Anti-Acne Vulgaris Effects of Pedunculagin from the Leaves of Quercus mongolica by Anti-Inflammatory Activity and 5α-Reductase Inhibition
Abstract
1. Introduction
2. Results
2.1. Isolation of PD
2.2. Inhibitory Activity on NO Production
2.3. Cytotoxic Activity
2.4. Inhibitory Activity on Cytokine Production
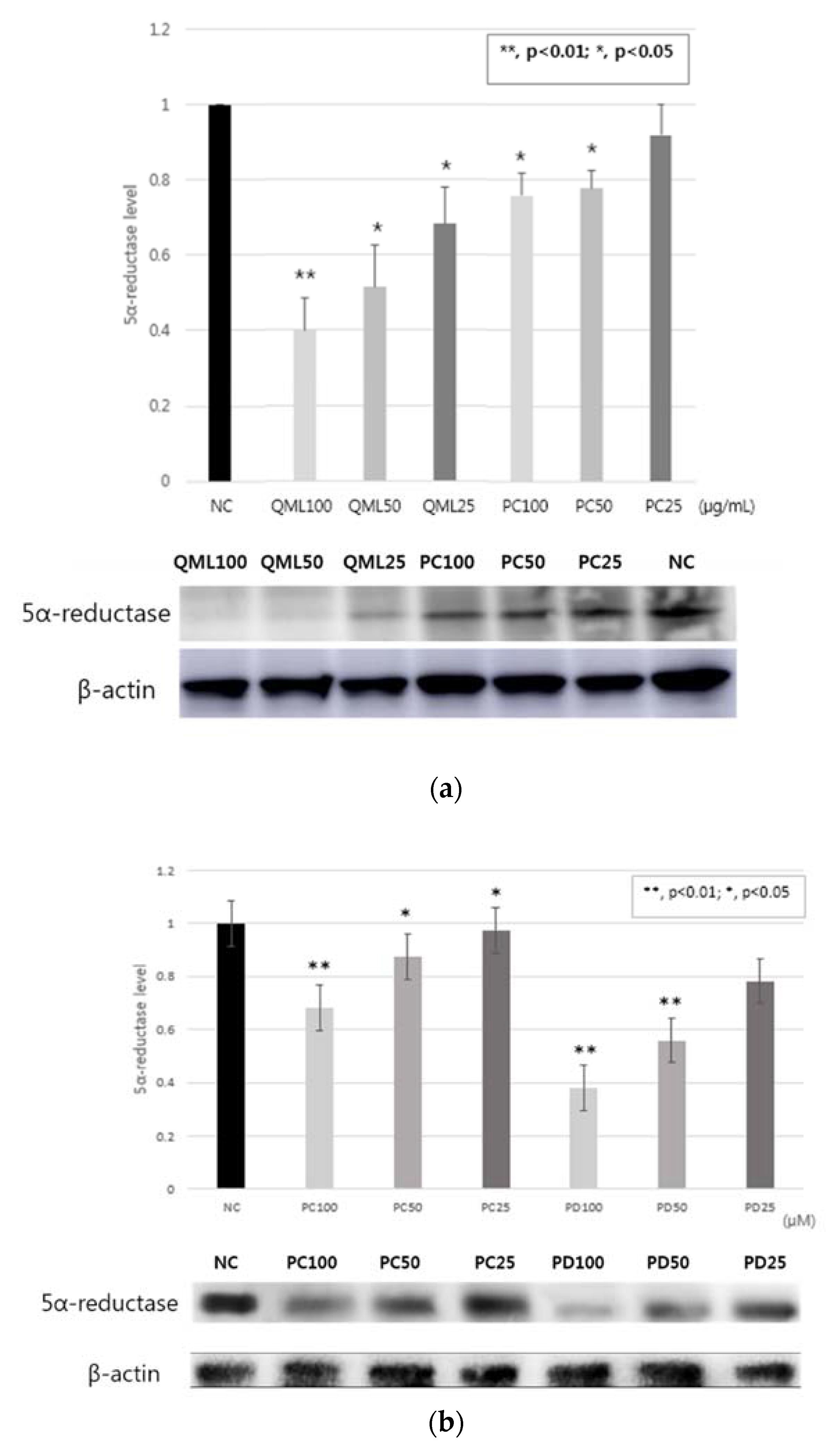
2.5. 5α-Reductase Inhibitory Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemical and Reagents
4.3. Cell Culture
4.4. MTT Assay
4.5. Measurement of Inhibitory Activity on NO Production
4.6. Measurement of Inhibitory Activity on Cytokine Production
4.7. Western Blot Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qin, M.; Landriscina, A.; Rosen, J.M.; Wei, G.; Kao, S.; Olcott, W.; Agak, G.W.; Paz, K.B.; Bonventre, J.; Clendaniel, A.; et al. Nitric Oxide-Releasing Nanoparticles Prevent Propionibacterium acnes-Induced Inflammation by Both Clearing the Organism and Inhibiting Microbial Stimulation of the Innate Immune Response. J. Investig. Dermatol. 2015, 135, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Grange, P.A.; Chereau, C.; Raingeaud, J.; Nicco, C.; Weill, B.; Dupin, N.; Batteux, F. Production of superoxide anions by keratinocytes initiates P. acnes-induced inflammation of the skin. PLoS Pathog. 2009, 5, e1000527. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Gans, E.H. Tretinoin: A Review of Its Anti-inflammatory Properties in the Treatment of Acne. J. Clin. Aesthet. Dermatol. 2011, 4, 22–29. [Google Scholar] [PubMed]
- Farrar, M.D.; Ingham, E. Acne: Inflammation. Clin. Dermatol. 2004, 22, 380–384. [Google Scholar] [CrossRef]
- Sarici, G.; Cinar, S.; Armutcu, F.; Altinyazar, C.; Koca, R.; Tekin, N.S. Oxidative stress in acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 763–767. [Google Scholar] [CrossRef]
- Al-Shobaili, H.A.; Alzolibani, A.A.; Al Robaee, A.A.; Meki, A.R.; Rasheed, Z. Biochemical markers of oxidative and nitrosative stress in acne vulgaris: Correlation with disease activity. J. Clin. Lab. Anal. 2013, 27, 45–52. [Google Scholar] [CrossRef]
- Grone, A. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 2002, 88, 1–12. [Google Scholar] [CrossRef]
- Kohda, F.; Koga, T.; Uchi, H.; Urabe, K.; Furue, M. Histamine-induced IL-6 and IL-8 production are differentially modulated by IFN-γ and IL-4 in human keratinocytes. J. Dermatol. Sci. 2002, 28, 34–41. [Google Scholar] [CrossRef]
- Kelhala, H.L.; Palatsi, R.; Fyhrquist, N.; Lehtimaki, S.; Vayrynen, J.P.; Kallioinen, M.; Kubin, M.E.; Greco, D.; Tasanen, K.; Alenius, H.; et al. IL-17/Th17 pathway is activated in acne lesions. PLoS ONE 2014, 9, e105238. [Google Scholar] [CrossRef]
- Agak, G.W.; Qin, M.; Nobe, J.; Kim, M.H.; Krutzik, S.R.; Tristan, G.R.; Elashoff, D.; Garban, H.J.; Kim, J. Propionibacterium acnes Induces an IL-17 Response in Acne Vulgaris that Is Regulated by Vitamin A and Vitamin D. J. Investig. Dermatol. 2014, 134, 366–373. [Google Scholar] [CrossRef]
- Takada, K.; Komine-Aizawa, S.; Hirohata, N.; Trinh, Q.D.; Nishina, A.; Kimura, H.; Hayakawa, S. Poly I:C induces collective migration of HaCaT keratinocytes via IL-8. BMC Immunol. 2017, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, W.R.; Kim, K.H.; An, H.J.; Chang, Y.C.; Han, S.M.; Park, Y.Y.; Pak, S.C.; Park, K.K. Effects of bee venom against Propionibacterium acnes-induced inflammation in human keratinocytes and monocytes. Int. J. Mol. Med. 2015, 35, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.; Gold, L.F.S.; Alexis, A.F.; Harper, J.C. Current concepts in acne pathogenesis: Pathways to inflammation. Semin. Cutan. Med. Surg. 2018, 37, S60–S62. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. p53: Key conductor of all anti-acne therapies. J. Transl. Med. 2017, 15, 195. [Google Scholar] [CrossRef]
- Kurokawa, I.; Danby, F.W.; Ju, Q.; Wang, X.; Xiang, L.F.; Xia, L.; Chen, W.; Nagy, I.; Picardo, M.; Suh, D.H.; et al. New developments in our understanding of acne pathogenesis and treatment. Exp. Dermatol. 2009, 18, 821–832. [Google Scholar] [CrossRef]
- Barros, B.; Thiboutot, D. Hormonal therapies for acne. Clin. Dermatol. 2017, 35, 168–172. [Google Scholar] [CrossRef]
- Thiboutot, D. Acne: Hormonal concepts and therapy. Clin. Dermatol. 2004, 22, 419–428. [Google Scholar] [CrossRef]
- Lakshmi, C. Hormone therapy in acne. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 322–337. [Google Scholar] [CrossRef]
- Koseki, J.; Matsumoto, T.; Matsubara, Y.; Tsuchiya, K.; Mizuhara, Y.; Sekiguchi, K.; Nishimura, H.; Watanabe, J.; Kaneko, A.; Hattori, T.; et al. Inhibition of Rat 5alpha-Reductase Activity and Testosterone-Induced Sebum Synthesis in Hamster Sebocytes by an Extract of Quercus acutissima Cortex. Evid. Based Complement. Alternat. Med. 2015, 2015, 853846. [Google Scholar] [CrossRef]
- Hiipakka, R.A.; Zhang, H.-Z.; Dai, W.; Dai, Q.; Liao, S. Structure–activity relationships for inhibition of human 5α-reductases by polyphenols. Biochem. Pharmacol. 2002, 63, 1165–1176. [Google Scholar] [CrossRef]
- Mahmood, T.; Akhtar, N.; Khan, B.A.; Khan, H.M.; Saeed, T. Outcomes of 3% green tea emulsion on skin sebum production in male volunteers. Bosn. J. Basic Med. Sci. 2010, 10, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Hwang, I.H.; Lee, M.W. Anti-acne vulgaris effect including skin barrier improvement and 5alpha-reductase inhibition by tellimagrandin I from Carpinus tschonoskii. BMC Complement. Altern. Med. 2019, 19, 323. [Google Scholar] [CrossRef] [PubMed]
- Mamedov, N.; Gardner, Z.; Craker, L.E. Medicinal Plants Used in Russia and Central Asia for the Treatment of Selected Skin Conditions. J. Herbs Spices Med. Plants 2005, 11, 191–222. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, M.; Zhou, W.; Li, G. CHEMICAL CONSTITUENTS OF THE STEM BARKS OF Quercus mongolica. Chem. Nat. Compd. 2018, 54, 973–974. [Google Scholar] [CrossRef]
- Eo, H.J.; Park, Y.; Kang, J.T.; Park, G.H. Anti-inflammatory Effect of Branches Extracts from Quercus mongolica in LPS-induced RAW264. 7 Cells. Korean J. Plant Res. 2019, 32, 698–704. [Google Scholar] [CrossRef]
- Kim, H.H.; Kim, D.H.; Oh, M.H.; Park, K.J.; Heo, J.H.; Lee, M.W. Inhibition of matrix metalloproteinase-1 and type-I procollagen expression by phenolic compounds isolated from the leaves of Quercus mongolica in ultraviolet-irradiated human fibroblast cells. Arch. Pharm. Res. 2015, 38, 11–17. [Google Scholar] [CrossRef]
- Yin, J.; Kim, H.H.; Hwang, I.H.; Kim, D.H.; Lee, M.W. Anti-Inflammatory Effects of Phenolic Compounds Isolated from Quercus Mongolica Fisch. ex Ledeb. on UVB-Irradiated Human Skin Cells. Molecules 2019, 24, 3094. [Google Scholar] [CrossRef]
- Khanbabaee, K.; Grosser, M. An efficient total synthesis of pedunculagin by using a twofold intramolecular double esterification strategy. Eur. J. Org. Chem. 2003, 2003, 2128–2131. [Google Scholar] [CrossRef]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef]
- Aslam, I.; Fleischer, A.; Feldman, S. Emerging drugs for the treatment of acne. Expert Opin. Emerg. Drugs 2015, 20, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, X.; Wu, C.; Jin, H. IL-36gamma inhibits differentiation and induces inflammation of keratinocyte via Wnt signaling pathway in psoriasis. Int. J. Med. Sci. 2017, 14, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Skroza, N.; Proietti, I.; Bernardini, N.; Aquila, E.; Balduzzi, V.; La Viola, G.; Mambrin, A.; Muscianese, M.; Tolino, E.; Zuber, S.; et al. Il-17 and Its Role in Psoriasis, Hidradenitis Suppurativa And Acne. Intern. Med. Care 2017, 1. [Google Scholar] [CrossRef]
- Nagy, I.; Pivarcsi, A.; Koreck, A.; Szell, M.; Urban, E.; Kemeny, L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J. Investig. Dermatol. 2005, 124, 931–938. [Google Scholar] [CrossRef]
- Basak, P.Y.; Gultekin, F.; Kilinc, I. The role of the antioxidative defense system in papulopustular acne. J. Dermatol. 2001, 28, 123–127. [Google Scholar] [CrossRef]
- Bissonnette, R.; Risch, J.E.; McElwee, K.J.; Marchessault, P.; Bolduc, C.; Nigen, S.; Maari, C. Changes in serum free testosterone, sleep patterns, and 5-alpha-reductase type I activity influence changes in sebum excretion in female subjects. Skin Res. Technol. 2015, 21, 47–53. [Google Scholar] [CrossRef]
- Leyden, J.; Bergfeld, W.; Drake, L.; Dunlap, F.; Goldman, M.P.; Gottlieb, A.B.; Heffernan, M.P.; Hickman, J.G.; Hordinsky, M.; Jarrett, M.; et al. A systemic type I 5 alpha-reductase inhibitor is ineffective in the treatment of acne vulgaris. J. Am. Acad. Dermatol. 2004, 50, 443–447. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Samples | NO Production IC50 (μg/mL) | NO Production IC50 (µM) |
|---|---|---|
| QML | 1.45 ± 0.25 a | – |
| PD | – | 53.52 ± 9.34 b |
| L-NMMA | 0.55 ± 0.19 b | 14.81 ± 12.76 a |
| Samples | IL-6 IC50 (μg/mL) | IL-6 IC50 (µM) | IL-8 IC50 (μg/mL) | IL-8 IC50 (µM) |
|---|---|---|---|---|
| QML | 9.37 ± 1.50 a | – | 6.38 ± 2.58 a | – |
| PD | – | 6.59 ± 1.66 b | – | 0.09 ± 0.41 b |
| EGCG | 2.98 ± 1.47 b | 6.68 ± 1.86 a | 0.74 ± 0.09 b | 0.56 ± 0.52 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Yin, J.; Hwang, I.H.; Park, D.H.; Lee, E.K.; Kim, M.J.; Lee, M.W. Anti-Acne Vulgaris Effects of Pedunculagin from the Leaves of Quercus mongolica by Anti-Inflammatory Activity and 5α-Reductase Inhibition. Molecules 2020, 25, 2154. https://doi.org/10.3390/molecules25092154
Kim M, Yin J, Hwang IH, Park DH, Lee EK, Kim MJ, Lee MW. Anti-Acne Vulgaris Effects of Pedunculagin from the Leaves of Quercus mongolica by Anti-Inflammatory Activity and 5α-Reductase Inhibition. Molecules. 2020; 25(9):2154. https://doi.org/10.3390/molecules25092154
Chicago/Turabian StyleKim, Min, Jun Yin, In Hyeok Hwang, Dong Hui Park, Eun Kyeong Lee, Min Ji Kim, and Min Won Lee. 2020. "Anti-Acne Vulgaris Effects of Pedunculagin from the Leaves of Quercus mongolica by Anti-Inflammatory Activity and 5α-Reductase Inhibition" Molecules 25, no. 9: 2154. https://doi.org/10.3390/molecules25092154
APA StyleKim, M., Yin, J., Hwang, I. H., Park, D. H., Lee, E. K., Kim, M. J., & Lee, M. W. (2020). Anti-Acne Vulgaris Effects of Pedunculagin from the Leaves of Quercus mongolica by Anti-Inflammatory Activity and 5α-Reductase Inhibition. Molecules, 25(9), 2154. https://doi.org/10.3390/molecules25092154





