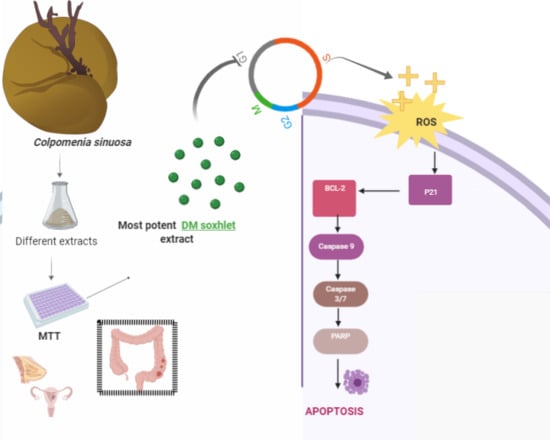
The Cytotoxic and Apoptotic Effects of the Brown Algae Colpomenia sinuosa are Mediated by the Generation of Reactive Oxygen Species
Abstract
1. Introduction
2. Results and Discussion
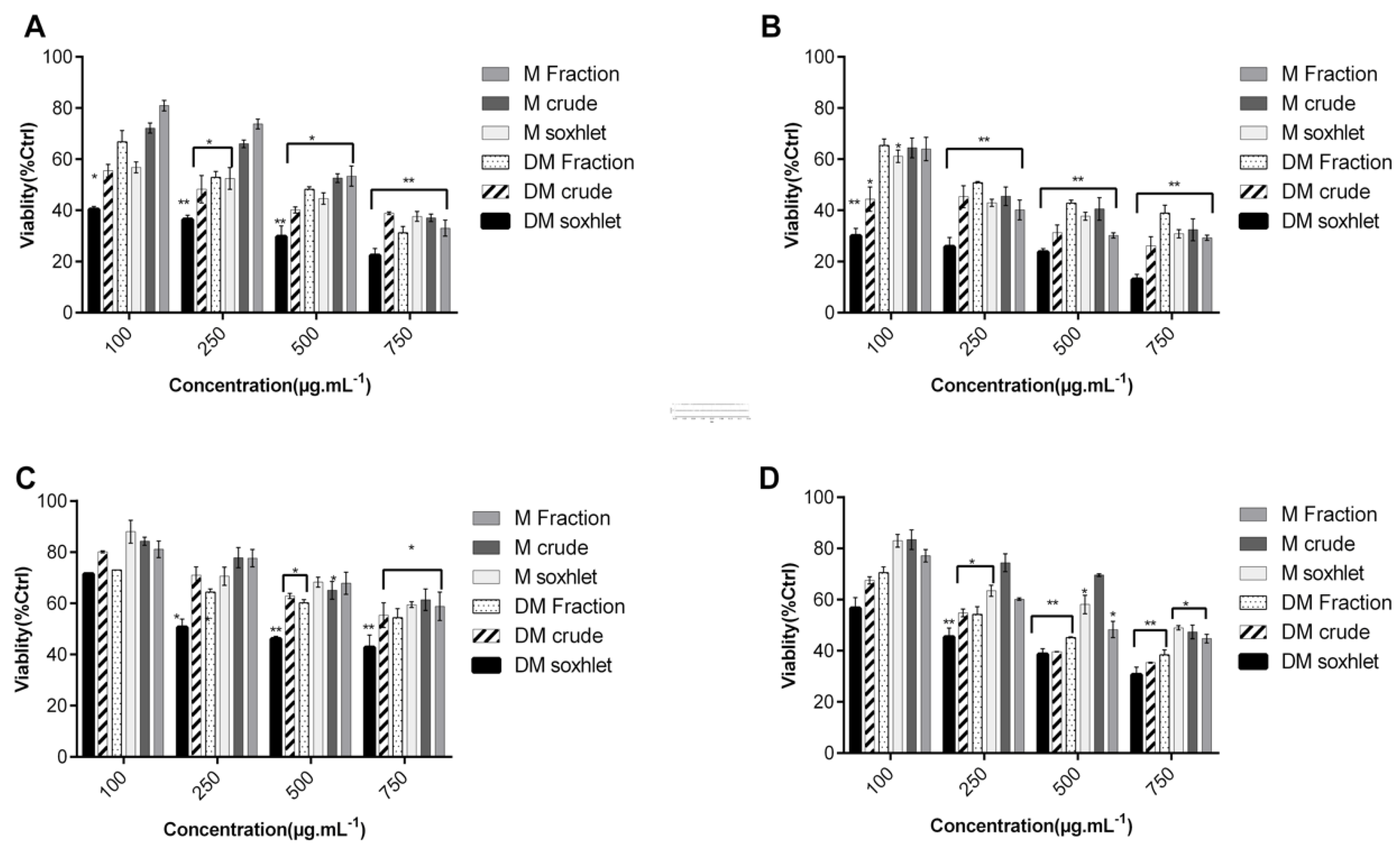
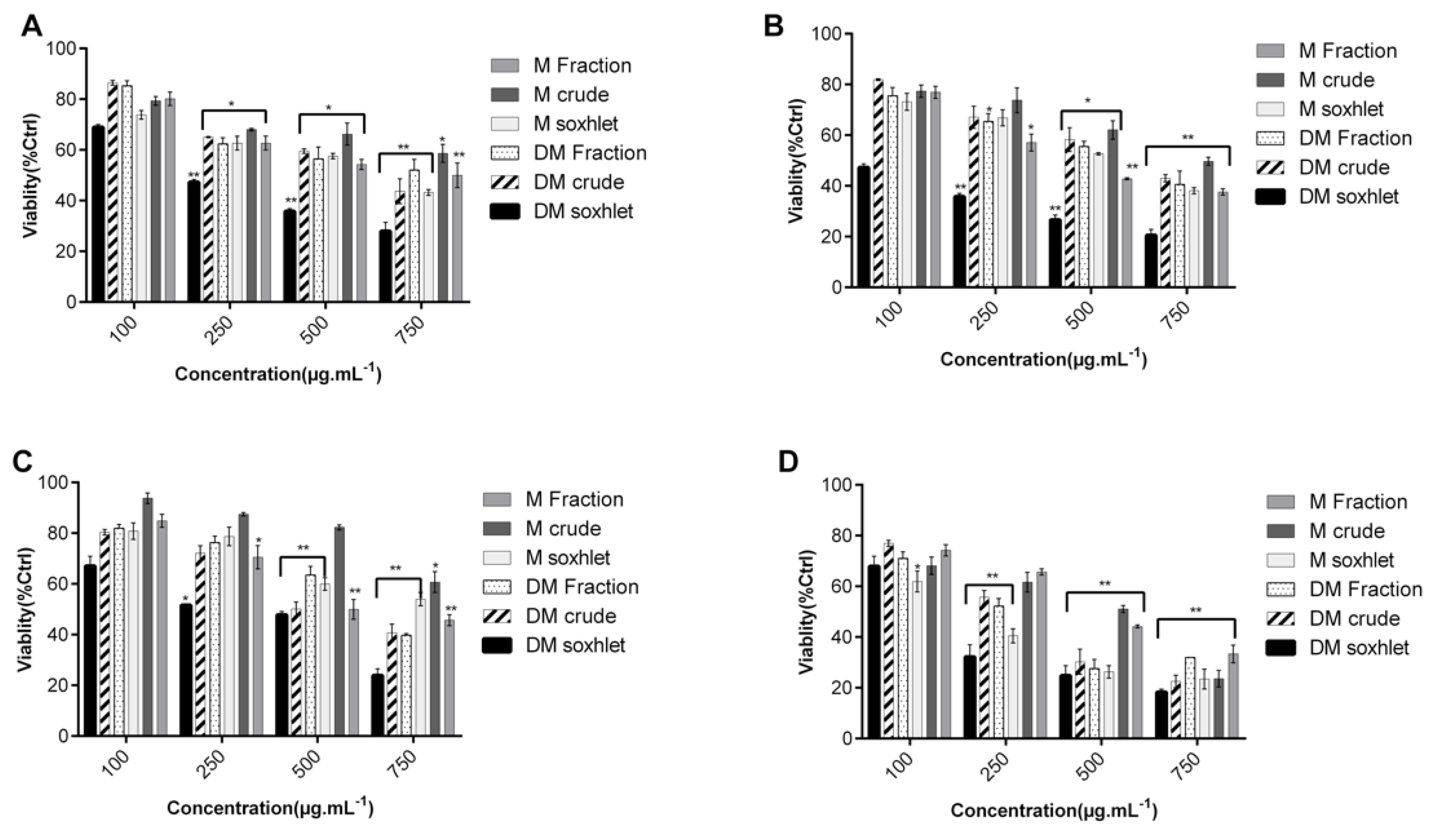
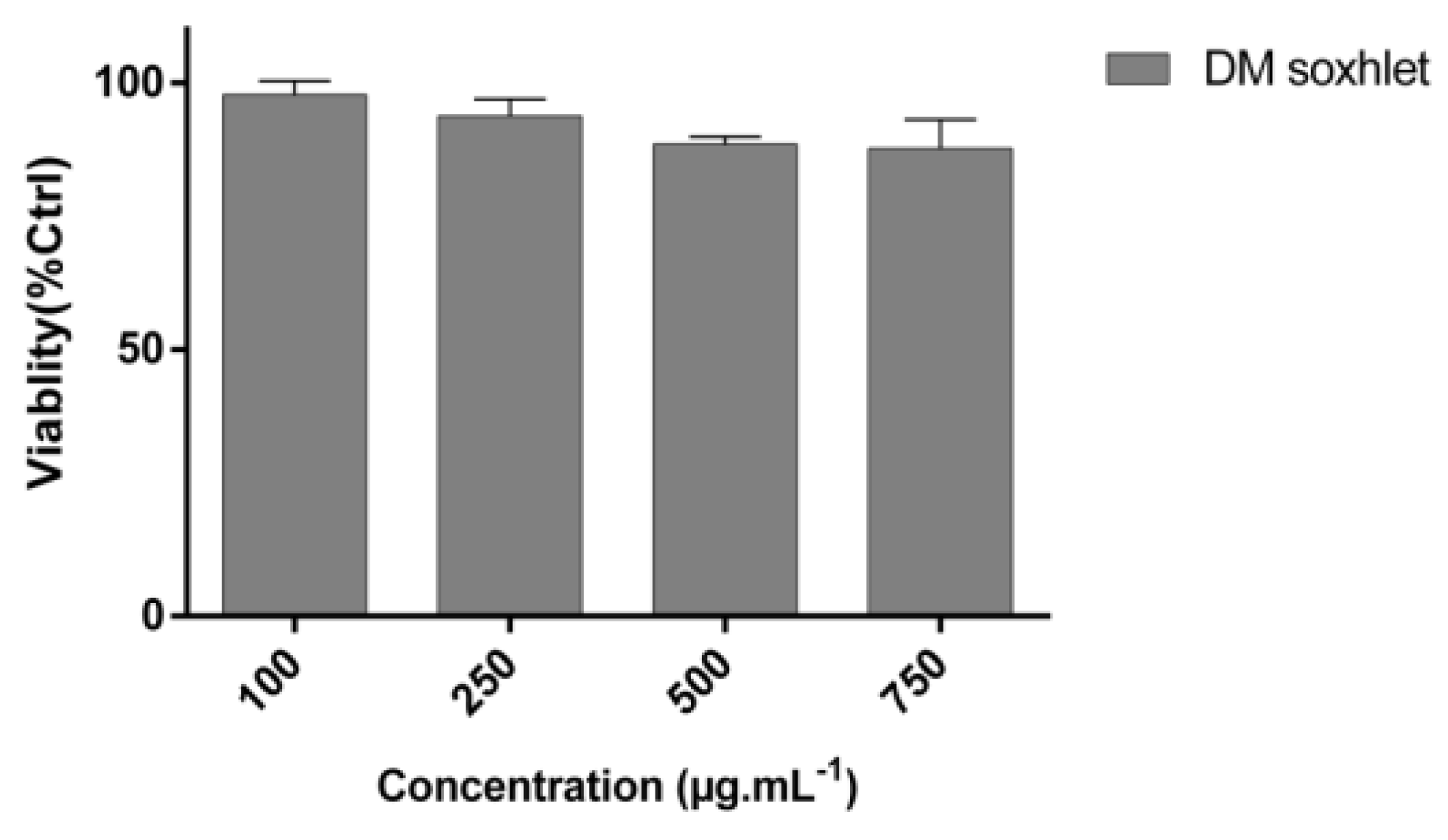
2.1. C. sinuosa Organic and Aqueous Extracts Inhibited Cancer Cell Viability
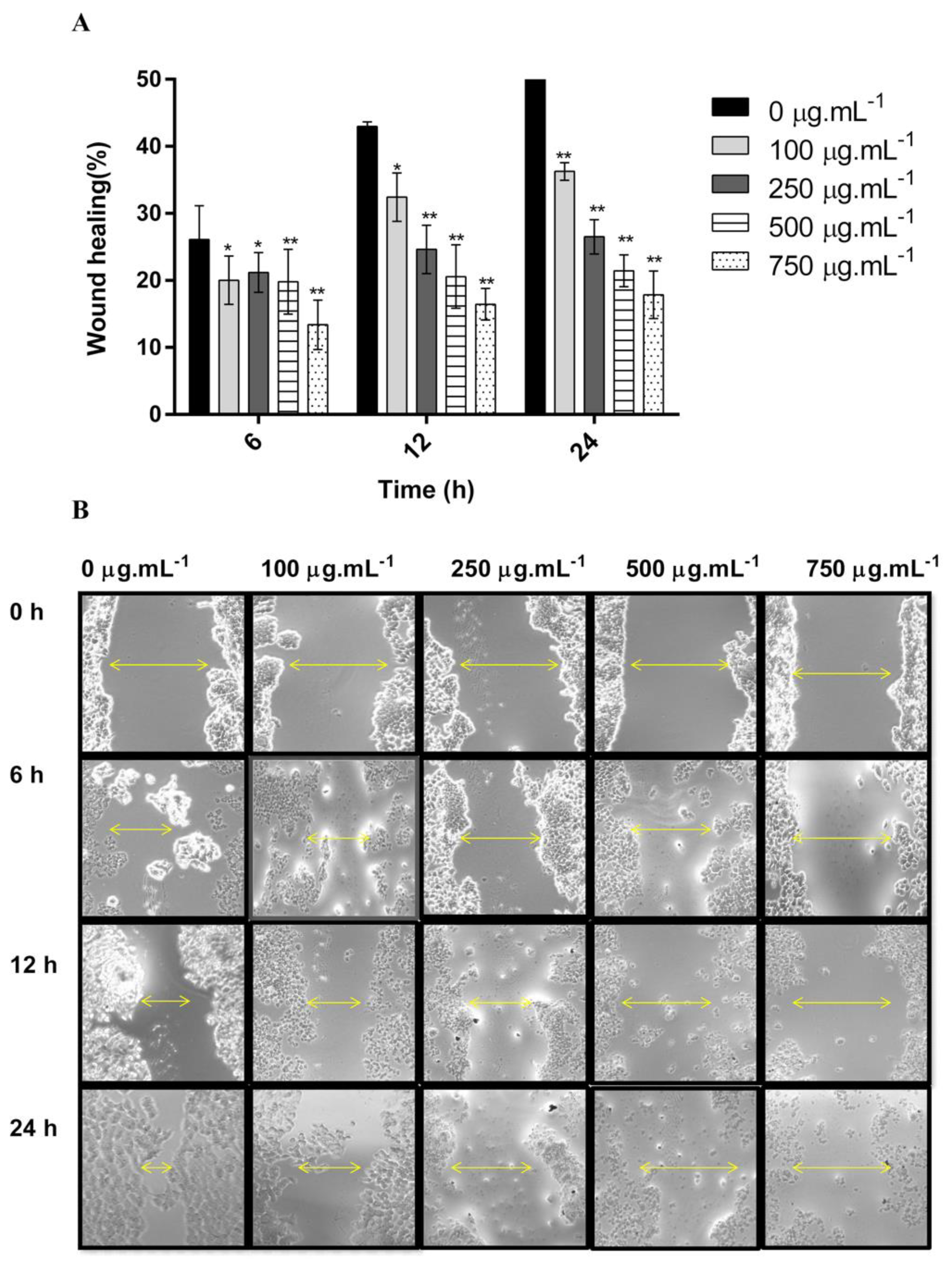
2.2. DM Soxhlet Extract Significantly Decreased the Migration of HCT-116 Cancer Cell Lines
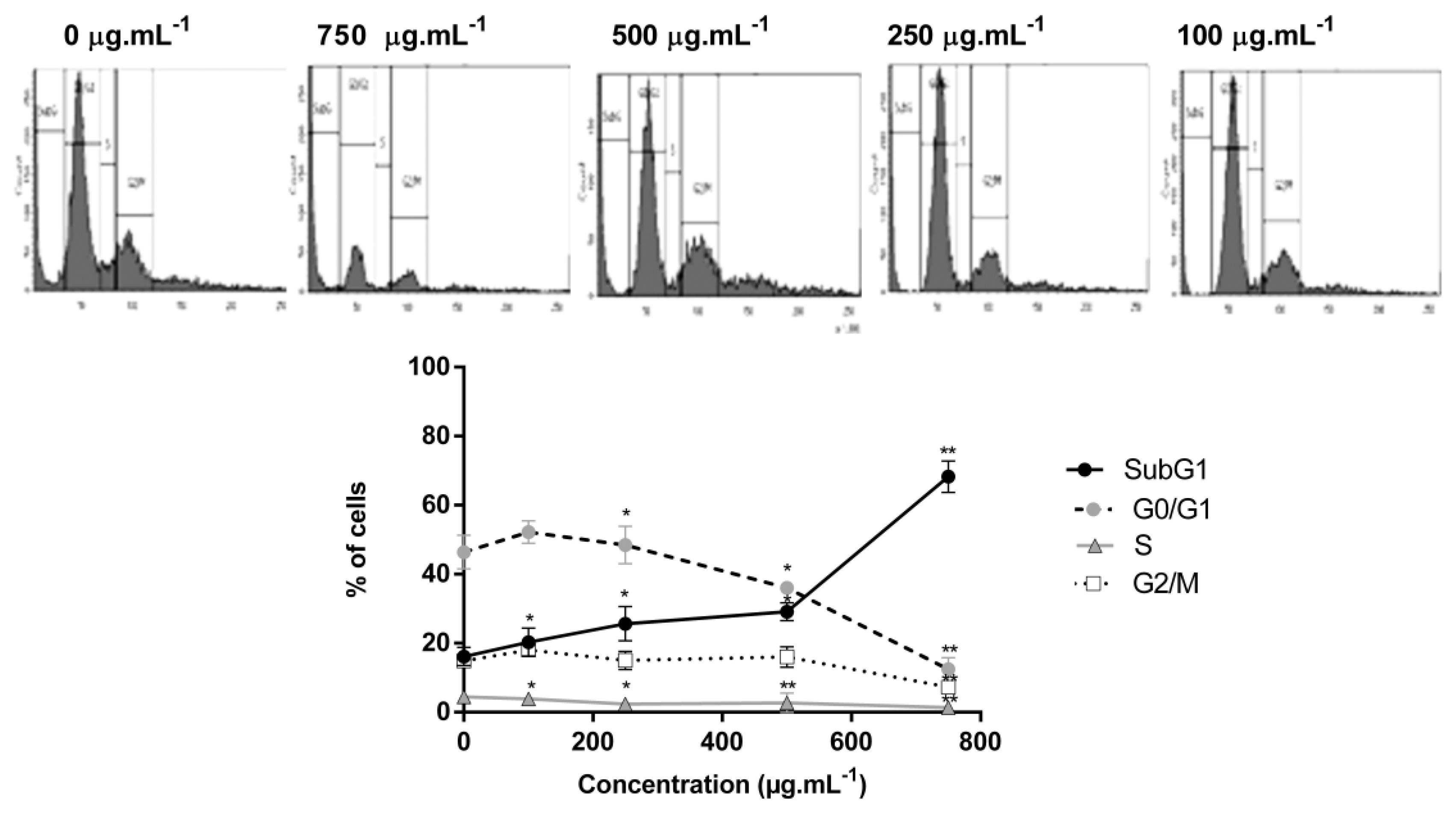
2.3. C. sinuosa Extract Induced A SubG1 Increase in HCT-116 Cancer Cells
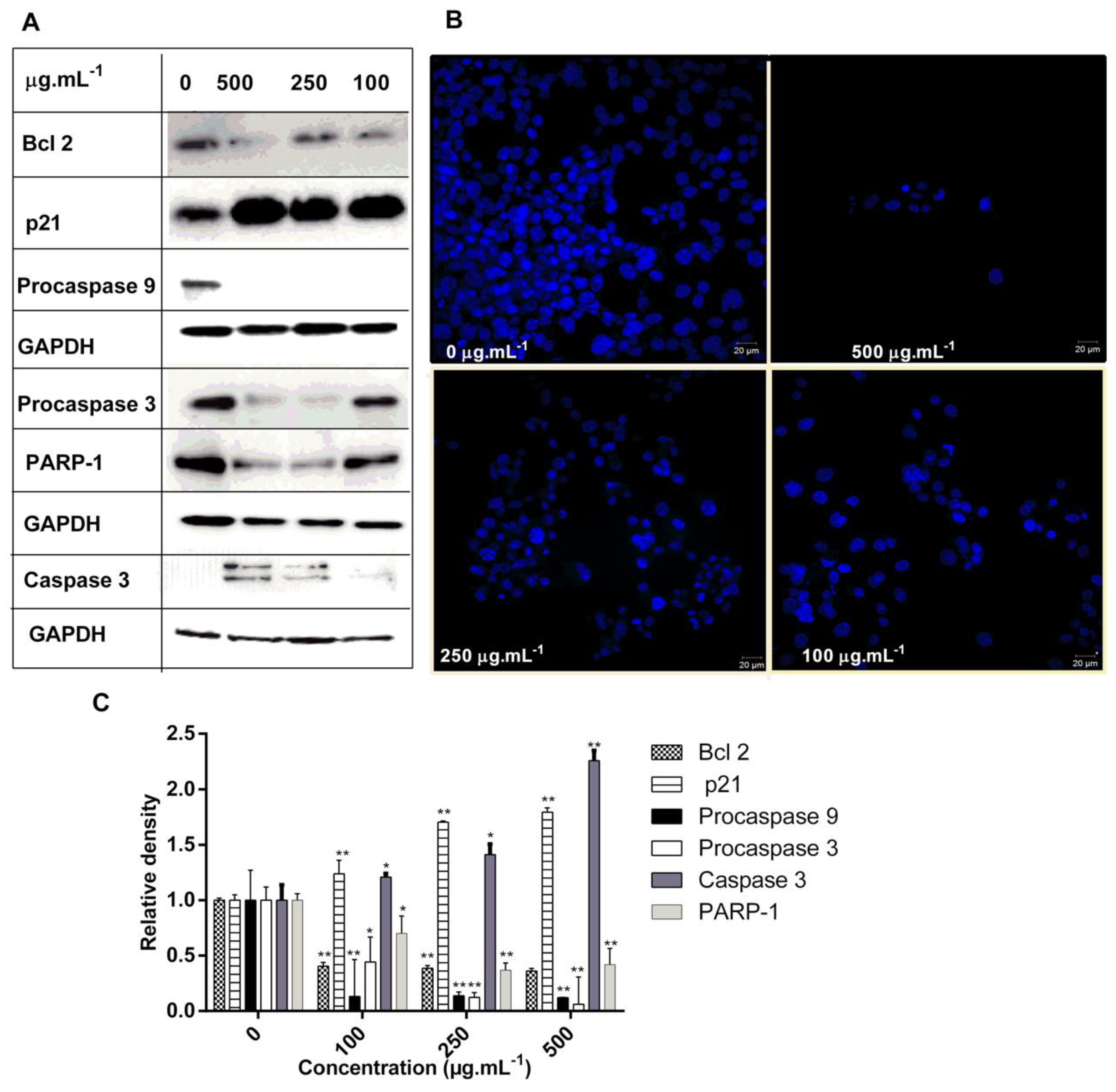
2.4. DM Soxhlet Extract Induced Apoptosis in HCT-116 Cell Line through the Regulation of Apoptotic Related Proteins
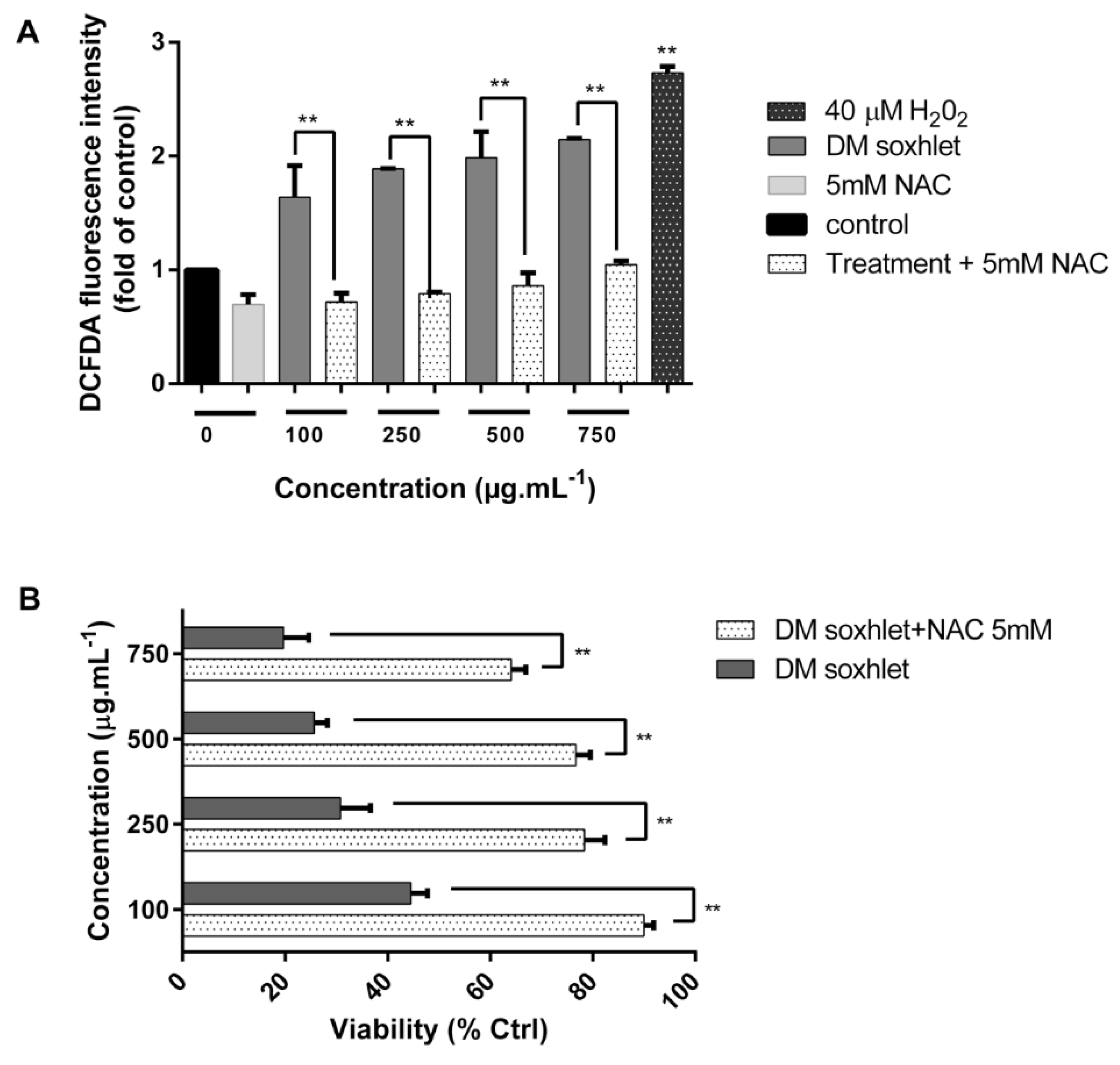
2.5. ROS Mediate the Cytostatic Effects of the DM Soxhlet Extract in HCT116 Cancer Cells
3. Materials and Methods
3.1. Marine Algal Material
3.2. Cell Lines and Cell Culture
3.3. Cell Viability Assay
3.4. Trypan Blue Test
3.5. Wound-Healing Migration Assay
3.6. Cell Cycle Analysis
3.7. DAPI Staining
3.8. Western Blot Analysis
3.9. Quantitative ROS Determination
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kranz, D.; Dobbelstein, M. A killer promoting survival: p53 as a selective means to avoid side effects of chemotherapy. Cell Cycle 2012, 11, 2053–2054. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Weng, Y.-P.; Lin, H.-Y.; Tang, S.-W.; Chen, C.-J.; Liang, C.-J.; Ku, C.-Y.; Lin, J.-Y. Aqueous extract of Polygonum bistorta modulates proteostasis by ROS-induced ER stress in human hepatoma cells. Sci. Rep. 2017, 7, 41437–41451. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Silva, A.M.; Matos, M.; Monteiro, S.M.; Álvaro, A.R. Copper induced apoptosis in Caco-2 and Hep-G2 cells: Expression of caspases 3, 8 and 9, AIF and p53. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 185, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Placzek, W.J. Post-Transcriptional Regulation of Anti-Apoptotic BCL2 Family Members. Int. J. Mol. Sci. 2018, 19, 308. [Google Scholar]
- Kim, C.; Kim, B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef]
- Ercolano, G.; De Cicco, P.; Ianaro, A. New Drugs from the Sea: Pro-Apoptotic Activity of Sponges and Algae Derived Compounds. Mar. Drugs 2019, 1, 31. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Y.; Xiao, Y.; Lu, Y. Upregulation of miR-21 expression is a valuable predicator of advanced clinicopathological features and poor prognosis in patients with renal cell carcinoma through the p53/p21-cyclin E2-Bax/caspase-3 signaling pathway. Oncol. Rep. 2017, 37, 1437–1444. [Google Scholar] [CrossRef]
- Voráčová, K.; Hájek, J.; Mareš, J.; Urajová, P.; Kuzma, M.; Cheel, J.; Villunger, A.; Kapuscik, A.; Bally, M.; Novák, P.; et al. The cyanobacterial metabolite nocuolina is a natural oxadiazine that triggers apoptosis in human cancer cells. PLoS ONE 2017, 12, e0172850. [Google Scholar] [CrossRef]
- Song, X.; Xiong, Y.; Qi, X.; Tang, W.; Dai, J.; Gu, Q.; Li, J. Molecular Targets of Active Anticancer Compounds Derived from Marine Sources. Mar. Drugs 2018, 16, 175. [Google Scholar] [CrossRef]
- Leal, M.C.; Munro, M.H.G.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat. Prod. Rep. 2013, 30, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.V.; Walsh, N.A. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol. 2006, 44, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Bañuelos, T.; Gutiérrez-Rodríguez, A.G.; Méndez-Bellido, R.; Tovar-Miranda, R.; Arroyo-Helguera, O.; Juárez-Portilla, C.; Meza-Menchaca, T.; Aguilar-Rosas, L.E.; Hernández-Kelly, L.C.R.; Ortega, A.; et al. Brown Seaweed Egregia menziesii’s Cytotoxic Activity against Brain Cancer Cell Lines. Molecules 2019, 24, 260. [Google Scholar] [CrossRef] [PubMed]
- Karaki, N.; Faour, T.; Sebaaly, C.; Chahine, N.; Zichenko, A.; Rachid, S.; Kanaan, H. The antioxidant and anticoagulant activities of polysaccharides isolated from the brown algae Dictyopteris polypodioides growing on the Lebanese coast. JAPS 2013, 3, 043–051. [Google Scholar]
- Haddad, M.; Zein, S.; Hazimeh, G.; Karaki, R.; Krivoruchko, E.; Makhour, Y.; Kassem, Z.; Kanaan, H. Structural Characteristics, Antitumor and Antioxidant Properties of Polysaccharides isolated from the brown algae Stypopodium schimperi growing on the Lebanese coast. ARJMD 2017, 17, 36–43. [Google Scholar]
- Sari-Chmayssem, N.; Taha, S.; Mawlawi, H.; Guégan, J.-P.; Jeftić, J.; Benvegnu, T. Extracted and depolymerized alginates from brown algae Sargassum vulgare of Lebanese origin: Chemical, rheological, and antioxidant properties. J. Appl. Phycol. 2016, 28, 1915–1929. [Google Scholar] [CrossRef]
- Saranraj, P. Pharmacological Importance of Seaweeds: A Review. WJFMS 2014, 6, 1–15. [Google Scholar]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.G.; Juárez-Portilla, C.; Olivares-Bañuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2018, 23, 434–447. [Google Scholar] [CrossRef]
- Kanaan, H.; Belous, O.; Chokr, A. Diversity Investigation of the Seaweeds Growing on the Lebanese Coast. J. Mar. Sci. Res. Dev. 2014, 5, 1–12. [Google Scholar]
- Harmelin, J.-G. High xenodiversity versus low native diversity in the south-eastern Mediterranean: Bryozoans from the coastal zone of Lebanon. Mediterr. Mar. Sci. 2016, 17, 417–439. [Google Scholar] [CrossRef][Green Version]
- Men’shova, R.V.; Ermakova, S.P.; Rachidi, S.M.; Al-Hajje, A.H.; Zvyagintseva, T.N.; Kanaan, H.M. Seasonal variations of the composition, structural features, and antitumor properties of polysaccharides from Padina pavonica (Lebanon) as a function of composition. Chem. Nat. Compd. 2012, 47, 870–875. [Google Scholar] [CrossRef]
- Tannoury, M.; Saab, A.M.; Elia, J.M.; Makhlouf, H.; Diab-Assaf, M. In Vitro Cytotoxic Activity of Laurencia papillosa, Marine Red Algae from the Lebanese Coast|Semantic Scholar. JAPS 2017, 7, 175–179. [Google Scholar]
- Tannoury, M.; Elia, J.M.; Saab, A.M.; Makhlouf, H.; Daouchabo, R.; Diab-Assaf, M. Evaluation of Cytotoxic Activity of Sargassum vulgare From the Lebanese Coast against Jurkat Cancer Cell Line. JAPS 2016, 6, 108–112. [Google Scholar] [CrossRef]
- El Asri, O.; Ramdani, M.; Latrach, L.; Haloui, B.; Ramdani, M.; Afilal, M.E. Comparison of energy recovery after anaerobic digestion of three Marchica lagoon algae (Caulerpa prolifera, Colpomenia sinuosa, Gracilaria bursa-pastoris). Sustain. Mater. Technol. 2017, 11, 47–52. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- Kanagasabhapathy, M.; Yamazaki, G.; Ishida, A.; Sasaki, H.; Nagata, S. Presence of quorum-sensing inhibitor-like compounds from bacteria isolated from the brown alga Colpomenia sinuosa. Lett. Appl. Microbiol. 2009, 49, 573–579. [Google Scholar] [CrossRef]
- Parsons, M.J. Colpomenia (Endlicher) Derbès et Solier (Phaecphyta) in New Zealand. N. Z. J. Bot. 1982, 20, 289–301. [Google Scholar] [CrossRef]
- Cotton, A.D. The Appearance of Colpomenia Sinuosa in Britain. Bull. Misc. Inform. Kew 1908, 1, 73–77. [Google Scholar] [CrossRef]
- Gyi, K.K.; Htun, S. The morphotaxonomy and phytogeographical distribution of Colpomenia sinuosa (Mertens ex Roth) Derbes & Solier (Scytosiphonales, Phaeophyta) from Myanmar. Univ. Res. J. 2012, 5, 1–21. [Google Scholar]
- Shaikh, W.; Shameel, M.; Ahmad, V.U.; Usmanghani, K. Phycochemical Studies on Colpomenia sinuosa (Scytosiphonales, Phaeophyta). Bot. Mar. 2009, 34, 77–80. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Waaland, J.R.; Rabiei, R. Fatty Acids, Amino Acids, Mineral Contents, and Proximate Composition of Some Brown Seaweeds1. J. Phycol. 2012, 48, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kanias, G.D.; Skaltsa, H.; Tsitsa, E.; Loukis, A.; Bitis, J. Study of the correlation between trace elements, sterols and fatty acids in brown algae from the Saronikos gulf of Greece. Fresenius J. Anal. Chem. 1992, 344, 334–339. [Google Scholar] [CrossRef]
- Hussain, M.M.; National, R.C.; Abdel-Aziz, A.; Saleem, H.M.; Cairo, U. Chemical composition of Colpomenia sinuosa as influenced by seasonal variation. Pak. J. Sci. Ind. Res. 1983, 26, 152–154. [Google Scholar]
- Karkhane Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghasempour, A.R. In vitro investigating of anticancer activity of focuxanthin from marine brown seaweed species. Glob. J. Environ. Sci. Manag. 2018, 4, 81–90. [Google Scholar]
- Rostami, Z.; Tabarsa, M.; You, S.; Rezaei, M. Structural characterization and RAW264.7 murine macrophage stimulating activity of a fucogalactoglucan from Colpomenia peregrina. J. Food Sci. Technol. 2018, 55, 4650–4660. [Google Scholar] [CrossRef]
- Green, D.; Kashman, Y.; Miroz, A. Colpol, a New Cytotoxic C6-C4-C6 Metabolite from the Alga Colpomenia sinuosa. J. Nat. Prod. 1993, 56, 1201–1202. [Google Scholar] [CrossRef]
- Khanavi, M.; Nabavi, M.; Sadati, N.; Shams Ardekani, M.; Sohrabipour, J.; Nabavi, S.M.B.; Ghaeli, P.; Ostad, S.N. Cytotoxic activity of some marine brown algae against cancer cell lines. Biol. Res. 2010, 43, 31–37. [Google Scholar] [CrossRef]
- Huang, H.-L.; Wu, S.; Liao, H.-F.; Jiang, C.-M.; Huang, R.-L.; Chen, Y.; Yang, Y.-C.; Chen, Y.-J. Induction of Apoptosis by Three Marine Algae through Generation of Reactive Oxygen Species in Human Leukemic Cell Lines. J. Agric. Food Chem. 2005, 53, 1776–1781. [Google Scholar] [CrossRef]
- Monla, R.; Dessouki, Z.; Gali-Muhtasib, H.; Mawalwi, H. Antioxidant, Anti-Inflammatory and Antimicrobial Activities with Chemical Analysis of Colpomenia Sinusa from the North Lebanese Coast. Pharmacogn. Res. 2020, in press. [Google Scholar]
- Mhadhebi, L.; Laroche-Clary, A.; Robert, J.; Bouraoui, A. Antioxidant, anti-inflammatory, and antiproliferative activities of organic fractions from the Mediterranean brown seaweed Cystoseira sedoides. Can. J. Physiol. Pharmacol. 2011, 89, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Ruiz, I.; López-Saiz, C.-M.; Burgos-Hernández, A.; Velázquez, C.; Nieves-Soto, M.; Hurtado-Oliva, M.A. Antioxidant, antimutagenic and antiproliferative activities in selected seaweed species from Sinaloa, Mexico. Pharm. Biol. 2016, 54, 2196–2210. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.A.; Lindequist, U.; Gruenert, R.; Bednarski, P.J. Studies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the island Soqotra. BMC Complement Altern. Med. 2009, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.V.; Scarlett, C.J.; Bowyer, M.C.; Ngo, P.D.; Vuong, Q.V. Impact of Different Extraction Solvents on Bioactive Compounds and Antioxidant Capacity from the Root of Salacia chinensis L. Available online: https://www.hindawi.com/journals/jfq/2017/9305047/ (accessed on 23 March 2020).
- Yang, N.J.; Hinner, M.J. Getting Across the Cell Membrane: An Overview for Small Molecules, Peptides, and Proteins. Methods Mol. Biol. 2015, 1266, 29–53. [Google Scholar] [PubMed]
- Bowe, C.L.; Mokhtarzadeh, L.; Venkatesan, P.; Babu, S.; Axelrod, H.R.; Sofia, M.J.; Kakarla, R.; Chan, T.Y.; Kim, J.S.; Lee, H.J.; et al. Design of compounds that increase the absorption of polar molecules. Proc. Natl. Acad. Sci. USA 1997, 94, 12218–12223. [Google Scholar] [CrossRef] [PubMed]
- Shirjang, S.; Mansoori, B.; Asghari, S.; Duijf, P.H.G.; Mohammadi, A.; Gjerstorff, M.; Baradaran, B. MicroRNAs in cancer cell death pathways: Apoptosis and necroptosis. Free Radic. Biol. Med. 2019, 139, 1–15. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Zeng, H.; Taussig, D.P.; Cheng, W.-H.; Johnson, L.K.; Hakkak, R. Butyrate Inhibits Cancerous HCT116 Colon Cell Proliferation but to a Lesser Extent in Noncancerous NCM460 Colon Cells. Nutrients 2017, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Sieprath, T.; Corne, T.D.J.; Willems, P.H.G.M.; Koopman, W.J.H.; De Vos, W.H. Integrated High-Content Quantification of Intracellular ROS Levels and Mitochondrial Morphofunction. Adv. Anat. Embryol. Cell Biol. 2016, 219, 149–177. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Monla, R.; Dassouki, Z.; Kouzayha, A.; Salma, Y.; Gali-Muhtasib, H.; Mawlawi, H. The Cytotoxic and Apoptotic Effects of the Brown Algae Colpomenia sinuosa are Mediated by the Generation of Reactive Oxygen Species. Molecules 2020, 25, 1993. https://doi.org/10.3390/molecules25081993
Al Monla R, Dassouki Z, Kouzayha A, Salma Y, Gali-Muhtasib H, Mawlawi H. The Cytotoxic and Apoptotic Effects of the Brown Algae Colpomenia sinuosa are Mediated by the Generation of Reactive Oxygen Species. Molecules. 2020; 25(8):1993. https://doi.org/10.3390/molecules25081993
Chicago/Turabian StyleAl Monla, Reem, Zeina Dassouki, Achraf Kouzayha, Yahya Salma, Hala Gali-Muhtasib, and Hiba Mawlawi. 2020. "The Cytotoxic and Apoptotic Effects of the Brown Algae Colpomenia sinuosa are Mediated by the Generation of Reactive Oxygen Species" Molecules 25, no. 8: 1993. https://doi.org/10.3390/molecules25081993
APA StyleAl Monla, R., Dassouki, Z., Kouzayha, A., Salma, Y., Gali-Muhtasib, H., & Mawlawi, H. (2020). The Cytotoxic and Apoptotic Effects of the Brown Algae Colpomenia sinuosa are Mediated by the Generation of Reactive Oxygen Species. Molecules, 25(8), 1993. https://doi.org/10.3390/molecules25081993