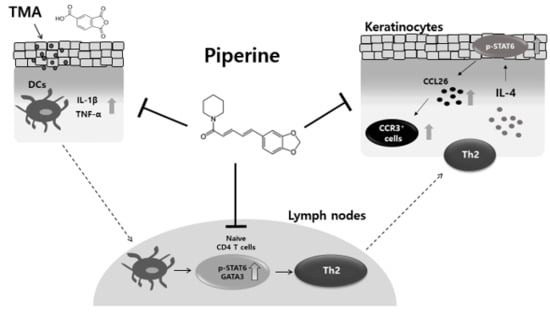
Piperine Ameliorates Trimellitic Anhydride-Induced Atopic Dermatitis-Like Symptoms by Suppressing Th2-Mediated Immune Responses via Inhibition of STAT6 Phosphorylation
Abstract
1. Introduction
2. Results
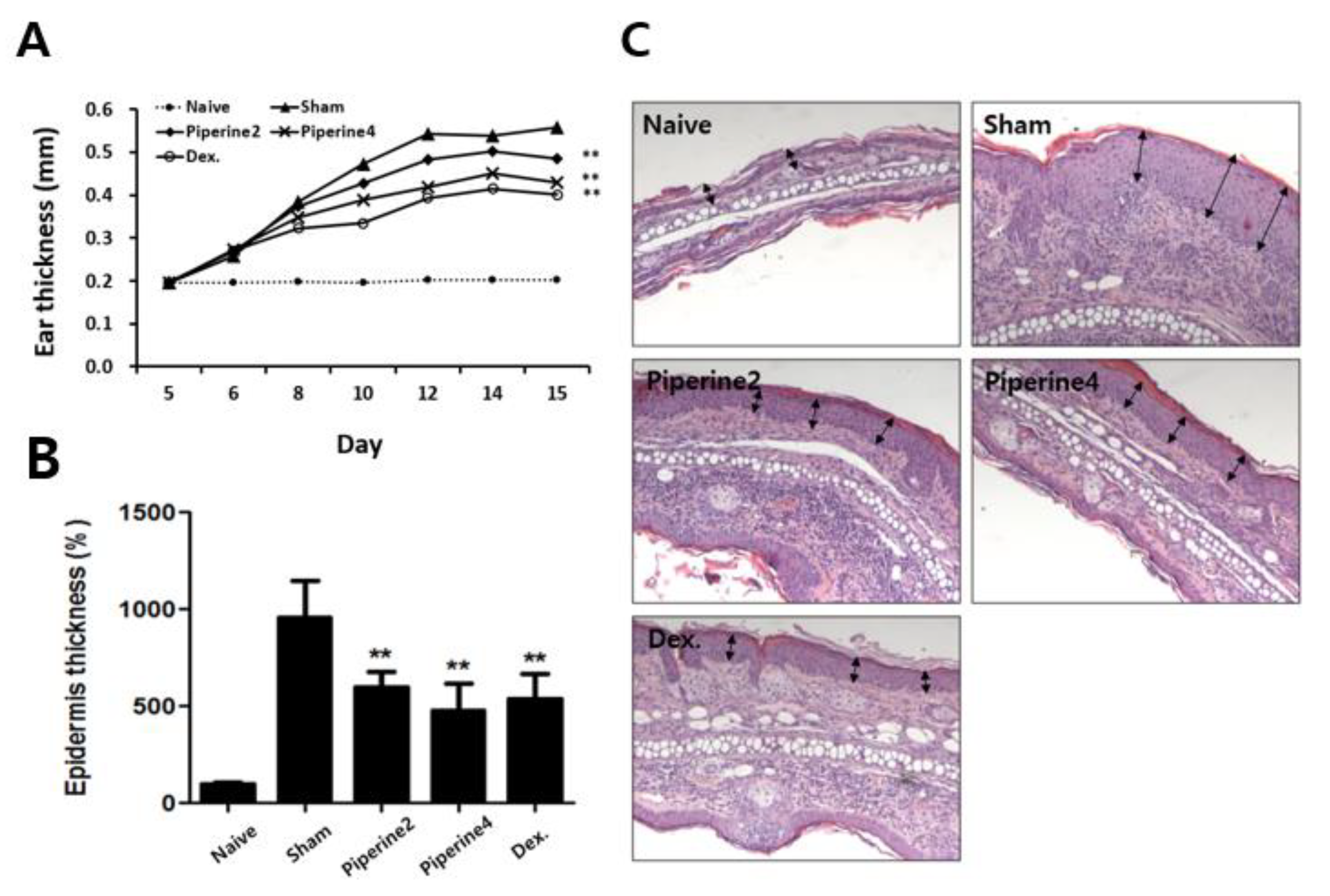
2.1. Effects of Piperine on the Symptoms in a TMA-Induced AD-Like Mouse Model
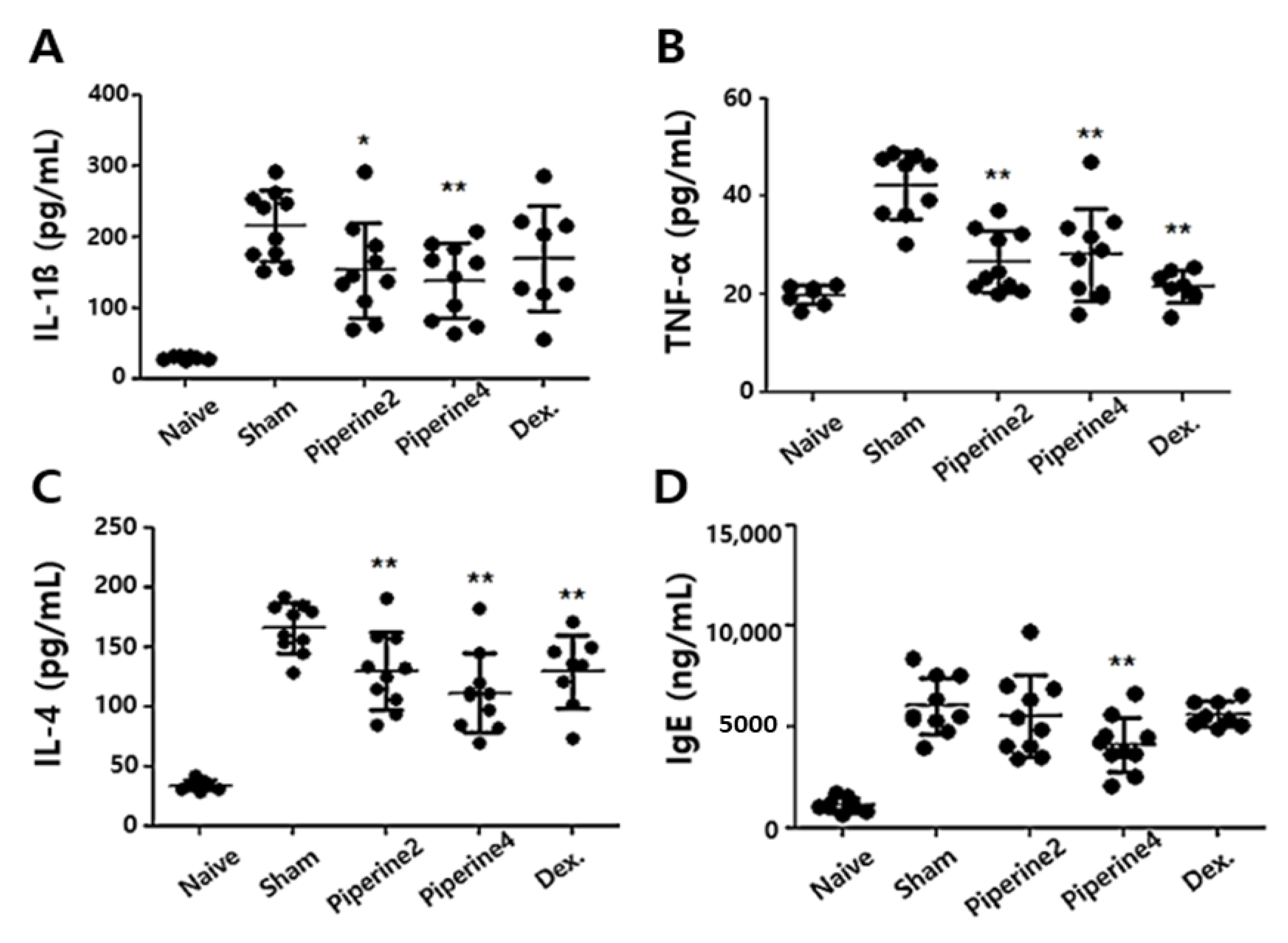
2.2. Effects of Piperine on Inflammatory and Allergic Factors in Ear Tissues
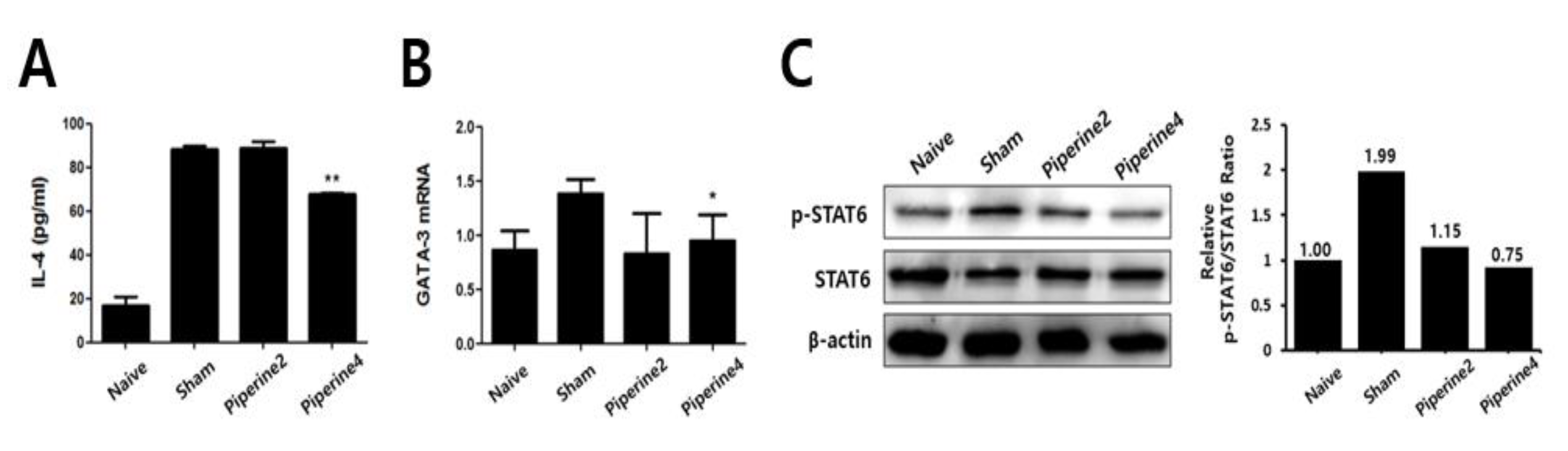
2.3. Effects of Piperine on Th2-Associated Immune Responses Induced by TMA
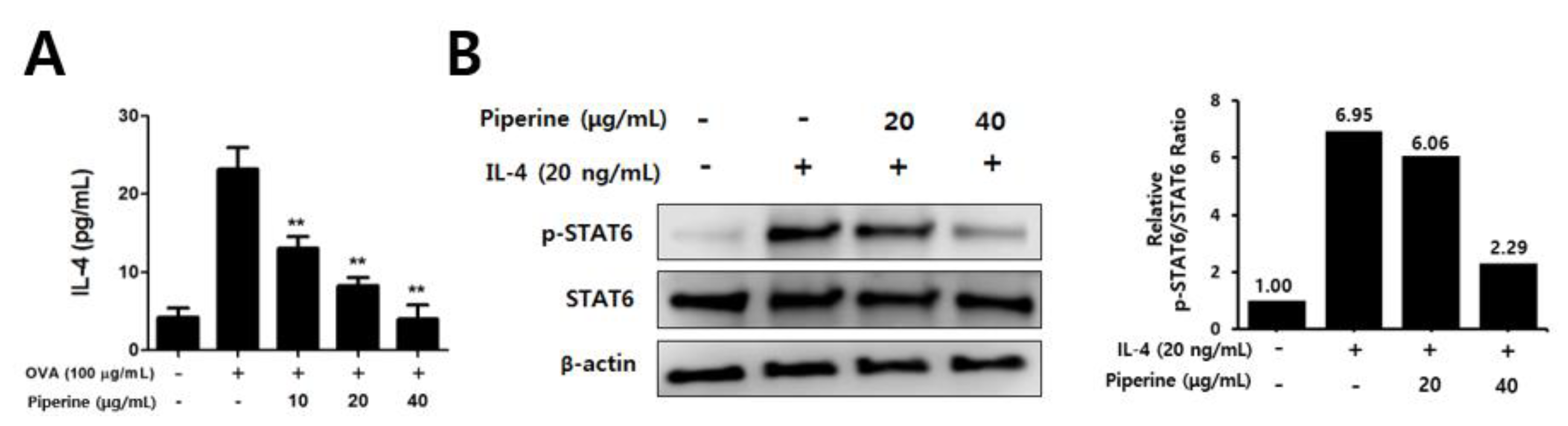
2.4. Effects of Piperine on Th2 Immune Responses in Splenocytes and CD4+ T Cells
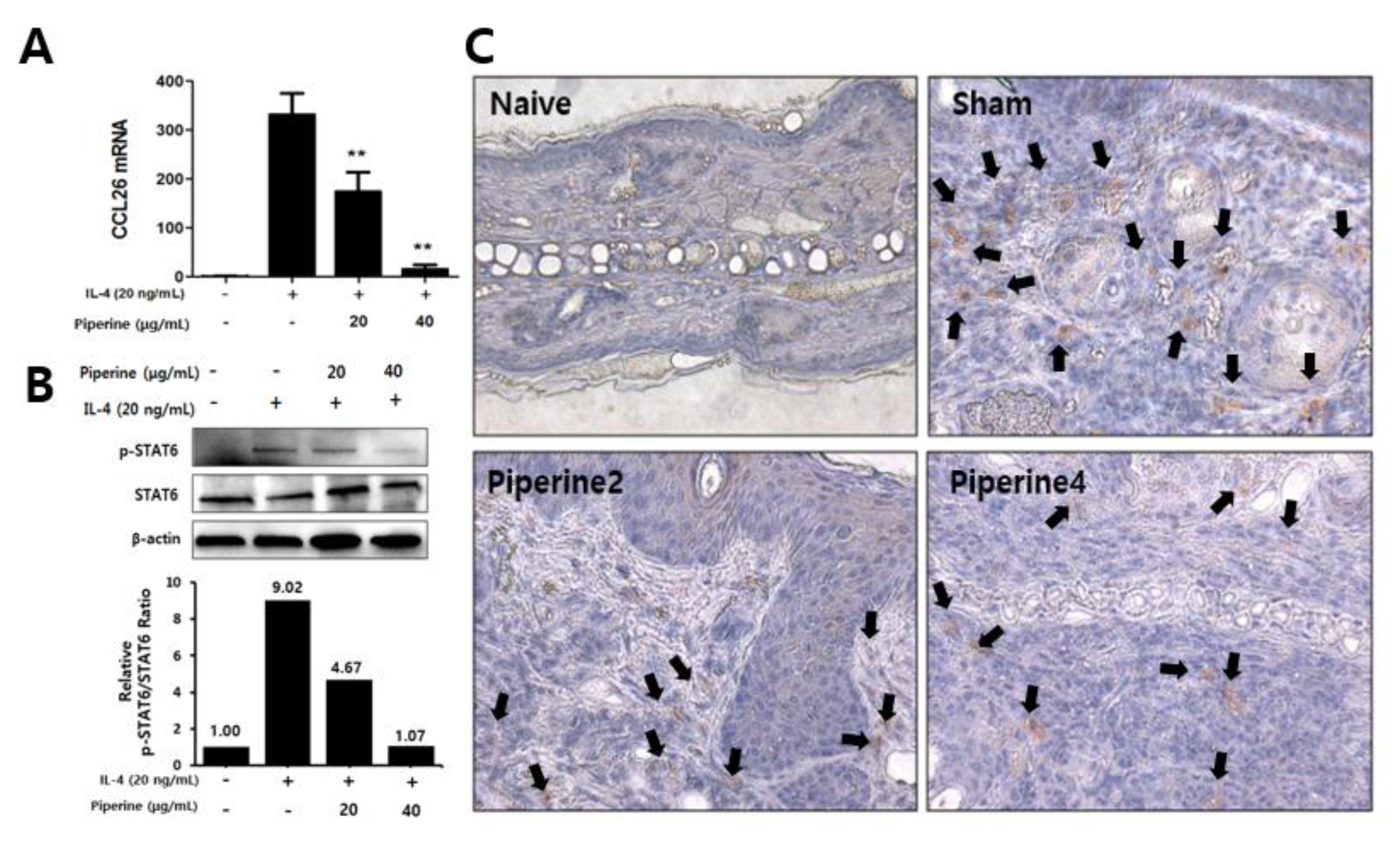
2.5. Effects of Piperine on CCR3 Cell Infiltration in Mouse Ear Tissues and IL-4-Induced STAT6 Phosphorylation in Keratinocytes
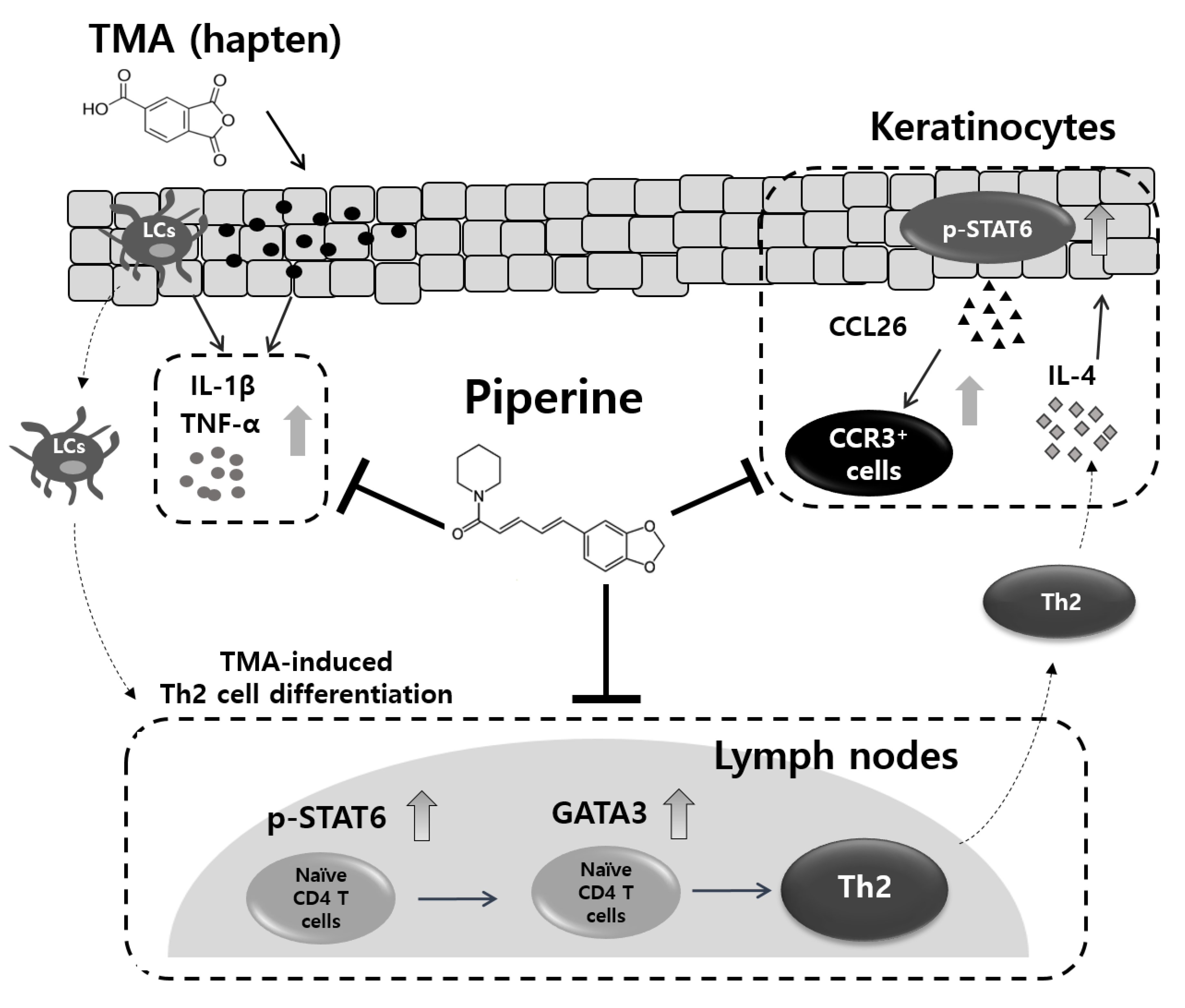
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
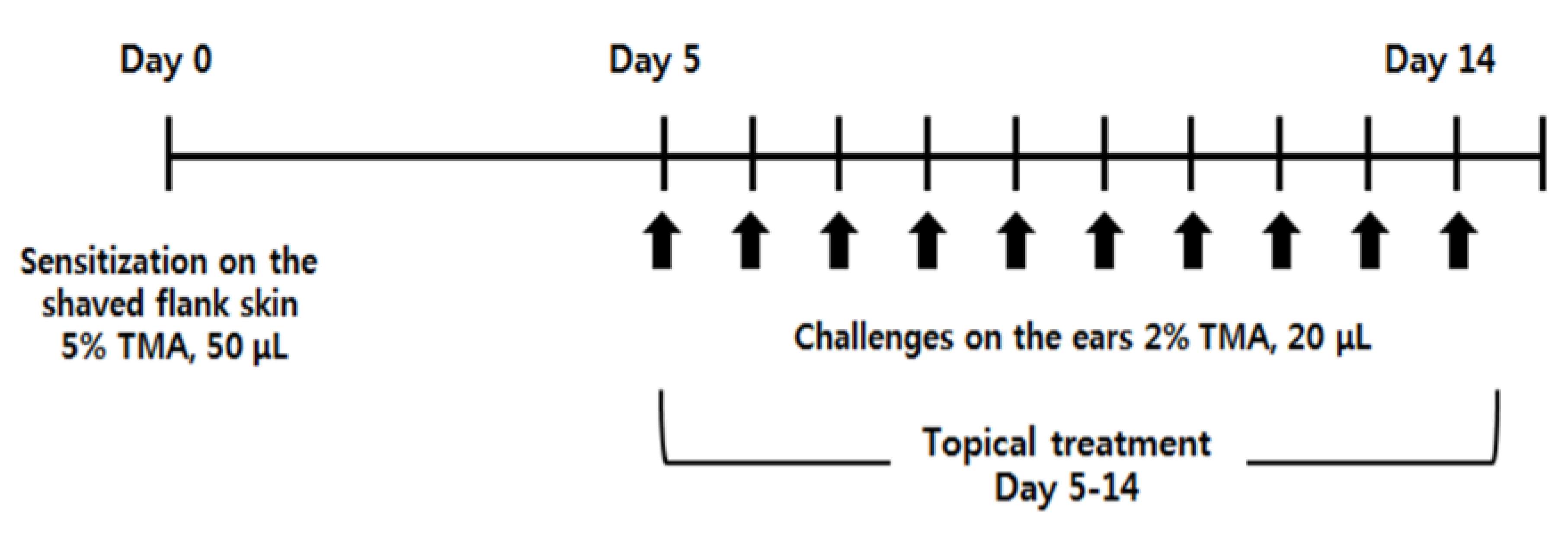
4.3. Induction of AD-Like Symptoms by TMA
4.4. Measurement of Epidermal Thickness
4.5. Preparation of Mouse Ears for Measurement of Cytokine Levels by ELISA
4.6. Culture of Draining Lymph Nodes
4.7. Induction of Th2-Dominant Immune Responses and Cell Culture
4.8. Measurement of Cytokine Levels by ELISA
4.9. CD4+ T Cell Isolation and Induction of STAT6 Phosphorylation Treated by IL-4
4.10. Induction of STAT6 Phosphorylation and CCL26 mRNA in HaCaT Cells Treated with IL-4
4.11. Western Blotting Analysis
4.12. Immunohistological Analysis
4.13. Quantitative RT-PCR Analysis and mRNA Isolation
4.14. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Czarnowicki, T.; Esaki, H.; Gonzalez, J.; Malajian, D.; Shemer, A.; Noda, S.; Talasila, S.; Berry, A.; Gray, J.; Becker, L. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)+ TH2/TH1 cell imbalance, whereas adults acquire CLA+ TH22/TC22 cell subsets. J. Allergy Clin. Immunol. 2015, 136, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Akdis, M.; Bieber, T.; Bindslev-Jensen, C.; Boguniewicz, M.; Eigenmann, P.; Hamid, Q.; Kapp, A.; Leung, D.Y.; Lipozencic, J. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. Allergy 2006, 61, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.D.; Conze, D.; Whitmarsh, A.J.; Barrett, T.; Davis, R.J.; Rincón, M.; Flavell, R.A. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity 1998, 9, 575–585. [Google Scholar] [CrossRef]
- Glimcher, L.H.; Murphy, K.M. Lineage commitment in the immune system: The T helper lymphocyte grows up. Genes Dev. 2000, 14, 1693–1711. [Google Scholar]
- Lee, K.; Gudapati, P.; Dragovic, S.; Spencer, C.; Joyce, S.; Killeen, N.; Magnuson, M.A.; Boothby, M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 2010, 32, 743–753. [Google Scholar] [CrossRef]
- Hamid, Q.; Naseer, T.; Minshall, E.M.; Song, Y.L.; Boguniewicz, M.; Leung, D.Y. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J. Allergy Clin. Immunol. 1996, 98, 225–231. [Google Scholar] [CrossRef]
- King, I.L.; Mohrs, M. IL-4–producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J. Exp. Med. 2009, 206, 1001–1007. [Google Scholar] [CrossRef]
- Kaplan, M.H.; Schindler, U.; Smiley, S.T.; Grusby, M.J. Stat6 is required for mediating responses to IL-4 and for the development of Th2 cells. Immunity 1996, 4, 313–319. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, L.; Watson, C.J.; Hu-Li, J.; Paul, W.E. Stat6 is necessary and sufficient for IL-4′s role in Th2 differentiation and cell expansion. J. Immunol. 2001, 166, 7276–7281. [Google Scholar] [CrossRef]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef]
- Benveniste, E.N.; Liu, Y.; McFarland, B.C.; Qin, H. Involvement of the janus kinase/signal transducer and activator of transcription signaling pathway in multiple sclerosis and the animal model of experimental autoimmune encephalomyelitis. J. Interf. Cytokine Res. 2014, 34, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Amerio, P.; Frezzolini, A.; Feliciani, C.; Verdolini, R.; Teofoli, P.; Pit, O.; Puddu, P. Eotaxins and CCR3 receptor in inflammatory and allergic skin diseases: Therapeutical implications. Curr. Drug Targets 2003, 2, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Hoeck, J.; Woisetschläger, M. Activation of Eotaxin-3/CCL26 gene expression in human dermal fibroblasts is mediated by STAT6. J. Immunol. 2001, 167, 3216–3222. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Bryce, P.J.; Humbles, A.A.; Laouini, D.; Yalcindag, A.; Alenius, H.; Friend, D.S.; Oettgen, H.C.; Gerard, C.; Geha, R.S. CCR3 is essential for skin eosinophilia and airway hyperresponsiveness in a murine model of allergic skin inflammation. J. Clin. Investig. 2002, 109, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Stirling, R.G.; Van Rensen, E.L.; Barnes, P.J.; Fan Chung, K. Interleukin-5 induces CD34+ eosinophil progenitor mobilization and eosinophil CCR3 expression in asthma. Am. J. Respir. Crit. Care Med. 2001, 164, 1403–1409. [Google Scholar] [CrossRef]
- Gleich, G.J. Mechanisms of eosinophil-associated inflammation. J. Allergy Clin. Immunol. 2000, 105, 651–663. [Google Scholar] [CrossRef]
- Daugherty, B.L.; Siciliano, S.J.; DeMartino, J.A.; Malkowitz, L.; Sirotina, A.; Springer, M.S. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J. Exp. Med. 1996, 183, 2349–2354. [Google Scholar] [CrossRef]
- Kagami, S.; Saeki, H.; Komine, M.; Kakinuma, T.; Tsunemi, Y.; Nakamura, K.; Sasaki, K.; Asahina, A.; Tamaki, K. Interleukin-4 and interleukin-13 enhance CCL26 production in a human keratinocyte cell line, HaCaT cells. Clin. Exp. Immunol. 2005, 141, 459–466. [Google Scholar] [CrossRef]
- Bao, L.; Shi, V.Y.; Chan, L.S. IL-4 regulates chemokine CCL26 in keratinocytes through the Jak1, 2/Stat6 signal transduction pathway: Implication for atopic dermatitis. Mol. Immunol. 2012, 50, 91–97. [Google Scholar] [CrossRef]
- Lee, E.B.; Shin, K.H.; Woo, W.S. Pharmacological study on piperine. Arch. Pharm. Res. 1984, 7, 127–132. [Google Scholar] [CrossRef]
- Rodgers, G.; Doucette, C.D.; Soutar, D.A.; Liwski, R.S.; Hoskin, D.W. Piperine impairs the migration and T cell-activating function of dendritic cells. Toxicol. Lett. 2016, 242, 23–33. [Google Scholar] [CrossRef]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef]
- Mittal, R.; Gupta, R. In vitro antioxidant activity of piperine. Methods Find. Exp. Clin. 2000, 22, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.S.; Choi, H.M.; Sur, B.J.; Lim, S.J.; Kim, J.Y.; Yang, H.-I.; Yoo, M.C.; Hahm, D.-H.; Kim, K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009, 11, R49. [Google Scholar] [CrossRef] [PubMed]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Aswar, U.; Shintre, S.; Chepurwar, S.; Aswar, M. Antiallergic effect of piperine on ovalbumin-induced allergic rhinitis in mice. Pharm. Biol. 2015, 53, 1358–1366. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, Y.C. Piperine inhibits eosinophil infiltration and airway hyperresponsiveness by suppressing T cell activity and Th2 cytokine production in the ovalbumin-induced asthma model. J. Pharm. Pharmacol. 2009, 61, 353–359. [Google Scholar] [CrossRef]
- Schneider, C.; Döcke, W.-D.F.; Zollner, T.M.; Röse, L. Chronic mouse model of TMA-induced contact hypersensitivity. J. Investig. Dermatol. 2009, 129, 899–907. [Google Scholar] [CrossRef]
- Hon, K.; Lee, V.W.; Leung, T.-F.; Lee, K.; Chan, A.; Fok, T.-F.; Leung, P.-C. Corticosteroids are not present in a traditional Chinese medicine formulation for atopic dermatitis in children. Ann. Acad. Med. Singap. 2006, 35, 759. [Google Scholar]
- Chapoval, S.; Dasgupta, P.; Dorsey, N.J.; Keegan, A.D. Regulation of the T helper cell type 2 (Th2)/T regulatory cell (Treg) balance by IL-4 and STAT6. J. Leukoc. Biol. 2010, 87, 1011–1018. [Google Scholar] [CrossRef]
- Tiplica, G.; Boralevi, F.; Konno, P.; Malinauskiene, L.; Kaszuba, A.; Laurens, C.; Saint-Aroman, M.; Delarue, A. The regular use of an emollient improves symptoms of atopic dermatitis in children: A randomized controlled study. J. Eur. Acad. Dermatol. 2018, 32, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Gelmetti, C.; Boralevi, F.; Seité, S.; Grimalt, R.; Humbert, P.; Luger, T.; Stalder, J.F.; Taïeb, A.; Tennstedt, D.; Garcia Diaz, R. Quality of life of parents living with a child suffering from atopic dermatitis before and after a 3-month treatment with an emollient. Pediatr. Dermatol. 2012, 29, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Arndt, J.; Smith, N.; Tausk, F. Stress and atopic dermatitis. Curr. Allergy Asthm 2008, 8, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Jones, S. Quality of life and childhood atopic dermatitis: The misery of living with childhood eczema. Int. J. Clin. Pract. 2006, 60, 984–992. [Google Scholar] [CrossRef]
- Fenner, J.; Silverberg, N.B. Skin diseases associated with atopic dermatitis. Clin. Dermatol. 2018, 36, 631–640. [Google Scholar] [CrossRef]
- Mamessier, E.; Magnan, A. Cytokines in atopic diseases: Revisiting the Th2 dogma. Eur. J. Dermatol. 2006, 16, 103–113. [Google Scholar]
- Arkwright, P.D.; Chase, J.M.; Babbage, S.; Pravica, V.; David, T.J.; Hutchinson, I.V. Atopic dermatitis is associated with a low-producer transforming growth factor β1 cytokine genotype. J. Allergy Clin. Immunol. 2001, 108, 281–284. [Google Scholar] [CrossRef]
- Leung, D.Y.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef]
- Suárez-Fariñas, M.; Dhingra, N.; Gittler, J.; Shemer, A.; Cardinale, I.; De Guzman Strong, C.; Krueger, J.G.; Guttman-Yassky, E. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 361–370. [Google Scholar] [CrossRef]
- Spergel, J.M.; Paller, A.S. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 2003, 112, S118–S127. [Google Scholar] [CrossRef]
- Novak, N.; Bieber, T.; Leung, D.Y. Immune mechanisms leading to atopic dermatitis. J. Allergy Clin. Immunol. 2003, 112, S128–S139. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, C.; Kuttan, G. Effect of Piperine on the Inhibition of Nitric Oxide (NO) and TNF-α Production. Immunopharm. Immunot. 2003, 25, 337–346. [Google Scholar]
- Ying, X.; Yu, K.; Chen, X.; Chen, H.; Hong, J.; Cheng, S.; Peng, L. Piperine inhibits LPS induced expression of inflammatory mediators in RAW 264.7 cells. Cell Immunol. 2013, 285, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Chen, X.; Cheng, S.; Shen, Y.; Peng, L.; Xu, H.Z. Piperine inhibits IL-β induced expression of inflammatory mediators in human osteoarthritis chondrocyte. Int. Immunopharmacol. 2013, 17, 293–299. [Google Scholar] [CrossRef]
- Liang, Y.D.; Bai, W.J.; Li, C.G.; Xu, L.-H.; Wei, H.-X.; Pan, H.; He, X.-H.; Ouyang, D.-Y. Piperine suppresses pyroptosis and interleukin-1β release upon ATP triggering and bacterial infection. Front. Pharmacol. 2016, 7, 390. [Google Scholar] [CrossRef]
- Cumberbatch, M.; Kimber, I. Tumour necrosis factor-alpha is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology 1995, 84, 31. [Google Scholar]
- Cumberbatch, M.; Dearman, R.; Kimber, I. Langerhans cells require signals from both tumour necrosis factor-α and interleukin-1β for migration. Immunology 1997, 92, 388–395. [Google Scholar] [CrossRef]
- Kimber, I.; Cumberbatch, M. Stimulation of Langerhans cell migration by tumor necrosis factor α (TNF-α). J. Investig. Dermatol. 1992, 99, S48–S50. [Google Scholar] [CrossRef]
- Vestergaard, C.; Johansen, C.; Christensen, U.; Just, H.; Hohwy, T.; Deleuran, M. TARC augments TNF-α-induced CTACK production in keratinocytes. Exp. Dermatol. 2004, 13, 551–557. [Google Scholar] [CrossRef]
- Eaton, L.H.; Roberts, R.A.; Kimber, I.; Dearman, R.J.; Metryka, A. Skin sensitization induced L angerhans’ cell mobilization: Variable requirements for tumour necrosis factor-α. Immunology 2015, 144, 139–148. [Google Scholar] [CrossRef]
- Ouwehand, K.; Santegoets, S.J.; Bruynzeel, D.P.; Scheper, R.J.; De Gruijl, T.D.; Gibbs, S. CXCL12 is essential for migration of activated Langerhans cells from epidermis to dermis. Eur. J. Immunol. 2008, 38, 3050–3059. [Google Scholar] [CrossRef] [PubMed]
- Saeki, H.; Moore, A.M.; Brown, M.J.; Hwang, S.T. Cutting edge: Secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 1999, 162, 2472–2475. [Google Scholar] [PubMed]
- Huang, J.; Zhang, T.; Han, S.; Cao, J.; Chen, Q.; Wang, S. The inhibitory effect of piperine from Fructus piperis extract on the degranulation of RBL-2H3 cells. Fitoterapia 2014, 99, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Piao, C.H.; Song, C.H.; Shin, H.S.; Shon, D.-H.; Chai, O.H. Piper nigrum extract ameliorated allergic inflammation through inhibiting Th2/Th17 responses and mast cells activation. Cell Immunol. 2017, 322, 64–73. [Google Scholar] [CrossRef]
- Jung, S.K.; Choi, D.W.; Jung, C.H.; Kim, Y.-J.; Jung, S.Y.; Shon, D.-H. Piper nigrum Fruit Extract Prevents TMA-Induced Allergic Contact Dermatitis by Regulating Th2 Cytokine Production. J. Agric. Sci. 2015, 7, 135. [Google Scholar] [CrossRef][Green Version]
- Kim, N.; Thatcher, T.H.; Sime, P.J.; Phipps, R.P. Corticosteroids inhibit anti-IgE activities of specialized proresolving mediators on B cells from asthma patients. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H. Topical application of fucoidan improves atopic dermatitis symptoms in NC/Nga mice. Phytother. Res. 2012, 26, 1898–1903. [Google Scholar] [CrossRef]
- Inagaki, N.; Shiraishi, N.; Igeta, K.; Itoh, T.; Chikumoto, T.; Nagao, M.; Kim, J.F.; Nagai, H. Inhibition of scratching behavior associated with allergic dermatitis in mice by tacrolimus, but not by dexamethasone. Eur. J. Pharmacol. 2006, 546, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.H.; Igyártó, B.Z.; Gaspari, A.A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 2012, 12, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.F. T lymphocyte-mediated immune responses to chemical haptens and metal ions: Implications for allergic and autoimmune disease. Int. Arch. Allergy Immunol. 2004, 134, 186–198. [Google Scholar] [CrossRef]
- Farraj, A.K.; Harkema, J.R.; Kaminski, N.E. Allergic rhinitis induced by intranasal sensitization and challenge with trimellitic anhydride but not with dinitrochlorobenzene or oxazolone in A/J mice. Toxicol. Sci. 2004, 79, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Aversa, G.; Punnonen, J.; Cocks, B.G.; De Waal Malefyt, R.; Vega Jr, F.; Zurawski, S.; Zurawski, G.; De Vries, J. An interleukin 4 (IL-4) mutant protein inhibits both IL-4 or IL-13-induced human immunoglobulin G4 (IgG4) and IgE synthesis and B cell proliferation: Support for a common component shared by IL-4 and IL-13 receptors. J. Exp. Med. 1993, 178, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Oki, S.; Otsuki, N.; Kohsaka, T.; Azuma, M. Stat6 activation and Th2 cell proliferation driven by CD28 signals. Eur. J. Immunol. 2000, 30, 1416–1424. [Google Scholar] [CrossRef]
- Chen, Z.; Lund, R.; Aittokallio, T.; Kosonen, M.; Nevalainen, O.; Lahesmaa, R. Identification of novel IL-4/Stat6-regulated genes in T lymphocytes. J. Immunol. 2003, 171, 3627–3635. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, M. Chemokines and skin diseases. Arch. Immunol. Ther. Exp. 2015, 63, 109–115. [Google Scholar] [CrossRef]
- Lv, J.; Xiong, Y.; Li, W.; Cui, X.; Cheng, X.; Leng, Q.; He, R. IL-37 inhibits IL-4/IL-13-induced CCL 11 production and lung eosinophilia in murine allergic asthma. Allergy 2018, 73, 1642–1652. [Google Scholar] [CrossRef]
- Zhou, L.; Chong, M.M.; Littman, D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef]
- Jung, S.; Choi, D.; Kwon, D.; Kim, M.; Seong, K.; Shon, D.H. Oral administration of achyranthis radix extract prevents TMA-induced allergic contact dermatitis by regulating th2 cytokine and chemokine production in vivo. Molecules 2015, 20, 21584–21596. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.W.; Jung, S.Y.; Shon, D.-H.; Shin, H.S. Piperine Ameliorates Trimellitic Anhydride-Induced Atopic Dermatitis-Like Symptoms by Suppressing Th2-Mediated Immune Responses via Inhibition of STAT6 Phosphorylation. Molecules 2020, 25, 2186. https://doi.org/10.3390/molecules25092186
Choi DW, Jung SY, Shon D-H, Shin HS. Piperine Ameliorates Trimellitic Anhydride-Induced Atopic Dermatitis-Like Symptoms by Suppressing Th2-Mediated Immune Responses via Inhibition of STAT6 Phosphorylation. Molecules. 2020; 25(9):2186. https://doi.org/10.3390/molecules25092186
Chicago/Turabian StyleChoi, Dae Woon, Sun Young Jung, Dong-Hwa Shon, and Hee Soon Shin. 2020. "Piperine Ameliorates Trimellitic Anhydride-Induced Atopic Dermatitis-Like Symptoms by Suppressing Th2-Mediated Immune Responses via Inhibition of STAT6 Phosphorylation" Molecules 25, no. 9: 2186. https://doi.org/10.3390/molecules25092186
APA StyleChoi, D. W., Jung, S. Y., Shon, D.-H., & Shin, H. S. (2020). Piperine Ameliorates Trimellitic Anhydride-Induced Atopic Dermatitis-Like Symptoms by Suppressing Th2-Mediated Immune Responses via Inhibition of STAT6 Phosphorylation. Molecules, 25(9), 2186. https://doi.org/10.3390/molecules25092186