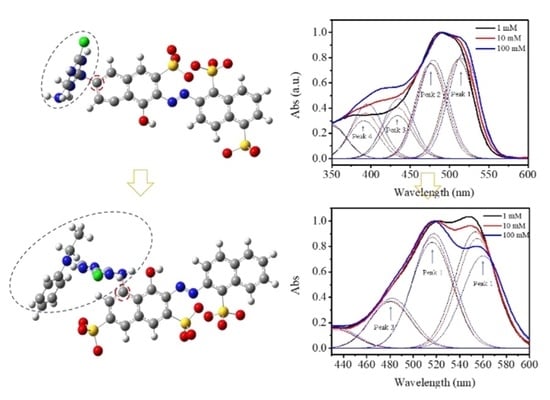
Systematically Exploring Molecular Aggregation and Its Impact on Surface Tension and Viscosity in High Concentration Solutions
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Aggregation of High Concentration Solutions
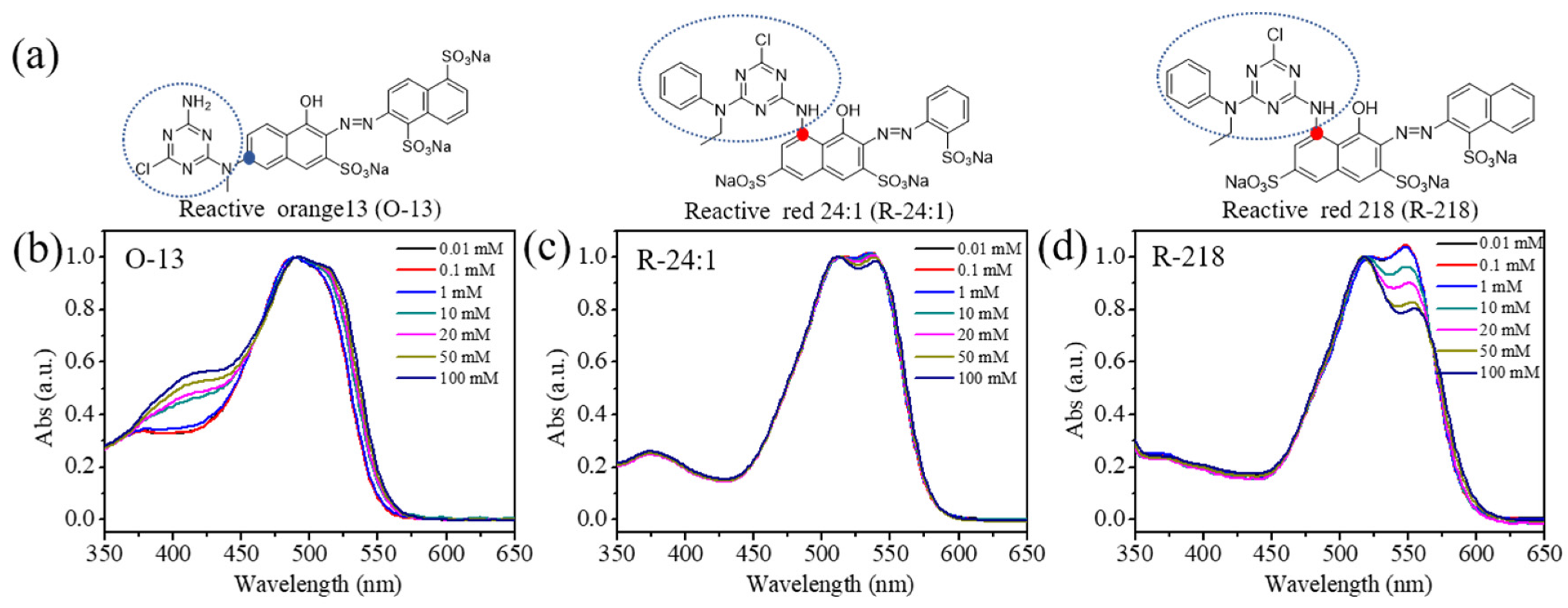
2.1.1. Absorption Spectra of the Three Dyes Solutions
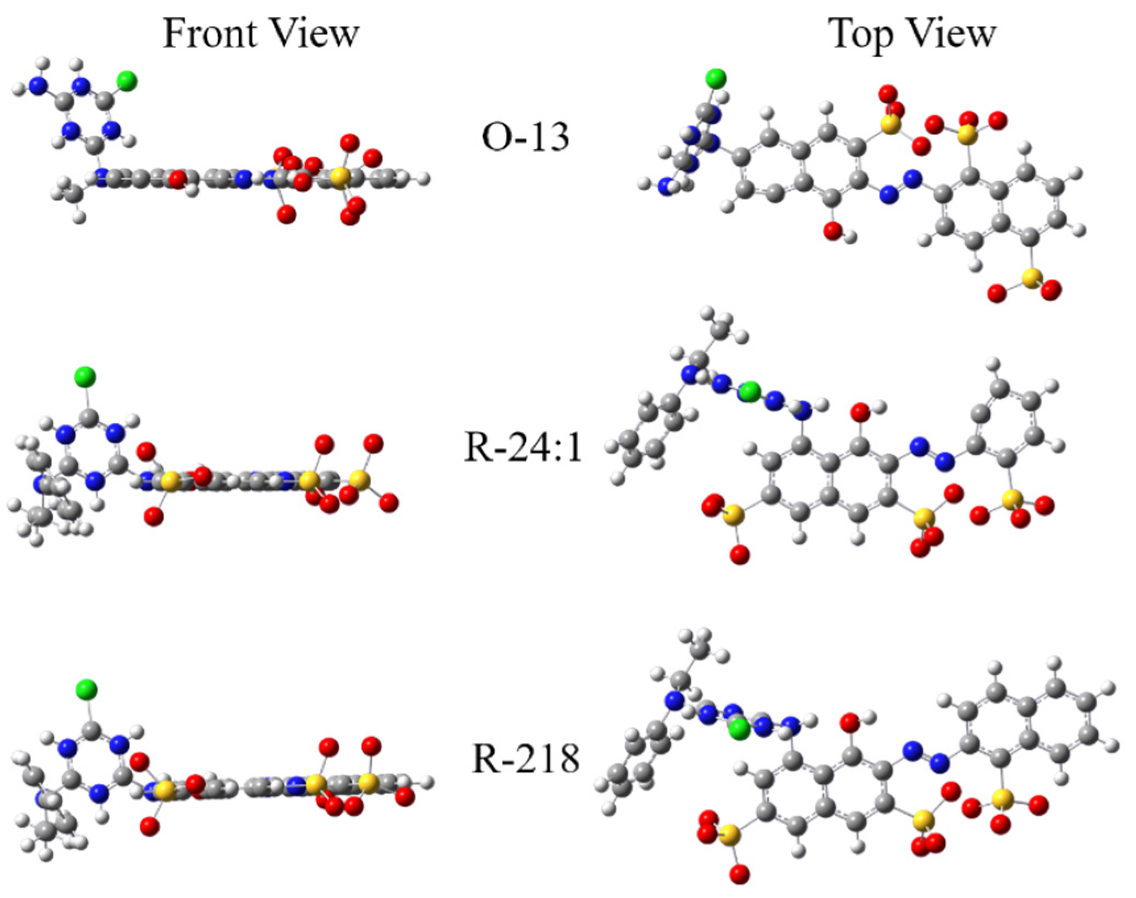
2.1.2. Molecular Structure of the Dye Molecules
2.1.3. The Aggregation Distributions of the Three Dye Solutions
2.2. The Rheology of High Concentration Solutions
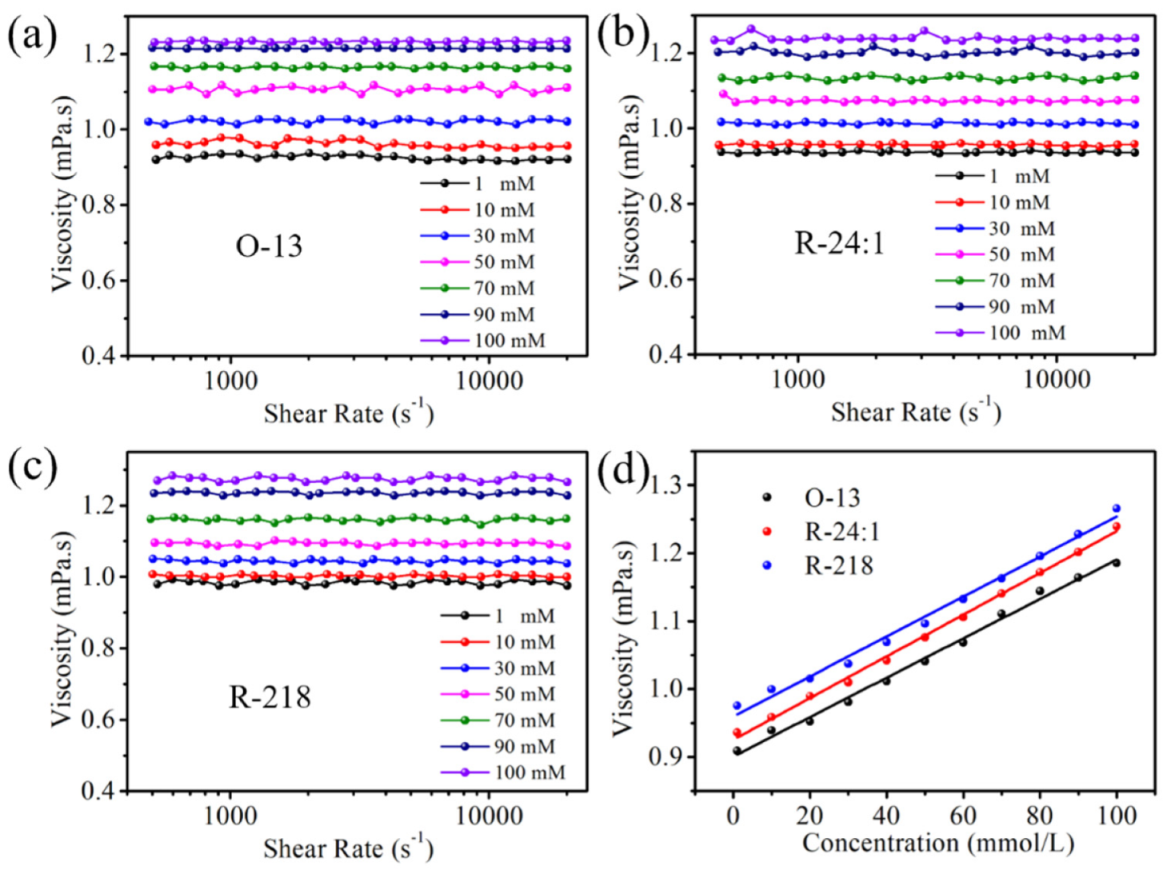
2.2.1. Viscosity of the Three Dye Solutions
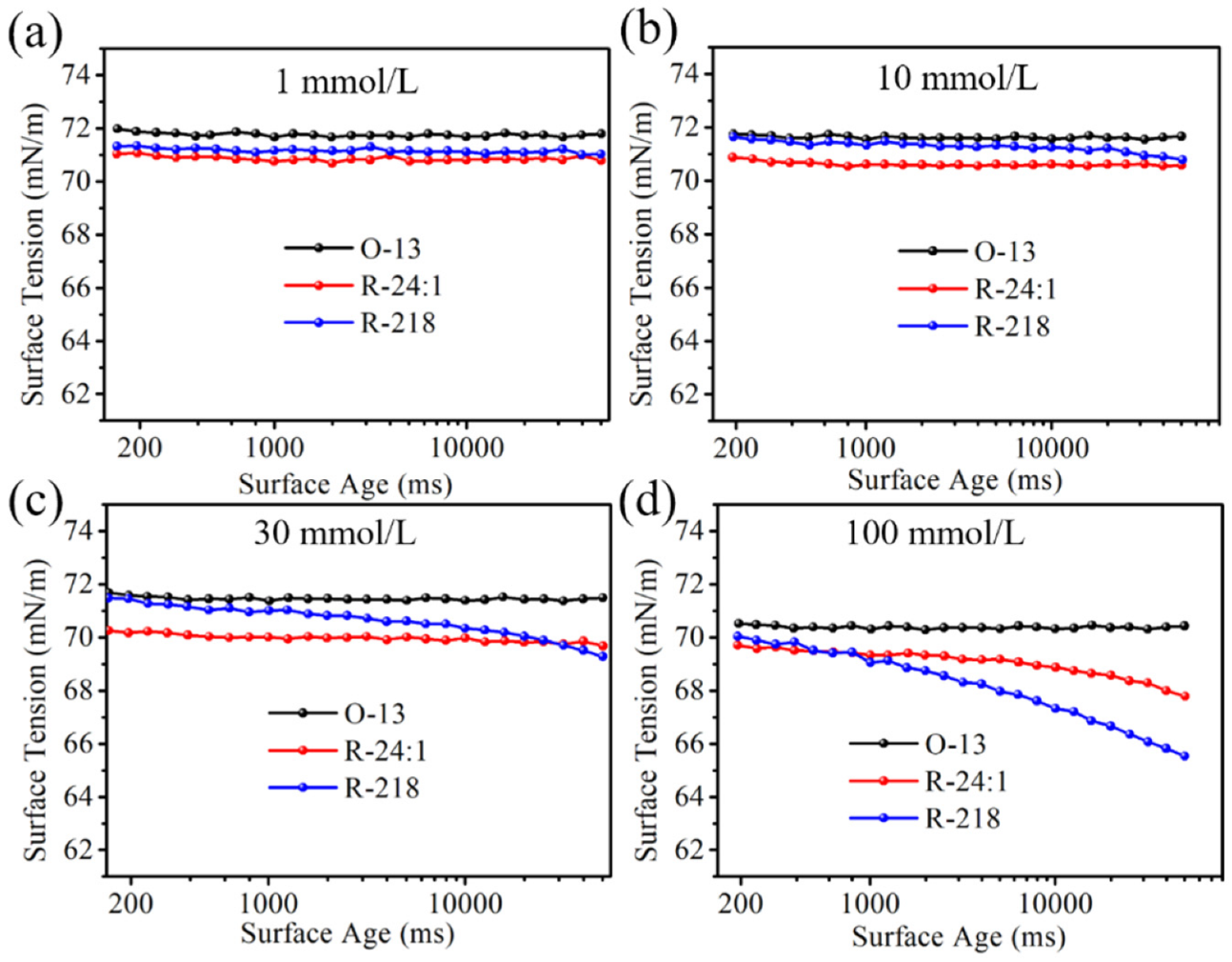
2.2.2. Surface Tension of the Three Dye Solutions
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duff, D.G.; Kirkwood, D.J.; Stevenson, D.M. The Behaviour of Dyes in Aqueous Solutions. I. The Influence of Chemical Structure on Dye Aggregation a Polarographic Study. J. Soc. Dyers Colour. 1977, 93, 303–306. [Google Scholar] [CrossRef]
- Grygorova, G.; Klochkov, V.; Yefimova, S.; Malyukin, Y. Effects of orthovanadate-based nanoparticles of various sizes on the aggregation behavior of polymethine dyes in aqueous solutions. Chem. Phys. Lett. 2015, 621, 46–51. [Google Scholar] [CrossRef]
- Navarro, A.; Sanz, F. Dye aggregation in solution: Study of C.I. direct red I. Dyes Pigments. 1999, 40, 131–139. [Google Scholar] [CrossRef]
- Herkstroeter, W.G.; Martic, P.A.; Farid, S. Inclusion by cyclodextrins to control dye aggregation equilibria in aqueous solution. J. Am. Chem. Soc. 1990, 112, 3583–3589. [Google Scholar] [CrossRef]
- Dawson, T.L.; Dunkerley, K.J.C.T. The Efficient Use of Steam and Water in the Continuous Dyeing and Printing of Carpets. Color. Technol. 2008, 94, 177–189. [Google Scholar] [CrossRef]
- Hamlin, J.D.; Whiting, A. An insight into the mechanism of the cellulose dyeing process, part 2: Simulation of aggregation, solvent and additive effects upon azo-linked aromatics and dyes. Mol. Simul. 2005, 31, 605–612. [Google Scholar] [CrossRef]
- Tang, A.; Wang, Y.; Lee, C.; Kan, C.-W. Computer Color Matching and Levelness of PEG-Based Reverse Micellar Decamethyl cyclopentasiloxane (D5) Solvent-Assisted Reactive Dyeing on Cotton Fiber. Appl. Sci. 2017, 7, 682. [Google Scholar] [CrossRef]
- Heidari, M.; Varma, R.; Ahmadian, M.; Pourkhosravani, M.; Asadzadeh, S.; Karimi, P.; Khatami, M. Photo-Fenton like Catalyst System: Activated Carbon/CoFe2O4 Nanocomposite for Reactive Dye Removal from Textile Wastewater. Appl. Sci. 2019, 9, 963. [Google Scholar] [CrossRef]
- Chen, W.; Shen, W.; Wang, H.; Liu, F.; Duan, L.; Xu, X.; Zhu, D.; Qiu, M.; Wang, E.; Yang, R. Enhanced efficiency of polymer solar cells by improving molecular aggregation and broadening the absorption spectra. Dyes Pigments. 2019, 166, 42–48. [Google Scholar] [CrossRef]
- Hu, X.; Tian, M.; Xu, T.; Sun, X.; Sun, B.; Sun, C.; Liu, X.; Zhang, X.; Qu, L. Multiscale Disordered Porous Fibers for Self-Sensing and Self-Cooling Integrated Smart Sportswear. ACS Nano. 2020, 14, 559–567. [Google Scholar] [CrossRef]
- Chen, W.; Huang, G.; Li, X.; Li, Y.; Wang, H.; Jiang, H.; Zhao, Z.; Yu, D.; Wang, E.; Yang, R. Revealing the Position Effect of an Alkylthio Side Chain in Phenyl-Substituted Benzodithiophene-Based Donor Polymers on the Photovoltaic Performance of Non-Fullerene Organic Solar Cells. ACS Appl. Mater. Inter. 2019, 11, 33173–33178. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tian, M.; Sun, X.; Pan, N.; Chen, F.; Zhu, S.; Zhang, X.; Chen, S. Highly sensitive wearable 3D piezoresistive pressure sensors based on graphene coated isotropic non-woven substrate. Compos. Part A-Appl. S 2019, 117, 202–210. [Google Scholar]
- Jiang, H.; Li, X.; Wang, H.; Ren, Z.; Zheng, N.; Wang, X.; Li, Y.; Chen, W.; Yang, R. Significantly Enhanced Molecular Stacking in Ternary Bulk Heterojunctions Enabled by an Appropriate Side Group on Donor Polymer. Adv. Sci. 2020, 1903455. [Google Scholar] [CrossRef]
- Li, X.; Liang, Z.; Wang, H.; Qiao, S.; Liu, Z.; Jiang, H.; Chen, W.; Yang, R. Fluorinated D1(0.5)–A–D2(0.5)–A model terpolymer: Ultrafast charge separation kinetics and electron transfer at the fluorinated D/A interface for power conversion. J. Mater. Chem. A 2020, 8, 1360–1367. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, B.; Wang, X.-X.; Cai, Z.-X.; Ning, X.; M. Alshehri, S.; Ahamad, T.; Xu, X.-T.; Yamauchi, Y.; Long, Y.-Z. Magnetic-Electrospinning Synthesis of γ-Fe2O3 Nanoparticle–Embedded Flexible Nanofibrous Films for Electromagnetic Shielding. Polymers 2020, 12, 695. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, R.; Fang, K.; Chen, W.; Shi, Z.; Ren, Y. Effects of reactive dye structures on surface tensions and viscosities of dye solutions. J. Mol. Liq. 2019, 287, 110932. [Google Scholar] [CrossRef]
- Tang, Z.; Fang, K.; Song, Y.; Sun, F. Jetting Performance of Polyethylene Glycol and Reactive Dye Solutions. Polymers 2019, 11, 739. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Xie, K.; Pei, L.; Hou, A. Synthesis of novel green reactive dyes and relationship between their structures and printing properties. Dyes Pigments. 2020, 174, 108079. [Google Scholar] [CrossRef]
- Wang, R.; Fang, K.; Ren, Y.; Song, Y.; Zhang, K.; Bukhari, M.N. Jetting performance of two lactam compounds in reactive dye solution. J. Mol. Liq. 2019, 294, 111668. [Google Scholar] [CrossRef]
- Batov, D.V.; Kustov, A.V.; Kruchin, S.O.; Makarov, V.V.; Berezin, D.B. Aggregation of Cationic Chlorin e6 Derivatives in Water and Aqueous Solutions of Polyvinilpyrrolidone. J. Struct. Chem. 2019, 60, 443–448. [Google Scholar] [CrossRef]
- Hamada, K.; Nonogaki, H.; Fukushima, Y.; Munkhbat, B.; Mitsuishi, M.J.D. Pigments. Effects of hydrating water molecules on the aggregation behavior of azo dyes in aqueous solutions. Dyes Pigments. 1991, 16, 111–118. [Google Scholar] [CrossRef]
- Pleşa Chicinaş, R.; Bedelean, H.; Stefan, R.; Măicăneanu, A. Ability of a montmorillonitic clay to interact with cationic and anionic dyes in aqueous solutions. J.Mol. Struct. 2018, 1154, 187–195. [Google Scholar] [CrossRef]
- Rivera, E.; Carreoncastro, M.; Buendia, I.; Cedillo, G. Optical properties and aggregation of novel azo-dyes bearing an end-capped oligo(ethylene glycol) side chain in solution, solid state and Langmuir–Blodgett films. Dyes Pigments. 2006, 68, 217–226. [Google Scholar] [CrossRef]
- Dong, W.H.; Liu, J.X.; Mou, X.J.; Liu, G.S.; Huang, X.W.; Yan, X.; Ning, X.; Russell, S.J.; Long, Y.Z. Performance of polyvinyl pyrrolidone-isatis root antibacterial wound dressings produced in situ by handheld electrospinner. Colloid. Surf. B 2020, 188, 110766. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Oda, H.; Izumi, T.; Hirohashi, R. Substituent effects on aggregation phenomena in aqueous solution of thiacarbocyanine dyes. Dyes Pigments. 2005, 66, 1–6. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Halina, S.; Stasyuk, O.A.; Justyna, D.; Marcin, P. Aromaticity from the viewpoint of molecular geometry: Application to planar systems. Chem. Rev. 2014, 114, 6383–6422. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Wang, W.; Yi, H.; Li, H.; Zhang, Q.; Song, S. A Novel Model of Aggregate Gradation for Autoclaved Bricks from Tailings. Minerals 2017, 7, 112. [Google Scholar] [CrossRef]
- Bračko, S.; Špan, J. Conductometric investigation of dye–surfactant ion pair formation in aqueous solution. Dyes Pigments. 2000, 45, 97–102. [Google Scholar] [CrossRef]
- Scholz, N.; Behnke, T.; Resch-Genger, U. Determination of the Critical Micelle Concentration of Neutral and Ionic Surfactants with Fluorometry, Conductometry, and Surface Tension-A Method Comparison. J. Fluoresc. 2018, 28, 465–476. [Google Scholar] [CrossRef]
- Safonova, E.A.; Martynov, A.G.; Zolotarevskii, V.I.; Nefedov, S.E.; Gorbunova, Y.G.; Tsivadze, A.Y. Design of UV-Vis-NIR panchromatic crown-phthalocyanines with controllable aggregation. Dalton T. 2015, 44, 1366–1378. [Google Scholar] [CrossRef]
- Ouyang, C.; Chen, S.; Che, B.; Xue, G. Aggregation of azo dye Orange I induced by polyethylene glycol in aqueous solution. Colloid. Surf. A 2007, 301, 346–351. [Google Scholar] [CrossRef]
- Ferus-Comelo, M.; Greaves, A.J. An investigation into direct dye aggregation. Color. Technol. 2006, 118, 15–19. [Google Scholar] [CrossRef]
- Yuan, M.-S.; Du, X.; Xu, F.; Wang, D.-E.; Wang, W.-J.; Li, T.-B.; Tu, Q.; Zhang, Y.; Du, Z.; Wang, J. Aggregation-induced bathochromic fluorescent enhancement for fluorenone dyes. Dyes Pigments. 2015, 123, 355–362. [Google Scholar] [CrossRef]
- Macchi, S.; Nifosi, R.; Signore, G.; Di Pietro, S.; Boccardi, C.; D’Autilia, F.; Beltram, F.; Cardarelli, F. Self-aggregation propensity of the Tat peptide revealed by UV-Vis, NMR and MD analyses. Phys. Chem. Chem. Phys. 2017, 19, 23910–23914. [Google Scholar] [CrossRef] [PubMed]
- Kanjanawarut, R.; Yuan, B.; XiaoDi, S. UV-vis spectroscopy and dynamic light scattering study of gold nanorods aggregation. Nucleic Acid Ther. 2013, 23, 273–280. [Google Scholar] [CrossRef]
- Aksenov, V.L. Study of fullerene aggregates in pyridine/water solutions. AIP Conf. Proc. 2001, 591, 66–69. [Google Scholar]
- Zhao, Y.; Wang, R.; Fang, K.; Tan, Y.; Chen, S.; Guan, Y.; Hao, L. Investigating the synergetic dispersing effect of hydrolyzed biomacromolecule Cellulase and SDS on CuPc pigment. Colloids Surf. B Biointerfaces 2019, 184, 110568. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, M.J. The self-aggregation of some 7H-indolo[1,2-a]quinolinium dyes in aqueous solution. J. Mol. Struct. 2015, 1098, 26–33. [Google Scholar] [CrossRef]
- Neumann, B.; Huber, K.; Pollmann, P. A comparative experimental study of the aggregation of Acid Red 266 in aqueous solution by use of 19F-NMR, UV/Vis spectroscopy and static light scattering. Phys. Chem. Chem. Phys. 2000, 2, 3687–3695. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, J.; Tang, Y.; Xu, G.; Yan, W. Aggregation behaviour of two thiacarbocyanine dyes in aqueous solution. Dyes Pigments. 2008, 76, 88–93. [Google Scholar] [CrossRef]
- Qiu, Y.; Hu, H.; Zhao, D.; Wang, J.; Wang, H.; Wang, Q.; Peng, H.; Liao, Y.; Xie, X. Concentration-dependent dye aggregation and disassembly triggered by the same artificial helical foldamer. Polymer 2019, 170, 7–15. [Google Scholar] [CrossRef]
- Ghanadzadeh Gilani, A.; Poormohammadi-Ahandani, Z.; Kian, R. Additive-induced aggregate changes of two structurally similar dyes in aqueous solutions: A comparative photophysical study. Spectrochim Acta A Mol. Biomol. Spectrosc. 2018, 189, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Berlepsch, H.V.; Kirstein, S.; Böttcher, C.J.L. Effect of Alcohols on J-Aggregation of a Carbocyanine Dye. Langmuir 2002, 18, 7699–7705. [Google Scholar] [CrossRef]
- Eisfeld, A.; Briggs, J.S. The J- and H-bands of organic dye aggregates. Chem. Phys. 2006, 324, 376–384. [Google Scholar] [CrossRef]
- Roden, J.; Eisfeld, A.; Briggs, J.S. The J- and H-bands of dye aggregate spectra: Analysis of the coherent exciton scattering (CES) approximation. Chem. Phys. 2008, 352, 258–266. [Google Scholar] [CrossRef][Green Version]
- Tokoro, Y.; Nagai, A.; Chujo, Y. Nanoparticles via H-aggregation of amphiphilic BODIPY dyes. Tetrahedron. Lett. 2010, 51, 3451–3454. [Google Scholar] [CrossRef]
- Verma, P.; Pal, H. Intriguing H-aggregate and H-dimer formation of coumarin-481 dye in aqueous solution as evidenced from photophysical studies. J. Phys. Chem. A. 2012, 116, 4473–4484. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A Gen. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Mishra, A.; Behera, R.K.; Mishra, B.K.; Behera, G.B. Dye–surfactant interaction: Chain folding during solubilization of styryl pyridinium dyes in sodium dodecyl sulfate aggregates. J. Photoch. Photobio. A 1999, 121, 63–73. [Google Scholar] [CrossRef]
- Karukstis, K.K.; Perelman, L.A.; Wong, W.K.J.L. Spectroscopic Characterization of Azo Dye Aggregation on Dendrimer Surfaces. Langmuir 2002, 18, 10363–10371. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
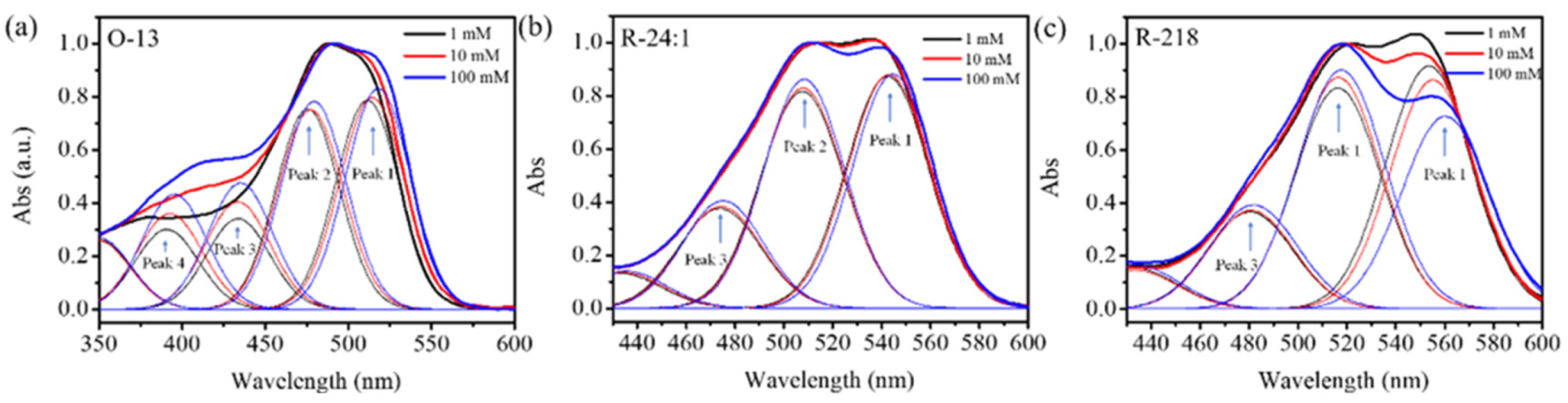
| Dyes | Concentrations (mM) | Curve 1 (%) | Curve 2 (%) | Curve 3 (%) | Curve 4 (%) |
|---|---|---|---|---|---|
| O-13 | 1 | 33.2 | 31.8 | 14.4 | 12.6 |
| 10 | 32.0 | 30.0 | 16.0 | 14.4 | |
| 100 | 30.6 | 28.8 | 17.5 | 16.0 | |
| R-24:1 | 1 | 40.8 | 38.1 | 17.4 | |
| 10 | 40.4 | 38.1 | 17.5 | ||
| 100 | 39.4 | 38.5 | 18.1 | ||
| R-218 | 1 | 41.4 | 37.8 | 16.6 | |
| 10 | 39.2 | 39.9 | 17.0 | ||
| 100 | 34.0 | 42.8 | 18.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Kong, H.; Zheng, J.; Peng, H.; Cao, C.; Qi, Y.; Fang, K.; Chen, W. Systematically Exploring Molecular Aggregation and Its Impact on Surface Tension and Viscosity in High Concentration Solutions. Molecules 2020, 25, 1588. https://doi.org/10.3390/molecules25071588
Wang H, Kong H, Zheng J, Peng H, Cao C, Qi Y, Fang K, Chen W. Systematically Exploring Molecular Aggregation and Its Impact on Surface Tension and Viscosity in High Concentration Solutions. Molecules. 2020; 25(7):1588. https://doi.org/10.3390/molecules25071588
Chicago/Turabian StyleWang, Huan, Han Kong, Jie Zheng, Hui Peng, Chuangui Cao, Yong Qi, Kuanjun Fang, and Weichao Chen. 2020. "Systematically Exploring Molecular Aggregation and Its Impact on Surface Tension and Viscosity in High Concentration Solutions" Molecules 25, no. 7: 1588. https://doi.org/10.3390/molecules25071588
APA StyleWang, H., Kong, H., Zheng, J., Peng, H., Cao, C., Qi, Y., Fang, K., & Chen, W. (2020). Systematically Exploring Molecular Aggregation and Its Impact on Surface Tension and Viscosity in High Concentration Solutions. Molecules, 25(7), 1588. https://doi.org/10.3390/molecules25071588