The Antiproliferative Effects of Flavonoid MAO Inhibitors on Prostate Cancer Cells
Abstract
1. Introduction
2. Results
2.1. KKRs Inhibited Monoamine Oxidase-A
2.1.1. KKRs High Throughput Screening for MAO-A Selective Inhibition
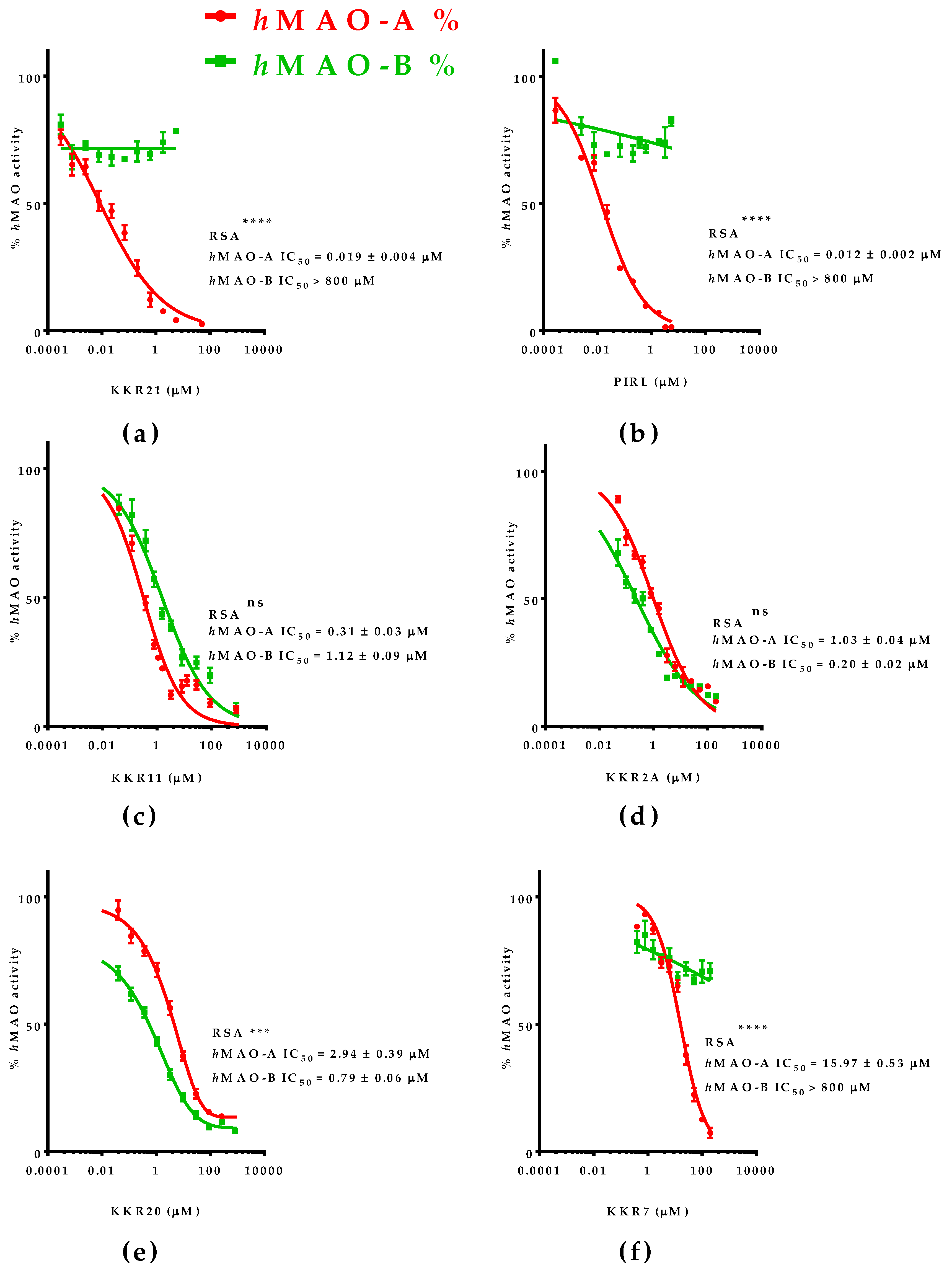
2.1.2. The Most Inhibitory Potency of KKR Compounds against hMAO-A
2.2. Mode of MAO-A Inhibition of KKR21 and KKR11
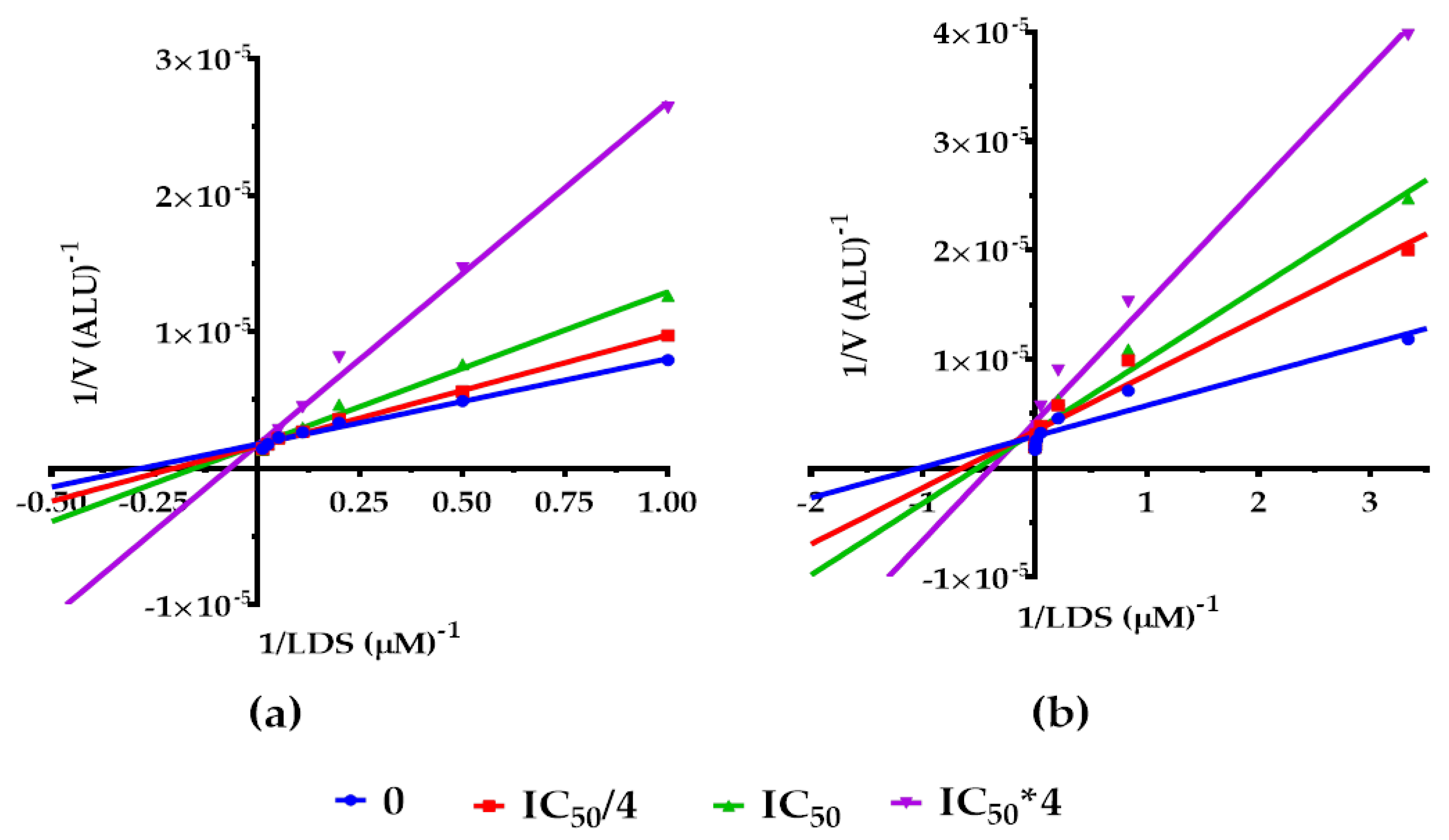
2.2.1. Effects on hMAO-A Michaelis–Menten Parameters
2.2.2. Molecular Docking at the MAO-A Active Site
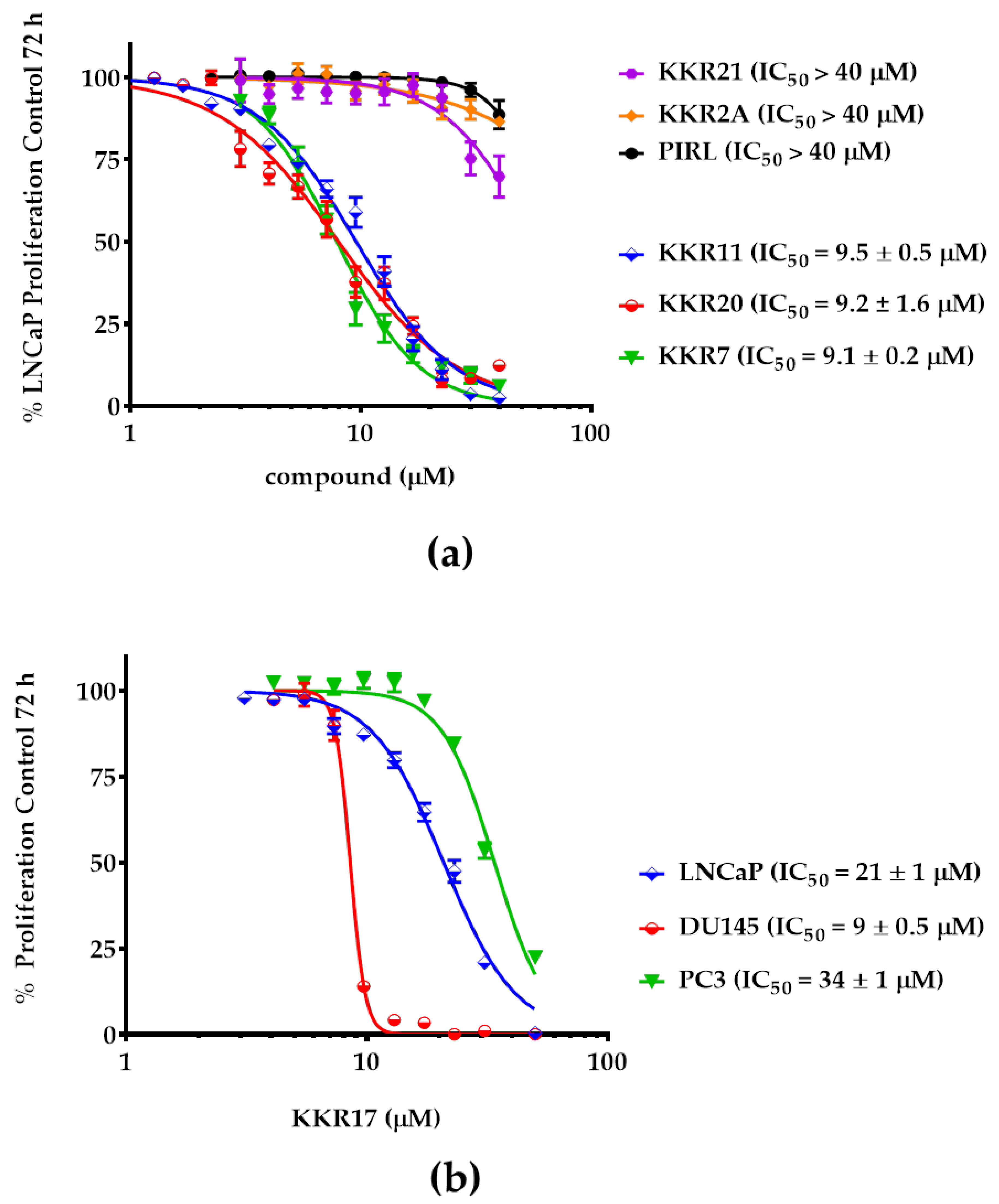
2.3. KKRs Cytotoxic and Antiproliferative Effects in PCa Cells
2.3.1. Standards Effects on PCa Cells Proliferation
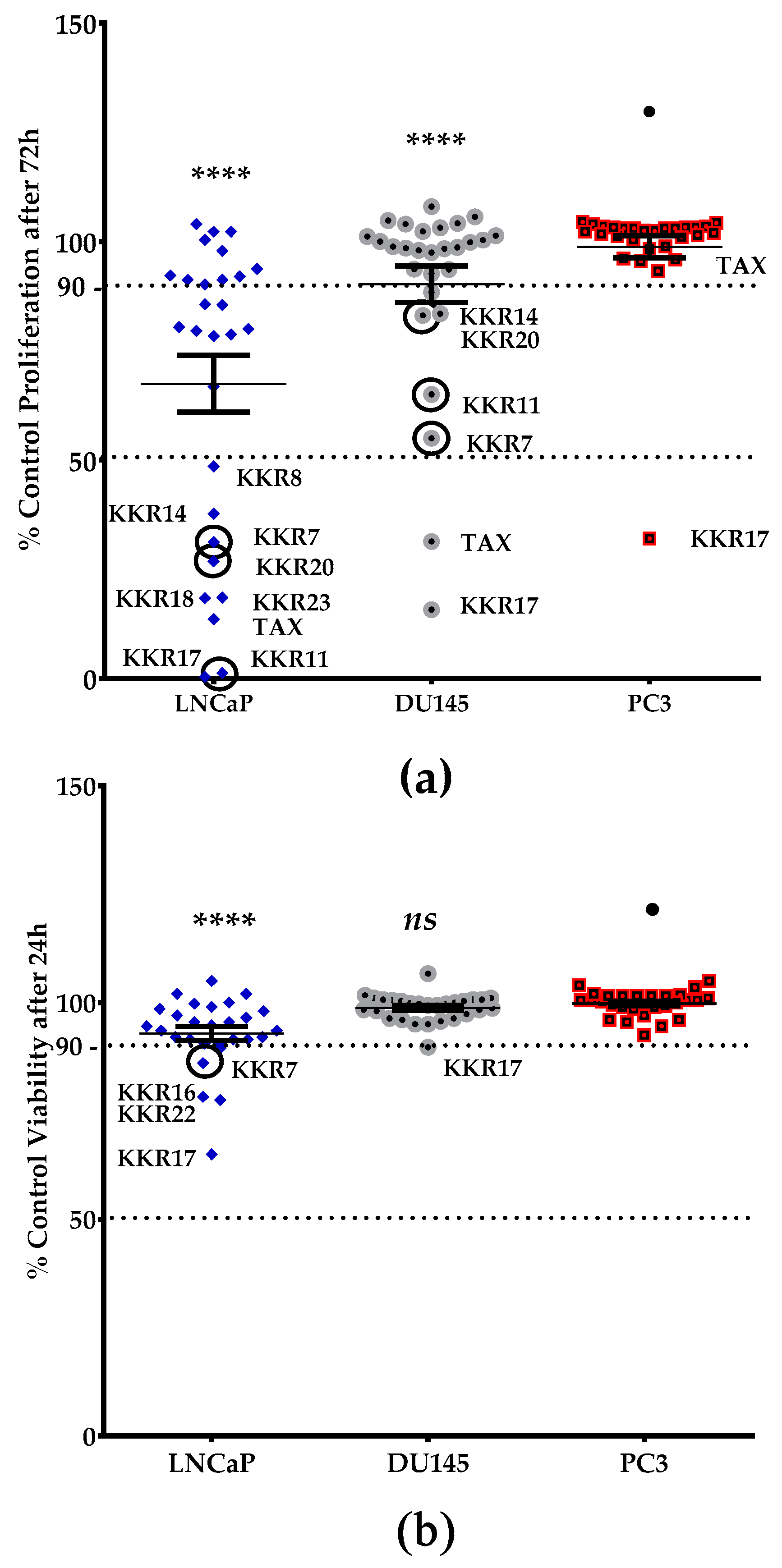
2.3.2. KKR Structures Affected the LNCaP Cells the Most
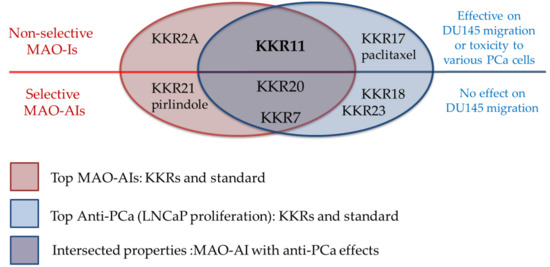
2.3.3. Three MAO-A Inhibiting KKRs Affected LNCaP Cells Proliferation Equally Potently
2.3.4. Three MAO-A Inhibiting KKRs Differently Affected the LNCaP Cells Morphology
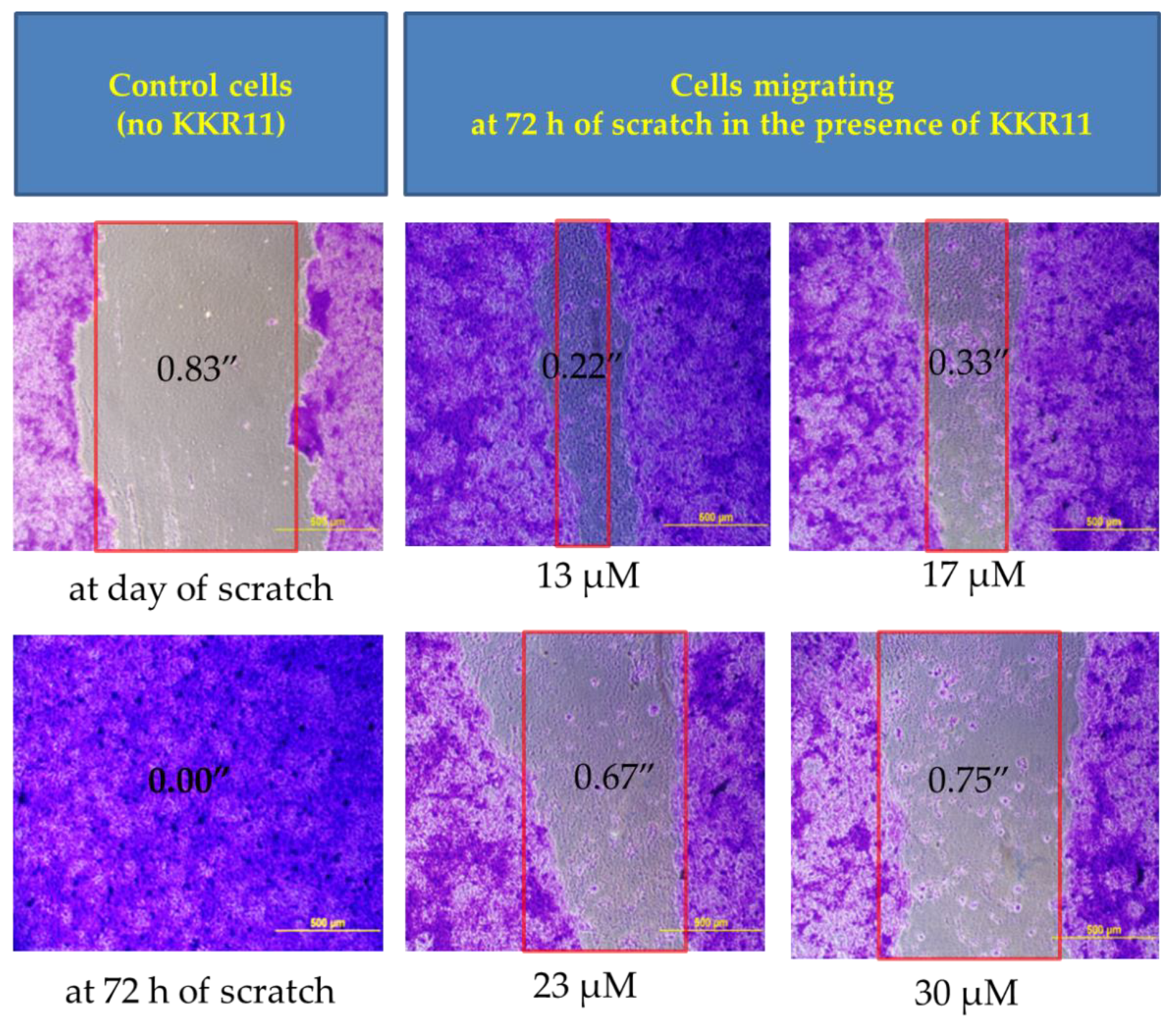
2.3.5. KKR11 Inhibited the DU145 Cells Migration
3. Discussion
4. Materials and Methods
4.1. Materials and Synthesis
4.1.1. Materials
4.1.2. KKR Synthesis
4.2. Investigating the Inhibition of Two Monoamine Oxidase Isozymes
4.2.1. Isozymes Reactions Optimization
4.2.2. Screening KKR Compounds for Inhibiting hMAO-A and hMAO-B Activities
4.2.3. Relative Selectivity against hMAO-A
4.2.4. Michaelis–Menten Kinetics Determination
4.2.5. Molecular Docking
4.3. Testing the Effects on Prostate Cancer Cell Proliferation and Metastasis
4.3.1. Cell-Culture
4.3.2. Screening for Effective KKRs against PCa-Cell Lines
The Viability Screen Assay
The Antiproliferation Screen Assay
4.3.3. Proliferation and Morphological Observations of the PCa Cells with Top Effective KKRs
Antiproliferation Dose-Response Assay for 72 h
Imaging the Proliferative LNCaP after Five-Days
4.3.4. Migration Assay, Fixing, and Imaging DU145 Cells
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Watts, S.; Leydon, G.; Birch, B.; Prescott, P.; Lai, L.; Eardley, S.; Lewith, G. Depression and anxiety in prostate cancer: A systematic review and meta-analysis of prevalence rates. BMJ Open 2014, 4, e003901. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, Z.; Arora, K.; Wilson, S.N.; King, S.A.; Madi, R.; Neal, D.E.; Kurdyak, P.; Kulkarni, G.S.; Lewis, R.W.; Terris, M.K. Decreasing suicide risk among patients with prostate cancer: Implications for depression, erectile dysfunction, and suicidal ideation screening. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Tombal, B. Prostate cancer, depression, and risk of suicide: Should we pay more attention? Eur. Urol. 2010, 57, 396–397. [Google Scholar] [CrossRef] [PubMed]
- Jayadevappa, R.; Malkowicz, S.B.; Chhatre, S.; Johnson, J.C.; Gallo, J.J. The burden of depression in prostate cancer. Psycho-Oncology 2012, 21, 1338–1345. [Google Scholar] [CrossRef]
- Sountoulides, P.; Rountos, T. Adverse effects of androgen deprivation therapy for prostate cancer: Prevention and management. ISRN Urol. 2013, 2013, 240108. [Google Scholar] [CrossRef]
- Crawford, E.D.; Moul, J.W. ADT risks and side effects in advanced prostate cancer: Cardiovascular and acute renal injury. Oncology 2015, 29, 55–58. [Google Scholar]
- Dinh, K.T.; Reznor, G.; Muralidhar, V.; Mahal, B.A.; Nezolosky, M.D.; Choueiri, T.K.; Hoffman, K.E.; Hu, J.C.; Sweeney, C.J.; Trinh, Q.-D.; et al. Association of androgen deprivation therapy with depression in localized prostate cancer. J. Clin. Oncol. 2016, 34, 1905–1912. [Google Scholar] [CrossRef]
- Nead, K.T.; Sinha, S.; Yang, D.D.; Nguyen, P.L. Association of androgen deprivation therapy and depression in the treatment of prostate cancer: A systematic review and meta-analysis. Urol. Oncol. 2017, 35, e661–e664. [Google Scholar] [CrossRef]
- Thomas, H.R.; Chen, M.-H.; D’Amico, A.V.; Bennett, C.L.; Kattan, M.W.; Sartor, O.; Stein, K.; Nguyen, P.L. Association between androgen deprivation therapy and patient-reported depression in men with recurrent prostate cancer. Clin. Genitourin. Cancer 2018, 16, 313–317. [Google Scholar] [CrossRef]
- Nouri, M.; Ratther, E.; Stylianou, N.; Nelson, C.C.; Hollier, B.G.; Williams, E.D. Androgen-targeted therapy-induced epithelial mesenchymal plasticity and neuroendocrine transdifferentiation in prostate cancer: An opportunity for intervention. Front. Oncol. 2014, 4, 370. [Google Scholar] [CrossRef]
- Wen, S.; Niu, Y.; Lee, S.O.; Chang, C. Androgen receptor (ar) positive vs negative roles in prostate cancer cell deaths including apoptosis, anoikis, entosis, necrosis and autophagic cell death. Cancer Treat. Rev. 2014, 40, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Sumanasuriya, S.; De Bono, J. Treatment of advanced prostate cancer-a review of current therapies and future promise. Cold Spring Harb. Perspect. Med. 2018, 8, a030635. [Google Scholar] [CrossRef]
- Nevedomskaya, E.; Baumgart, S.J.; Haendler, B. Recent advances in prostate cancer treatment and drug discovery. Int. J. Mol. Sci. 2018, 19, 1359. [Google Scholar] [CrossRef] [PubMed]
- Drozak, J.; Kozlowski, M. monoamine oxidase as a target for drug action. Postepy Hig. I Med. Dosw. 2006, 60, 498–515. [Google Scholar]
- Shih, J.C. Monoamine oxidase isoenzymes: Genes, functions and targets for behavior and cancer therapy. J. Neural Transm. 2018, 125, 1553–1566. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Type a monoamine oxidase and serotonin are coordinately involved in depressive disorders: From neurotransmitter imbalance to impaired neurogenesis. J. Neural Transm. 2018, 125, 53–66. [Google Scholar] [CrossRef]
- Peehl, D.M.; Coram, M.; Khine, H.; Reese, S.; Nolley, R.; Zhao, H. The significance of monoamine oxidase-a expression in high grade prostate cancer. J. Urol. 2008, 180, 2206–2211. [Google Scholar] [CrossRef]
- Zhao, H.; Nolley, R.; Chen, Z.; Reese, S.W.; Peehl, D.M. Inhibition of monoamine oxidase a promotes secretory differentiation in basal prostatic epithelial cells. Differ. Res. Biol. Divers. 2008, 76, 820–830. [Google Scholar] [CrossRef][Green Version]
- Wu, J.B.; Shao, C.; Li, X.; Li, Q.; Hu, P.; Shi, C.; Li, Y.; Chen, Y.T.; Yin, F.; Liao, C.P.; et al. Monoamine oxidase a mediates prostate tumorigenesis and cancer metastasis. J. Clin. Investig. 2014, 124, 2891–2908. [Google Scholar] [CrossRef]
- Gordon, R.R.; Wu, M.; Huang, C.Y.; Harris, W.P.; Sim, H.G.; Lucas, J.M.; Coleman, I.; Higano, C.S.; Gulati, R.; True, L.D.; et al. Chemotherapy-induced monoamine oxidase expression in prostate carcinoma functions as a cytoprotective resistance enzyme and associates with clinical outcomes. PLoS ONE 2014, 9, e104271. [Google Scholar] [CrossRef]
- Youdim, M.B.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Sturza, A.; Leisegang, M.S.; Babelova, A.; Schroder, K.; Benkhoff, S.; Loot, A.E.; Fleming, I.; Schulz, R.; Muntean, D.M.; Brandes, R.P. Monoamine oxidases are mediators of endothelial dysfunction in the mouse aorta. Hypertension 2013, 62, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kaludercic, N.; Mialet-Perez, J.; Paolocci, N.; Parini, A.; Di Lisa, F. Monoamine oxidases as sources of oxidants in the heart. J. Mol. Cell. Cardiol. 2014, 73, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Maker, G.L.; Hood, S.D.; Drummond, P.D. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Khandrika, L.; Kumar, B.; Koul, S.; Maroni, P.; Koul, H.K. Oxidative stress in prostate cancer. Cancer Lett. 2009, 282, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Asnis, G.M.; Henderson, M.A. Emsam (deprenyl patch): How a promising antidepressant was underutilized. Neuropsychiatr. Dis. Treat. 2014, 10, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chang, Y.T.; Campbell, M.; Lin, T.P.; Pan, C.C.; Lee, H.C.; Shih, J.C.; Chang, P.C. Maoa-a novel decision maker of apoptosis and autophagy in hormone refractory neuroendocrine prostate cancer cells. Sci. Rep. 2017, 7, 46338. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Vue, B.; Zhang, S.; Chen, Q.H. Flavonoids with therapeutic potential in prostate cancer. Anti-Cancer Agents Med. Chem. 2016, 16, 1205–1229. [Google Scholar] [CrossRef]
- Miron, A.; Aprotosoaie, A.C.; Trifan, A.; Xiao, J. Flavonoids as modulators of metabolic enzymes and drug transporters. Ann. N. Y. Acad. Sci. 2017, 1398, 152–167. [Google Scholar] [CrossRef]
- Mathew, B.; Haridas, A.; Suresh, J.; Mathew, G.E.; Ucar, G.; Jayaprakash, V. Monoamine oxidase inhibitory action of chalcones: A mini review. Cent. Nerv. Syst. Agents Med. Chem. 2016, 16, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Leon-Gonzalez, A.J.; Acero, N.; Munoz-Mingarro, D.; Navarro, I.; Martin-Cordero, C. Chalcones as promising lead compounds on cancer therapy. Curr. Med. Chem. 2015, 22, 3407–3425. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Moorthy, N.S.; Ramasamy, S.; Vanam, U.; Manivannan, E.; Karunagaran, D.; Trivedi, P. Advances in chalcones with anticancer activities. Recent Pat. Anti-Cancer Drug Discov. 2015, 10, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef]
- Russo, G.L.; Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Iannitti, R.; Palumbo, R. Quercetin: A pleiotropic kinase inhibitor against cancer. Cancer Treat. Res. 2014, 159, 185–205. [Google Scholar]
- Yanagihara, N.; Zhang, H.; Toyohira, Y.; Takahashi, K.; Ueno, S.; Tsutsui, M.; Takahashi, K. New insights into the pharmacological potential of plant flavonoids in the catecholamine system. J. Pharmacol. Sci. 2014, 124, 123–128. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yuan, B.; Hu, X.; Okazaki, M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am. J. Cancer Res. 2019, 9, 1517–1535. [Google Scholar]
- Chaurasiya, N.D.; Ibrahim, M.A.; Muhammad, I.; Walker, L.A.; Tekwani, B.L. Monoamine oxidase inhibitory constituents of propolis: Kinetics and mechanism of inhibition of recombinant human mao-a and mao-b. Molecules 2014, 19, 18936–18952. [Google Scholar] [CrossRef]
- Margret, A.A.; Begum, T.N.; Parthasarathy, S.; Suvaithenamudhan, S. A strategy to employ clitoria ternatea as a prospective brain drug confronting monoamine oxidase (mao) against neurodegenerative diseases and depression. Nat. Prod. Bioprospect. 2015, 5, 293–306. [Google Scholar] [CrossRef]
- Zarmouh, N.O.; Eyunni, S.K.; Soliman, K.F. The benzopyrone biochanin-a as a reversible, competitive, and selective monoamine oxidase b inhibitor. BMC Complementary Altern. Med. 2017, 17, 34. [Google Scholar] [CrossRef]
- Zarmouh, N.O.; Messeha, S.S.; Elshami, F.M.; Soliman, K.F. Evaluation of the isoflavone genistein as reversible human monoamine oxidase-a and -b inhibitor. Evid.-Based Complementary Altern. Med. eCAM 2016, 2016, 1423052. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Guo, S.; Rajaram, P.; Lee, M.; Chen, G.; Fong, R.; Gonzalez, A.; Zhang, Q.; Zheng, S.; et al. Structure-activity relationship and pharmacokinetic studies of 3-O-substitutedflavonols as anti-prostate cancer agents. Eur. J. Med. Chem. 2018, 157, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.Q.; Venkateswaran, V.; Viswanathan, L.; Teahan, S.J.; Fleshner, N.E.; Klotz, L.H. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2006, 9, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M.I. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007, 18, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Majewska-Wierzbicka, M.; Czeczot, H. Anticancer activity of flavonoids. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2012, 33, 364–369. [Google Scholar]
- Gupta-Elera, G.; Garrett, A.R.; Robison, R.A.; O’Neill, K.L. The role of oxidative stress in prostate cancer. Eur. J. Cancer Prev. 2012, 21, 155–162. [Google Scholar] [CrossRef]
- Inaba-Hasegawa, K.; Shamoto-Nagai, M.; Maruyama, W.; Naoi, M. Type B and A monoamine oxidase and their inhibitors regulate the gene expression of Bcl-2 and neurotrophic factors in human glioblastoma u118mg cells: Different signal pathways for neuroprotection by selegiline and rasagiline. J. Neural Transm. 2017, 124, 1055–1066. [Google Scholar] [CrossRef]
- Johnson, S.; Stockmeier, C.A.; Meyer, J.H.; Austin, M.C.; Albert, P.R.; Wang, J.; May, W.L.; Rajkowska, G.; Overholser, J.C.; Jurjus, G.; et al. The reduction of r1, a novel repressor protein for monoamine oxidase a, in major depressive disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011, 36, 2139–2148. [Google Scholar] [CrossRef]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.Z.; Huang, J. Pc3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.M.; Chresta, C.M.; Davies, S.M.; Walker, M.C.; Harris, A.L.; Hartley, J.A.; Masters, J.R.; Hickson, I.D. Relationship between topoisomerase ii level and chemosensitivity in human tumor cell lines. Cancer Res. 1991, 51, 6592–6595. [Google Scholar] [PubMed]
- van Brussel, J.P.; van Steenbrugge, G.J.; Romijn, J.C.; Schroder, F.H.; Mickisch, G.H. Chemosensitivity of prostate cancer cell lines and expression of multidrug resistance-related proteins. Eur. J. Cancer 1999, 35, 664–671. [Google Scholar] [CrossRef]
- Larsen, A.K.; Escargueil, A.E.; Skladanowski, A. Catalytic topoisomerase ii inhibitors in cancer therapy. Pharmacol. Ther. 2003, 99, 167–181. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Busselberg, D. Flavonoids in cancer and apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Flamand, V.; Zhao, H.; Peehl, D.M. Targeting monoamine oxidase a in advanced prostate cancer. J. Cancer Res. Clin. Oncol. 2010, 136, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Shetnev, A.; Osipyan, A.; Baykov, S.; Sapegin, A.; Chirkova, Z.; Korsakov, M.; Petzer, A.; Engelbrecht, I.; Petzer, J.P. Novel monoamine oxidase inhibitors based on the privileged 2-imidazoline molecular framework. Bioorg. Med. Chem. Lett. 2018, 29, 40–46. [Google Scholar] [CrossRef]
- Chirkova, Z.V.; Kabanova, M.V.; Filimonov, S.I.; Abramov, I.G.; Petzer, A.; Engelbrecht, I.; Petzer, J.P.; Yu Suponitsky, K.; Veselovsky, A.V. An investigation of the monoamine oxidase inhibition properties of pyrrolo[3,4-f]indole-5,7-dione and indole-5,6-dicarbonitrile derivatives. Drug Dev. Res. 2018, 79, 81–93. [Google Scholar] [CrossRef]
- Thorp, A.A.; Sinn, N.; Buckley, J.D.; Coates, A.M.; Howe, P.R. Soya isoflavone supplementation enhances spatial working memory in men. Br. J. Nutr. 2009, 102, 1348–1354. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Ahmad, A.; Bao, B.; Sarkar, F.H. Antioxidant function of isoflavone and 3,3′-diindolylmethane: Are they important for cancer prevention and therapy? Antioxid. Redox Signal. 2013, 19, 139–150. [Google Scholar] [CrossRef]
- Wei, H.; Bowen, R.; Zhang, X.; Lebwohl, M. Isoflavone genistein inhibits the initiation and promotion of two-stage skin carcinogenesis in mice. Carcinogenesis 1998, 19, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Raschke, M.; Rowland, I.R.; Magee, P.J.; Pool-Zobel, B.L. Genistein protects prostate cells against hydrogen peroxide-induced DNA damage and induces expression of genes involved in the defence against oxidative stress. Carcinogenesis 2006, 27, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Biersack, B.; Li, Y.; Bao, B.; Kong, D.; Ali, S.; Banerjee, S.; Sarkar, F.H. Perspectives on the role of isoflavones in prostate cancer. AAPS J. 2013, 15, 991–1000. [Google Scholar] [CrossRef]
- Ajdzanovic, V.; Medigovic, I.; Zivanovic, J.; Mojic, M.; Milosevic, V. Membrane steroid receptor-mediated action of soy isoflavones: Tip of the iceberg. J. Membr. Biol. 2015, 248, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Prins, L.H.; Petzer, J.P.; Malan, S.F. Inhibition of monoamine oxidase by indole and benzofuran derivatives. Eur. J. Med. Chem. 2010, 45, 4458–4466. [Google Scholar] [CrossRef]
- Youdim, M.B.; Bakhle, Y.S. Monoamine oxidase: Isoforms and inhibitors in parkinson’s disease and depressive illness. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S287–S296. [Google Scholar] [CrossRef]
- Vezina, P.; Mohr, E.; Grimes, D. Deprenyl in parkinson’s disease: Mechanisms, neuroprotective effect, indications and adverse effects. Can. J. Neurol. Sci. Le J. Can. Des Sci. Neurol. 1992, 19, 142–146. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Khatkar, A. Docking-related survey on natural-product-based new monoamine oxidase inhibitors and their therapeutic potential. Comb. Chem. High Throughput Screen. 2017, 20, 474–491. [Google Scholar] [CrossRef]
- Tripathi, A.C.; Upadhyay, S.; Paliwal, S.; Saraf, S.K. Privileged scaffolds as mao inhibitors: Retrospect and prospects. Eur. J. Med. Chem. 2018, 145, 445–497. [Google Scholar] [CrossRef]
- Bonnet, U. Moclobemide: Therapeutic use and clinical studies. CNS Drug Rev. 2003, 9, 97–140. [Google Scholar] [CrossRef]
- Anderson, M.C.; Hasan, F.; McCrodden, J.M.; Tipton, K.F. Monoamine oxidase inhibitors and the cheese effect. Neurochem. Res. 1993, 18, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Iacovino, L.G.; Magnani, F.; Binda, C. The structure of monoamine oxidases: Past, present, and future. J. Neural Transm. 2018, 125, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Son, S.Y.; Ma, J.; Kondou, Y.; Yoshimura, M.; Yamashita, E.; Tsukihara, T. Structure of human monoamine oxidase a at 2.2-a resolution: The control of opening the entry for substrates/inhibitors. Proc. Natl. Acad. Sci. USA 2008, 105, 5739–5744. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Su, Z.; Long, W.; Liu, Y.; Chang, X.; Zhang, H.; Ding, G.; Feng, Y.; Cai, D.; Zou, Z. Metabonomics combined with uplc-ms chemical profile for discovery of antidepressant ingredients of a traditional chinese medicines formula, chaihu-shu-gan-san. Evid.-Based Complementary Altern. Med. eCAM 2013, 2013, 487158. [Google Scholar] [CrossRef] [PubMed]
- Mateeva, N.N.; Kode, R.N.; Redda, K.K. Synthesis of novel flavonoid derivatives as potential hiv- integrase inhibitors. J. Heterocycl. Chem. 2002, 39, 1251–1258. [Google Scholar] [CrossRef]
- Mills, C.; Mateeva, N.; Redda, K. Synthesis of novel flavonoid derivatives and evaluation of their anti-cancer activities. Cancer Res. 2006, 66, 267. [Google Scholar]
- Valley, M.P.; Zhou, W.; Hawkins, E.M.; Shultz, J.; Cali, J.J.; Worzella, T.; Bernad, L.; Good, T.; Good, D.; Riss, T.L.; et al. A bioluminescent assay for monoamine oxidase activity. Anal. Biochem. 2006, 359, 238–246. [Google Scholar] [CrossRef]
- Hawkins, P.C.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Conformer generation with omega: Algorithm and validation using high quality structures from the protein databank and cambridge structural database. J. Chem. Inf. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef]
Sample Availability: Samples of the KKR compounds are available from the author K.K.R. |




| Code | Structure | IUPAC Name |
|---|---|---|
| KKR-1 | 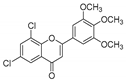 | 6,8-dichloro-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one |
| KKR-2A |  | (E)-3-hydroxy-5,7-dimethoxy-2-(3,4,5-trimethoxystyryl)-4H-chromen-4-one |
| KKR-3 | 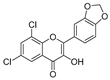 | 2-(benzo[d][1,3] dioxol-5-yl)-6,8-dichloro-3-hydroxy-4H-chromen-4-one |
| KKR-4 | 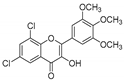 | 6,8-dichloro-3-hydroxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one |
| KKR-5 | 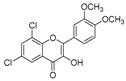 | 6,8-dichloro-2-(3,4-dimethoxyphenyl)-3-hydroxy-4H-chromen-4-one |
| KKR-7 |  | 6,8-dichloro-3-hydroxy-2-phenyl-4H-chromen-4-one |
| KKR-8 | 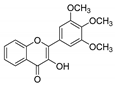 | 3-hydroxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one |
| KKR-9 | 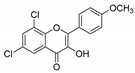 | 6,8-dichloro-3-hydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one |
| KKR-10 | 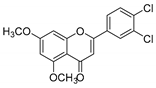 | 2-(3,4-dichlorophenyl)-5,7-dimethoxy-4H-chromen-4-one |
| KKR-11 | 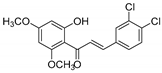 | (E)-3-(3,4-dichlorophenyl)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one |
| KKR-12 | 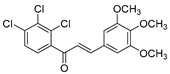 | (E)-1-(2,3,4-trichlorophenyl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one |
| KKR-14 | 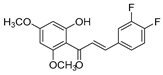 | (E)-3-(3,4-difluorophenyl)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one |
| KKR-16 | 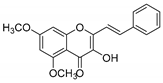 | (E)-3-hydroxy-5,7-dimethoxy-2-styryl-4H-chromen-4-one |
| KKR-17 |  | (E)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one |
| KKR-18 | 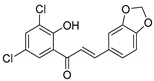 | (E)-3-(benzo[d][1,3]dioxol-5-yl)-1-(3,5-dichloro-2-hydroxyphenyl)prop-2-en-1-one |
| KKR-19 | 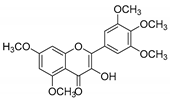 | 3-hydroxy-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one |
| KKR-20 | 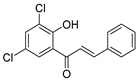 | (E)-1-(3,5-dichloro-2-hydroxyphenyl)-3-phenylprop-2-en-1-one |
| KKR-21 | 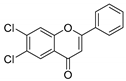 | 6,7-dichloro-2-phenyl-4H-chromen-4-one |
| KKR-22 | 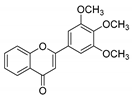 | 2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one |
| KKR-23 | 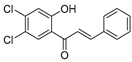 | (E)-1-(4,5-dichloro-2-hydroxyphenyl)-3-phenylprop-2-en-1-one |
| Inhibitor | KKR21 | KKR11 | ||||
|---|---|---|---|---|---|---|
| Parameter | Average (μM) | %R2 | p Value | Average (μM) | %R2 | p Value |
| Km (μM) | ||||||
| 0 | 3.85 ± 0.44 | 99 | 0.98 ± 0.05 | 93 | ||
| IC50/4 | 5.88 ± 0.35 | 99 | ** | 1.59 ± 0.10 | 95 | ** |
| IC50 | 7.69 ± 0.59 | 99 | *** | 1.64 ± 0.03 | 95 | *** |
| IC50 × 4 | 14.29 ± 2.04 | 99 | **** | 2.44 ± 0.24 | 97 | *** |
| Vmax(U/mL/h) | 1.38 × 10−6–1.43 × 10−6 | ns | 3 × 10−6–4 × 10−6 | ns | ||
| Alpha | >>1 | >>1 | ||||
| Ki (μM) | 0.0017 | 0.0074 | ||||
| Non-Covalent Interaction | Shared in KKR21, KKR11 and 2Z5X | KKR21 and KKR11 | with KKR11 | with 2Z5X |
|---|---|---|---|---|
| Amino acid residue | ILE180A, ILE325A, ILE335A | ASN181A | ALA111A | THR336A |
| LEU337A, PHE208A, PHE352A | LEU97A | |||
| TYR69A, TYR407A, TYR444A * | ||||
| GLN215A, CYS323A, MET350A | ||||
| other molecules | HOH726, HOH739, HOH746 | FAD 600A | ||
| HOH766, HOH805 | HOH710 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarmouh, N.O.; Messeha, S.S.; Mateeva, N.; Gangapuram, M.; Flowers, K.; Eyunni, S.V.K.; Zhang, W.; Redda, K.K.; Soliman, K.F.A. The Antiproliferative Effects of Flavonoid MAO Inhibitors on Prostate Cancer Cells. Molecules 2020, 25, 2257. https://doi.org/10.3390/molecules25092257
Zarmouh NO, Messeha SS, Mateeva N, Gangapuram M, Flowers K, Eyunni SVK, Zhang W, Redda KK, Soliman KFA. The Antiproliferative Effects of Flavonoid MAO Inhibitors on Prostate Cancer Cells. Molecules. 2020; 25(9):2257. https://doi.org/10.3390/molecules25092257
Chicago/Turabian StyleZarmouh, Najla O., Samia S. Messeha, Nelly Mateeva, Madhavi Gangapuram, Kacy Flowers, Suresh V. K. Eyunni, Wang Zhang, Kinfe K. Redda, and Karam F. A. Soliman. 2020. "The Antiproliferative Effects of Flavonoid MAO Inhibitors on Prostate Cancer Cells" Molecules 25, no. 9: 2257. https://doi.org/10.3390/molecules25092257
APA StyleZarmouh, N. O., Messeha, S. S., Mateeva, N., Gangapuram, M., Flowers, K., Eyunni, S. V. K., Zhang, W., Redda, K. K., & Soliman, K. F. A. (2020). The Antiproliferative Effects of Flavonoid MAO Inhibitors on Prostate Cancer Cells. Molecules, 25(9), 2257. https://doi.org/10.3390/molecules25092257