Purification, Identification, and Characterization of an Endo-1,4-β-Xylanase from Wheat Malt
Abstract
1. Introduction
2. Results and Discussion
2.1. Wheat Malt Endo-1,4-β-Xylanase
2.1.1. Wheat Malt Endo-1,4-β-Xylanase Purification
2.1.2. LC–MS/MS Proteomic Identification of a Purified Enzyme
2.2. Degradation Effect of the Purified Endo-1,4-β-Xylanase on Wheat-Derived WEAX
2.2.1. Changes of WEAX Content, avDP, and AXOS Content
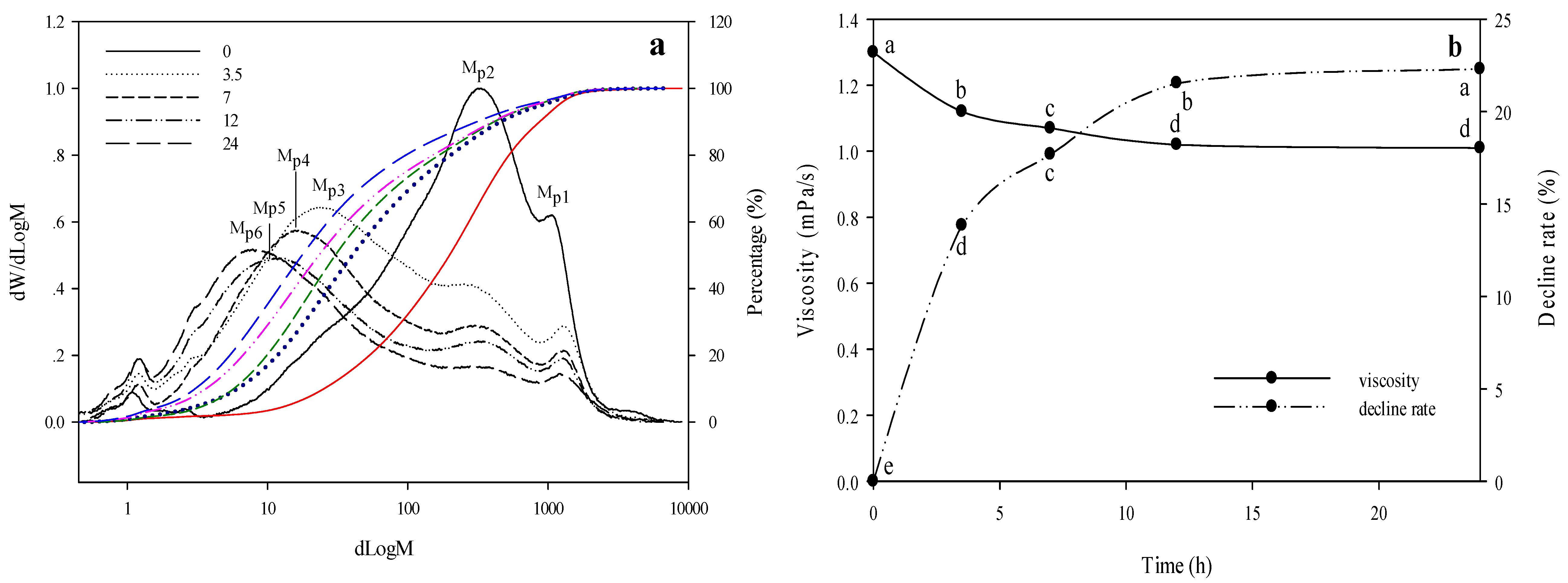
2.2.2. Changes of WEAX Mw and Viscosity
2.2.3. Changes of WEAX Surface Morphological
3. Materials and Methods
3.1. Experimental Materials
3.2. Purification of the Wheat Malt Endo-1,4-β-Xylanase
3.2.1. Crude Enzyme Extraction and Fractional Ammonium Sulfate Precipitation
3.2.2. SP-Sepharose Fast Flow Cation Exchange Chromatography
3.2.3. Q-Sepharose Fast Flow Anion Exchange Chromatography
3.2.4. Source 30Q Anion Exchange Chromatography
3.3. Analysis Techniques
3.3.1. Measurement of Endo-1,4-β-Xylanase Activity
3.3.2. β-D-Xylosidase Activity Assay
3.3.3. Arabinofuranosidase Activity Assay
3.3.4. Protein Content Determination
3.3.5. SDS-PAGE
3.3.6. LC–MS/MS of Purified Enzyme
3.4. Degradation Ability on Wheat-Derived Arabinoxylans
3.4.1. Degradation Method
3.4.2. Arabinoxylan-Oligosaccharides (AXOS) Content Determination
3.4.3. The Content and avDP Analysis of WEAX
3.4.4. Determination of the Mw
3.4.5. Viscosity Determination
3.4.6. Scanning Electron Microscopy (SEM)
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mastanjević, K.; Šarkanj, B.; Krska, R.; Sulyok, M.; Warth, B.; Mastanjević, K.; Šarkanj, B.; Krstanović, V. From malt to wheat beer: A comprehensive multi-toxin screening, transfer assessment and its influence on basic fermentation parameters. Food Chem. 2018, 254, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Vietor, R.J.; Voragen, A.G.J.; Angelino, S.A.G.F. Composition of non-starch polysaccharides in wort and spent grain from brewing trials with malt from a good malting variety and a feed variety. J. Inst. Brew. 1993, 99, 243–248. [Google Scholar] [CrossRef]
- Rakszegi, M.; Lovegrove, A.; Balla, K.; Láng, L.; Bedő, Z.; Veisz, O.; Shewry, P.R. Effect of heat and drought stress on the structure and composition of arabinoxylan and β-glucan in wheat grain. Carbohydr. Polym. 2014, 102, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Revanappa, S.B.; Nandini, C.D.; Salimath, P.V. Structural variations of arabinoxylans extracted from different wheat (Triticum aestivum) cultivars in relation to chapati-quality. Food Hydrocoll. 2015, 43, 736–742. [Google Scholar] [CrossRef]
- Sajib, M. Preparation and Evaluation of Arabinoxylan Based Prebiotics. Master’s Thesis, Lund University, Lund, Sweden, 2017. Available online: https://www.researchgate.net/publication/315769547_Preparation_and_evaluation_of_arabinoxylan_based_prebiotics (accessed on 23 January 2020).
- Li, S.B.; Morris, C.F.; Bettge, A.D. Genotype and environment variation for arabinoxylans in hard winter and spring wheats of the U.S. Pacific Northwest. Cereal Chem. 2009, 86, 88–95. [Google Scholar] [CrossRef]
- Guo, M.M.; Du, J.H.; Zhang, K.L.; Jin, Y.H. Content and molecular weight of water-extractable arabinoxylans in wheat malt and wheat malt-based wort with different Kolbach indices. J. Sci. Food Agric. 2014, 94, 2794–2800. [Google Scholar] [CrossRef]
- Mendis, M.; Simsek, S. Production of structurally diverse wheat arabinoxylan hydrolyzates using combinations of xylanase and arabinofuranosidase. Carbohydr. Polym. 2015, 132, 452–459. [Google Scholar] [CrossRef]
- Jin, Y.H.; Du, J.H.; Zhang, K.L.; Xie, L.; Li, P.P. Relationship between Kolbach Index and other quality parameters of wheat malt. J. Inst. Brew. 2012, 118, 57–62. [Google Scholar] [CrossRef]
- Han, J.-Y. Structural characteristics of arabinoxylan in barley, malt, and beer. Food Chem. 2000, 70, 131–138. [Google Scholar] [CrossRef]
- Evans, D.E.; Sheehan, M.C.; Stewart, D.C. The impact of malt derived proteins on beer foam quality. Part II. The influence of malt foam-positive proteins and non-starch polysaccharides on beer foam quality. J. Inst. Brew. 1999, 105, 171–177. [Google Scholar] [CrossRef]
- Mendis, M.; Simsek, S. Arabinoxylans and human health. Food Hydrocoll. 2014, 42, 239–243. [Google Scholar] [CrossRef]
- Song, H.Y.; Lim, H.K.; Kim, D.R.; Lee, K.I.; Hwang, I.T. A new bi-modular endo-β-1,4-xylanase KRICT PX-3 from whole genome sequence of Paenibacillus terrae HPL-003. Enzyme Microb. Technol. 2014, 54, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Souza, A.R.; de Araújo, G.C.; Zanphorlin, L.M.; Ruller, R.; Franco, F.C.; Torres, F.A.; Mertens, J.A.; Bowman, M.J.; Gomes, E.; Da Silva, R. Engineering increased thermostability in the GH-10 endo-1,4-β-xylanase from Thermoascus aurantiacus CBMAI 756. Int. J. Biol. Macromol. 2016, 93, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Debyser, W.; Derdelinckx, G.; Delcour, J.A. Arabinoxylan and arabinoxylan hydrolysing activities in barley malts and worts derived from them. J. Cereal Sci. 1997, 26, 67–74. [Google Scholar] [CrossRef]
- Viëtor, R.J.; Hoffmann, R.A.; Angelino, S.A.G.F.; Voragen, A.G.J.; Kamerling, J.P.; Vliegenthart, J.F.G. Structures of small oligomers liberated from barley arabinoxylans by endoxylanase from Aspergillus awamori. Carbohydr. Res. 1994, 254, 245–255. [Google Scholar] [CrossRef]
- Cleemput, G.; Hessing, M.; van Oort, M.; Deconynck, M.; Delcour, J.A. Purification and Characterization of a β-D-xylosidase and an endo-xylanase from wheat flour. Plant Physiol. 1997, 113, 377–386. [Google Scholar] [CrossRef]
- Cleemput, G.; Van Laere, K.; Hessing, M.; Van Leuven, F.; Torrekens, S.; Delcour, J.A. Identification and characterization of a nove1 arabinoxylanase from wheat flour. Plant Physiol. 1997, 115, 1619–1627. [Google Scholar] [CrossRef][Green Version]
- Dornez, E.; Gebruers, K.; Delcour, J.A.; Courtin, C.M. Grain-associated xylanases: Occurrence, variability, and implications for cereal processing. Trends Food Sci. Technol. 2009, 20, 495–510. [Google Scholar] [CrossRef]
- Benjavongkulchai, E.; Spencer, M.S. Purification and characterization of barley-aleurone xylanase. Planta 1986, 169, 415–419. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Verma, P. A detailed overview of xylanases: An emerging biomolecule for current and future prospective. Bioresour. Bioprocess. 2019, 6, 40. [Google Scholar] [CrossRef]
- Silva, L.A.O.; Terrasan, C.R.F.; Carmona, E.C. Purification and characterization of xylanases from Trichoderma inhamatum, Electron. J. Biotechnol. 2015, 18, 307–313. [Google Scholar]
- Xie, L.; Jin, Y.H.; Du, J.H.; Zhang, K.L. Water-soluble protein molecular weight distribution and effects on wheat malt quality during malting. J. Inst. Brew. 2014, 120, 399–403. [Google Scholar] [CrossRef]
- Guo, X.; Jin, Y.H.; Du, J.H. Extraction and purification of an endo-1,4-β-xylanase from wheat malt. J. Cereal Sci. 2017, 74, 218–223. [Google Scholar] [CrossRef]
- Peng, Z.J.; Jin, Y.H.; Du, J.H. Enzymatic Properties of endo-1,4-β-xylanase from Wheat Malt. Protein Pept. Lett. 2019, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.N.; Jin, Y.H.; Du, J.H.; Li, J.; Zhang, K.L. Partial characterization of β-D-xylosidase from wheat malts. J. Inst. Brew. 2015, 121, 338–342. [Google Scholar] [CrossRef]
- Sozzi, G.O.; Fraschina, A.A.; Navarro, A.A.; Cascone, O.; Greve, L.C.; Labavitch, J.M. α-L-Arabino furanosidase activity during development and ripening of normal and ACC synthase antisense tomato fruit. HortScience 2002, 37, 564–566. [Google Scholar] [CrossRef]
- Kong, F.J.; Oyanagi, A.; Komatsu, S. Cell wall proteome of wheat roots under flooding stress using gel-based and LC MS/MS-based proteomics approaches. Biochim. Biophys. Acta Biomembr. 2010, 1804, 124–136. [Google Scholar] [CrossRef]
- Loosveld, A.A.; Grobet, P.J.; Delcour, J.A. Contents and structural features of water-extractable arabinogalactan in wheat flour fractions. J. Agric. Food Chem. 1997, 45, 1998–2002. [Google Scholar] [CrossRef]
- Englyst, H.N.; Cummings, J.H. Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1984, 109, 937–942. [Google Scholar] [CrossRef]
- Courtin, C.M.; Den Broeck, H.V.; Delcour, J.A. Determination of reducing end sugar residues in oligo- and polysaccharides by gas-liquid chromatography. J. Chromatogr. A 2000, 866, 97–104. [Google Scholar] [CrossRef]
- Li, M.M.; Du, J.H.; Han, Y.Y.; Li, J.; Bao, J.; Zhang, K.L. Non-starch polysaccharides in commercial beers on China market: Mannose polymers content and its correlation with beer physicochemical indices. J. Food Compos. Anal. 2019, 79, 122–127. [Google Scholar] [CrossRef]
- Jiang, Y.; Du, J.H.; Zhang, L.G.; Li, W.Q. Properties of pectin extracted from fermented and steeped hawthorn wine pomace: A comparison. Carbohydr. Polym. 2018, 197, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Rascón-Chu, A.; Martínez-Lópe, A.L.; Carvajal-Millán, E.; León-Renova, N.P.; Márquez-Escalante, J.A.; Romo-Chacón, A. Pectin from low quality ‘Golden Delicious’ apples: Composition and gelling capability. Food Chem. 2009, 116, 101–110. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
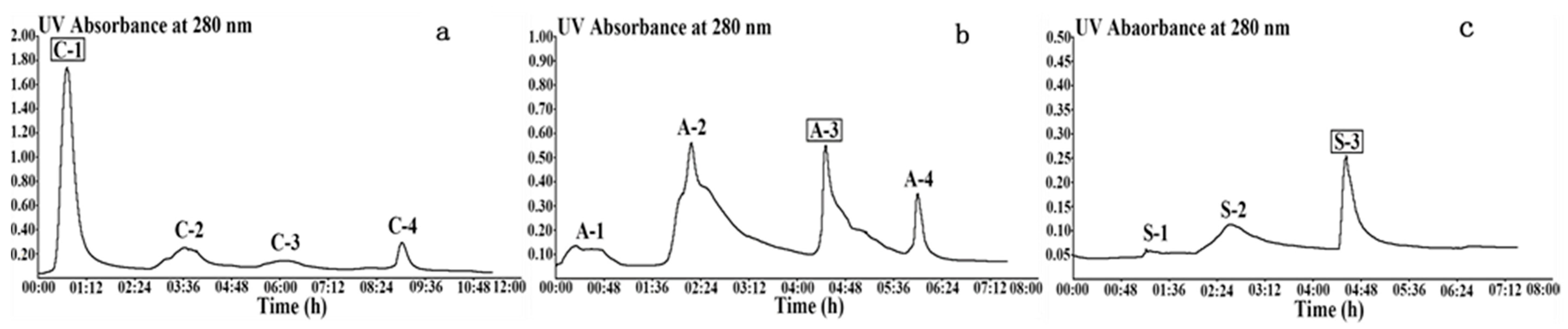
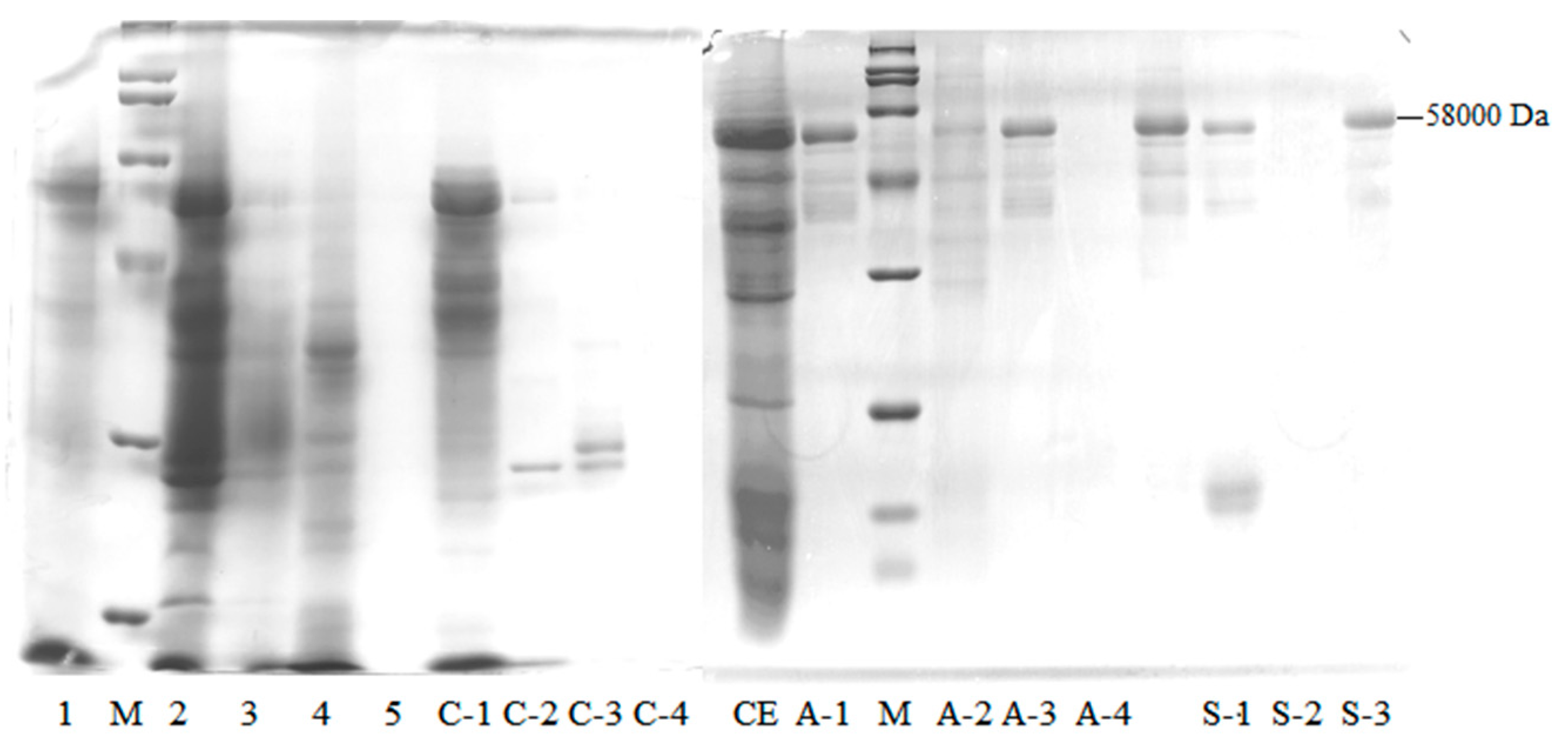

| Fraction | Total Protein (mg) | Endo-1,4-β-Xylanase Activity (u) | Specific Activity (u/mg) | Purification Fold | Recovery Rate (%) | Arabinofuranosidase Activity (u) | β-d-xylosidase Activity (u) |
|---|---|---|---|---|---|---|---|
| Crude enzyme | 8231 ± 286.18 | 3538 ± 106.33 | 0.43 ± 0.02 | 100 | 23445 ± 497.00 | 131825 ± 70.76 | |
| AS0-20 | 857 ± 4.23 | 55 ± 0.80 | 0.06 ± 0.00 | 0.1 | 2 | 129 ± 2.74 | 877 ± 2.74 |
| AS20-40 | 1270 ± 11.06 | 125 ± 0.78 | 0.10 ± 0.00 | 0.2 | 4 | 2588 ± 47.74 | 12043 ± 0.00 |
| C-1 | 1405 ± 42.38 | 649 ± 11.47 | 0.46 ± 0.01 | 1 | 18 | 513 ± 8.55 | 2466 ± 75.62 |
| A-3 | 234 ± 12.93 | 490 ± 0.00 | 2.09 ± 0.12 | 5 | 14 | 36 ± 0.00 | 160 ± 24.25 |
| S-3 | 6.08 ± 0.00 | 23.95 ± 1.33 | 3.94 ± 0.22 | 9 | 0.7 | 0.27 ± 0.07 | 1.47 ± 0.23 |
| Num | Prot_Access | Prot_Describe | Prot-Score | Prot-Mass | Matches_Sig | Sequences_Sig | Prot-Cover | emPAI |
|---|---|---|---|---|---|---|---|---|
| 1 | tr|A0A3B6MY89|A0A3B6MY89_WHEAT | GH10 domain-containing protein OS=Triticum aestivum OX=4565 PE=4 SV=1 | 585 | 60827 | 26 | 23 | 45.5 | 2.36 |
| 2 | tr|A0A3B6LV77|A0A3B6LV77_WHEAT | GH10 domain-containing protein OS=Triticum aestivum OX=4565 PE=4 SV=1 | 465 | 61183 | 21 | 18 | 41.5 | 1.57 |
| 3 | tr|A0A3B6KNQ8|A0A3B6KNQ8_WHEAT | GH10 domain-containing protein OS=Triticum aestivum OX=4565 PE=4 SV=1 | 437 | 61298 | 21 | 19 | 43.2 | 1.84 |
| 4 | tr|Q9ZR33|Q9ZR33_WHEAT | Glycosyltransferase 75 OS=Triticum aestivum OX=4565 GN=rgp PE=2 SV=1 | 153 | 41985 | 6 | 6 | 43.2 | 0.58 |
| 5 | tr|O04869|O04869_HORVU | Phenylalanine ammonia-lyase (Fragment) OS=Hordeum vulgare OX=4513 GN=PAL PE=2 SV=1 | 135 | 54553 | 5 | 5 | 23.5 | 0.34 |
| 6 | tr|W5C539|W5C539_WHEAT | GT75-3 OS=Triticum aestivum OX=4565 PE=2 SV=1 | 132 | 41291 | 5 | 5 | 23.9 | 0.47 |
| 7 | tr|A0A3B6LUY6|A0A3B6LUY6_WHEAT | GH10 domain-containing protein OS=Triticum aestivum OX=4565 PE=4 SV=1 | 112 | 64488 | 7 | 6 | 15.5 | 0.35 |
| 9 | tr|Q94G07|Q94G07_HORVU | 1,4-beta-D xylan xylanohydrolase (Fragment) OS=Hordeum vulgare OX=4513 PE=2 SV=1 | 103 | 55951 | 8 | 8 | 20.7 | 0.58 |
| 8 | tr|P93185|P93185_HORVU | (1,4)-beta-xylan endohydrolase, isoenzyme X-I OS=Hordeum vulgare OX=4513 PE=2 SV=1 | 103 | 48388 | 8 | 8 | 21.5 | 0.69 |
| 10 | tr|A0A3B6KR49|A0A3B6KR49_WHEAT | GH10 domain-containing protein OS=Triticum aestivum OX=4565 PE=4 SV=1 | 100 | 60806 | 7 | 7 | 16.8 | 0.45 |
| 11 | tr|P93187|P93187_HORVU | Xylan endohydrolase isoenzyme X-I OS=Hordeum vulgare OX=4513 PE=4 SV=1 | 100 | 48273 | 7 | 7 | 22.7 | 0.59 |
| 12 | tr|A0A3B6LTQ3|A0A3B6LTQ3_WHEAT | GH10 domain-containing protein OS=Triticum aestivum OX=4565 PE=4 SV=1 | 80 | 61306 | 6 | 5 | 14.9 | 0.3 |
| Num | Entry | Protein Names | Identity |
|---|---|---|---|
| 1 | A0A061FEZ9 | Endo-1,4-beta-xylanase Z (Aegilops tauschii) | 98.70% |
| 2 | Q9XGT8 | (1,4)-beta-xylan endohydrolase (Triticum aestivum) | 94.80% |
| 3 | P93187 | Xylan endohydrolase isoenzyme X-I (Hordeum vulgare) | 90.70% |
| 4 | P93185 | (1,4)-beta-xylan endohydrolase, isoenzyme X-I (Hordeum vulgare) | 90.70% |
| 5 | Q94G07 | 1,4-beta-d xylan xylanohydrolase (Hordeum vulgare) | 89.00% |
| 6 | Q4H019 | Endo-1,4-beta-xylanase (Hordeum vulgare) | 87.90% |
| 7 | Q94G06 | 1,4-beta-d xylan xylanohydrolase (Hordeum vulgare) | 87.40% |
| 8 | Q94G05 | 1,4-beta-d xylan xylanohydrolase (Hordeum vulgare) | 87.00% |
| Num | Sequence | Num | Sequence | Num | Sequence |
|---|---|---|---|---|---|
| 1 | GAVVGGIGLQGHVQNPVGEVIGAAIDRLAK | 11 | DGVRLPIPVGVLKPGITYR | 21 | GNVDGDGDFKFR |
| 2 | GAVVGGIGLQGHVQNPVGEVIGAAIDR | 12 | GHCVFWSVDGDVQQWVK | 22 | HYDVNNEMLHGR |
| 3 | LGDEDIPAYMFK | 13 | LRTEPR | 23 | AKDLEVVLR |
| 4 | GNDPNATPEKYAK | 14 | LEGLVSR | 24 | VFPVDHKAR |
| 5 | LDPEPALFVNDYNVER | 15 | YILVAGR | 25 | TFTVEK |
| 6 | LGDEDIPAYMFKEVAR | 16 | VFPVDHK | 26 | DQLRSAMQSR |
| 7 | FKHYDVNNEMLHGR | 17 | DLEVVLR | 27 | LEGLVSRYAGR |
| 8 | DRLGDEDIPAYMFK | 18 | LGAGAAASVR | 28 | VGGWISLGAAR |
| 9 | YVVEVTTATGKQMLK | 19 | AVEKDGVR | 29 | NLNR DQLR |
| 10 | LPIPVGVLKPGITYR | 20 | QVAWLQGR | 30 | GNVDGDGDFK |
| Degradation Time (h) | 0 | 3.5 | 7 | 12 | 24 |
|---|---|---|---|---|---|
| avDP of WEAX | ND | 25.29 | 21.24 | 19.12 | 16.22 |
| Content of WEAX (mg/mL) | 1.00 | 0.98 | 0.94 | 0.91 | 0.90 |
| Free Ara | ND | ||||
| Free Xyl | ND | ||||
| AXOS2 (μg/mL) | ND | 1.38 | 4.10 | 4.28 | 4.63 |
| AXOS3 (μg/mL) | ND | 2.96 | 7.50 | 10.33 | 11.51 |
| AXOS4 (μg/mL) | ND | 2.57 | 6.68 | 9.70 | 13.87 |
| AXOS5 (μg/mL) | ND | 0.31 | 3.68 | 5.23 | 8.74 |
| AXOS6 (μg/mL) | ND | 1.48 | 5.17 | 9.18 | 14.46 |
| Fixed Modifications | Carbamidomethyl (C) |
|---|---|
| Variable modifications | Oxidation (M) |
| Enzyme | Trypsin |
| Maximum Missed Cleavages | 1 |
| Peptide Mass Tolerance | 20 ppm |
| Fragment Mass Tolerance | 0.6 Da |
| Mass values | Monoisotopic |
| Significance threshold | 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Jin, Y. Purification, Identification, and Characterization of an Endo-1,4-β-Xylanase from Wheat Malt. Molecules 2020, 25, 1572. https://doi.org/10.3390/molecules25071572
Peng Z, Jin Y. Purification, Identification, and Characterization of an Endo-1,4-β-Xylanase from Wheat Malt. Molecules. 2020; 25(7):1572. https://doi.org/10.3390/molecules25071572
Chicago/Turabian StylePeng, Zhaojun, and Yuhong Jin. 2020. "Purification, Identification, and Characterization of an Endo-1,4-β-Xylanase from Wheat Malt" Molecules 25, no. 7: 1572. https://doi.org/10.3390/molecules25071572
APA StylePeng, Z., & Jin, Y. (2020). Purification, Identification, and Characterization of an Endo-1,4-β-Xylanase from Wheat Malt. Molecules, 25(7), 1572. https://doi.org/10.3390/molecules25071572




