Rapid and Scalable Wire-bar Strategy for Coating of TiO2 Thin-films: Effect of Post-Annealing Temperatures on Structures and Catalytic Dye-Degradation
Abstract
1. Introduction
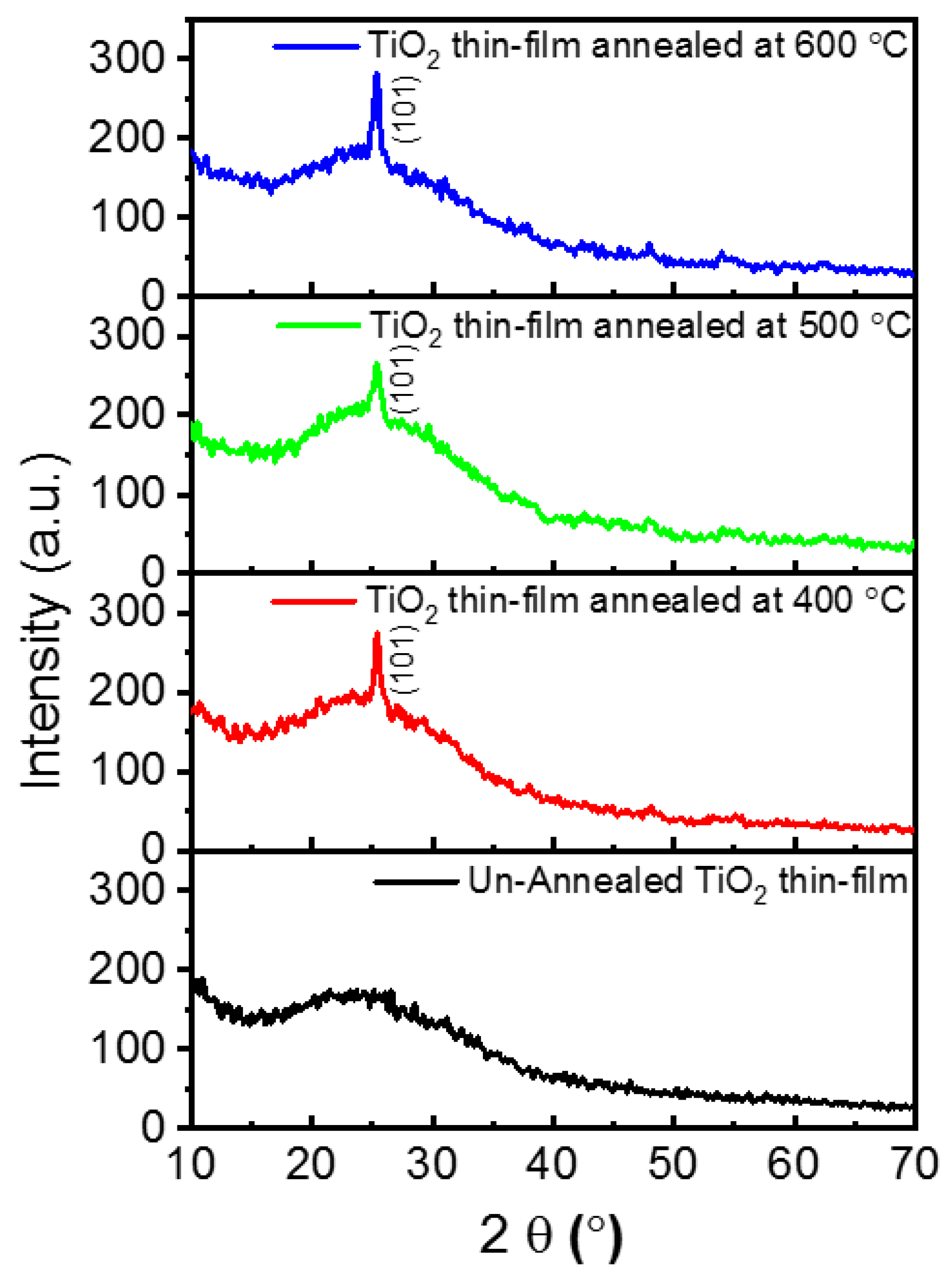
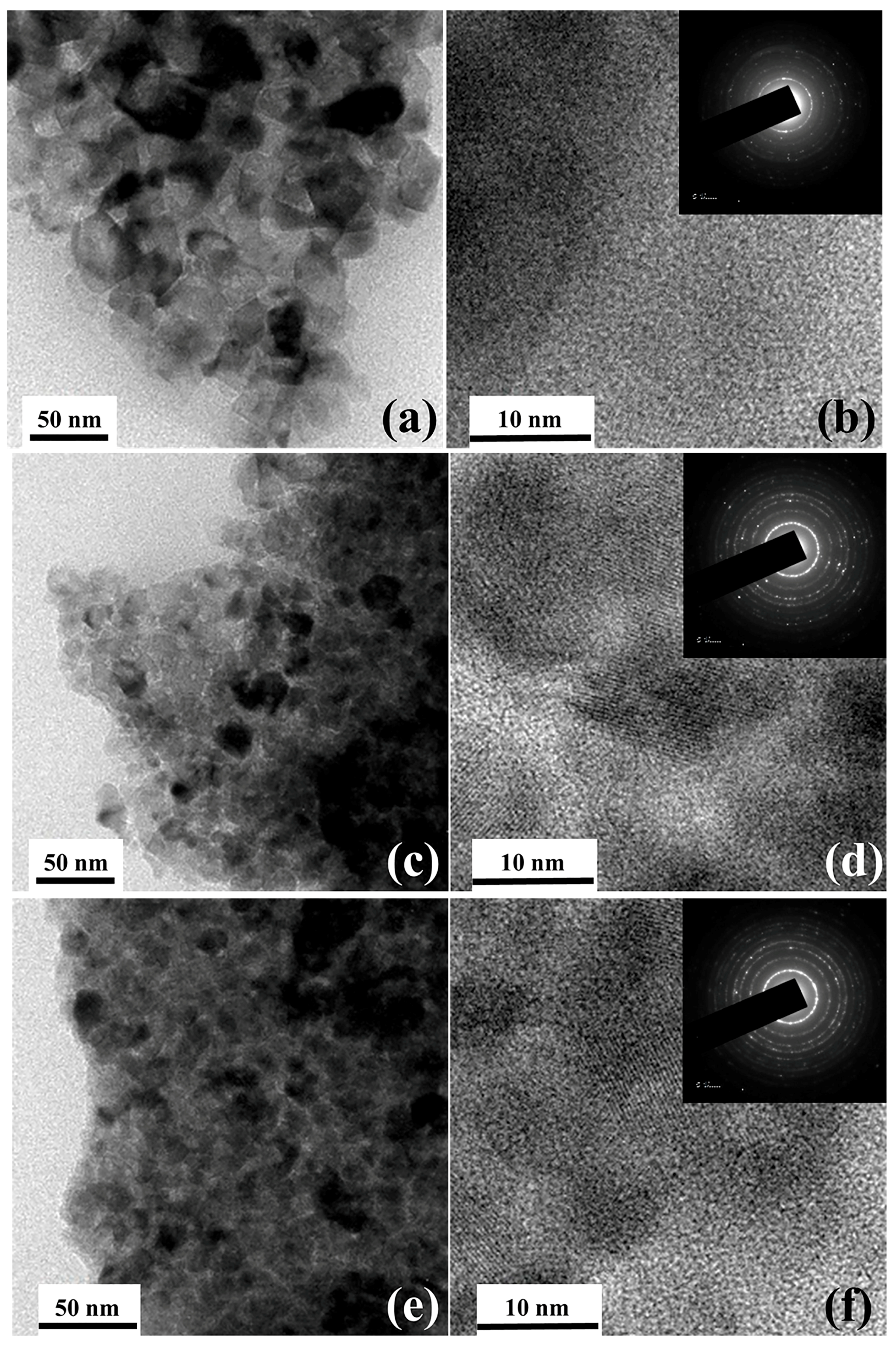
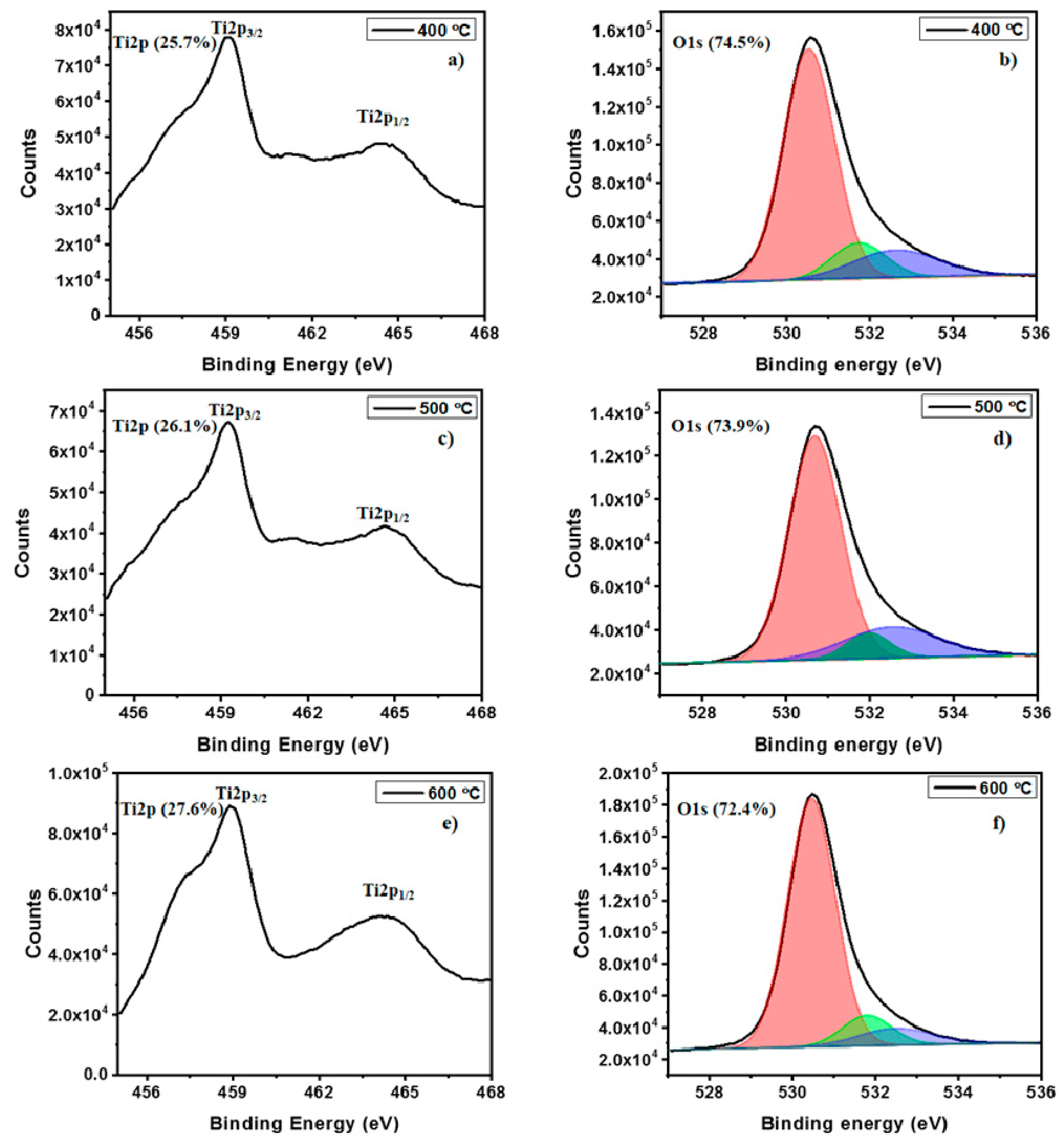
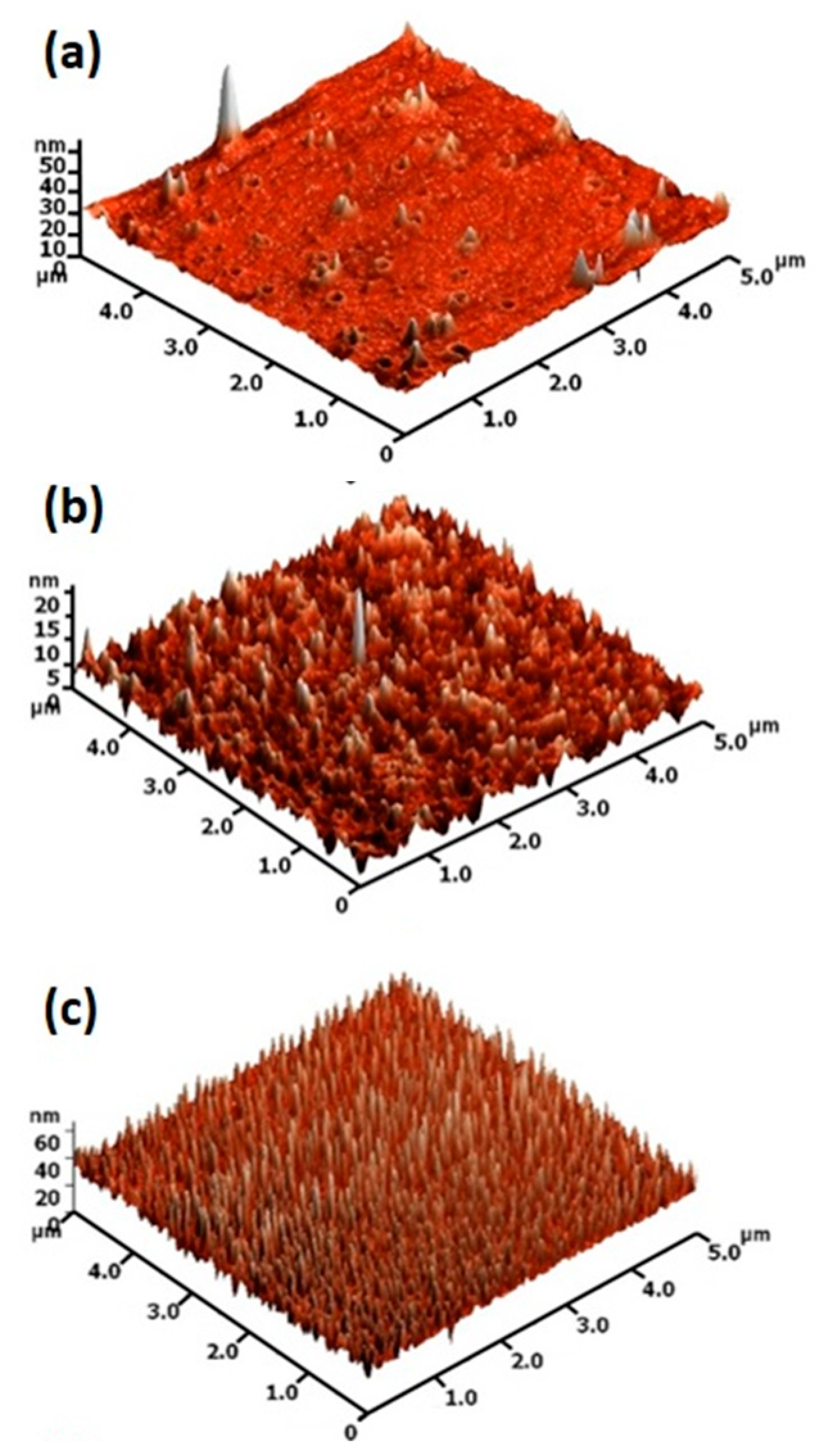
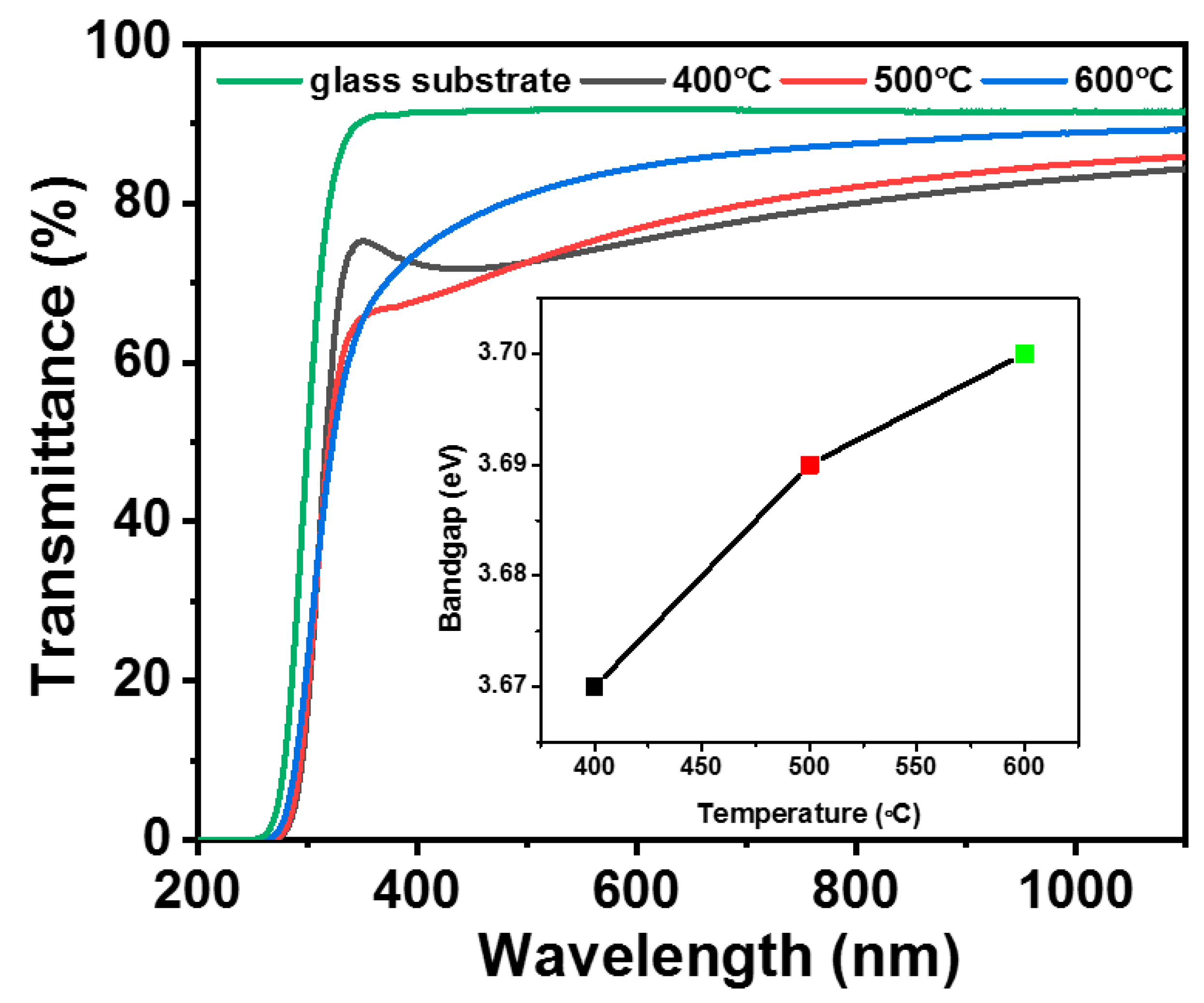
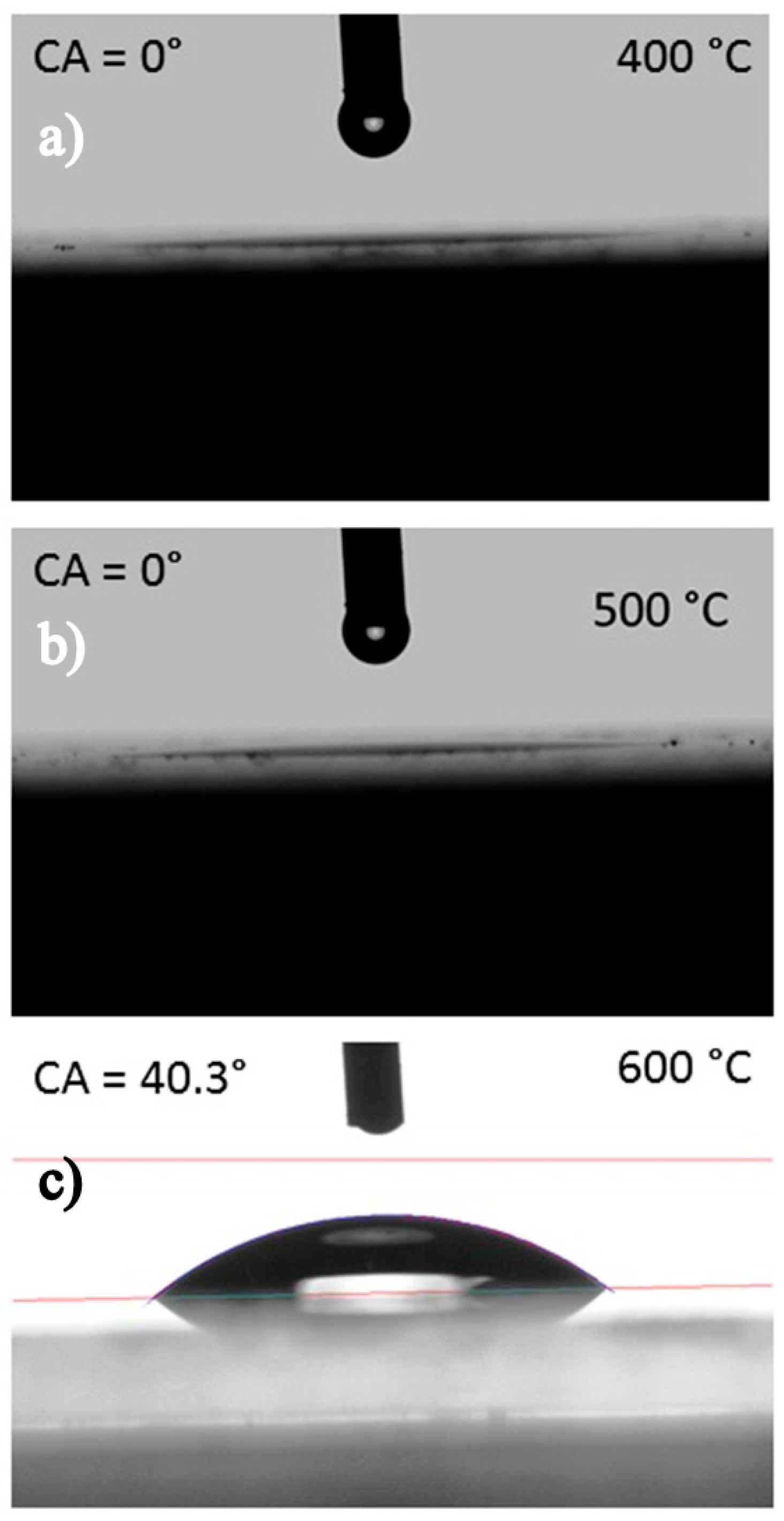
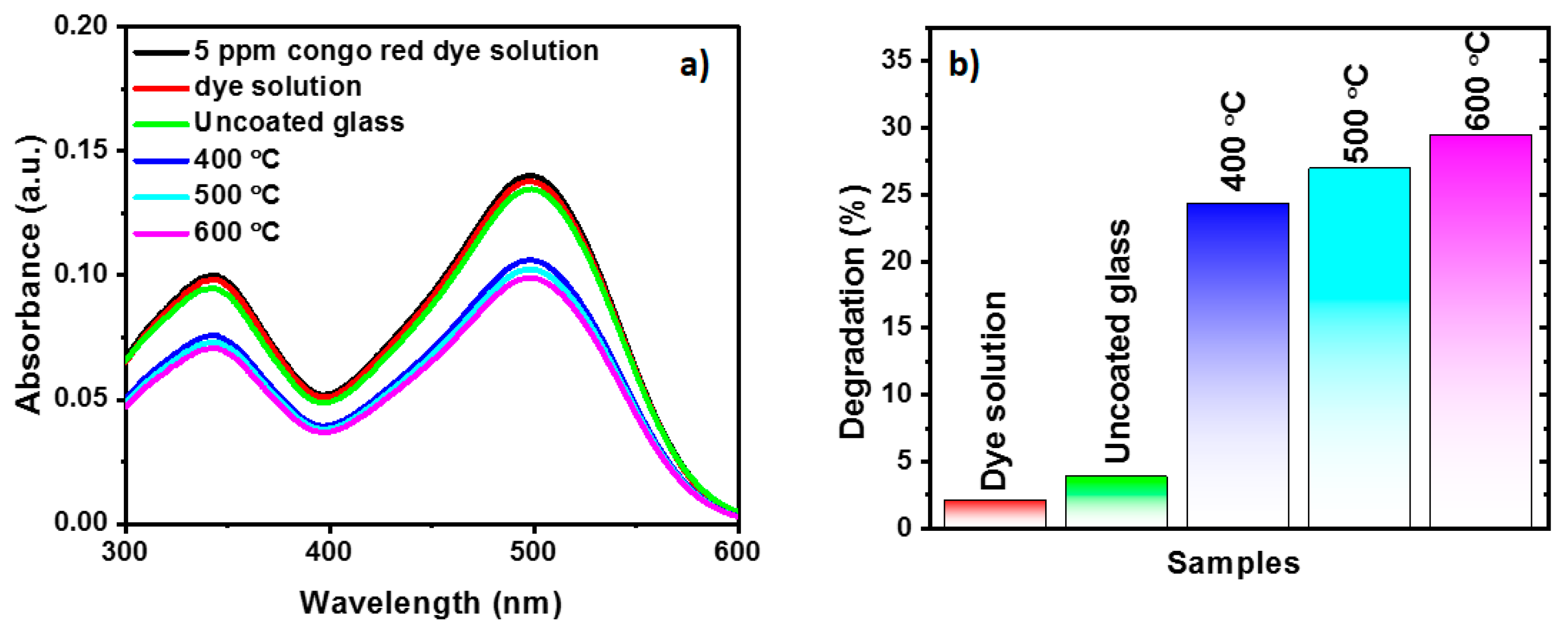
2. Results and Discussions
3. Experimental
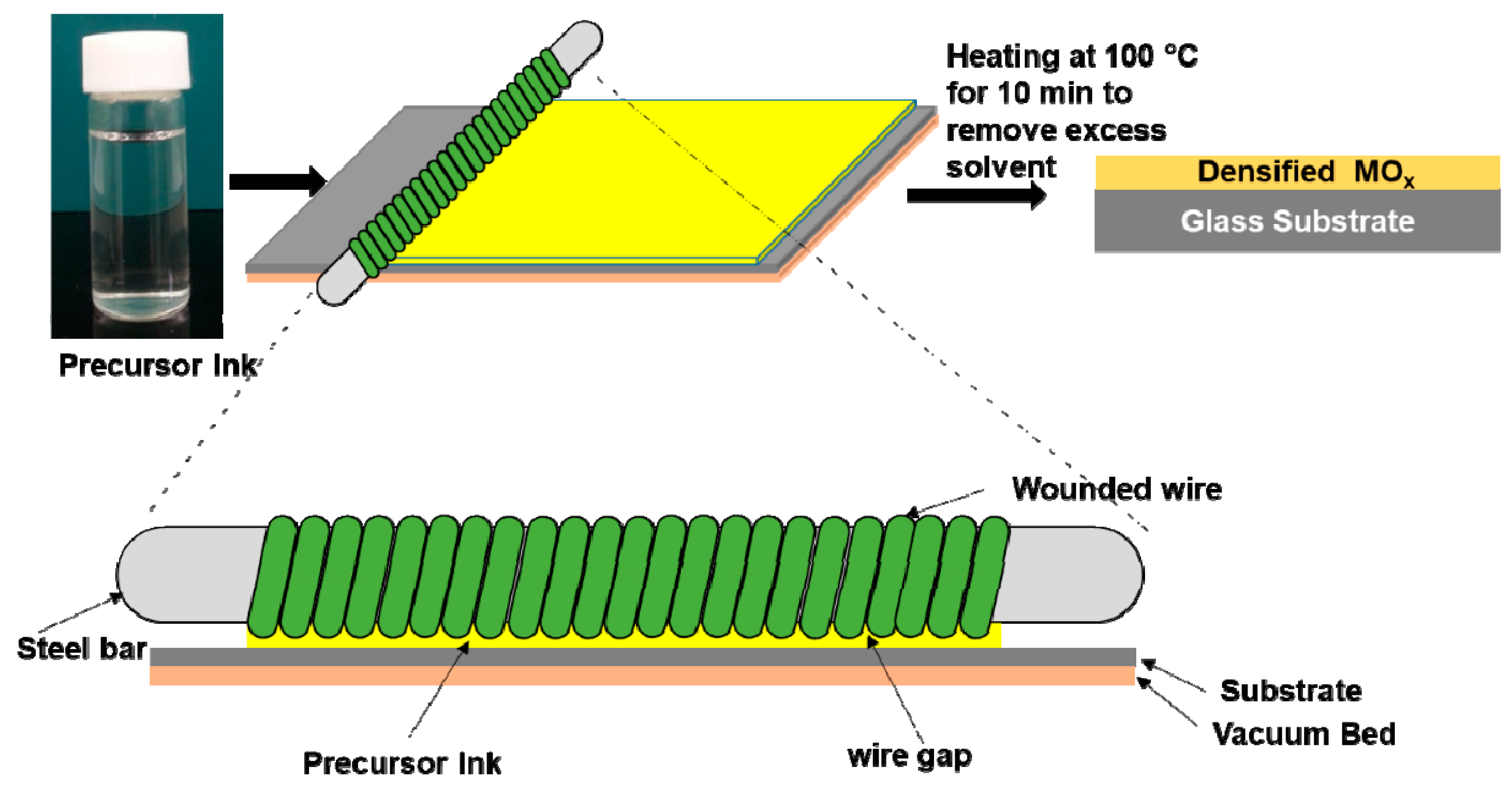
3.1. TiO2 Ink Synthesis
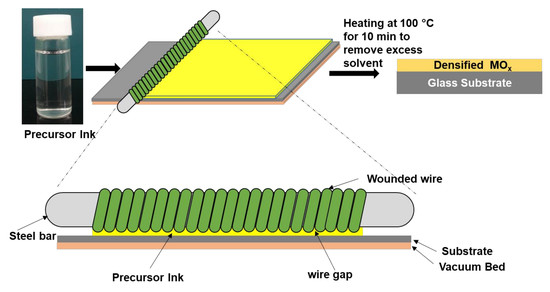
3.2. Wire-Bar Coating Process
3.3. Characterization
3.4. Photocatalytic Activity Testing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Dillert, R.; Bahnemann, D.; Vormoor, M. Photo-induced hydrophilicity and self-cleaning: models and reality. Energy Environ. Sci. 2012, 5, 7491. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Parkin, I.P.; Palgrave, R.G. Self-cleaning coatings. J. Mater. Chem. 2005, 15, 1689. [Google Scholar] [CrossRef]
- Mills, A.; Lepre, A.; Elliott, N.; Bhopal, S.; Parkin, I.P.; O’Neill, S.A. Characterisation of the photocatalyst Pilkington ActivTM: A reference film photocatalyst? J. Photochem. Photobiol. A 2003, 160, 213–224. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, J.; Cui, Z.; Ge, M.; Zhang, K.; Chen, Z.; Chi, L. Recent Advances in TiO2-Based Nanostructured Surfaces with Controllable Wettability and Adhesion. Small 2015, 12, 2203–2224. [Google Scholar] [CrossRef]
- Liu, K.; Cao, M.; Fujishima, A.; Jiang, L. Bio-Inspired Titanium Dioxide Materials with Special Wettability and Their Applications. Chem. Rev. 2014, 114, 10044–10094. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176, 396–428. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO 2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Fleisch, M.; Bahnemann, D.W. Surface-grafted WO3/TiO2 photocatalysts: Enhanced visible-light activity towards indoor air purification. Catal. Today 2018, 313, 63–71. [Google Scholar] [CrossRef]
- Huang, C.; Ding, Y.; Chen, Y.; Li, P.; Zhu, S.; Shen, S. Highly efficient Zr doped-TiO2/glass fiber photocatalyst and its performance in formaldehyde removal under visible light. J. Environ. Sci. 2017, 60, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase – A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Ortelli, S.; Barentin, C.; Dondi, M. TiO2 Nanosols Applied Directly on Textiles Using Different Purification Treatments. Materials 2015, 8, 7988–7996. [Google Scholar] [CrossRef] [PubMed]
- Garlisi, C.; Palmisano, G. Radiation-free superhydrophilic and antifogging properties of e-beam evaporated TiO 2 films on glass. Appl. Surf. Sci. 2017, 420, 83–93. [Google Scholar] [CrossRef]
- Garlisi, C.; Lai, C.-Y.; George, L.; Chiesa, M.; Palmisano, G. Relating Photoelectrochemistry and Wettability of Sputtered Cu- and N-Doped TiO2 Thin Films via an Integrated Approach. J. Phys. Chem. C 2018, 122, 12369–12376. [Google Scholar] [CrossRef]
- Jiang, H.-Q.; Wei, Q.; Cao, Q.-X.; Yao, X. Spectroscopic ellipsometry characterization of TiO2 thin films prepared by the sol–gel method. Ceram. Int. 2008, 34, 1039–1042. [Google Scholar] [CrossRef]
- Mohite, V.S.; Mahadik, M.A.; Kumbhar, S.S.; Kothavale, V.P.; Moholkar, A.V.; Rajpure, K.Y.; Bhosale, C.H. Photoelctrocatalytic degradation of benzoic acid using sprayed TiO2 thin films. Ceram. Int. 2015, 41, 2202–2208. [Google Scholar] [CrossRef]
- Ashith, V.K.; Rao, K.G.; Smitha, R.; Moger, S.N. Study of micro-structural, optical and electrical properties of TiO2 films obtained from micro-controller based SILAR method. Ceram. Int. 2018, 44, 17623–17629. [Google Scholar]
- Park, J.-J.; Kim, -Y.; Latthe, S.S.; Lee, J.-G.; Swihart, M.T.; Yoon, S.S. Thermally Induced Superhydrophilicity in TiO2 Films Prepared by Supersonic Aerosol Deposition. ACS Appl. Mater. Interfaces 2013, 5, 6155–6160. [Google Scholar] [CrossRef]
- Khim, D.; Han, H.; Baeg, K.-J.; Kim, J.; Kwak, S.-W.; Kim, D.-Y.; Noh, Y.-Y. Simple Bar-Coating Process for Large-Area, High-Performance Organic Field-Effect Transistors and Ambipolar Complementary Integrated Circuits. Adv. Mater. 2013, 25, 4302–4308. [Google Scholar] [CrossRef]
- Khim, D.; Ryu, G.-S.; Park, W.-T.; Kim, H.; Lee, M.; Noh, Y.-Y. Precisely Controlled Ultrathin Conjugated Polymer Films for Large Area Transparent Transistors and Highly Sensitive Chemical Sensors. Adv. Mater. 2016, 28, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, M.; Fang, Y.; Qiu, T.; Zhang, J.; Zhi, L. Rod-coating: Towards large-area fabrication of uniform reduced grapheme oxide films for flexible touch screens. Adv. Mater. 2012, 24, 2874. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, V.; Kondaiah, P. Influence of silver nanoparticles on titanium oxide and nitrogen doped titanium oxide thin films for sun light photocatalysis. Appl. Surf. Sci. 2018, 436, 708–719. [Google Scholar]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley: Boston, MA, USA, 1978. [Google Scholar]
- Tauc, J.; Menth, A. States in the gap. J. Non Cryst. Solids 1972, 8, 569–585. [Google Scholar] [CrossRef]
- Alamelu, K.; Raja, V.; Shiamala, L.; Kaliyaperumal, A. Biphasic TiO 2 nanoparticles decorated graphene nanosheets for visible light driven photocatalytic degradation of organic dyes. Appl. Surf. Sci. 2018, 430, 145–154. [Google Scholar] [CrossRef]
- Lin, H.-J.; Yang, T.-S.; Hsi, C.-S.; Wang, M.-C.; Lee, K.-C. Optical and photocatalytic properties of Fe3+-doped TiO2 thin films prepared by a sol–gel spin coating. Ceram. Int. 2014, 40, 10633–10640. [Google Scholar] [CrossRef]
- Morselli, D.; Campagnolo, L.; Prato, M.; Papadopoulou, E.L.; Scarpellini, A.; Athanassiou, A.; Fragouli, D. Ceria/Gold Nanoparticles in Situ Synthesized on Polymeric Membranes with Enhanced Photocatalytic and Radical Scavenging Activity. ACS Appl. Nano Mater. 2018, 1, 5601–5611. [Google Scholar] [CrossRef]
- Campagnolo, L.; Lauciello, S.; Athanassiou, A.; Fragouli, D. Au/ZnO Hybrid Nanostructures on Electrospun Polymeric Mats for Improved Photocatalytic Degradation of Organic Pollutants. Water 2019, 11, 1787. [Google Scholar] [CrossRef]
- Morselli, D.; Bondioli, F.; Fiorini, M.; Messori, M. Poly(methyl methacrylate)–TiO2 nanocomposites obtained by non-hydrolytic sol–gel synthesis: The innovative tert-butyl alcohol route. J. Mater. Sci. 2012, 47, 7003–7012. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J.Y.; Park, S.H.; Kim, S.H.; Cho, S.; Heeger, A.J. Air-Stable Polymer Electronic Devices. Adv. Mater. 2007, 19, 2445–2449. [Google Scholar] [CrossRef]
- Cho, S.; Lee, K.; Heeger, A.J. Extended Lifetime of Organic Field-Effect Transistors Encapsulated with Titanium Sub-Oxide as an ‘Active’ Passivation/Barrier Layer. Adv. Mater. 2009, 21, 1941–1944. [Google Scholar] [CrossRef]
- Arulkumar, S.; Parthiban, S.; Goswami, A.; Varma, R.S.; Naushad, M.; Gawande, M.B.; Shanmugam, P.; Rajender, V. Low temperature processed titanium oxide thin-film using scalable wire-bar coating. Mater. Res. Express 2019, 6, 126427. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Material | Dye used and Duration | Degradation % | Reference |
|---|---|---|---|
| TiO2 thin-film (wire-bar coating) | Congo red | 29.4% at 5 h for TiO2 thin-film annealed at 600 °C. | This work |
| Ceria/Au membrane (electrospinning) | Methylene blue | 90% in 24 h | [28] |
| Au/ZnO mats (electrospinning) | Methylene blue | 35% at 5 h | [29] |
| TiO2 Nanopowder | Methylene blue | ~30% at 5 h for TiO2 | [30] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Divya, P.; Arulkumar, S.; Parthiban, S.; Goswami, A.; Ahamad, T.; Gawande, M.B. Rapid and Scalable Wire-bar Strategy for Coating of TiO2 Thin-films: Effect of Post-Annealing Temperatures on Structures and Catalytic Dye-Degradation. Molecules 2020, 25, 1683. https://doi.org/10.3390/molecules25071683
Divya P, Arulkumar S, Parthiban S, Goswami A, Ahamad T, Gawande MB. Rapid and Scalable Wire-bar Strategy for Coating of TiO2 Thin-films: Effect of Post-Annealing Temperatures on Structures and Catalytic Dye-Degradation. Molecules. 2020; 25(7):1683. https://doi.org/10.3390/molecules25071683
Chicago/Turabian StyleDivya, P., S. Arulkumar, S. Parthiban, Anandarup Goswami, Tansir Ahamad, and Manoj B. Gawande. 2020. "Rapid and Scalable Wire-bar Strategy for Coating of TiO2 Thin-films: Effect of Post-Annealing Temperatures on Structures and Catalytic Dye-Degradation" Molecules 25, no. 7: 1683. https://doi.org/10.3390/molecules25071683
APA StyleDivya, P., Arulkumar, S., Parthiban, S., Goswami, A., Ahamad, T., & Gawande, M. B. (2020). Rapid and Scalable Wire-bar Strategy for Coating of TiO2 Thin-films: Effect of Post-Annealing Temperatures on Structures and Catalytic Dye-Degradation. Molecules, 25(7), 1683. https://doi.org/10.3390/molecules25071683