New Acidic Precursor and Acetone-Based Solvent for Fast Perovskite Processing via Proton-Exchange Reaction with Methylamine
Abstract
1. Introduction
2. Results and Discussion
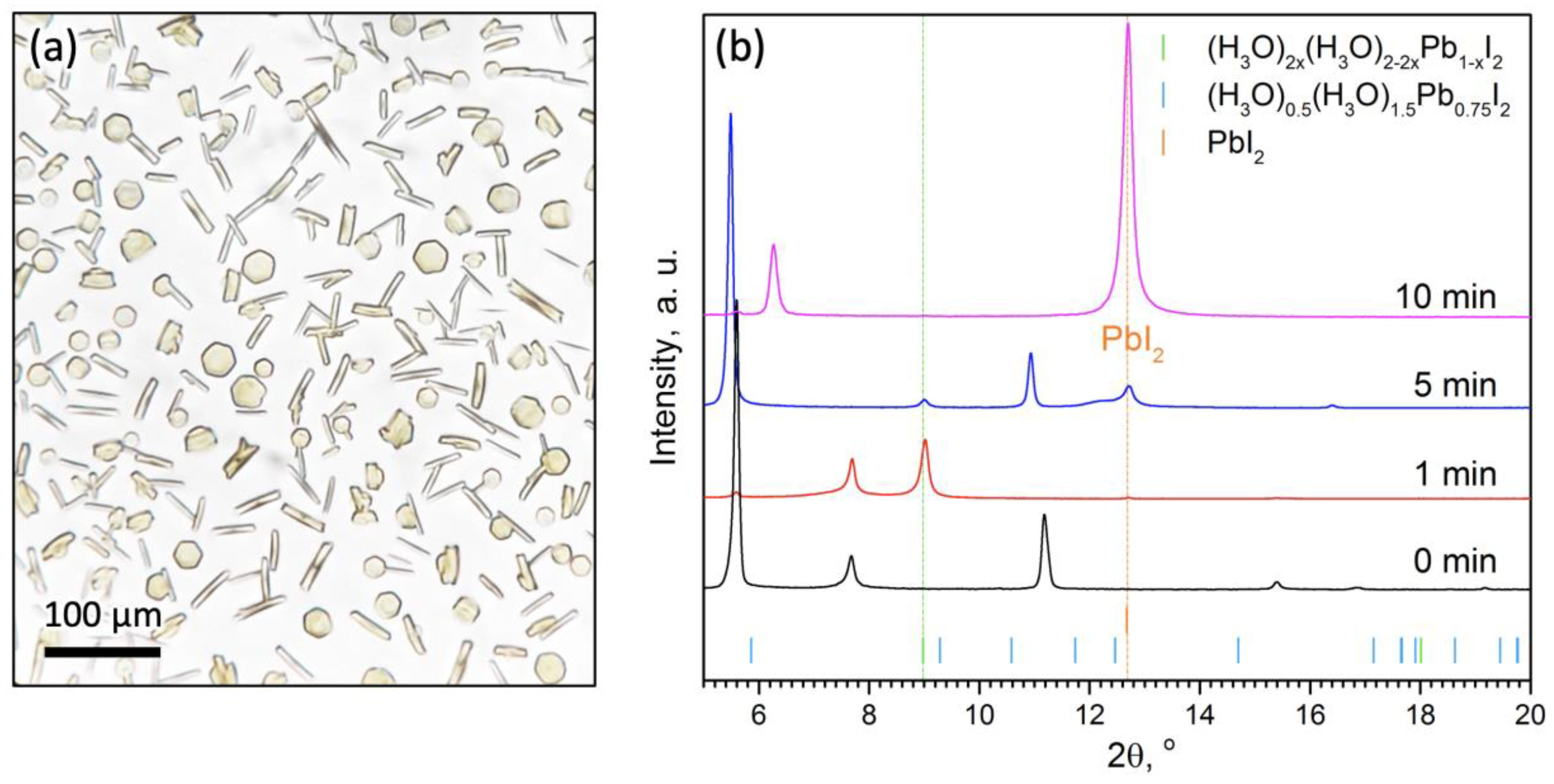
2.1. Solvation and Crystallization from Acetone–HI(aq.) System
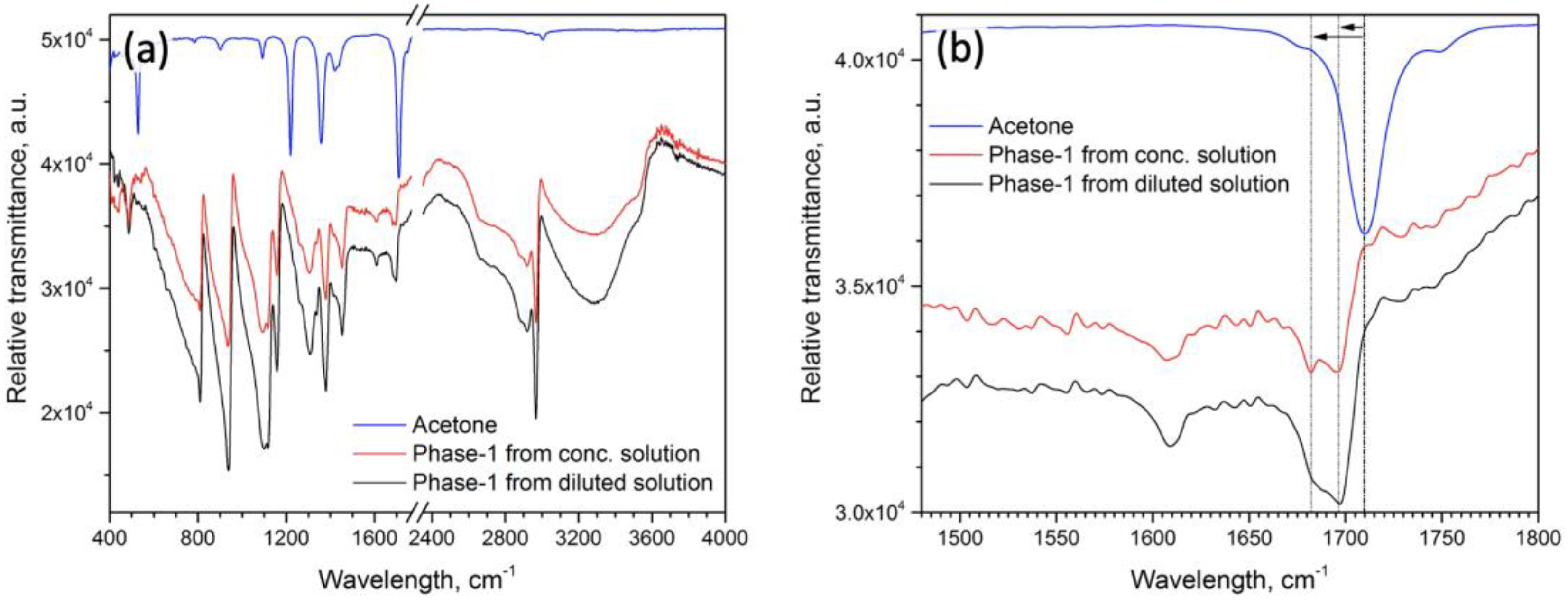
2.2. Characterization of the Phase-1
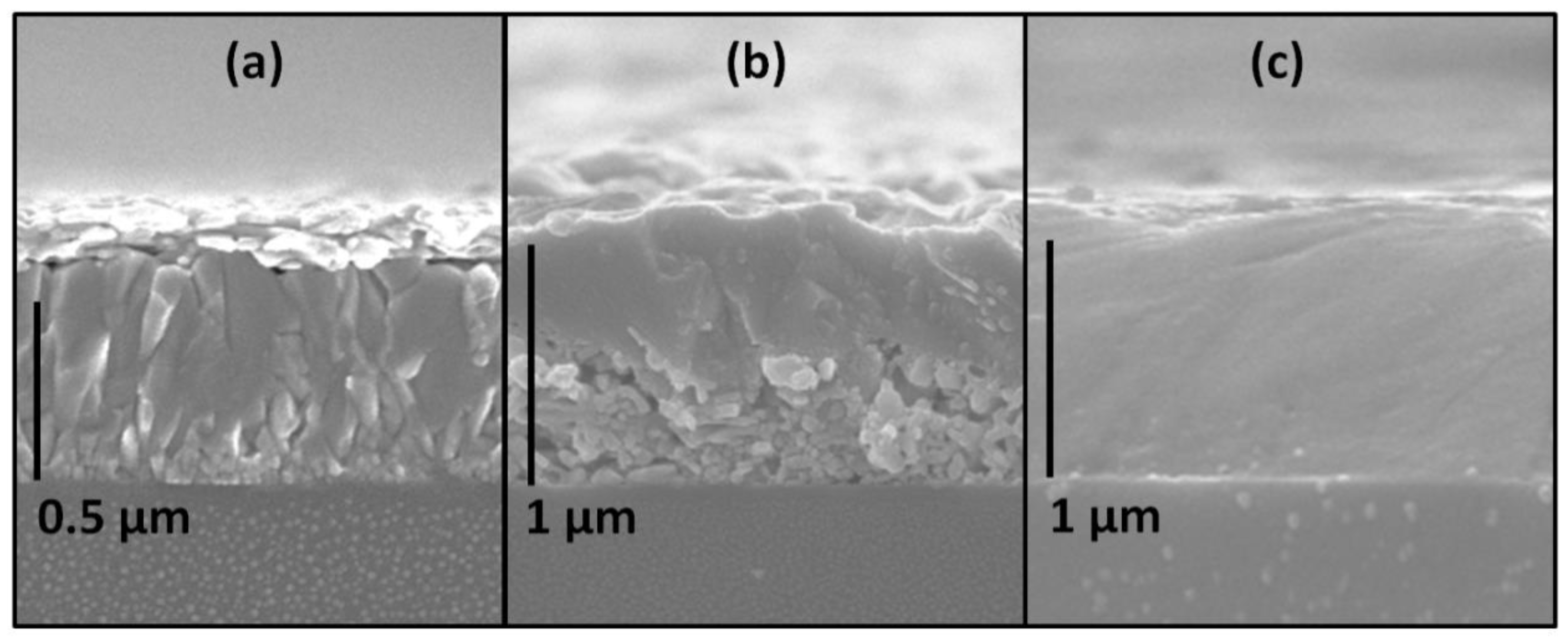
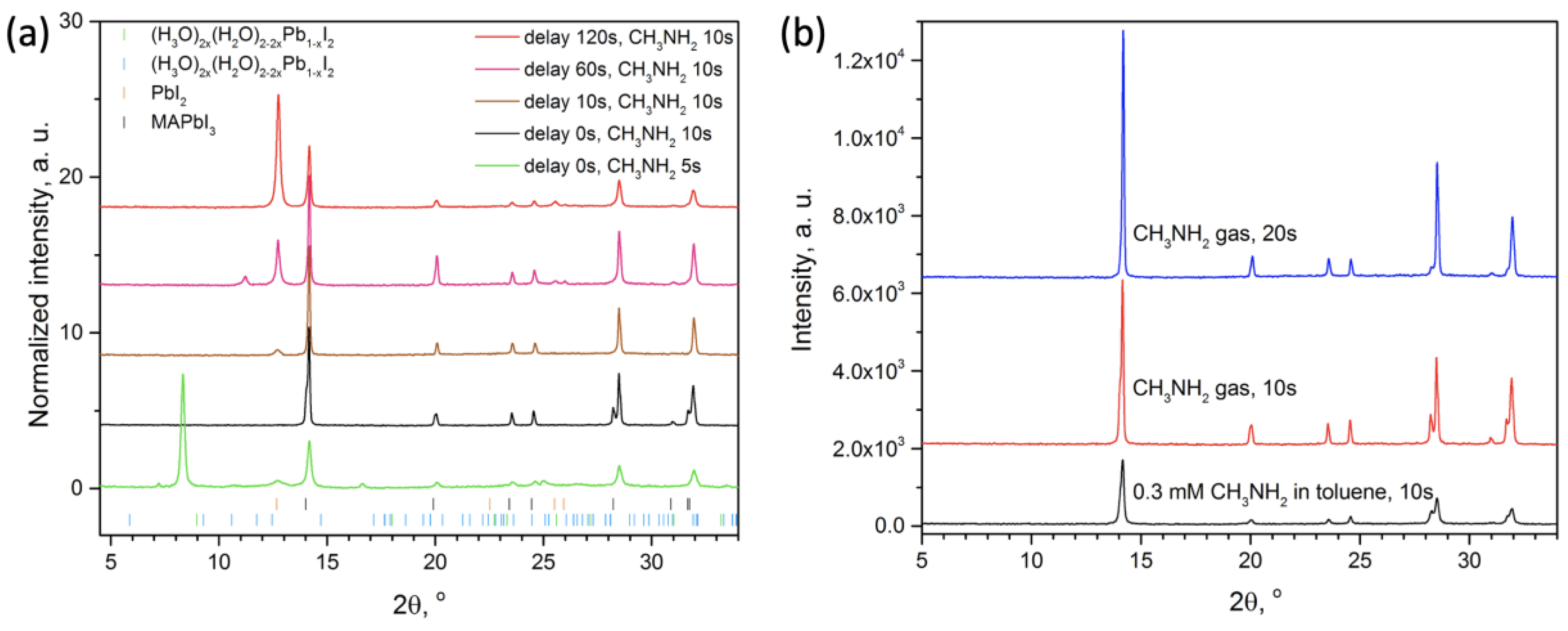
2.3. Perovskite Films Processing
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- NREL Best-Research Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html. (accessed on 6 April 2020).
- Dai, X.; Deng, Y.; Van Brackle, C.H.; Chen, S.; Rudd, P.N.; Xiao, X.; Lin, Y.; Chen, B.; Huang, J. Scalable Fabrication of Efficient Perovskite Solar Modules on Flexible Glass Substrates. Adv. Energy Mater. 2020, 10, 1903108. [Google Scholar] [CrossRef]
- Nagabhushana, G.P.; Shivaramaiah, R.; Navrotsky, A. Direct calorimetric verification of thermodynamic instability of lead halide hybrid perovskites. Proc. Natl. Acad. Sci. USA 2016, 113, 7717–7721. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.L.; Steparuk, A.S.; Bolyachkina, M.S.; Tsvetkov, D.S.; Safronov, A.P.; Zuev, A.Y. Thermodynamics of formation of hybrid perovskite-type methylammonium lead halides. J. Chem. Thermodyn. 2018, 116, 253–258. [Google Scholar] [CrossRef]
- Hsieh, T.Y.; Huang, C.K.; Su, T.-S.; Hong, C.-Y.; Wei, T.-C. Crystal Growth and Dissolution of Methylammonium Lead Iodide Perovskite in Sequential Deposition: Correlation between Morphology Evolution and Photovoltaic Performance. ACS Appl. Mater. Interfaces 2017, 9, 8623–8633. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, H.; Xu, H.; Zhao, N. HPbI3: A new precursor compound for highly efficient solution-processed perovskite solar cells. Adv. Funct. Mater. 2015, 25, 1120–1126. [Google Scholar] [CrossRef]
- Zhang, T.; Dar, M.I.; Li, G.; Xu, F.; Guo, N.; Grätzel, M.; Zhao, Y. Bication lead iodide 2D perovskite component to stabilize inorganic α-CsPbI3 perovskite phase for high-efficiency solar cells. Sci. Adv. 2017, 3, e1700841. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Padture, N.P. Gas-Induced Formation/Transformation of Organic–Inorganic Halide Perovskites. ACS Energy Lett. 2017, 2, 2166–2176. [Google Scholar] [CrossRef]
- Long, M.; Zhang, T.; Chai, Y.; Ng, C.-F.; Mak, T.C.W.; Xu, J.; Yan, K. Nonstoichiometric acid–base reaction as reliable synthetic route to highly stable CH3NH3PbI3 perovskite film. Nat. Commun. 2016, 7, 13503. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Zhou, Y.; Wang, Z.; Yang, M.; Krause, A.R.; Zhou, Z.; Zhu, K.; Padture, N.P.; Cui, G. Transformative Evolution of Organolead Triiodide Perovskite Thin Films from Strong Room-Temperature Solid-Gas Interaction between HPbI3-CH3NH2 Precursor Pair. J. Am. Chem. Soc. 2016, 138, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Eppel, S.; Fridman, N.; Frey, G. Amide-Templated Iodoplumbates: Extending Lead-Iodide Based Hybrid Semiconductors. Cryst. Growth Des. 2015, 15, 4363–4371. [Google Scholar] [CrossRef]
- Ke, W.; Spanopoulos, I.; Stoumpos, C.C.; Kanatzidis, M.G. Myths and reality of HPbI3 in halide perovskite solar cells. Nat. Commun. 2018, 9, 4785. [Google Scholar] [CrossRef] [PubMed]
- Daub, M.; Hillebrecht, H. On the Demystification of “HPbI3” and the Peculiarities of the Non-innocent Solvents H2O and DMF. Z. Anorg. Allg. Chem. 2018, 644, 1393–1400. [Google Scholar] [CrossRef]
- Lee, M.V.; Raga, S.R.; Kato, Y.; Leyden, M.R.; Ono, L.K.; Wang, S.; Qi, Y. Transamidation of dimethylformamide during alkylammonium lead triiodide film formation for perovskite solar cells. J. Mater. Res. 2017, 32, 45–55. [Google Scholar] [CrossRef]
- Noel, N.K.; Habisreutinger, S.N.; Wenger, B.; Klug, M.T.; Hörantner, M.T.; Johnston, M.B.; Nicholas, R.J.; Moore, D.T.; Snaith, H.J. A low viscosity, low boiling point, clean solvent system for the rapid crystallisation of highly specular perovskite films. Energy Environ. Sci. 2017, 10, 145–152. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Wang, Z.; Li, Z.; Fan, Y.; Meng, H.; Liu, R.; Wang, Y.; Hagfeldt, A.; Cui, G.; Pang, S. A Scalable Methylamine Gas Healing Strategy for High-Efficiency Inorganic Perovskite Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 5587–5591. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Fateev, S.; I. Marchenko, E.; A. Petrov, A.; A. Goodilin, E.; B. Tarasov, A. New Acidic Precursor and Acetone-Based Solvent for Fast Perovskite Processing via Proton-Exchange Reaction with Methylamine. Molecules 2020, 25, 1856. https://doi.org/10.3390/molecules25081856
A. Fateev S, I. Marchenko E, A. Petrov A, A. Goodilin E, B. Tarasov A. New Acidic Precursor and Acetone-Based Solvent for Fast Perovskite Processing via Proton-Exchange Reaction with Methylamine. Molecules. 2020; 25(8):1856. https://doi.org/10.3390/molecules25081856
Chicago/Turabian StyleA. Fateev, Sergey, Ekaterina I. Marchenko, Andrey A. Petrov, Eugene A. Goodilin, and Alexey B. Tarasov. 2020. "New Acidic Precursor and Acetone-Based Solvent for Fast Perovskite Processing via Proton-Exchange Reaction with Methylamine" Molecules 25, no. 8: 1856. https://doi.org/10.3390/molecules25081856
APA StyleA. Fateev, S., I. Marchenko, E., A. Petrov, A., A. Goodilin, E., & B. Tarasov, A. (2020). New Acidic Precursor and Acetone-Based Solvent for Fast Perovskite Processing via Proton-Exchange Reaction with Methylamine. Molecules, 25(8), 1856. https://doi.org/10.3390/molecules25081856






