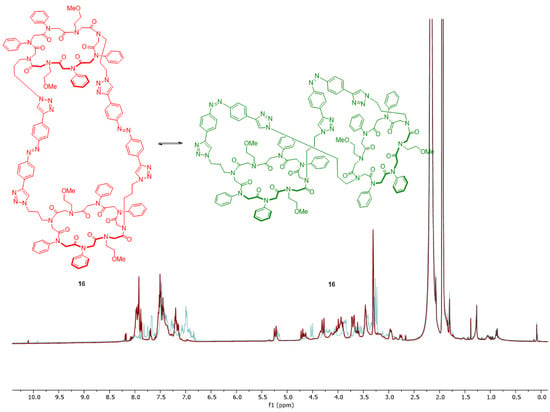
Figure 6.
Switch experiment of the tricyclic structure 16, whose conformation change from trans (red) to cis (green) after irradiation with UV light was monitored via NMR.
Additionally, UV-Vis spectra of compounds 15 and 16 were recorded before and after irradiation with UV light (Figure 7 and Supplementary Materials).
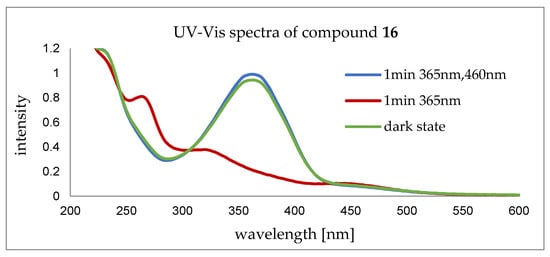
Figure 7.
UV-Vis spectra of compound 16 before (green) and after irradiation with UV light (365 nm) for 1 sec (red), indicating a conformational change from trans to cis. The reverse conformational change was induced via irradiation with UV light (365 nm), followed by irradiation with visible light (460 nm) for 1 min each (blue).
Trans-conformation is indicated by the dark state trace. Conformational change towards the cis-conformation was triggered by irradiation with UV light (365 nm) for 1 min. Reverse conformational change from cis to trans was monitored after irradiation with UV light (365 nm) first to force the azobenzene into cis, with subsequent irradiation with visible light (460 nm) for 1 min each.
When changing conformation from trans to cis, a significant increase in intensity is observable at around 280 nm. A decrease occurs simultaneously at around 360 nm. The trace of the probe irradiated with UV light first and with visible light next corroborates the transfer from cis to trans, showing the reversibility of the system.
These results give evidence of a reversible conformational switch of the tricyclic peptoid 16. With this, the tube-like structure can open and close, rendering it applicable for storage or host–guest chemistry.
3. Materials and Methods
General procedure for the synthesis of cyclic peptoids: In a fritted syringe, a 2-chlorotrityl-chloride resin (300 mg, 0.480 mmol, 1.60 mmol/mg loading density, 100–200 mesh, 1.00 equiv.) was swollen in 3 mL of methylene chloride (DCM) for 2 h. After filtration, a freshly prepared solution of bromoacetic acid (2.59 mmol, 5.40 equiv.) and N,N′-diisopropylethylamine (DIPEA, 2.59 mmol, 5.40 equiv.) in 2.5 mL of DCM was added and shaken for 1 h at 21 °C. The resin was extensively washed with peptide-grade N,N′-dimethyl-formamide (pDMF). For the following substitution reaction, a solution of the corresponding amine (3.98 mmol, 8.30 equiv.) in 2.5 mL of pDMF was added to the resin and shaken for 30 min at room temperature (overnight in the case of aniline). Following extensive washing with pDMF, a solution of bromoacetic acid (4.80 mmol, 10.0 equiv.) and N,N′-diisopropylcarbodiimide (DIC, 4.80 mmol, 10.0 equiv.) in 2.5 mL pDMF were added and shaken for 30 min at room temperature (2 h in the case of aniline). The acetylation and substitution steps were alternated repeatedly until the desired peptoid length was achieved. For cleavage, a solution of 33% hexafluoroisopropanol in DCM was added and the mixture was shaken overnight. The solvent was removed under reduced pressure.
For cyclisation, the activating agent benzotriazol-1-yl-oxytripyrrolidino-phosphonium hexafluorophosphate (PyBOP, 1.44 mmol, 3.00 equiv.) and DIPEA (2.88 mmol, 6.00 equiv.) were added to a solution of the respective peptoid in 100 mL of DCM. The solution was stirred overnight at room temperature, and the solvent was removed under reduced pressure. The residue was dissolved in a mixture of acetonitrile/water (1:1), lyophilised, and purified via preparative reverse-phase HPLC.
General procedure for the CuAAC of the two peptoids: Both the peptoid decorated with azide (1.00 equiv.) and the one decorated with alkyne moieties (1.00 equiv.) were dissolved in dry DCM and degassed with argon. Afterwards, 2,6-lutidine (6.00 equiv.) was added and the solution was stirred for 5 min. Finally, tetrakis(acetonitrile)copper(I) hexafluorophosphate (Cu(CH3CN)4PF6, 1.00 equiv.) was added, and the mixture was stirred for 3 days at room temperature. The solvent was removed under reduced pressure and the residue was purified via preparative reverse-phase HPLC.
General procedure for the CuAAC of a linker and a peptoid: The desired peptoid (1.00 equiv.) and the respective linker (10.0 equiv.) were dissolved in dry DCM and degassed with argon for 10 min. Afterwards, 2,6-lutidine (8.00 equiv.) was added and the solution was stirred for 5 min. Finally, Cu(CH3CN)4PF6 (1.00 equiv.) was added and the mixture was stirred for 3 days at room temperature. The solvent was removed under reduced pressure and the residue was purified via preparative reverse-phase HPLC.
Switch experiments via NMR: The sample was dissolved in deuterated acetonitrile and stored under exclusion of light. Measurements were performed on a Bruker 500 spectrometer at 500 MHz. Spectra were recorded before and after irradiation with UV light (365 nm).
Switch experiments via UV-Vis: 20 μM solutions of the single samples were stored in the dark. Measurements were performed on a PerkinElmer Lambda 750 UV/Vis/NIR spectrometer and monitored in the range from 200 to 800 nm wavelength. Spectra were monitored before and after irradiation with UV and visible light, respectively.
Crystal Structure Determinations
The single-crystal X-ray diffraction study was carried out on a Bruker D8 Venture diffractometer with a Photon100 detector at 123(2) K (7b) and an Agilent SuperNova-Dual diffractometer with an Atlas CCD-detector at 120(2) K (7b) using Cu-Kα radiation (λ = 1.54178 Å). (Bruker AXS GmbH, Karlsruhe, Germany] Agilent Technologies, Oxford, United Kingdom) Dual-Space Methods (SHELXD) (for 7b) or Direct methods (SHELXS-97) (for 7c) (SHELXD and SHELXS [58]) were used for the structure solution, and refinement was carried out using SHELXL-2013 or SHELXL-2014 (full-matrix least-squares on F2) (SHELXL [59]). Hydrogen atoms were refined using a riding model (H(water) free). Semi-empirical absorption corrections were applied. For 7b, an extinction correction was applied. In 7b, the solvent water and the two 3-azidopropyl groups were disordered (see cif-file for details). In 7c, the absolute structure could not be determined reliably (Parsons x-parameter, x = 0.5(2); see cif-file for details) [60].
7b: colourless crystals, C52H62N14O10 − 0.5 H2O, Mr = 1052.16, crystal size 0.36 × 0.12 × 0.06 mm, monoclinic, space group C2/c (No. 15), a = 23.0990(10) Å, b = 18.4326(8) Å, c = 25.4929(12) Å, β = 105.159(2)°, V = 10476.5(8) Å3, Z = 8, ρ = 1.334 Mg/m−3, µ(Cu-Kα) = 0.789 mm−1, F(000) = 4456, 2θmax = 145.4°, 50,224 reflections, of which 10,350 were independent (Rint = 0.073), 687 parameters, 51 restraints, R1 = 0.090 (for 8336 I > 2σ(I)), wR2 = 0.228 (all data), S = 1.04, largest diff. peak/hole = 1.196 (in disordered 3-azidopropyl)/−0.772 e Å−3.
7c: colourless crystals, C56H64N8O12, Mr = 1041.15, crystal size 0.19 × 0.08 × 0.04 mm, orthorhombic, space group Pca21 (No. 29), a = 36.3213(8) Å, b = 9.1548(3) Å, c = 32.2512(7) Å, V = 10724.0(5) Å3, Z = 8, ρ = 1.290 Mg/m−3, µ(Cu-Kα) = 0.754 mm−1, F(000) = 4416, 2θmax = 152.4°, 24,059 reflections, of which 15,570 were independent (Rint = 0.043), 1369 parameters, 1 restraint, R1 = 0.079 (for 12810 I > 2σ(I)), wR2 = 0.214 (all data), S = 1.03, largest diff. peak/hole = 0.851/−0.306 e Å−3.
Cambridge Chrystallographic Data Centre (CCDC) 1446764 (7b) and 1446765 (7c) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
4. Conclusions
Herein, we report the successful synthesis of tube-like tricyclic peptoids via CuAAC, giving access to novel secondary structures. An azobenzene linker system was successfully incorporated as a spacer between two macrocycles. The opportunity to modify the linkage makes the structures tuneable in size and enables the introduction of functional centres. The azobenzene changed its conformation reversibly from trans to cis after irradiation with UV and visible light, respectively. With this, the tricyclic structure was forced into bot an open and a closed conformation. This switch was verified using NMR experiments and UV-Vis measurements. Hence, this new class of tube-like peptoid structures gives access to host–guest applications or controllable storage of small molecules.
Supplementary Materials
The following are available online: synthetic procedure, switch experiments.
Author Contributions
Conceptualisation, C.N.H. and K.S.; methodology, K.S.; validation, S.B.; formal analysis, K.S., P.W., and M.N.; investigation, K.S.; resources, S.B.; data curation, C.N.H., K.S., and M.N.; writing—original draft preparation, C.N.H. and K.S.; writing—review and editing, C.N.H. and S.B.; visualisation, C.N.H.; supervision, S.B.; project administration, S.B.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge support from the KIT, the DAAD, and the DFG (SFB 1176, EXC-2082/1–390761711).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in the Chemotion repository: www.chemotion-repository.net.
Acknowledgments
We thank Kent Kirshenbaum (New York) for fruitful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
The molecules are deposited at the Compound Platform of the KIT. Selected molecules are available on request.
References
- Shin, S.B.; Yoo, B.; Todaro, L.J.; Kirshenbaum, K. Cyclic peptoids. J. Am. Chem. Soc. 2007, 129, 3218–3225. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.; Shin, S.B.Y.; Huang, M.L.; Kirshenbaum, K. Peptoid macrocycles: Making the rounds with peptidomimetic oligomers. Chem. Eur. J. 2010, 16, 5528–5537. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.M.; Cobb, S.L. Recent advances in the synthesis of peptoid macrocycles. Chem. Eur. J. 2018, 24, 7560–7573. [Google Scholar] [CrossRef]
- Baldauf, C.; Günther, R.; Hofmann, H.-J. Helices in peptoids of α-and β-peptides. Phys. Biol. 2006, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, T.J.; Wu, C.W.; Zuckermann, R.N.; Barron, A.E. Extreme stability of helices formed by water-soluble poly-n-substituted glycines (polypeptoids) with α-chiral side chains. Biopolymers 2002, 63, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Stringer, J.R.; Crapster, J.A.; Guzei, I.A.; Blackwell, H.E. Extraordinarily robust polyproline type i peptoid helices generated via the incorporation of α-chiral aromatic n-1-naphthylethyl side chains. J. Am. Chem. Soc. 2011, 133, 15559–15567. [Google Scholar] [CrossRef]
- Mannige, R.V.; Haxton, T.K.; Proulx, C.; Robertson, E.J.; Battigelli, A.; Butterfoss, G.L.; Zuckermann, R.N.; Whitelam, S. Peptoid nanosheets exhibit a new secondary-structure motif. Nature 2015, 526, 415–420. [Google Scholar] [CrossRef]
- Robertson, E.J.; Oliver, G.K.; Qian, M.; Proulx, C.; Zuckermann, R.N.; Richmond, G.L. Assembly and molecular order of two-dimensional peptoid nanosheets through the oil-water interface. Proc. Natl. Acad. Sci. USA 2014, 111, 13284–13289. [Google Scholar] [CrossRef]
- Kudirka, R.; Tran, H.; Sanii, B.; Nam, K.T.; Choi, P.H.; Venkateswaran, N.; Chen, R.; Whitelam, S.; Zuckermann, R.N. Folding of a single-chain, information-rich polypeptoid sequence into a highly ordered nanosheet. Biopolymers 2011, 96, 586–595. [Google Scholar] [CrossRef]
- Sanii, B.; Kudirka, R.; Cho, A.; Venkateswaran, N.; Olivier, G.K.; Olson, A.M.; Tran, H.; Harada, R.M.; Tan, L.; Zuckermann, R.N. Shaken, not stirred: Collapsing a peptoid monolayer to produce free-floating, stable nanosheets. J. Am. Chem. Soc. 2011, 133, 20808–20815. [Google Scholar] [CrossRef]
- Smith, P.T.; Huang, M.L.; Kirshenbaum, K. Osmoprotective polymer additives attenuate the membrane pore-forming activity of antimicrobial peptoids. Biopolymers 2015, 103, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.; Shin, S.B.Y.; Benson, M.A.; Torres, V.J.; Kirshenbaum, K. A comparison of linear and cyclic peptoid oligomers as potent antimicrobial agents. ChemMedChem 2012, 7, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Chongsiriwatana, N.P.; Patch, J.A.; Czyzewski, A.M.; Dohm, M.T.; Ivankin, A.; Gidalevitz, D.; Zuckermann, R.N.; Barron, A.E. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2008, 105, 2794–2799. [Google Scholar] [CrossRef] [PubMed]
- Bolt, H.L.; Eggimann, G.A.; Jahoda, C.A.B.; Zuckermann, R.N.; Sharples, G.J.; Cobb, S.L. Exploring the links between peptoid antibacterial activity and toxicity. MedChemComm 2017, 8, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Mojsoska, B.; Jenssen, H. Peptides and peptidomimetics for antimicrobial drug design. Pharmaceuticals 2015, 8, 366–415. [Google Scholar] [CrossRef]
- Mojsoska, B.; Zuckermann, R.N.; Jenssen, H. Structure-activity relationship study of novel peptoids that mimic the structure of antimicrobial peptides. Antimicrob. Agents Chemother. 2015, 59, 4112–4120. [Google Scholar] [CrossRef]
- Czyzewski, A.M.; Jenssen, H.; Fjell, C.D.; Waldbrook, M.; Chongsiriwatana, N.P.; Yuen, E.; Hancock, R.E.; Barron, A.E. In vivo, in vitro, and in silico characterization of peptoids as antimicrobial agents. PLoS ONE 2016, 11, e0135961. [Google Scholar] [CrossRef]
- Jahnsen, R.D.; Frimodt-Moller, N.; Franzyk, H. Antimicrobial activity of peptidomimetics against multidrug-resistant Escherichia coli: A comparative study of different backbones. J. Med. Chem. 2012, 55, 7253–7261. [Google Scholar] [CrossRef]
- Mojsoska, B.; Carretero, G.; Larsen, S.; Mateiu, R.V.; Jenssen, H. Peptoids successfully inhibit the growth of gram negative E. coli causing substantial membrane damage. Sci. Rep. 2017, 7, 42332. [Google Scholar] [CrossRef]
- Bicker, K.L.; Cobb, S.L. Recent advances in the development of anti-infective peptoids. Chem. Comm. 2020, 56, 11158–11168. [Google Scholar] [CrossRef]
- Molchanova, N.; Nielsen, J.E.; Sørensen, K.B.; Prabhala, B.K.; Hansen, P.R.; Lund, R.; Barron, A.E.; Jenssen, H. Halogenation as a tool to tune antimicrobial activity of peptoids. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Choi, J.; Kumar, S.D.; Nielsen, J.E.; Kyeong, M.; Wang, S.; Kang, D.; Lee, Y.; Lee, J.; Yoon, M.-H. Helicity modulation improves the selectivity of antimicrobial peptoids. ACS Infect. Dis. 2020, 6, 2732–2744. [Google Scholar] [CrossRef] [PubMed]
- Khara, J.S.; Mojsoska, B.; Mukherjee, D.; Langford, P.R.; Robertson, B.D.; Jenssen, H.; Ee, P.L.R.; Newton, S.M. Ultra-short antimicrobial peptoids show propensity for membrane activity against multi-drug resistant mycobacterium tuberculosis. Front. Microbol. 2020, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Dohm, M.T.; Kapoor, R.; Barron, A.E. Peptoids: Bio-inspired polymers as potential pharmaceuticals. Curr. Pharm. Des. 2011, 17, 2732–2747. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, R.N.; Kodadek, T. Peptoids as potential therapeutics. Curr. Opin. Mol. Ther. 2009, 11, 299–307. [Google Scholar]
- Rhodes, C.A.; Pei, D. Bicyclic peptides as next-generation therapeutics. Chem. Eur. J. 2017, 23, 12690–12703. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y.; Wang, M.; Jian, T.; Ding, S.; Mu, P.; Liao, Z.; Shi, Q.; Cai, X.; Jin, H. Bioinspired peptoid nanotubes for targeted tumor cell imaging and chemo-photodynamic therapy. Small 2019, 15, 1902485. [Google Scholar] [CrossRef]
- Maayan, G.; Ward, M.D.; Kirshenbaum, K. Folded biomimetic oligomers for enantioselective catalysis. Proc. Natl. Acad. Sci. USA 2009, 106, 13679–13684. [Google Scholar] [CrossRef]
- Drapaneni, C.; Ghosh, P.; Ghosh, T.; Maayan, G. Unique β-turn peptoid structures and their application as asymmetric catalysts. Chem. Eur. J. 2020, 26, 9573–9579. [Google Scholar] [CrossRef]
- Olivier, G.K.; Cho, A.; Sanii, B.; Connolly, M.D.; Tran, H.; Zuckermann, R.N. Antibody-mimetic peptoid nanosheets for molecular recognition. ACS Nano 2013, 7, 9276–9286. [Google Scholar] [CrossRef]
- Liu, J.; Cai, B.; Cui, L.; Chen, C.-L. Peptoid-based hierarchically-structured biomimetic nanomaterials: Synthesis, characterization and applications. Sci. China Mater. 2020, 63, 1099–1112. [Google Scholar] [CrossRef]
- Battigelli, A.; Kim, J.H.; Dehigaspitiya, D.C.; Proulx, C.; Robertson, E.J.; Murray, D.J.; Rad, B.; Kirshenbaum, K.; Zuckermann, R.N. Glycosylated peptoid nanosheets as a multivalent scaffold for protein recognition. ACS Nano 2018, 12, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.C.; Kline, M.A.; Grzincic, E.M.; Tresca, B.W.; Cardiel, J.; Karbaschi, M.; Dehigaspitiya, D.C.; Chen, Y.; Udumula, V. Discovery of stable and selective antibody mimetics from combinatorial libraries of polyvalent, loop-functionalized peptoid nanosheets. ACS Nano 2019, 14, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Song, Y.; Mu, P.; Cai, X.; Lin, Y.; Chen, C.-L. Peptoid-based programmable 2d nanomaterial sensor for selective and sensitive detection of h2s in live cells. ACS Appl. Bio Mater. 2020, 3, 6039–6048. [Google Scholar] [CrossRef]
- Zuckermann, R.N.; Kerr, J.M.; Kent, S.B.H.; Moos, W.H. Efficient method for the preparation of peptoids [oligo(n-substituted glycines)] by submonomer solid-phase synthesis. J. Am. Chem. Soc. 1992, 114, 10646–10647. [Google Scholar] [CrossRef]
- Butterfoss, G.L.; Renfrew, P.D.; Kuhlman, B.; Kirshenbaum, K.; Bonneau, R. A preliminary survey of the peptoid folding landscape. J. Am. Chem. Soc. 2009, 131, 16798–16807. [Google Scholar] [CrossRef]
- Vollrath, S.B.; Bräse, S.; Kirshenbaum, K. Twice tied tight: Enforcing conformational order in bicyclic peptoid oligomers. Chem. Sci. 2012, 3, 2726–2731. [Google Scholar] [CrossRef]
- Vollrath, S.B.; Hu, C.; Bräse, S.; Kirshenbaum, K. Peptoid nanotubes: An oligomer macrocycle that reversibly sequesters water via single-crystal-to-single-crystal transformations. Chem. Comm. 2013, 49, 2317–2319. [Google Scholar] [CrossRef]
- Shah, N.H.; Butterfoss, G.L.; Nguyen, K.; Yoo, B.; Bonneau, R.; Rabenstein, D.L.; Kirshenbaum, K. Oligo(n-aryl glycines): A new twist on structured peptoids. J. Am. Chem. Soc. 2008, 130, 16622–16632. [Google Scholar] [CrossRef]
- Wijaya, A.W.; Nguyen, A.I.; Roe, L.T.; Butterfoss, G.L.; Spencer, R.K.; Li, N.K.; Zuckermann, R.N. Cooperative intramolecular hydrogen bonding strongly enforces cis-peptoid folding. J. Am. Chem. Soc. 2019, 141, 19436–19447. [Google Scholar] [CrossRef]
- Knight, A.S.; Zhou, E.Y.; Francis, M.B.; Zuckermann, R.N. Sequence programmable peptoid polymers for diverse materials applications. Adv. Mater. 2015, 27, 5665–5691. [Google Scholar] [CrossRef] [PubMed]
- Kolaskar, A.; Lakshminarayanan, A.; Sarathy, K.; Sasisekharan, V. The nonplanar peptide unit iii. Quantum chemical calculations for related compounds and experimental x-ray diffraction data. Biopolymers 1975, 14, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.; Pierri, G.; Tedesco, C.; Della Sala, G.; Izzo, I.; Costabile, C.; De Riccardis, F. Reverse turn and loop secondary structures in stereodefined cyclic peptoid scaffolds. J. Org. Chem. 2019, 84, 10911–10928. [Google Scholar] [CrossRef] [PubMed]
- De Riccardis, F. The challenge of conformational isomerism in cyclic peptoids. Eur. J. Org. Chem. 2020, 2020, 2981–2994. [Google Scholar] [CrossRef]
- D’Amato, A.; Della Sala, G.; Izzo, I.; Costabile, C.; Masuda, Y.; De Riccardis, F. Cyclic octamer peptoids: Simplified isosters of bioactive fungal cyclodepsipeptides. Molecules 2018, 23, 1779. [Google Scholar] [CrossRef]
- Tedesco, C.; Erra, L.; Izzo, I.; De Riccardis, F. Solid state assembly of cyclic α-peptoids. CrystEngComm 2014, 16, 3667–3687. [Google Scholar] [CrossRef]
- Schettini, R.; Costabile, C.; Della Sala, G.; Iuliano, V.; Tedesco, C.; Izzo, I.; De Riccardis, F. Cation-induced molecular switching based on reversible modulation of peptoid conformational states. J. Org. Chem. 2018, 83, 12648–12663. [Google Scholar] [CrossRef]
- Yoo, B.; Kirshenbaum, K. Peptoid architectures: Elaboration, actuation, and application. Curr. Opin. Chem. Biol. 2008, 12, 714–721. [Google Scholar] [CrossRef]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-catalysed azide–alkyne cycloadditions (cuaac): An update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(i)-catalyzed alkyne-azide cycloaddition (cuaac) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Holub, J.M.; Jang, H.; Kirshenbaum, K. Fit to be tied: Conformation-directed macrocyclization of peptoid foldamers. Org. Lett. 2007, 9, 3275–3278. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, R.; Holub, J.M.; Bollinger, M.; Kirshenbaum, K.; Finn, M. Peptide cyclization and cyclodimerization by cui-mediated azide− alkyne cycloaddition. J. Org. Chem. 2009, 74, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Kordel, C.; Popeney, C.S.; Haag, R. Photoresponsive amphiphiles based on azobenzene-dendritic glycerol conjugates show switchable transport behavior. Chem. Comm. 2011, 47, 6584–6586. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-X.; Stellacci, F.; McGrath, D.V. Photoswitchable flexible and shape-persistent dendrimers: Comparison of the interplay between a photochromic azobenzene core and dendrimer structure. J. Am. Chem. Soc. 2004, 126, 2181–2185. [Google Scholar] [CrossRef] [PubMed]
- Zeitouny, J.; Aurisicchio, C.; Bonifazi, D.; De Zorzi, R.; Geremia, S.; Bonini, M.; Palma, C.-A.; Samorì, P.; Listorti, A.; Belbakra, A. Photoinduced structural modifications in multicomponent architectures containing azobenzene moieties as photoswitchable cores. J. Mater. Chem. 2009, 19, 4715–4724. [Google Scholar] [CrossRef]
- Shah, N.H.; Kirshenbaum, K. Photoresponsive peptoid oligomers bearing azobenzene side chains. Org. Biomol. Chem. 2008, 6, 2516–2521. [Google Scholar] [CrossRef]
- Kimoto, A.; Iwasaki, K.; Abe, J. Formation of photoresponsive gold nanoparticle networks via click chemistry. Photochem. Photobiol. Sci. 2010, 9, 152–156. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with shelxl. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H. Precise absolute-structure determination in light-atom crystals. Acta Crystallogr. Sect. A Found. Crystallogr. 2004, 60, 61. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).