Anti-Virulence Potential and In Vivo Toxicity of Persicaria maculosa and Bistorta officinalis Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Antibacterial Effect of the Extracts
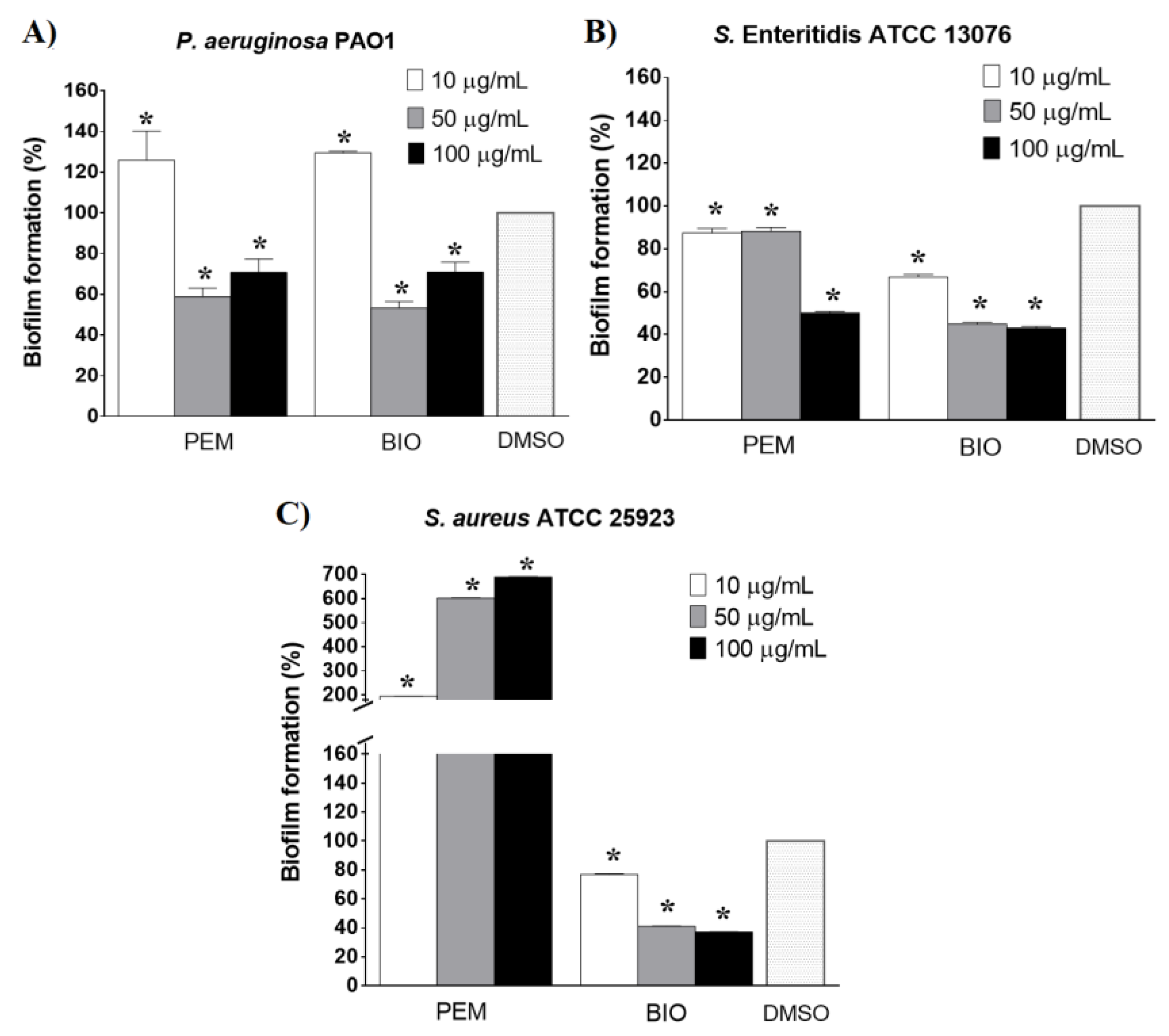
2.2. Antibiofilm Activity
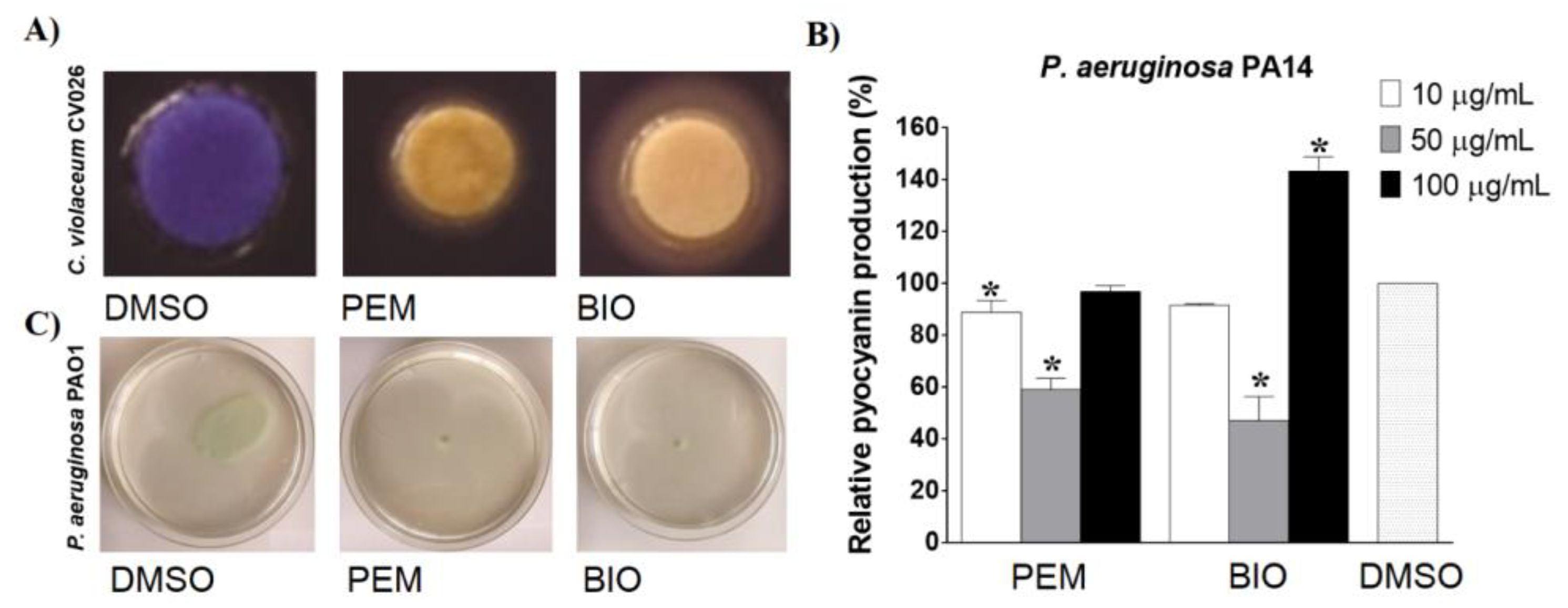
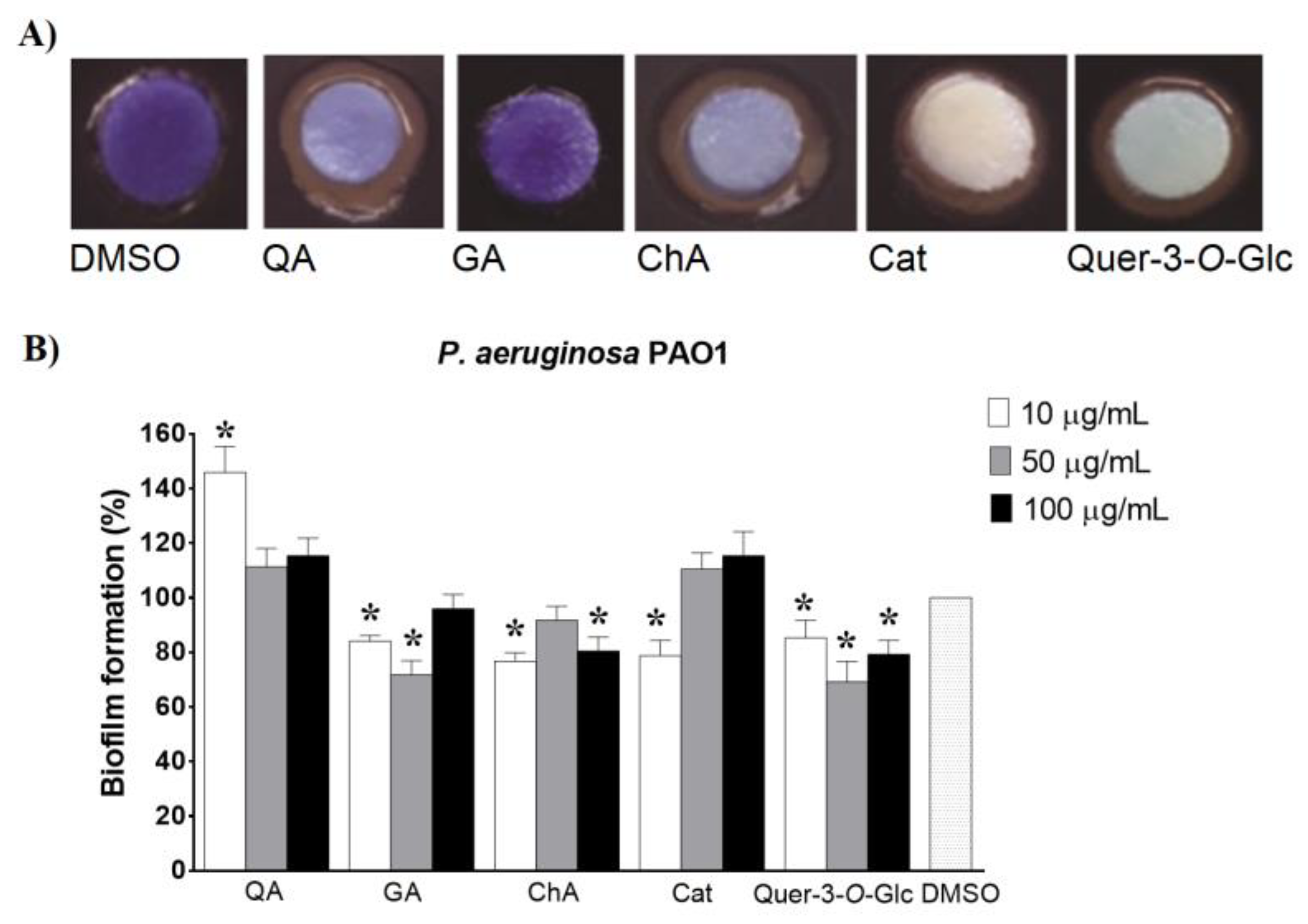
2.3. Detection of Anti-QS Activity of the Extracts and Their Effects on Selected Virulence Factors in Gram-Negative Bacteria
2.4. In Vitro Cytotoxicity Assessment
2.5. Identification of Compounds in the Extracts
2.6. Identification of QS Signalling Pathways Affected by Extracts and Selected Phenolic Components
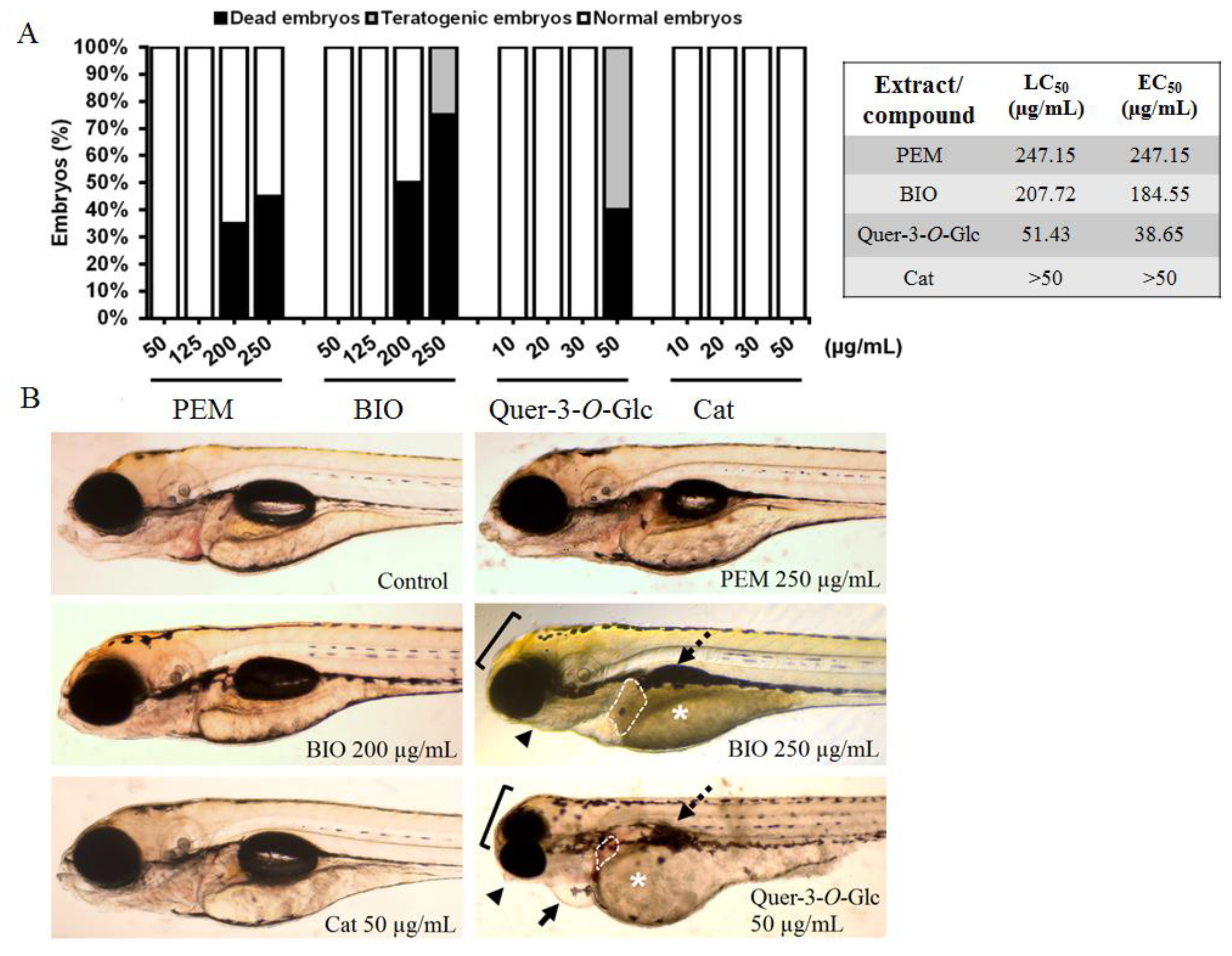
2.7. Toxicity Assessment in the Zebrafish Model
3. Materials and Methods
3.1. Chemicals and Media
3.2. Plant Material, Extract Preparation and Chemical Analysis
3.3. Microbial Strains and Growth Conditions
3.4. Antibacterial Susceptibility Test for Planktonic Cells
3.5. Quorum Sensing Inhibition Assays
3.5.1. Static Biofilm Formation Inhibition Assay
3.5.2. Chromobacterium violaceum Assay
3.5.3. Pyocyanin Production
3.5.4. Swarming Assay
3.5.5. Interference of Extracts and Components with QS Pathways
3.6. Cytotoxicity Assay
3.7. In Vivo Toxicity Evaluation
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Balcázar, J.L.; Subirats, J.; Borrego, C.M. The role of biofilms as environmental reservoirs of antibiotic resistance. Front Microbiol. 2015, 6, 1216. [Google Scholar] [CrossRef]
- Borges, A.; Serra, S.; Cristina Abreu, A.; Saavedra, M.J.; Salgado, A.; Simões, M. Evaluation of the effects of selected phytochemicals on quorum sensing inhibition and in vitro cytotoxicity. Biofouling 2014, 30, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Saavedra, M.J.; Simões, M. Antibiofilm activity of plant molecules against Pseudomonas aeruginosa and Staphylococcus aureus. Planta Med. 2014, 80, P1L120. [Google Scholar] [CrossRef]
- Nkuété, A.H.; Migliolo, L.; Wabo, H.K.; Tane, P.; Franco, O.L. Evaluation of multiple functions of Polygonum genus compounds. Eur. J. Med. Plants 2015, 6, 1. [Google Scholar] [CrossRef]
- Liu, X.Q.; Hua, H.M.; Liu, J.; Chen, F.K.; Wu, L.J. A new tannin-related compound from the rhizome of Polygonum bistorta L. J. Asian Nat. Prod. Res. 2006, 8, 299–302. [Google Scholar] [CrossRef]
- Derita, M.; Zacchino, S. Validation of the ethnopharmacological use of Polygonum persicaria for its antifungal properties. Nat. Prod. Commun. 2011, 6. [Google Scholar] [CrossRef]
- Demiray, S.; Pintado, M.; Castro, P. Evaluation of phenolic profiles and antioxidant activities of Turkish medicinal plants: Tilia argentea, Crataegi folium leaves and Polygonum bistorta roots. World Acad. Sci. Eng. Technol. 2009, 54, 312–317. [Google Scholar]
- Paoletti, M.G.; Dreon, A.; Lorenzoni, G. Pistic, traditional food from western Friuli, NE Italy. Econ. Bot. 1995, 49, 26–30. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Li, X.; Wang, B.; Cao, S.; Zhang, J.; Zhang, H.; Zhao, Y.; Ni, J. Pharmacological and other Bioactivities of the Genus Polygonum—A Review. Trop. J. Pharm. Res. 2014, 13, 1749–1759. [Google Scholar]
- Satish, S.; Mohana, D.; Raghavendra, M.; BABU, S.; Raveesha, K. Antibacterial evaluation of some Iranian medicinal plants against some human pathogenic bacteria. Asian J. Microbiol. Biotech. Environ. Sci. 2009, 11, 735–738. [Google Scholar]
- Hussain, F.; Ahmad, B.; Hameed, I.; Dastagir, G.; Sanaullah, P.; Azam, S. Antibacterial, antifungal and insecticidal activities of some selected medicinal plants of Polygonaceae. Afr. J. Biotechnol. 2010, 9, 5032–5036. [Google Scholar]
- Khalid, A.; Waseem, A.; Saadullah, M.; Khiljee, S.; Sethi, A.; Asad, M.H.H.B.; Rasool, F.; Waqas, M.K.; Murtaza, G. Antibacterial activity analysis of extracts of various plants against gram-positive and-negative bacteria. Afr. J. Pharm. Pharmacol. 2011, 5, 887–893. [Google Scholar]
- Cecotti, R.; Carpana, E.; Falchero, L.; Paoletti, R.; Tava, A. Determination of the volatile fraction of Polygonum bistorta L. at different growing stages and evaluation of its antimicrobial activity against two major honeybee (Apis mellifera) pathogens. Chem. Biodivers. 2012, 9, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Magrini, N.; Kahlmeter, G.; Singh, N. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017; Volume 27. [Google Scholar]
- Duan, K.; Surette, M.G. Environmental regulation of Pseudomonas aeruginosa PAO1 Las and Rhl quorum-sensing systems. J. Bacteriol. 2007, 189, 4827–4836. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.J.; Forbes, A.; Perkins, A.V.; Davey, A.K.; Chess-Williams, R.; Kiefel, M.J.; Arora, D.; et al. Cellular effects of pyocyanin, a secreted virulence factor of Pseudomonas aeruginosa. Toxins 2016, 8, 236. [Google Scholar] [CrossRef]
- Rada, B.; Leto, T.L. Pyocyanin effects on respiratory epithelium: Relevance in Pseudomonas aeruginosa airway infections. Trends microbiol. 2013, 21, 73–81. [Google Scholar] [CrossRef]
- Rossi, E.; Paroni, M.; Landini, P. Biofilm and motility in response to environmental and host-related signals in Gram negative opportunistic pathogens. J. Appl. Microbiol. 2018, 125, 1587–1602. [Google Scholar] [CrossRef]
- Annapoorani, A.; Umamageswaran, V.; Parameswari, R.; Pandian, S.K.; Ravi, A.V. Computational discovery of putative quorum sensing inhibitors against LasR and RhlR receptor proteins of Pseudomonas aeruginosa. J. Comput. Aided Mol. Des. 2012, 26, 1067–1077. [Google Scholar] [CrossRef]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; El Jaziri, M.; Baucher, M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.; Subramaniam, S.; Christena, L.R.; Muthuraman, M.S.; Subramanian, N.S.; Pemiah, B.; Sivasubramanian, A. Antimicrobial flavonoids isolated from Indian medicinal plant Scutellaria oblonga inhibit biofilms formed by common food pathogens. Nat. Prod. Res. 2016, 30, 2002–2006. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, I.; Ristivojevic, P.; Pavic, A.; Radojević, I.; Čomić, L.R.; Vasiljevic, B.; Opsenica, D.; Milojković-Opsenica, D.; Senerovic, L. Anti-quorum sensing activity, toxicity in zebrafish (Danio rerio) embryos and phytochemical characterization of Trapa natans leaf extracts. J. Ethnopharmacol. 2018, 222, 148–158. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Barros, T.P.; Alderton, W.K.; Reynolds, H.M.; Roach, A.G.; Berghmans, S. Zebrafish: An emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br. J. Pharmacol. 2008, 154, 1400–1413. [Google Scholar] [CrossRef]
- Orčić, D.; Francišković, M.; Bekvalac, K.; Svirčev, E.; Beara, I.; Lesjak, M.; Mimica-Dukić, N. Quantitative Determination of Plant Phenolics in Urtica dioica Extracts by High-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometric Detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef]
- Wikler, M.A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard; CLSI (NCCLS): Wayne, PA, USA, 2006; Volume 26, pp. M7–A7. [Google Scholar]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and analyzing static biofilms. Curr Protoc. Microbiol. 2011, 22, 1B.1.1–1B.1.18. [Google Scholar] [CrossRef]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef]
- O’Loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Drescher, K.; Semmelhack, M.F.; Bassler, B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar] [CrossRef]
- Ha, D.G.; Kuchma, S.L.; O’Toole, G.A. Plate-based assay for swarming motility in Pseudomonas aeruginosa. Methods Mol. Biol. 2014, 1149, 67–72. [Google Scholar]
- Fletcher, M.P.; Diggle, S.P.; Crusz, S.A.; Chhabra, S.R.; Camara, M.; Williams, P. A dual biosensor for 2-alkyl-4-quinolone quorum-sensing signal molecules. Environ. Microbiol. 2007, 9, 2683–2693. [Google Scholar] [CrossRef]
- Massai, F.; Imperi, F.; Quattrucci, S.; Zennaro, E.; Visca, P.; Leoni, L. A multitask biosensor for micro-volumetric detection of N-3-oxo-dodecanoyl-homoserine lactone quorum sensing signal. Biosens. Bioelectron. 2011, 26, 3444–3449. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Srdić-Rajić, T.; Svirčev, E.; Jasnić, N.; Nikolić, B.; Bojić, S.; Stević, T.; Knežević-Vukčević, J.; Mitić-Ćulafić, D. Evaluation of anticancer and antimicrobial activities of the Polygonum maritimum ethanol extract. Arch. Biol. Sci. 2018, 70, 665–673. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemicals, Test No. 236; Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013. [Google Scholar]
- Pavic, A.; Glišić, B.Đ.; Vojnovic, S.; Warżajtis, B.; Savić, N.D.; Antić, M.; Radenković, S.; Janjić, G.V.; Nikodinovic-Runic, J.; Rychlewska, U. Mononuclear gold (III) complexes with phenanthroline ligands as efficient inhibitors of angiogenesis: A comparative study with auranofin and sunitinib. J. Inorg. Biochem. 2017, 174, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Pavic, A.; Mitic-Culafic, D.; Jasnic, N.; Nikolic, B.; Simin, N.; Vasiljevic, B.; Knezevic-Vukcevic, J. Wild edible onions—Allium flavum and Allium carinatum—successfully prevent adverse effects of chemotherapeutic drug doxorubicin. Biomed. Pharmacother. 2019, 109, 2482–2491. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Compound | Content (mg/g dw) a | |
|---|---|---|
| PEM | BIO | |
| Phenolic acids | ||
| Quinic acid | 12.8 ± 1.28 | 1.14 ± 0.11 |
| Gallic acid | 2.47 ± 0.22 | 0.97 ± 1.69 |
| Chlorogenic acid | 6.41 ± 0.32 | 33.89 ± 1.69 |
| Protocatechuic acid | 0.21 ± 0.02 | 0.01 ± 0.00 |
| 2,5-dihydroxybenzoic acid | 0.01 ± 0.00 | Nd |
| p-hydroxybenzoic acid | 0.04 ± 0.00 | Nd |
| Caffeic acid | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Syringic acid | 0.01 ± 0.00 | Nd |
| Coumaric acid | 0.06 ± 0.01 | Nd |
| Ferulic acid | 0.05 ± 0.01 | Nd |
| Flavonoids | ||
| Catechin | 0.19 ± 0.02 | 14.92 ± 1.49 |
| Epicatechin | 0.15 ± 0.02 | 1.67 ± 0.17 |
| Hyperoside | 4.93 ± 0.30 | Nd |
| Rutin | 2.65 ± 0.08 | Nd |
| Quercetin-3-O-glucoside | 11.71 ± 0.35 | Nd |
| Quercetin-3-O-L-rhamnoside | 2.10 ± 0.13 | Nd |
| Kaempferol-3-O-glucoside | 1.44 ± 0.06 | Nd |
| Epigallocatechin gallate | 0.30 ± 0.09 | Nd |
| Vitexin | 0.03 ± 0.00 | Nd |
| Apigenin-7-O-glucoside | 0.22 ± 0.01 | Nd |
| Myricetin | 0.02 ± 0.01 | Nd |
| Luteolin-7-O-glucoside | 0.14 ± 0.00 | Nd |
| Quercetin | 0.27 ± 0.08 | 0.01 ± 0.00 |
| Naringenin | 0.08 ± 0.01 | Nd |
| Luteolin | 0.13 ± 0.01 | Nd |
| Apigenin | 0.56 ± 0.04 | Nd |
| Extract or Compound | Relative Receptor Activity (%) * | ||
|---|---|---|---|
| LasR | RhlR | PqsR | |
| PEM | 77 ± 1 | 88 ± 1 | 153 ± 4 |
| BIO | 70 ± 1 | 90 ± 2 | 120 ± 10 |
| QA | 120 ± 2 | 103 ± 4 | 108 ± 8 |
| GA | 92 ± 3 | 95 ± 3 | 150 ± 35 |
| ChA | 108 ± 2 | 119 ± 2 | 125 ± 5 |
| Cat | 82 ± 2 | 98 ± 6 | 116 ± 7 |
| Quer-3-O-Glc | 81 ± 4 | 93 ± 4 | 98 ± 10 |
| Category | Developmental Endpoints | Exposure Time (hpf) | ||||
|---|---|---|---|---|---|---|
| 24 | 48 | 72 | 96 | 120 | ||
| Lethal effect | Coagulated egg a | ● | ||||
| Tail not detachment | ● | |||||
| No somite formation | ● | |||||
| Lack of heart beat | ● | |||||
| Teratogenic effect | Malformation of head | ● | ● | ● | ● | |
| Malformation of eyes b | ● | ● | ● | ● | ||
| Malformation of jaw | ● | ● | ● | ● | ||
| Malformation of sacculi/otoliths c | ● | ● | ● | ● | ||
| Malformation of chorda | ● | ● | ● | ● | ||
| Malformation of tail d | ● | ● | ● | ● | ||
| Scoliosis | ● | ● | ● | ● | ||
| Yolk oedema | ● | ● | ● | ● | ||
| Yolk deformation | ● | ● | ● | ● | ||
| Growth retardation e | ● | ● | ● | |||
| Hatching | ● | ● | ||||
| Cardiotoxicity | Pericardial oedema | ● | ● | ● | ● | |
| Heart morphology | ● | ● | ||||
| Heart beating rate (beats/min) | ● | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, M.; Morić, I.; Nikolić, B.; Pavić, A.; Svirčev, E.; Šenerović, L.; Mitić-Ćulafić, D. Anti-Virulence Potential and In Vivo Toxicity of Persicaria maculosa and Bistorta officinalis Extracts. Molecules 2020, 25, 1811. https://doi.org/10.3390/molecules25081811
Jovanović M, Morić I, Nikolić B, Pavić A, Svirčev E, Šenerović L, Mitić-Ćulafić D. Anti-Virulence Potential and In Vivo Toxicity of Persicaria maculosa and Bistorta officinalis Extracts. Molecules. 2020; 25(8):1811. https://doi.org/10.3390/molecules25081811
Chicago/Turabian StyleJovanović, Marina, Ivana Morić, Biljana Nikolić, Aleksandar Pavić, Emilija Svirčev, Lidija Šenerović, and Dragana Mitić-Ćulafić. 2020. "Anti-Virulence Potential and In Vivo Toxicity of Persicaria maculosa and Bistorta officinalis Extracts" Molecules 25, no. 8: 1811. https://doi.org/10.3390/molecules25081811
APA StyleJovanović, M., Morić, I., Nikolić, B., Pavić, A., Svirčev, E., Šenerović, L., & Mitić-Ćulafić, D. (2020). Anti-Virulence Potential and In Vivo Toxicity of Persicaria maculosa and Bistorta officinalis Extracts. Molecules, 25(8), 1811. https://doi.org/10.3390/molecules25081811







