Phloroglucinol Derivatives from Dryopteris crassirhizoma as Potent Xanthine Oxidase Inhibitors
Abstract
1. Introduction
2. Results and Discussion
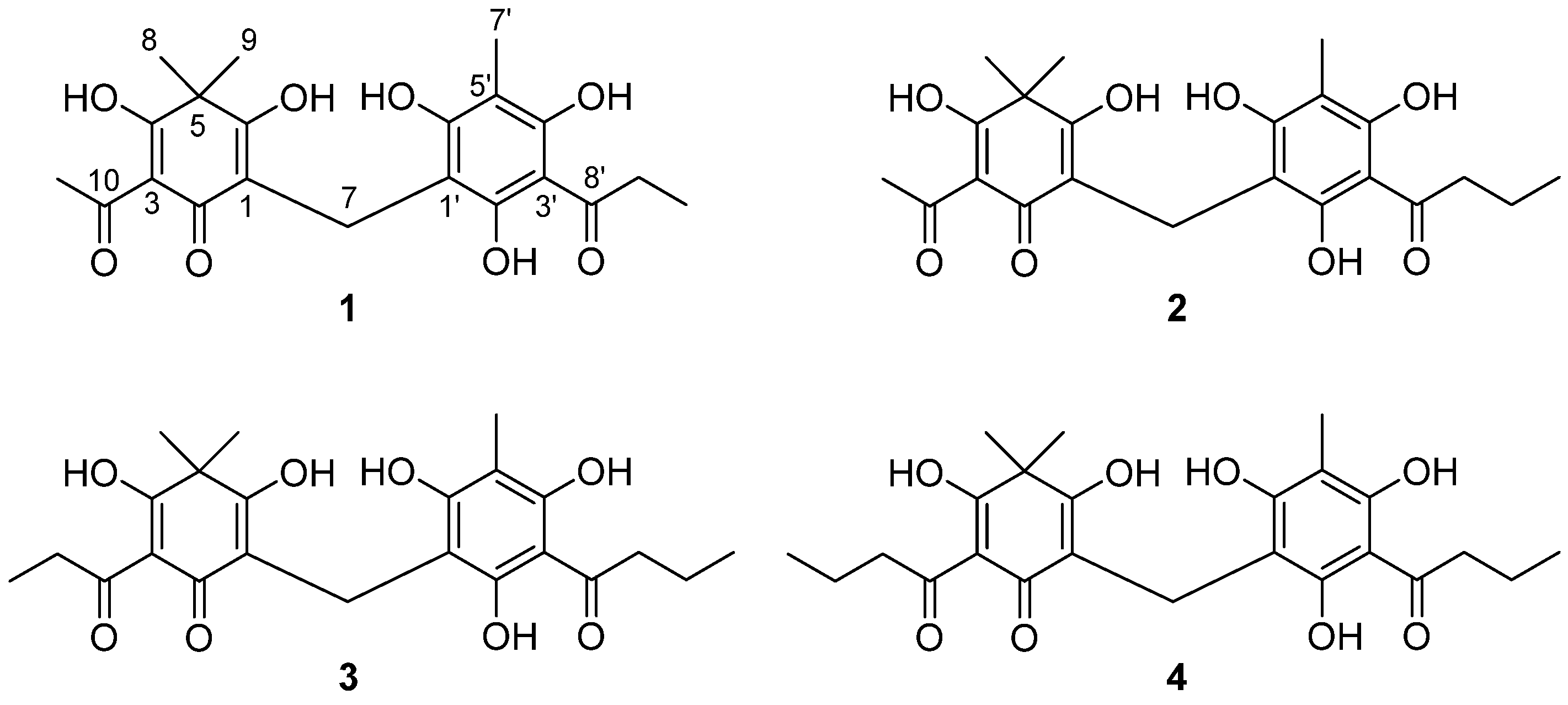
2.1. Bioassay-Guided Isolation and Structural Identification of XO Inhibitors
2.2. Contribution of the Identified Compounds to XO Inhibitory Activity
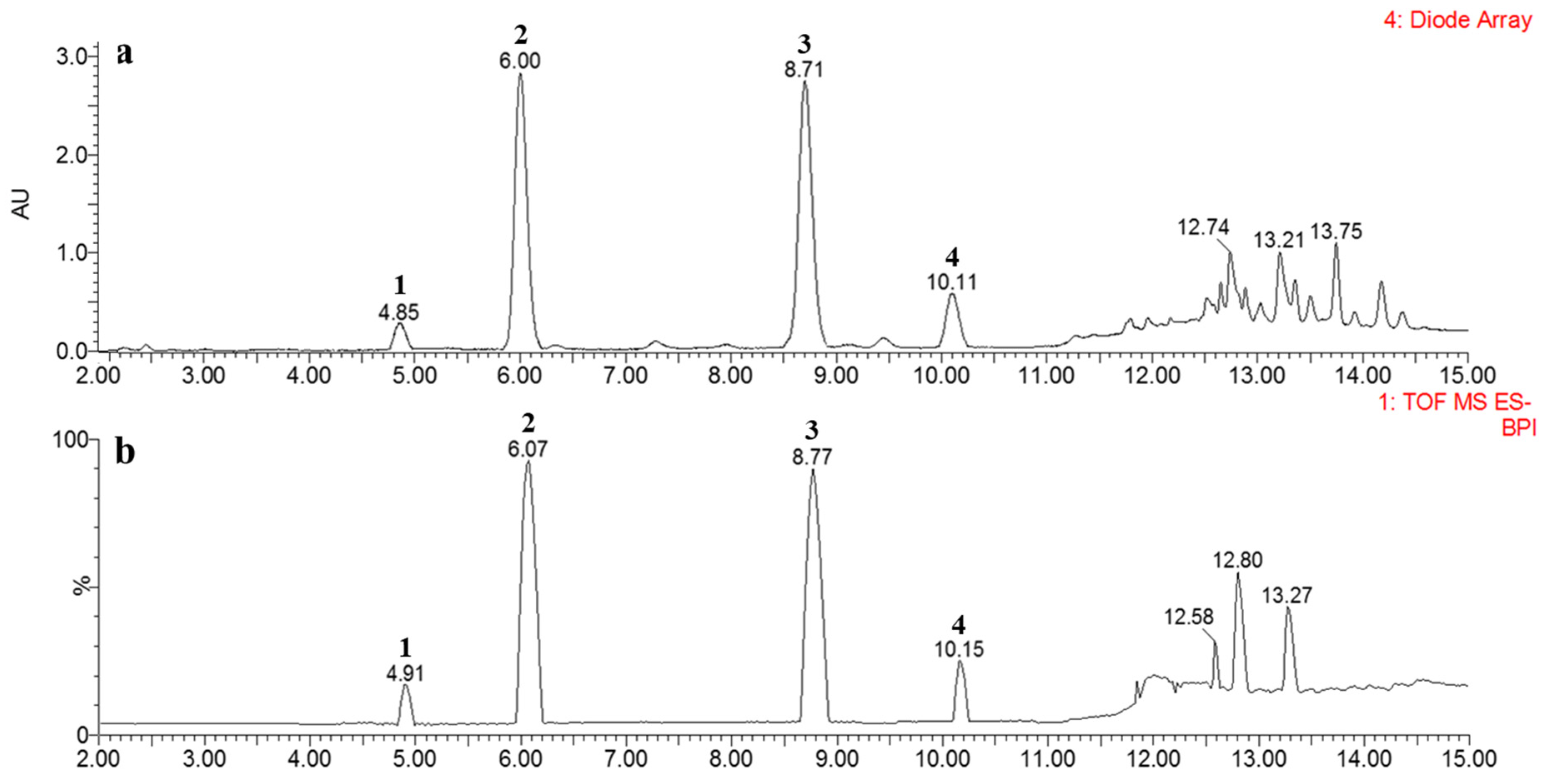
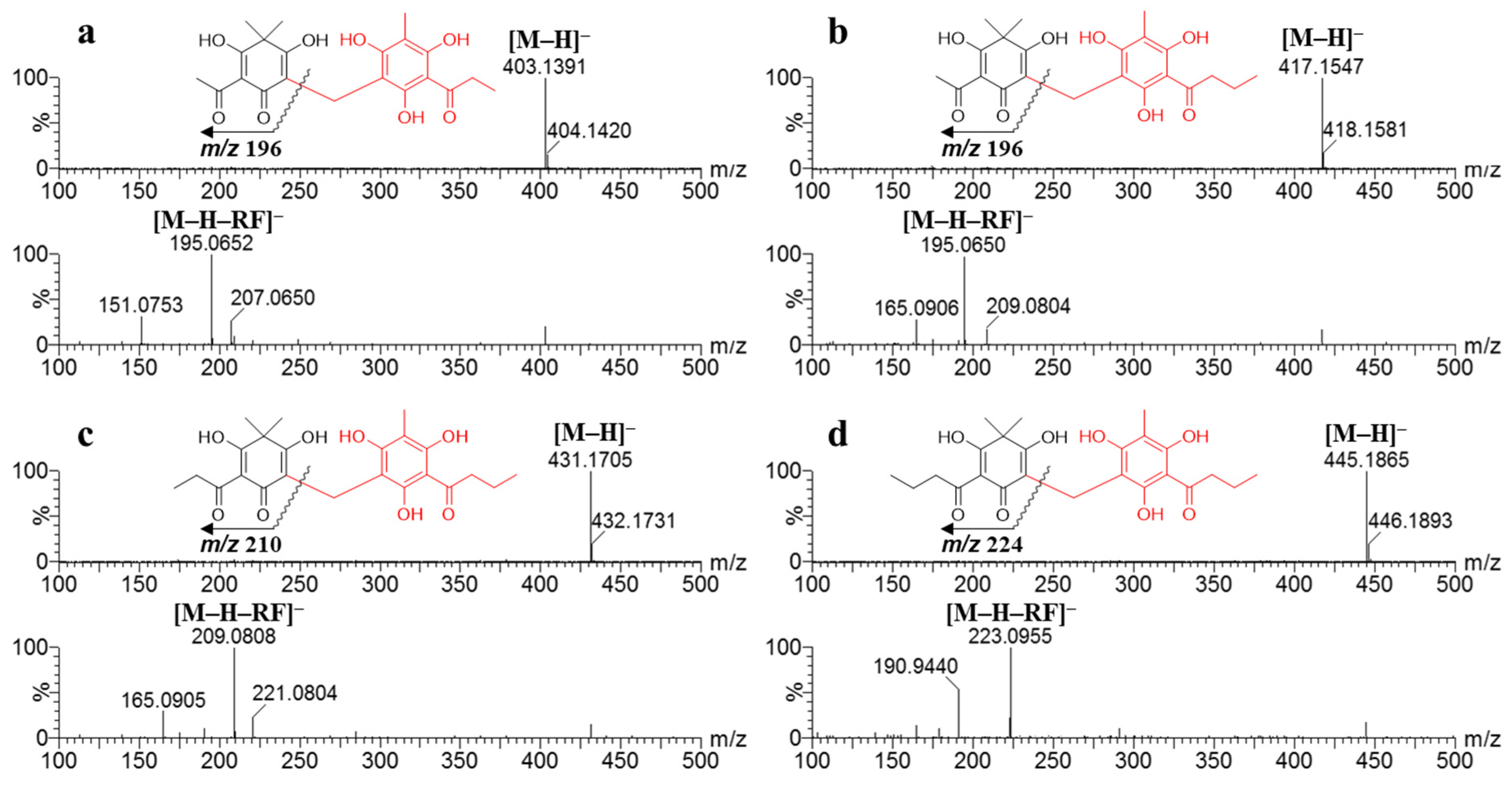
2.3. Ultra-Performance Liquid Chromatography (UPLC)-QTOF-MS Profiles
3. Materials and Methods
3.1. Plant Materials and Sample Preparation
3.2. Instruments
3.3. Sample Extraction, Fractionation, and Isolation
3.4. UPLC-QTOF-MS Analysis
3.5. Assay of XO-Inhibitory Activity and Kinetics
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gibson, T. Hyperuricemia, gout and the kidney. Curr. Opin. Rheumatol. 2012, 24, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Bitik, B.; Öztürk, M.A. An old disease with new insights: Update on diagnosis and treatment of gout. Eur. J. Rheumatol. 2014, 1, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zheng, A.; Xu, P.; Wang, J.; Xue, T.; Dai, S.; Pan, S.; Guo, Y.; Xie, X.; Li, L.; et al. High-protein diet induces hyperuricemia in a new animal model for studying human gout. Int. J. Mol. Sci. 2020, 21, 2147. [Google Scholar] [CrossRef]
- Fukunari, A.; Okamoto, K.; Nishino, T.B.; Eger, T.; Pai, E.F.; Kamezawa, M.; Yamada, I.; Kato, N. Y-700 [1-[3-Cyano-4-(2,2-dimethylpropoxy)phenyl]-1H-pyrazole-4-carboxylic acid]: A potent xanthine oxidoreductase inhibitor with hepatic excretion. J. Pharmacol. Exp. Ther. 2004, 311, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Martillo, M.A.; Nazzal, L.; Crittenden, D.B. The crystallization of monosodium urate. Curr. Rheumatol. Rep. 2014, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.-N.; Wang, W.; Zhang, C.-Y.; Shi, H.-B.; Liu, Y.; Liu, X.H.; Guo, B.-H.; Zhao, D.-M.; Gao, H. Bioassy-guided isolation and identification of xanthine oxidase inhibitory constituents from the leaves of Perilla frutescens. Molecules 2015, 20, 17848–17859. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Nivorozhkin, A.; Szabo, C. Therapeutic effects of xanthine oxidase inhibitor: Renaissance half a century after the discovery of allopurinol. Pharmacol. Rev. 2006, 58. [Google Scholar] [CrossRef] [PubMed]
- Namba, T. The Encyclopedia of Wakan-Yaku (Traditional Sino-Japanese Medicines) with Color Pictures; New completely revised edition; Hoikusha Publishing Co. Ltd.: Osaka, Japan, 1994; Volume II, pp. 53–57. [Google Scholar]
- Ren, Q.; Quan, X.-G.; Wang, Y.-L.; Wang, H.-Y. Isolation and identification of phloroglucinol derivatives from Dryopteris crassirhizoma by HPLC-LTQ-Orbitrap Mass Spectrometry. Chem. Nat. Compd. 2016, 52, 1137–1140. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Y.-T.; Fu, S.-Z.; Peng, B.; Bao, L.-L.; Zhang, Y.-L.; Hu, J.-H.; Zeng, Z.-P.; Geng, D.-H.; Gao, Z.P. Anti-influenza virus (H5N1) activity screening on the phloroglucinols from rhizomes of Dryopteris crassirhizoma. Molecules 2017, 22, 431. [Google Scholar] [CrossRef] [PubMed]
- Na, M.-K.; Jang, J.-P.; Min, B.-S.; Lee, S.-J.; Lee, M.S.; Kim, B.-Y.; Oh, W.-K.; Ahn, J.-S. Fatty acid synthase inhibitory activity of acylphloroglucinols isolated from Dryopteris crassirhizoma. Bioorg. Med. Chem. Lett. 2016, 16, 4738–4742. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.-C.; Kim, O.-H.; Lee, J.-H.; Min, B.-S.; Kim, J.-A. Inhibitory effects of phloroglucinols from the roots of Dryopteris crassirhizoma on melanogenesis. Phytochem. Lett. 2017, 21, 51–56. [Google Scholar] [CrossRef]
- Gao, Z.-P.; Ali, Z.; Zhao, J.-P.; Qiao, L.; Lei, H.-M.; Lu, Y.R.; Khan, I.-A. Phytochemical investigation of the rhizomes of Dryopteris crassirhizoma. Phytochem. Lett. 2008, 1, 188–190. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Lee, G.-J.; Yoon, D.-H.; Yu, T.; Oh, J.-E.; Jeong, D.-O.; Lee, J.-S.; Kim, S.-H.; Kim, T.; Cho, J.Y. ERK1- and TBK1-targeted anti-inflammatory activity of an ethanol extract of Dryopteris crassirhizoma. Phytochem. Lett. 2013, 145, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Na, M.-K.; Na, R.-B.; Min, B.-S.; Lee, H.-K. Antioxidant activity of two phloroglucinol derivatives from Dryopteris crassirhizoma. Biol. Pharm. Bull. 2003, 26, 1354–1356. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.-C.; Lee, S.-M. Antibacterial activity of two phloroglucinols, flavaspidic acids AB and PB, from Dryopteris crassirhizoma. Arch. Pharm. Res. 2009, 32, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, W.; Guo, B.-H.; Gao, H.; Liu, Y.; Liu, X.-H.; Yao, H.-L.; Cheng, K. Chemical evidence for potent xanthine oxidase inhibitory activity of ethyl acetate extract of Citrus aurantium L. dried immature fruits. Molecules 2016, 21, 302. [Google Scholar] [CrossRef] [PubMed]
- Lounasmaa, M. Dérivés Phloroglucinoliques des Fougères du Genre Dryopteris. Planta Med. 1978, 33, 173–176. [Google Scholar] [CrossRef]
- Chu, Y.-H.; Chen, C.-J.; Wu, S.-H.; Hsieh, J.-F. Inhibition of xanthine oxidase by Rhodiola crenulata extracts and their phytochemicals. J. Agric. Food Chem. 2014, 62, 3742–3749. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuk, H.-J.; Kim, J.-Y.; Kim, D.-W.; Song, Y.-H.; Tan, X.-F.; Curtis-Long, M.-J.; Park, K.-H. Novel chromenedione derivatives displaying inhibition of protein tyrosine phosphatase 1B (PTP1B) from Flemingia philippinensis. Bioorg. Med. Chem. Lett. 2016, 26, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.-J.; Lee, Y.-S.; Ryu, H.-W.; Kim, S.-H.; Kim, D.-S. Effects of Toona sinensis leaf extract and its chemical constituents on xanthine oxidase activity and serum uric acid levels in potassium oxonate-induced hyperuricemic rats. Molecules 2018, 23, 3254. [Google Scholar] [CrossRef] [PubMed]

| Compound | Extraction Yield (%) 1 | Xanthine Oxidase | ||
|---|---|---|---|---|
| IC50 2 | Inhibition % 3 | Kinetic Mode (Ki 4, µM) | ||
| EtOAc | 11.3 | 48.7 ± 1.4 ppm | 76.5 ± 1.5 | NT5 |
| MeOH | 22.9 | 54.4 ± 1.1 ppm | 72.4 ± 1.3 | NT |
| 50% MeOH | 21.6 | 134.5 ± 1.7 ppm | 46.8 ± 1.2 | NT |
| H2O | 14.5 | >500 ppm | 7.5 ± 1.0 | NT |
| 1 | - | 6.3 ± 0.4 µM | 79.6 ± 1.6 | Noncompetitive (7.8) |
| 2 | - | 10.2 ± 0.5 µM | 68.1 ± 1.3 | Noncompetitive (11.7) |
| 3 | - | 13.2 ± 0.6 µM | 62.1 ± 0.9 | Noncompetitive (15.3) |
| 4 | - | 20.9 ± 0.9 µM | 54.4 ± 1.5 | Noncompetitive (21.1) |
| Allopurinol | - | 5.7 ± 0.2 µM | 82.5 ± 1.7 | Competitive |
| Oxypurinol | - | 43.1 ± 0.8 µM | 42.2 ± 1.2 | NT |
| Peak | tR | λmax | Dried Rhizomes (mg/g) 1 | [M − H]− (m/z) | Molecular Formula | Identification | |||
|---|---|---|---|---|---|---|---|---|---|
| (no.) | (min) | (nm) | EtOAc | MeOH | 50%MeOH | H2O | (ESI-HR-MS) | (ppm Error) | |
| 1 | 4.85 | 294 | 0.33 | 0.32 | 0.21 | tr | 403.1391 | C21H23O8 (−0.5) | Flavaspidic acid AP |
| 2 | 6.00 | 296 | 4.41 | 4.12 | 3.46 | tr | 417.1547 | C22H25O8 (−0.5) | Flavaspidic acid AB |
| 3 | 8.71 | 296 | 4.49 | 4.47 | 3.26 | tr | 431.1705 | C23H27O8 (−0.2) | Flavaspidic acid PB |
| 4 | 10.11 | 295 | 0.72 | 0.71 | 0.56 | tr | 445.1865 | C24H29O8 (0.7) | Flavaspidic acid BB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuk, H.J.; Kim, J.-Y.; Sung, Y.-Y.; Kim, D.-S. Phloroglucinol Derivatives from Dryopteris crassirhizoma as Potent Xanthine Oxidase Inhibitors. Molecules 2021, 26, 122. https://doi.org/10.3390/molecules26010122
Yuk HJ, Kim J-Y, Sung Y-Y, Kim D-S. Phloroglucinol Derivatives from Dryopteris crassirhizoma as Potent Xanthine Oxidase Inhibitors. Molecules. 2021; 26(1):122. https://doi.org/10.3390/molecules26010122
Chicago/Turabian StyleYuk, Heung Joo, Ji-Yul Kim, Yoon-Young Sung, and Dong-Seon Kim. 2021. "Phloroglucinol Derivatives from Dryopteris crassirhizoma as Potent Xanthine Oxidase Inhibitors" Molecules 26, no. 1: 122. https://doi.org/10.3390/molecules26010122
APA StyleYuk, H. J., Kim, J.-Y., Sung, Y.-Y., & Kim, D.-S. (2021). Phloroglucinol Derivatives from Dryopteris crassirhizoma as Potent Xanthine Oxidase Inhibitors. Molecules, 26(1), 122. https://doi.org/10.3390/molecules26010122







