Electrochemistry of Inorganic OCT-PbS/HDA and OCT-PbS Photosensitizers Thermalized from Bis(N-diisopropyl-N-octyldithiocarbamato) Pb(II) Molecular Precursors
Abstract
1. Introduction
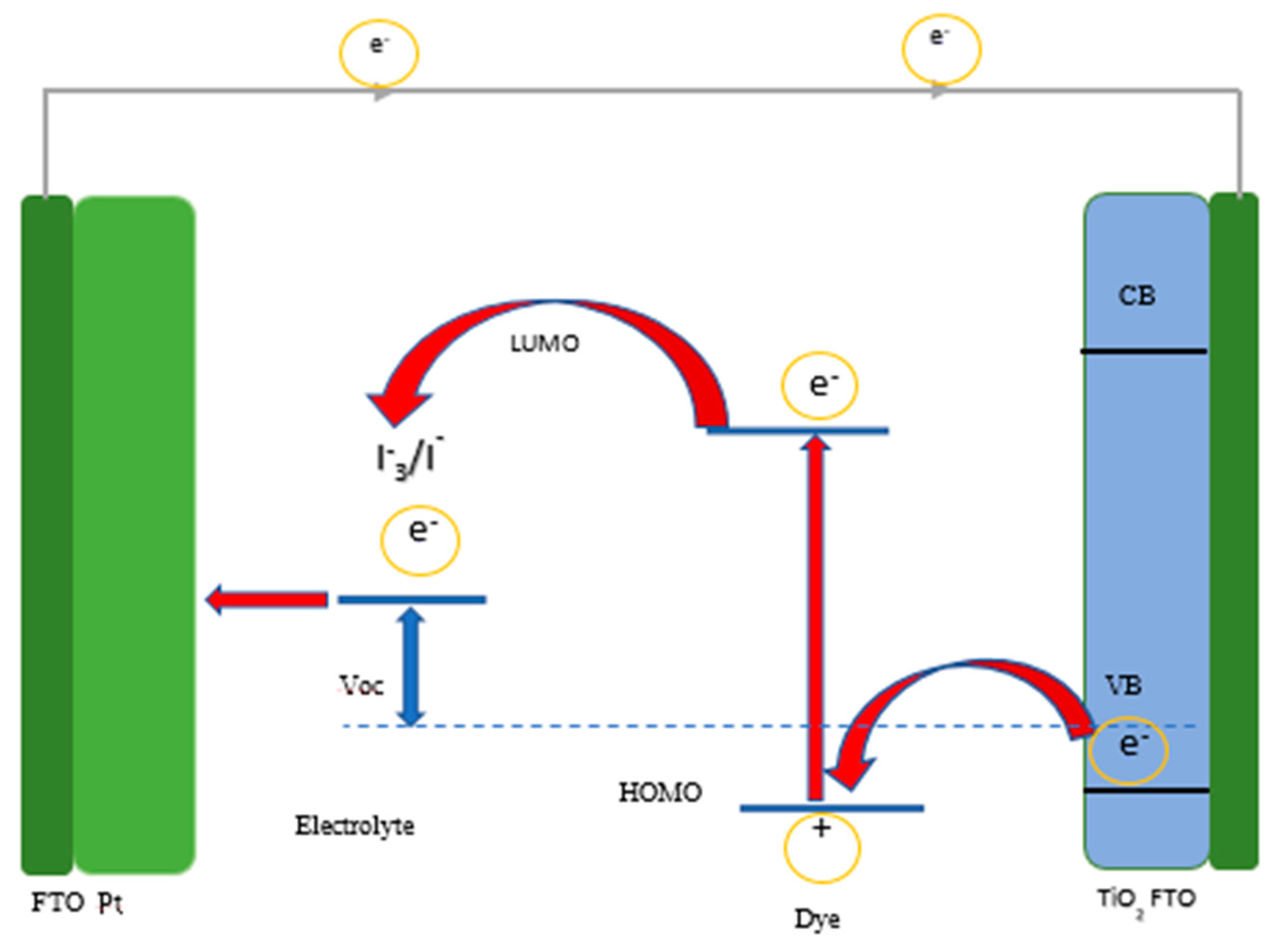
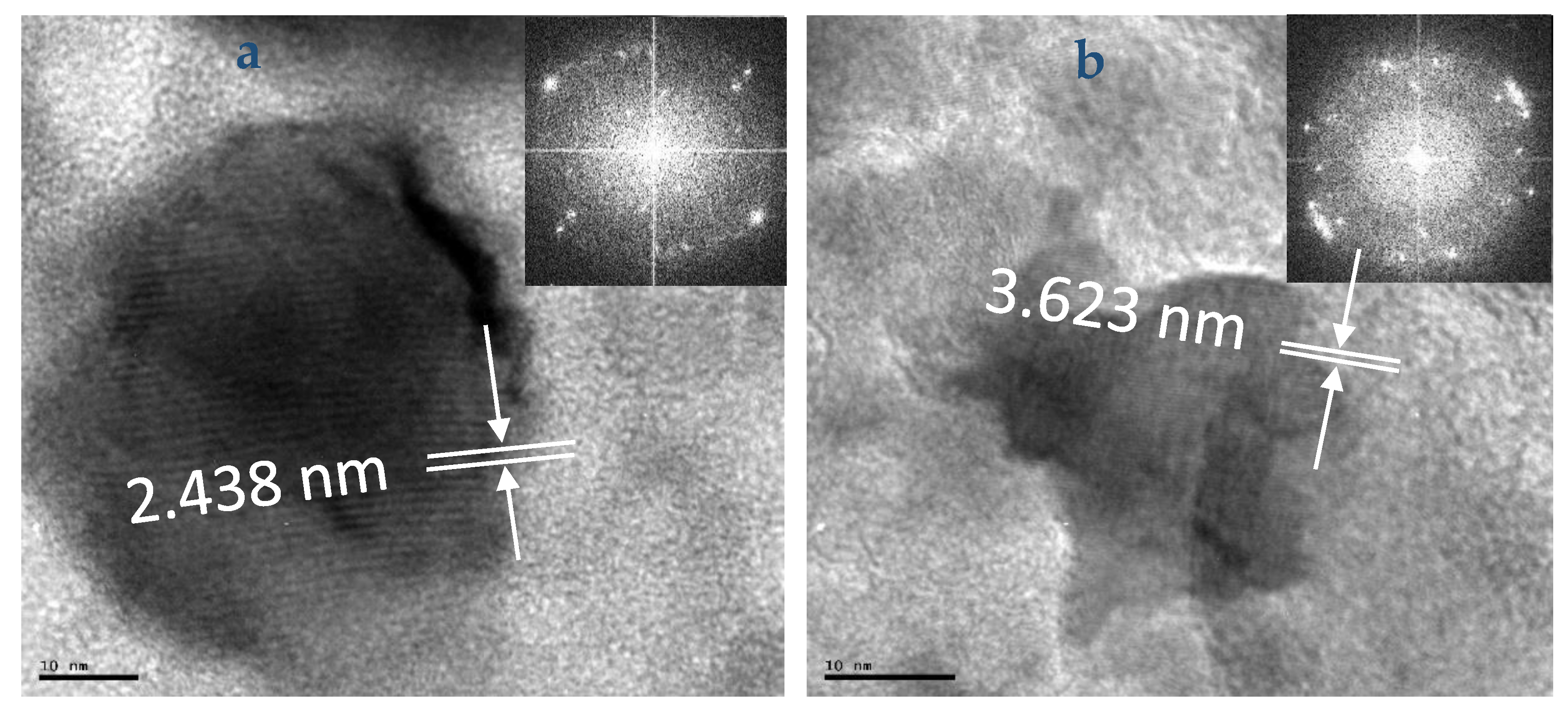
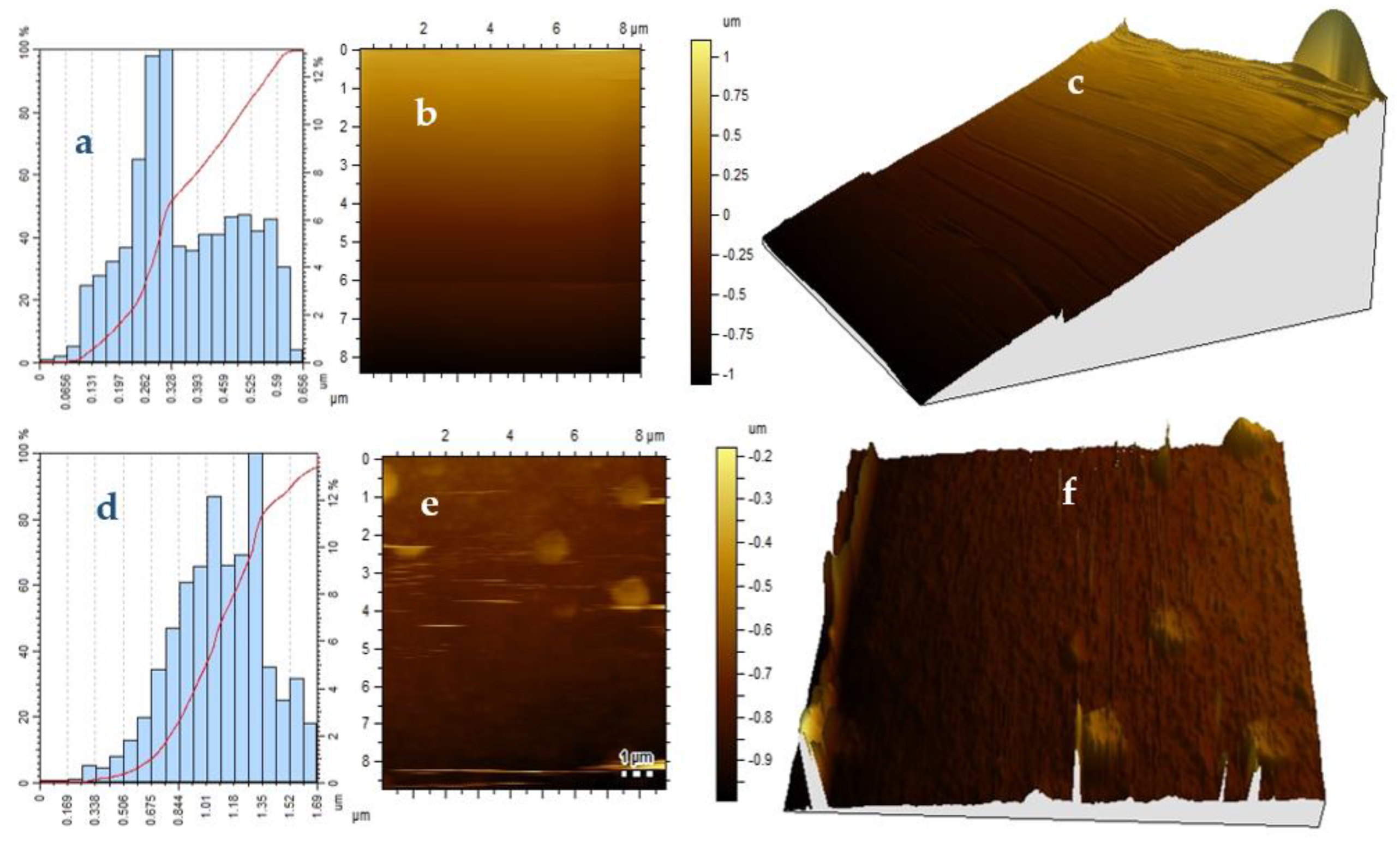
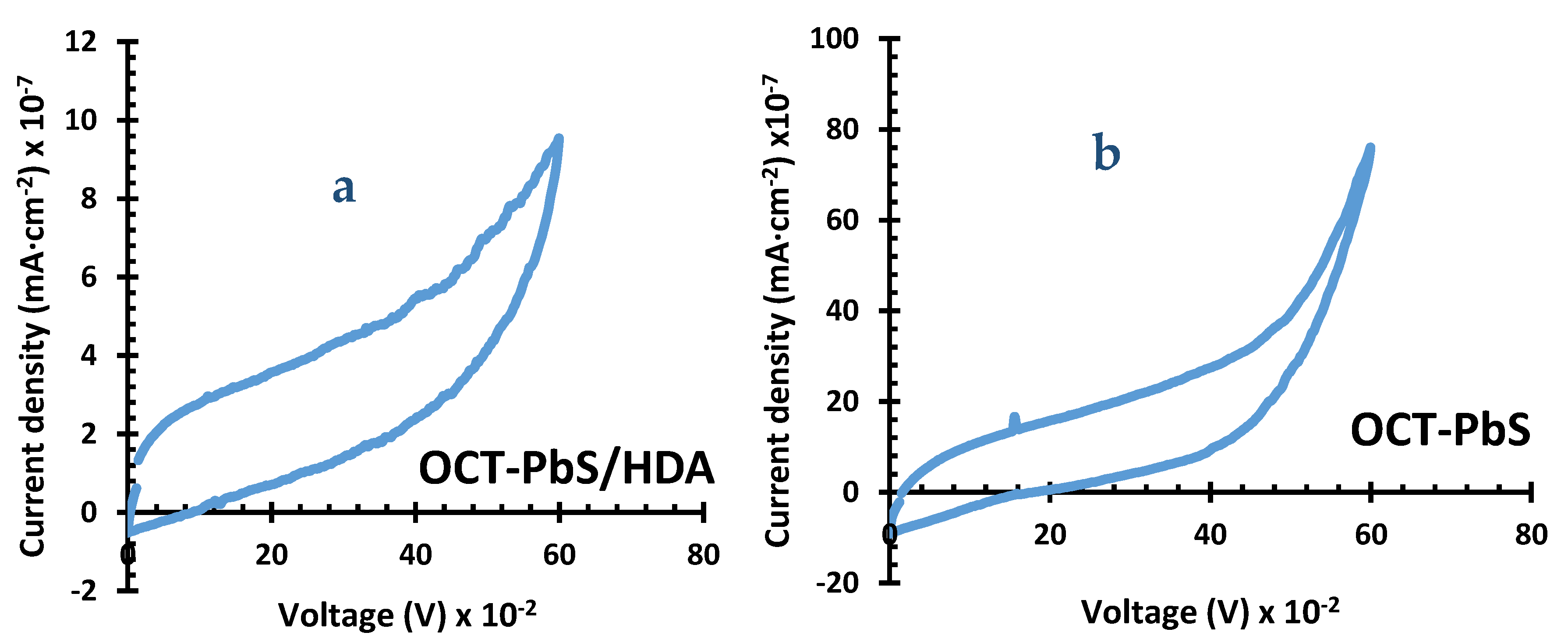
2. Results and Discussion
2.1. X-Ray Diffraction
2.2. HRTEM
2.3. Atomic Force Microscopy
2.4. Cyclic Voltammetry
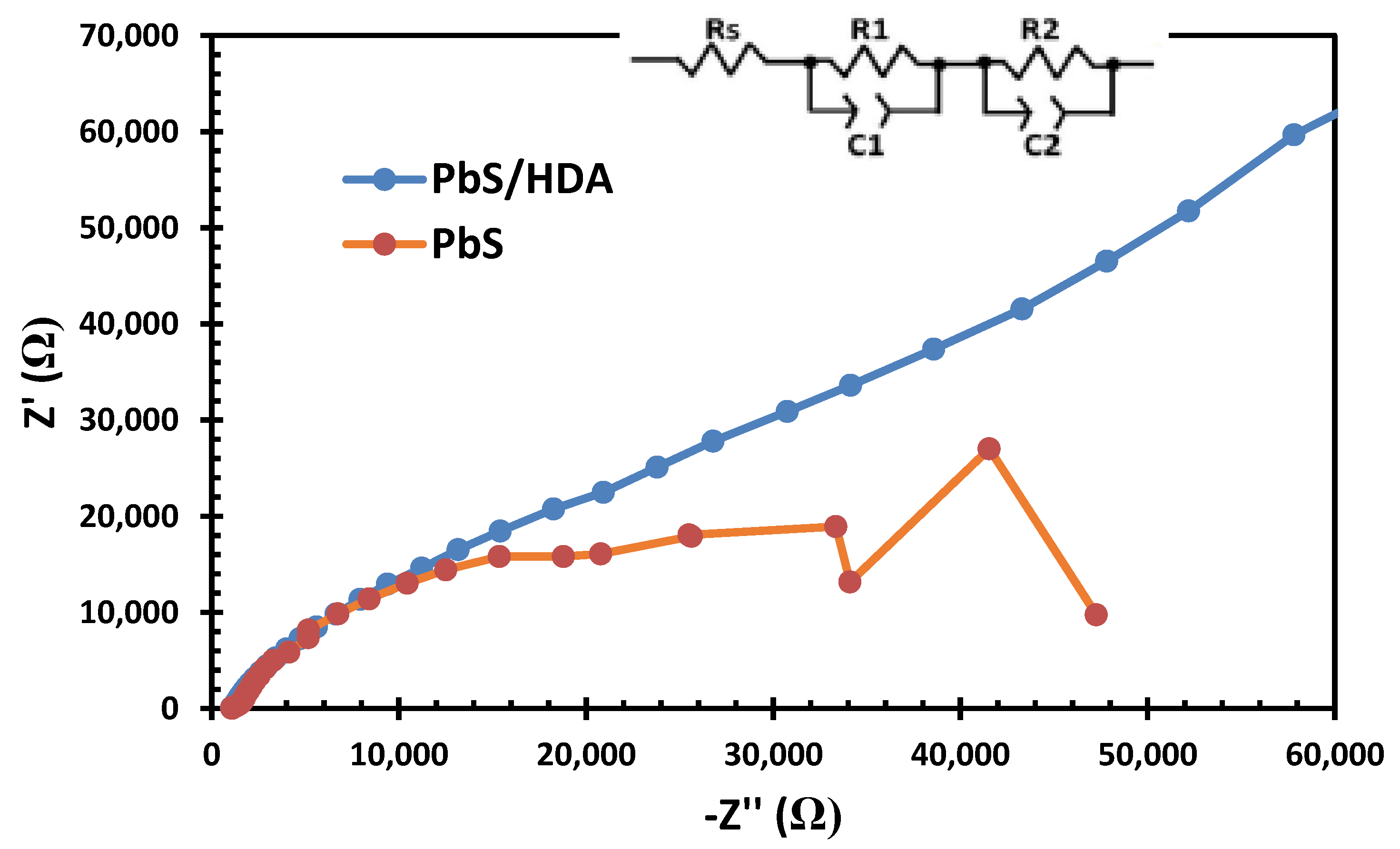
2.5. Electrochemical Impedance Spectroscopy Results
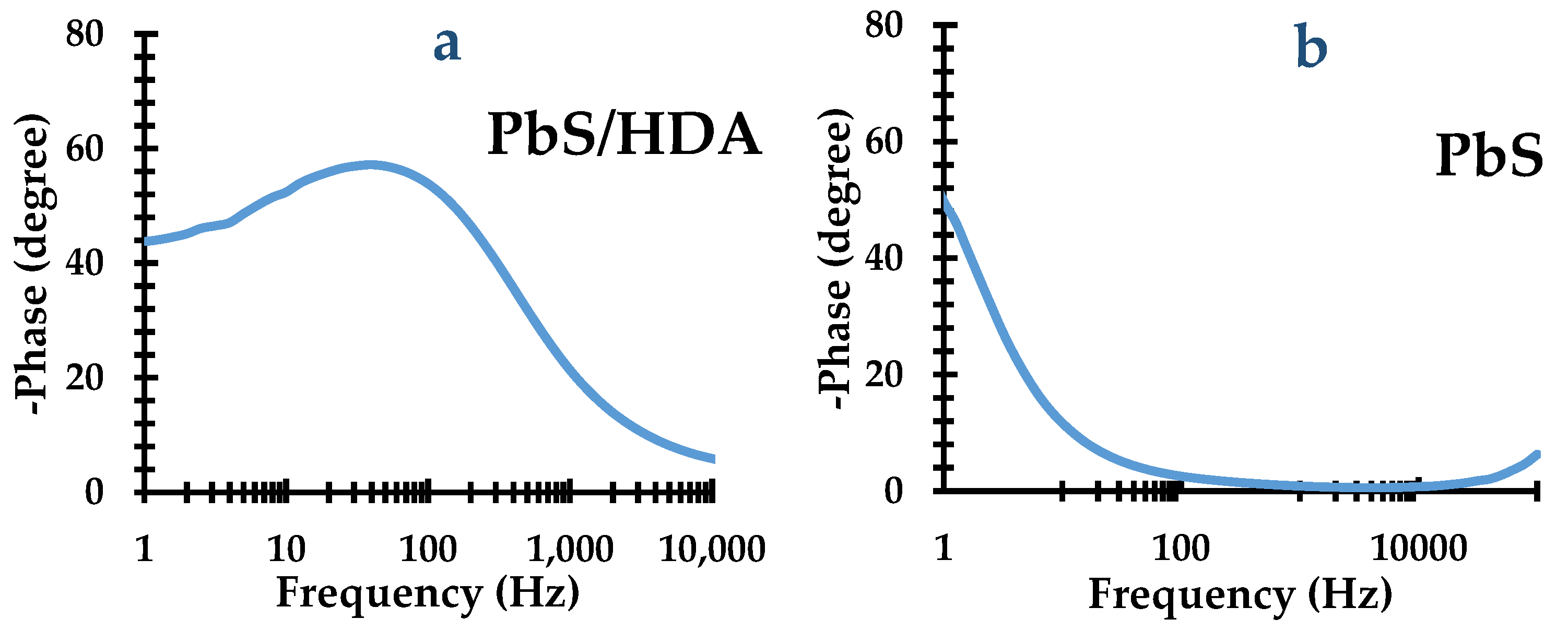
2.6. Bode Plot Results of Metal Sulfides Nanoparticles
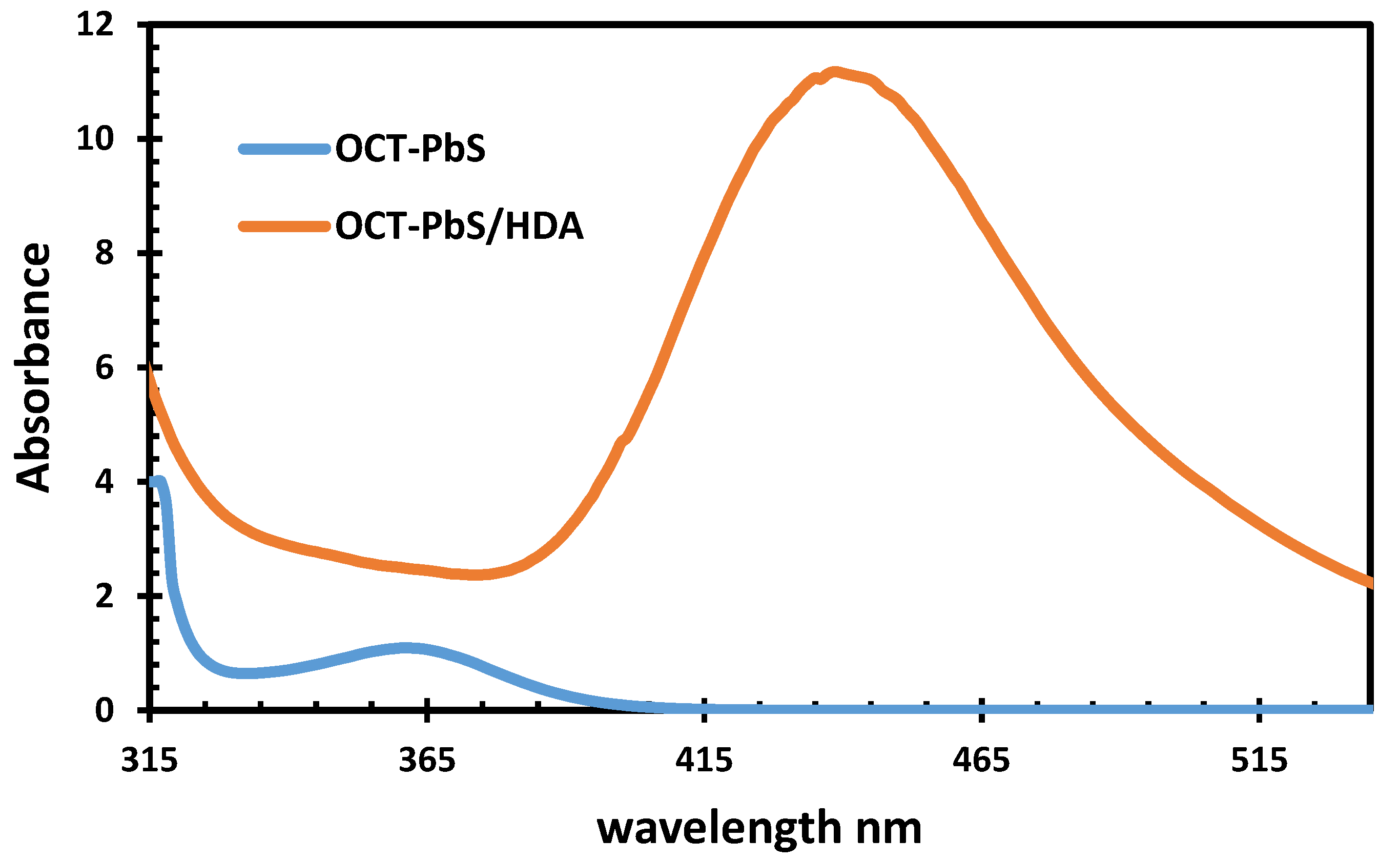
2.7. UV−Vis
2.8. J–V Results
3. Materials and Methods
3.1. Material
3.2. Synthesis of OCT-PbS/HDA and OCT-PbS Nanoparticles
3.3. Fabrication and Assembling of Solar Cells
3.4. Physical Measurements
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rath, T.; Trimmel, G. In situ syntheses of semiconducting nanoparticles in conjugated polymer matrices and their application in photovoltaics. Hybrid Mater. 2015, 1. [Google Scholar] [CrossRef][Green Version]
- Meyer, E.L.; Mbese, J.Z.; Agoro, M.A. The Frontiers of Nanomaterials (SnS, PbS and CuS) for Dye-Sensitized Solar Cell Applications: An Exciting New Infrared Material. Molecules 2019, 24, 4223. [Google Scholar] [CrossRef]
- Reynolds, L.X.; Lutz, T.; Dowland, S.; MacLachlan, A.; King, S.; Haque, S.A. Charge photogeneration in hybrid solar cells: A comparison between quantum dots and in situ grown CdS. Nanoscale 2012, 4, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Eck, M.; Men, C.; Rauscher, F.; Niyamakom, P.; Yilmaz, S.; Dumsch, I.; Allard, S.; Scherf, U.; Krüger, M. Efficient polymer nanocrystal hybrid solar cells by improved nanocrystal composition. Sol. Energy Mater. Sol. Cells 2011, 95, 3227–3232. [Google Scholar] [CrossRef]
- Luo, K.; Wu, W.; Xie, S.; Jiang, Y.; Liao, S.; Qin, D. Building Solar Cells from Nanocrystal Inks. Appl. Sci. 2019, 9, 1885. [Google Scholar] [CrossRef]
- Chen, H.C.; Lai, C.W.; Wu, I.C.; Pan, H.R.; Chen, I.W.; Peng, Y.K.; Liu, C.L.; Chen, C.H.; Chou, P.T. Enhanced performance and air stability of 3.2% hybrid solar cells: How the functional polymer and CdTe nanostructure boost the solar cell efficiency. Adv. Mater. 2011, 23, 5451–5455. [Google Scholar] [CrossRef]
- Rong, Z.; Guo, X.; Lian, S.; Liu, S.; Qin, D.; Mo, Y.; Xu, W.; Wu, H.; Zhao, H.; Hou, L. Interface Engineering for Both Cathode and Anode Enables Low-Cost Highly Efficient Solution-Processed CdTe Nanocrystal Solar Cells. Adv. Funct. Mater. 2019, 29, 1904018. [Google Scholar] [CrossRef]
- Dogan, S.; Bielewicz, T.; Lebedeva, V.; Klinke, C. Photovoltaic effect in individual asymmetrically contacted lead sulfide nanosheets. Nanoscale 2015, 7, 4875–4883. [Google Scholar] [CrossRef]
- Zhao, N.; Osedach, T.P.; Chan, L.Y.; Geyer, S.M.; Wanger, D.; Binda, M.T.; Arango, A.C.; Bawendi, M.G.; Bulovic, V. Colloidal PbS quantum dot solar cells with high fill factor. ACS Nano 2010, 4, 3743–3752. [Google Scholar] [CrossRef]
- Onwudiwe, D.C. Microwave-assisted synthesis of PbS nanostructures. Heliyon 2019, 5, e01413. [Google Scholar] [CrossRef]
- Davar, F.; Mohammadikish, M.; Loghman-Estarki, M.R.; Masteri-Farahani, M. Synthesis of micro-and nanosized PbS with different morphologies by the hydrothermal process. Ceram. Int. 2014, 40, 8143–8148. [Google Scholar] [CrossRef]
- Mamiyev, Z.Q.; Balayeva, N.O. Preparation and optical studies of PbS nanoparticles. Opt. Mater. 2015, 46, 522–525. [Google Scholar] [CrossRef]
- Goswami, S.K.; Oh, E. Morphology control and photovoltaic application of solvothermally synthesized PbS nanostructures. Mater. Lett. 2014, 117, 138–141. [Google Scholar]
- Zhao, Z.; Zhang, J.; Dong, F.; Yang, B. Supercrystal structures of polyhedral PbS nanocrystals. J. Colloid Interface Sci. 2011, 359, 351–358. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, K.; Zhang, J.; Yang, K.; He, C.; Dong, F.; Yang, B. Synthesis of size and shape-controlled PbS nanocrystals and their self-assembly. Colloids Surf. A Physicochem. Eng. Asp. 2010, 355, 114–120. [Google Scholar] [CrossRef]
- Kumar, D.; Agarwal, G.; Tripathi, B.; Vyas, D.; Kulshrestha, V. Characterization of PbS nanoparticles synthesized by chemical bath deposition. J. Alloys Compd. 2009, 484, 463–466. [Google Scholar] [CrossRef]
- Kowshik, M.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Microbial synthesis of semiconductor PbS nanocrystallites. Adv. Mater. 2002, 14, 815–818. [Google Scholar] [CrossRef]
- Zhengping, Q.; Yi, X.; Yingjie, Z.; Yitai, Q. Synthesis of PbS/poly (vinyl acetate) nanocomposites by γ-irradiation. Mater. Sci. Eng. B 2000, 77, 144–146. [Google Scholar] [CrossRef]
- Nyamen, L.D.; Pullabhotla, V.S.; Nejo, A.A.; Ndifon, P.; Revaprasadu, N. Heterocyclic dithiocarbamates: Precursors for shape-controlled growth of CdS nanoparticles. New J. Chem. 2011, 35, 1133–1139. [Google Scholar] [CrossRef]
- Nyamen, L.D.; Revaprasadu, N.; Pullabhotla, R.V.; Nejo, A.A.; Ndifon, P.T.; Malik, M.A.; O’Brien, P. Synthesis of multi-podal CdS nanostructures using heterocyclic dithiocarbamato complexes as precursors. Polyhedron 2013, 56, 62–70. [Google Scholar] [CrossRef]
- Kun, W.N.; Mlowe, S.; Nyamen, L.D.; Ndifon, P.T.; Malik, M.A.; Munro, O.Q.; Revaprasadu, N. Heterocyclic Bismuth(III) Dithiocarbamato Complexes as Single-Source Precursors for the Synthesis of Anisotropic Bi2S3 Nanoparticles. Chem. Eur. J. 2016, 22, 13127–13135. [Google Scholar] [CrossRef] [PubMed]
- Roffey, A.; Hollingsworth, N.; Islam, H.U.; Mercy, M.; Sankar, G.; Catlow, C.R.; Hogarth, G.; de Leeuw, N.H. Phase control during the synthesis of nickel sulfide nanoparticles from dithiocarbamate precursors. Nanoscale 2016, 8, 11067–11075. [Google Scholar] [CrossRef] [PubMed]
- Uflyand, I.E.; Dzhardimalieva, G.I. Nanomaterials Preparation by Thermolysis of Metal Chelates. In Thermolysis of Metal Chelates in Polymer Matrices; Springer: Berlin, Germany, 2018; pp. 425–458. ISBN 978-3-319-93404-4. [Google Scholar]
- Ficai, D.; Grumezescu, A.M. Dendrimers and dendronized materials as nanocarriers. In Nanostructures for Novel Therapy: Synthesis, Characterization and Applications; Elsevier: Amsterdam, The Netherlands, 2017; p. 429. ISBN 9780323461481. [Google Scholar]
- Dzhardimalieva, G.I.; Uflyand, I.E. Chalcogen-containing metal chelates as single-source precursors of nanostructured materials: Recent advances and future development. J. Coord. Chem. 2019, 72, 1425–1465. [Google Scholar] [CrossRef]
- Agoro, M.A.; Meyer, E.L.; Mbese, J.Z.; Manu, K. Electrochemical Fingerprint of CuS-Hexagonal Chemistry from (Bis(N-1,4-Phenyl-N-(4-Morpholinedithiocarbamato) Copper(II) Complexes) as Photon Absorber in Quantum-Dot/Dye-Sensitised Solar Cells. Catalysts 2020, 10, 300. [Google Scholar] [CrossRef]
- Mbese, J.Z.; Meyer, E.L.; Agoro, M.A. Electrochemical Performance of Photovoltaic Cells using HDA Capped-SnS Nanocrystal from bis (N-1,4-Phenyl-N-Morpho-Dithiocarbamato) Sn(II) Complexes. Nanomaterials 2020, 10, 414. [Google Scholar] [CrossRef]
- Moreno, O.P.; Pérez, R.G.; Merino, R.P.; Portillo, M.C.; Téllez, G.H.; Rosas, E.R. Optical and structural properties of PbSIn3+ nanocrystals grown by chemical bath. Thin Solid Films 2016, 616, 800–807. [Google Scholar] [CrossRef]
- Portillo, M.C.; Moreno, O.P.; Pérez, R.G.; Merino, R.P.; Juarez, H.S.; Cuapa, S.T.; Rosas, E.R. Characterization and growth of doped-PbS in situ with Bi3+, Cd2+ and Er3+ ions by chemical bath. Mater. Sci. Semicond. Process. 2017, 72, 22–31. [Google Scholar] [CrossRef]
- Dasgupta, N.P.; Lee, W.; Prinz, F.B. Atomic layer deposition of lead sulfide thin films for quantum confinement. Chem. Mater. 2009, 21, 3973–3978. [Google Scholar] [CrossRef]
- Portillo-Moreno, O.; Gutiérrez-Pérez, R.; Chávez Portillo, M.; Specia, M.; Hernández-Téllez, G.; Lazcano Hernández, M.; Moreno Rodríguez, A.; Palomino-Merino, R.; Rubio Rosas, E. Growth of doped PbS: Co2+ nanocrystals by Chemical Bath. Rev. Mex. Fis. 2016, 62, 456–460. [Google Scholar]
- Palomino Merino, R.; Gutiérrez Pérez, R.; Trejo García, P.; Chaltel Lima, L.; Portillo Moreno, O.; Araiza García, M.E.; Moreno Rodriguez, A.; Rubio Rosas, E. Influence of L-Tryptophan on Growth and Optical Properties of PbS Nanocrystalline Thin Films. J. Nanomater. 2018, 3431942. [Google Scholar] [CrossRef]
- Dong, Y.; Wen, J.; Pang, F.; Luo, Y.; Peng, G.D.; Chen, Z.; Wang, T. Formation and photoluminescence property of PbS quantum dots in silica optical fiber based on atomic layer deposition. Opt. Mater. Express. 2015, 5, 712–719. [Google Scholar] [CrossRef]
- Khan, A.H.; Maji, S.; Chakraborty, S.; Manik, N.B.; Acharya, S. Multidimensional self-assembly of peanut shaped PbS nanostructures. RSC Adv. 2012, 2, 186–191. [Google Scholar] [CrossRef]
- Saraidarov, T.; Gevorgian, A.; Reisfeld, R.; Sashchiuk, A.; Bashouti, M.; Lifshitz, E. Synthesis, structural and electrical characterization of PbS NCs in titania sol–gel films. J. Sol-Gel Sci. Technol. 2007, 44, 87–95. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.; Zhao, J.; Gao, J.; Hagfeldt, A.; Sun, L. Efficient organic dye sensitized solar cells based on modified sulfide/polysulfide electrolyte. J. Mater. Chem. 2011, 21, 5573–5575. [Google Scholar] [CrossRef]
- Lee, Y.L.; Chang, C.H. Efficient polysulfide electrolyte for CdS quantum dot-sensitized solar cells. J. Power Sources 2008, 185, 584–588. [Google Scholar] [CrossRef]
- Radich, J.G.; Dwyer, R.; Kamat, P.V. Cu2S reduced graphene oxide composite for high-efficiency quantum dot solar cells. Overcoming the redox limitations of S2–/Sn2– at the counter electrode. J. Phys. Chem. Lett. 2011, 2, 2453–2460. [Google Scholar] [CrossRef]
- Gopi, C.V.; Bae, J.H.; Venkata-Haritha, M.; Kim, S.K.; Lee, Y.S.; Sarat, G.; Kim, H.J. One-step synthesis of solution processed time-dependent highly efficient and stable PbS counter electrodes for quantum dot-sensitized solar cells. RSC Adv. 2015, 5, 107522–107532. [Google Scholar] [CrossRef]
- Han, L.; Koide, N.; Chiba, Y.; Islam, A.; Komiya, R.; Fuke, N.; Fukui, A.; Yamanaka, R. Improvement of efficiency of dye-sensitized solar cells by reduction of internal resistance. Appl. Phys. Lett. 2005, 86, 213501. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Tao, Q.; Fu, W.; Yang, H.; Su, S.; Mu, Y.; Zhou, L.; Li, M. High catalytic activity of a PbS counter electrode prepared via chemical bath deposition for quantum dots-sensitized solar cells. RSC Adv. 2015, 5, 1835–1840. [Google Scholar] [CrossRef]
- Punnoose, D.; Suh, S.M.; Kim, B.J.; Kumar, C.S.P.; Rao, S.S.; Thulasi-Varma, C.V.; Reddy, A.E.; Chung, S.H.; Kim, H.J. The influence of in situ deposition techniques on PbS seeded CdS/CdSe for enhancing the photovoltaic performance of quantum dot sensitized solar cells. J. Electroanal. Chem. 2016, 773, 27–38. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Ghanbari, D.; Loghman-Estarki, M.R. Star-shaped PbS nanocrystals prepared by hydrothermal process in the presence of thioglycolic acid. Polyhedron 2012, 35, 149–153. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Pal, A.; Chatterjee, K.; Rana, T.H.; Bhattacharya, G.; Roy, S.S.; Chowdhury, P.; Sharma, G.D.; Biswas, S. Significant enhancement of power conversion efficiency of dye-sensitized solar cells by the incorporation of TiO2–Au nanocomposite in TiO2 photoanode. J. Mater. Sci. 2018, 53, 8460–8473. [Google Scholar]
- Bhardwaj, S.; Pal, A.; Chatterjee, K.; Rana, T.H.; Bhattacharya, G.; Roy, S.S.; Chowdhury, P.; Sharma, G.D.; Biswas, S. Enhanced efficiency of PbS quantum dot-sensitized solar cells using plasmonic photoanode. J. Nanopart. Res. 2018, 20, 198. [Google Scholar] [CrossRef]
- Tachan, Z.; Shalom, M.; Hod, I.; Ruhle, S.; Tirosh, S.; Zaban, A. PbS as a highly catalytic counter electrode for polysulfide-based quantum dot solar cells. J. Phys. Chem. Lett. 2011, 115, 6162–6166. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.W.; Gopi, C.V.; Kim, S.K.; Rao, S.S.; Jeong, M.S. Improved performance of quantum dot-sensitized solar cells adopting a highly efficient cobalt sulfide/nickel sulfide composite thin film counter electrode. J. Power Sources 2014, 268, 163–170. [Google Scholar] [CrossRef]
- Mbese, J.Z.; Ajibade, P.A.; Matebese, F.; Agoro, M.A. Optical and Structural Properties of TOPO/HDA Capped Cus Nanocrystals via Thermal Decomposition of Bis(N-Diisopropyldithiocarbamate) Cu(II) Complex. J. Nano Res. 2019, 59, 161–165. [Google Scholar] [CrossRef]
- Meyer, E.L.; Mbese, J.Z.; Agoro, M.A.; Taziwa, T. Optical and structural-chemistry of SnS nanocrystals prepared by thermal decomposition of bis(N-di-isopropyl-N-octyldithiocarbamato)tin(II) complex for promising materials in solar cell applications. Opt. Quant. Electron. 2020, 52, 2–11. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Dye | Photoanode | Electrolyte | CEs | JSC (mA/cm2) | VOC (V) | FF | η (%) |
|---|---|---|---|---|---|---|---|
| OCT-PbS/HDA | TiO2 | HI-30 | Pt | 11 | 0.52 | 0.33 | 1.89 |
| OCT-PbS | TiO2 | HI-30 | Pt | 2.4 | 0.48 | 0.74 | 0.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agoro, M.A.; Mbese, J.Z.; Meyer, E.L. Electrochemistry of Inorganic OCT-PbS/HDA and OCT-PbS Photosensitizers Thermalized from Bis(N-diisopropyl-N-octyldithiocarbamato) Pb(II) Molecular Precursors. Molecules 2020, 25, 1919. https://doi.org/10.3390/molecules25081919
Agoro MA, Mbese JZ, Meyer EL. Electrochemistry of Inorganic OCT-PbS/HDA and OCT-PbS Photosensitizers Thermalized from Bis(N-diisopropyl-N-octyldithiocarbamato) Pb(II) Molecular Precursors. Molecules. 2020; 25(8):1919. https://doi.org/10.3390/molecules25081919
Chicago/Turabian StyleAgoro, Mojeed A., Johannes Z. Mbese, and Edson L. Meyer. 2020. "Electrochemistry of Inorganic OCT-PbS/HDA and OCT-PbS Photosensitizers Thermalized from Bis(N-diisopropyl-N-octyldithiocarbamato) Pb(II) Molecular Precursors" Molecules 25, no. 8: 1919. https://doi.org/10.3390/molecules25081919
APA StyleAgoro, M. A., Mbese, J. Z., & Meyer, E. L. (2020). Electrochemistry of Inorganic OCT-PbS/HDA and OCT-PbS Photosensitizers Thermalized from Bis(N-diisopropyl-N-octyldithiocarbamato) Pb(II) Molecular Precursors. Molecules, 25(8), 1919. https://doi.org/10.3390/molecules25081919







