Indirect Competitive Determination of Tetracycline Residue in Honey Using an Ultrasensitive Gold-Nanoparticle-Linked Aptamer Assay
Abstract
1. Introduction
2. Results and Discussion
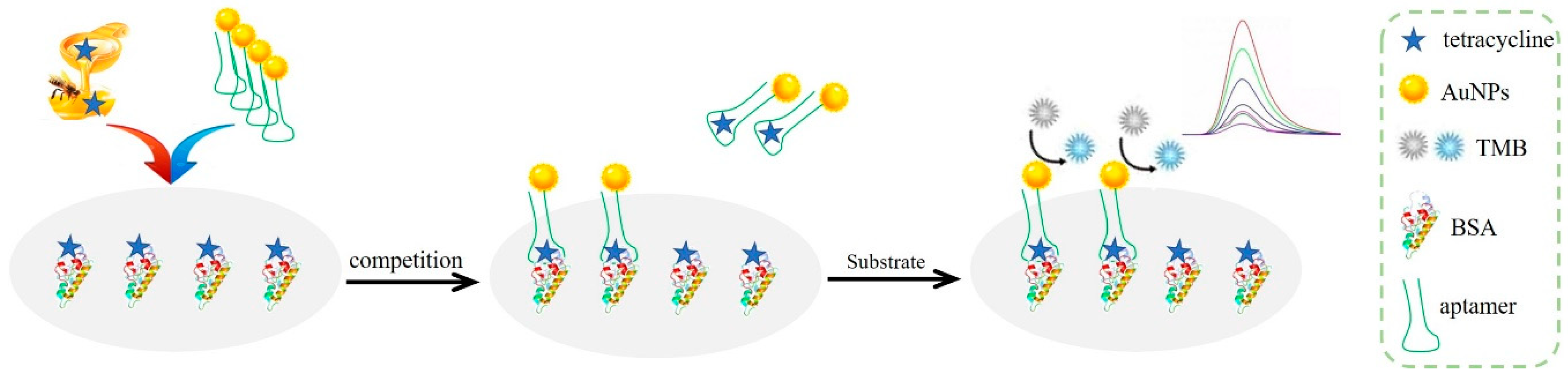
2.1. Principle of Analysis
2.2. Characterization of Tetracycline-BSA and Aptamer-AuNPs
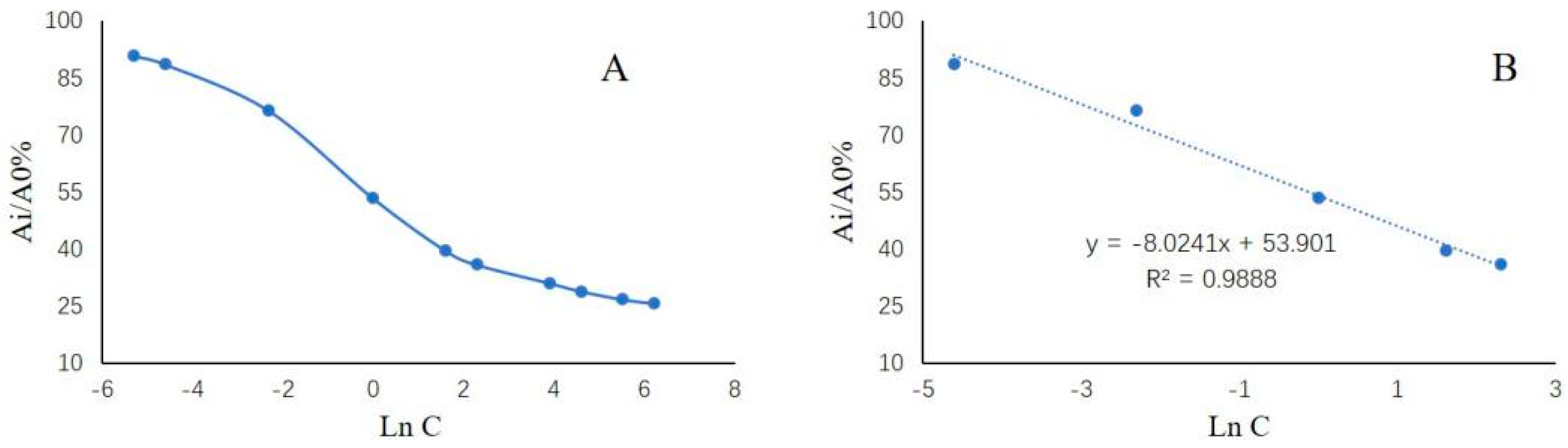
2.3. Analytical Performance of the Present Method for Tetracycline Determination
2.4. Detection of Tetracycline in Real Samples
3. Materials and Method
3.1. Reagents and Instruments
3.2. Preparation of the Conjugate of Tetracycline with Bovine Serum Protein (Tetracycline-BSA)
3.3. Preparation of the Tetracycline-BSA-Coated Microplate
3.4. Preparation of Tetracycline Aptamer Labeled Gold Nanoparticles (Aptamer-AuNPs)
3.5. Determination of Tetracycline in Honey
3.6. Validation Procedure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- di Cerbo, A.; Pezzuto, F.; Guidetti, G.; Canello, S.; Corsi, L. Tetracyclines: Insights and updates of their use in human and animal pathology and their potential toxicity. Open Biochem. J. 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Cherkashina, K.; Vakh, C.; Lebedinets, S.; Pochivalov, A.; Moskvin, L.; Lezov, A.; Bulatov, A. An automated salting-out assisted liquid-liquid microextraction approach using 1-octylamine: On-line separation of tetracycline in urine samples followed by HPLC-UV determination. Talanta 2018, 184, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, P.; Bedi, J.S.; Aulakh, R.S.; Gill, J.P.S.; Kumar, A. Validation of HPLC multi-residue method for determination of fluoroquinolones, tetracycline, sulphonamides and chloramphenicol residues in bovine milk. Food Anal. Methods 2019, 12, 338–346. [Google Scholar] [CrossRef]
- Saridal, K.; Ulusoy, H.I. A simple methodology based on cloud point extraction prior to HPLC-PDA analysis for tetracycline residues in food samples. Microchem. J. 2019, 150. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, S.; Yang, D.; Jiang, Y.; Li, Y.; Zhou, C.; Sun, C. Carboxyl Fe3O4 magnetic nanoparticle-based SPE and HPLC method for the determination of six tetracyclines in water. Anal. Bioanal. Chem. 2019, 411, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Dai, Y.; Xu, K.; Qi, M.; Wang, W.; Wu, L.; Wang, A. Determination of tetracycline in water and honey by iron(II, III)/aptamer-based magnetic solid-phase extraction with high-performance liquid chromatography analysis. Anal. Lett. 2019, 52, 1653–1669. [Google Scholar] [CrossRef]
- Cammilleri, G.; Pulvirenti, A.; Vella, A.; Macaluso, A.; Lo Dico, G.M.; Giaccone, V.; Giordano, V.; Vinciguerra, M.; Cicero, N.; Cicero, A.; et al. Tetracycline residues in bovine muscle and liver samples from Sicily (Southern Italy) by LC-MS/MS method: A six-year study. Molecules 2019, 24, 695. [Google Scholar] [CrossRef]
- de Almeida, M.P.; Rezende, C.P.; Ferreira, F.D.; de Souza, L.F.; Sampaio de Assis, D.C.; de Figueiredo, T.C.; Leite, M.d.O.; Cancado, S.d.V. Optimization and validation method to evaluate the residues of beta-lactams and tetracyclines in kidney tissue by UPLC-MS/MS. Talanta 2015, 144, 922–932. [Google Scholar] [CrossRef]
- Jansen, L.J.M.; Bolck, Y.J.C.; Rademaker, J.; Zuidema, T.; Berendsen, B.J.A. The analysis of tetracyclines, quinolones, macrolides, lincosamides, pleuromutilins, and sulfonamides in chicken feathers using UHPLC-MS/MS in order to monitor antibiotic use in the poultry sector. Anal. Bioanal. Chem. 2017, 409, 4927–4941. [Google Scholar] [CrossRef]
- Nebot, C.; Guarddon, M.; Seco, F.; Iglesias, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Monitoring the presence of residues of tetracyclines in baby food samples by HPLC-MS/MS. Food Control 2014, 46, 495–501. [Google Scholar] [CrossRef]
- Ajibola, A.S.; Tisler, S.; Zwiener, C. Simultaneous determination of multiclass antibiotics in sewage sludge based on QuEChERS extraction and liquid chromatography-tandem mass spectrometry. Anal. Methods 2020, 12, 576–586. [Google Scholar] [CrossRef]
- El Alami El Hassani, N.; Baraket, A.; Boudjaoui, S.; Neto, E.T.T.; Bausells, J.; El Bari, N.; Bouchikhi, B.; Elaissari, A.; Errachid, A.; Zine, N. Development and application of a novel electrochemical immunosensor for tetracycline screening in honey using a fully integrated electrochemical BioMEMS. Biosens. Bioelectron. 2019, 130, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Starzec, K.; Cristea, C.; Tertis, M.; Feier, B.; Wieczorek, M.; Koscielniak, P.; Kochana, J. Employment of electrostriction phenomenon for label-free electrochemical immunosensing of tetracycline. Bioelectrochemistry (Amst. Neth.) 2020, 132, 107405. [Google Scholar] [CrossRef] [PubMed]
- Berlina, A.N.; Bartosh, A.V.; Zherdev, A.V.; Xu, C.; Dzantiev, B.B. Development of immunochromatographic assay for determination of tetracycline in human serum. Antibiotics 2018, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Q.; Han, M.; Liu, J.; Zhao, P.; He, L.; Zhang, Y.; Niu, Y.; Yang, W.; Zhang, L. Near-infrared fluorescence-based multiplex lateral flow immunoassay for the simultaneous detection of four antibiotic residue families in milk. Biosens. Bioelectron. 2016, 79, 430–434. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, S.; Hu, Y.; Li, Z.; Luo, F.; He, Z. Electrochemical immunosensor based on the chitosan-magnetic nanoparticles for detection of tetracycline. Food Anal. Methods 2016, 9, 2972–2978. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Roshani, S.; Bagheri, Z.; Yaghoubi-Avini, M. Carbon dots—Sodium alginate hydrogel: A novel tetracycline fluorescent sensor and adsorber. J. Environ. Chem. Eng. 2019, 7, 103419. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, D.; Liu, L.; Song, S.; Kuang, H.; Xu, C. Development of an ELISA and immunochromatographic assay for tetracycline, oxytetracycline, and chlortetracycline residues in milk and honey based on the class-specific monoclonal antibody. Food Anal. Methods 2016, 9, 905–914. [Google Scholar] [CrossRef]
- Taranova, N.A.; Kruhlik, A.S.; Zvereva, E.A.; Shmanai, V.V.; Vashkevich, I.I.; Semyonov, D.A.; Eremin, S.A.; Zherdev, A.V.; Dzantiev, B.B. Highly sensitive immunochromatographic identification of tetracycline antibiotics in milk. Int. J. Anal. Chem. 2015, 2015, 347621. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Y.; Liang, X. Development of a SPR aptasensor containing oriented aptamer for direct capture and detection of tetracycline in multiple honey samples. Biosens. Bioelectron. 2018, 109, 1–7. [Google Scholar] [CrossRef]
- Gong, X.; Li, X.; Qing, T.; Zhang, P.; Feng, B. Amplified colorimetric detection of tetracycline based on an enzyme-linked aptamer assay with multivalent HRP-mimicking DNAzyme. Analyst 2019, 144, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, J.; Yong, W.; Chen, Q.; Zhang, L.; Dong, Y.; Su, H.; Tan, T. A direct competitive assay-based aptasensor for sensitive determination of tetracycline residue in Honey. Talanta 2015, 131, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yong, W.; Liu, J.; Zhang, L.; Chen, Q.; Dong, Y. Development of an indirect competitive assay-based aptasensor for highly sensitive detection of tetracycline residue in honey. Biosens. Bioelectron. 2014, 57, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Benvidi, A.; Yazdanparast, S.; Rezaeinasab, M.; Tezerjani, M.D.; Abbasi, S. Designing and fabrication of a novel sensitive electrochemical aptasensor based on poly (L-glutamic acid)/MWCNTs modified glassy carbon electrode for determination of tetracycline. J. Electroanal. Chem. 2018, 808, 311–320. [Google Scholar] [CrossRef]
- Jahanbani, S.; Benvidi, A. Comparison of two fabricated aptasensors based on modified carbon paste/oleic acid and magnetic bar carbon paste/Fe3O4@oleic acid nanoparticle electrodes for tetracycline detection. Biosens. Bioelectron. 2016, 85, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, J.; Liu, J.-H.; Gapparov, I.; Wang, S.; Dong, Y.; Su, H.; Tan, T. The development of a graphene oxide-based aptasensor used for the detection of tetracycline in honey. Anal. Methods 2017, 9, 1133–1140. [Google Scholar] [CrossRef]
- He, L.; Luo, Y.; Zhi, W.; Wu, Y.; Zhou, P. A colorimetric aptamer biosensor based on gold nanoparticles for the ultrasensitive and specific detection of tetracycline in milk. Aust. J. Chem. 2013, 66, 485–490. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, J.; Li, Y.; Gao, H.; Guo, J.; Shen, F.; Sun, C. A novel colorimetric aptasensor using cysteamine-stabilized gold nanoparticles as probe for rapid and specific detection of tetracycline in raw milk. Food Control 2015, 54, 7–15. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Li, J.; Li, X.-D.; Ding, L.-S.; Xie, J.; Qing, L.-S. A simple nano-SiO2-based ELISA method for residue detection of 2,4-dichlorophenoxyacetic acid in bean sprouts. Food Anal. Methods 2017, 10, 1500–1506. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Li, J.; Li, X.-D.; Tao, W.-J.; Ding, L.-S.; Luo, P.; Qing, L.-S. An efficient direct competitive nano-ELISA for residual BSA determination in vaccines. Anal. Bioanal. Chem. 2017, 409, 4607–4614. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Xie, J.; Li, X.-D.; Ding, L.-S.; Liang, J.; Luo, P.; Qing, L.-S. Development of a nano-SiO2 based enzyme-linked ligand binding assay for the determination of ibuprofen in human urine. Talanta 2017, 167, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Taron, W.; Jamnongkan, W.; Techasen, A.; Phetcharaburanin, J.; Namwat, N.; Sithithaworn, P.; Khuntikeo, N.; Mukdasai, S.; Sayasone, S.; Loilome, W.; et al. AuNPs-LISA, an efficient detection assay for Opisthorchis viverrini (Ov) antigen in urine. Talanta 2020, 209, 120592. [Google Scholar] [CrossRef] [PubMed]
- Haider, W.; Hayat, A.; Raza, Y.; Anwar Chaudhry, A.; Rehman, I.-U.; Marty, J.L. Gold nanoparticle decorated single walled carbon nanotube nanocomposite with synergistic peroxidase like activity for d-alanine detection. RSC Adv. 2015, 5, 24853–24858. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Huang, W.-X.; Zhang, P.-J.; Chen, L.; Lio, C.-K.; Zhou, H.; Qing, L.-S.; Luo, P. Colorimetric determination of the early biomarker hypoxia-inducible factor-1 alpha (HIF-1α) in circulating exosomes by using a gold seed-coated with aptamer-functionalized Au@Au core-shell peroxidase mimic. Microchim. Acta 2020, 187, 61. [Google Scholar] [CrossRef]
- Wang, T.-T.; Lio, C.k.; Huang, H.; Wang, R.-Y.; Zhou, H.; Luo, P.; Qing, L.-S. A feasible image-based colorimetric assay using a smartphone RGB camera for point-of-care monitoring of diabetes. Talanta 2020, 206, 120211. [Google Scholar] [CrossRef]
- Burkin, M.A.; Galvidis, I.A. Improved group determination of tetracycline antibiotics in competitive enzyme-linked immunosorbent assay. Food Agric. Immunol. 2009, 20, 245–252. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV–vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Pavlov, V.; Xiao, Y.; Shlyahovsky, B.; Willner, I. Aptamer-functionalized Au nanoparticles for the amplified optical detection of thrombin. J. Am. Chem. Soc. 2004, 126, 11768–11769. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Spike (ng/mL) | Intra-Day Precision | Inter-Day Precision | ||
|---|---|---|---|---|
| Detected (ng/mL) | CV | Detected (ng/mL) | CV | |
| 0.1 | 0.10 ± 0.00 | 3.46% | 0.10 ± 0.01 | 7.17% |
| 1 | 0.97 ± 0.03 | 3.11% | 0.97 ± 0.05 | 5.20% |
| 5 | 4.76 ± 0.12 | 2.55% | 4.75 ± 0.20 | 4.25% |
| Spike (ng/mL) | Detected (ng/mL) | Recovery | Mean Recovery | SD | CV | |
|---|---|---|---|---|---|---|
| 1 | 0.1 | 0.0893 | 89.30% | 91.50% | 4.34% | 4.74% |
| 2 | 0.1 | 0.0965 | 96.50% | |||
| 3 | 0.1 | 0.0887 | 88.70% | |||
| 4 | 1 | 0.914 | 91.40% | 93.00% | 1.42% | 1.52% |
| 5 | 1 | 0.935 | 93.60% | |||
| 6 | 1 | 0.941 | 94.10% | |||
| 7 | 5 | 4.76 | 95.20% | 95.33% | 3.20% | 3.36% |
| 8 | 5 | 4.61 | 92.20% | |||
| 9 | 5 | 4.93 | 98.60% |
| Method | Application | Apparatus | Range | LOD | Reference |
|---|---|---|---|---|---|
| SPR aptasensor containing oriented aptamer | honey | Biacore T200 SPR instrument | 0.01–1000 μg/kg | 0.0069 μg/kg | [20] |
| Enzyme-linked aptamer assay with multivalent HRP-mimicking DNAzyme | honey | UV–vis spectrophotometer | 1.0 × 10−2 to 1.0 × 104 ng/mL | 8.1 × 10−2 ng/mL | [21] |
| direct competitive assay-based aptasensor | honey | microplate reader | 0.1–1000 ng/mL | 0.0978 ng/mL | [22] |
| Indirect competitive assay-based aptasensor | honey | microplate reader | 0.01–100 ng/mL | 9.6 × 10−3 ng/mL | [23] |
| electrochemical aptasensor based on poly (L-glutamic acid)/MWCNTs modified glassy carbon electrode | honey | potentiostat/galvanostat | 1.0 × 10−16–1.0 × 10−6 M | 3.7 × 10−17 M | [24] |
| Two aptasensors based on modified carbon paste/oleic acid and magnetic bar carbon paste/Fe3O4@oleic acid nanoparticle electrodes | drug, milk, honey and serum | potentiostat/galvanostat | 1.0 × 10−12–1.0 × 10−7 M; 1.0 × 10−10–1.0 × 10−7 M; | 3 × 10−13 M; 2.9 × 10−11 M; | [25] |
| Graphene oxide-based aptasensor | honey | UV–vis spectrophotometer | 0.002–20 ng/mL | 0.001 ng/mL | [26] |
| Colorimetric aptamer biosensor | milk | Microplate Spectrophotometer | 122 nM | [27] | |
| Colorimetric aptasensor | milk | UV–vis spectrophotometer | 0.20–2.0 μg/mL | 0.039 μg/mL | [28] |
| Electrochemical immunosensor | milk | electrochemical workstation | 0.08–1 ng/mL | 0.0321 ng/mL | [16] |
| Near-infrared fluorescence-based multiplex lateral flow immunoassay | milk | odyssey infrared imaging system | 0.04–0.98 ng/mL | 0.04 ng/mL | [15] |
| Immunochromatographic assay | serum | microplate reader | 0.7–26 ng/mL | 0.2 ng/mL | [14] |
| Aptamer-based magnetic solid-phase extraction | water and honey | HPLC/UV | 10–3000 µg/L | 2.5 µg/L | [6] |
| gold nanoparticle-linked aptamer assay | honey | microplate reader | 0.01–10 ng/mL | 0.0027 ng/mL | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Y.-M.; Liang, J.; Xie, J. Indirect Competitive Determination of Tetracycline Residue in Honey Using an Ultrasensitive Gold-Nanoparticle-Linked Aptamer Assay. Molecules 2020, 25, 2144. https://doi.org/10.3390/molecules25092144
Sheng Y-M, Liang J, Xie J. Indirect Competitive Determination of Tetracycline Residue in Honey Using an Ultrasensitive Gold-Nanoparticle-Linked Aptamer Assay. Molecules. 2020; 25(9):2144. https://doi.org/10.3390/molecules25092144
Chicago/Turabian StyleSheng, Yan-Mei, Jian Liang, and Jing Xie. 2020. "Indirect Competitive Determination of Tetracycline Residue in Honey Using an Ultrasensitive Gold-Nanoparticle-Linked Aptamer Assay" Molecules 25, no. 9: 2144. https://doi.org/10.3390/molecules25092144
APA StyleSheng, Y.-M., Liang, J., & Xie, J. (2020). Indirect Competitive Determination of Tetracycline Residue in Honey Using an Ultrasensitive Gold-Nanoparticle-Linked Aptamer Assay. Molecules, 25(9), 2144. https://doi.org/10.3390/molecules25092144





