Sex-Related Differences of the Effect of Lipoproteins and Apolipoproteins on 10-Year Cardiovascular Disease Risk; Insights from the ATTICA Study (2002–2012)
Abstract
:1. Introduction
2. Results
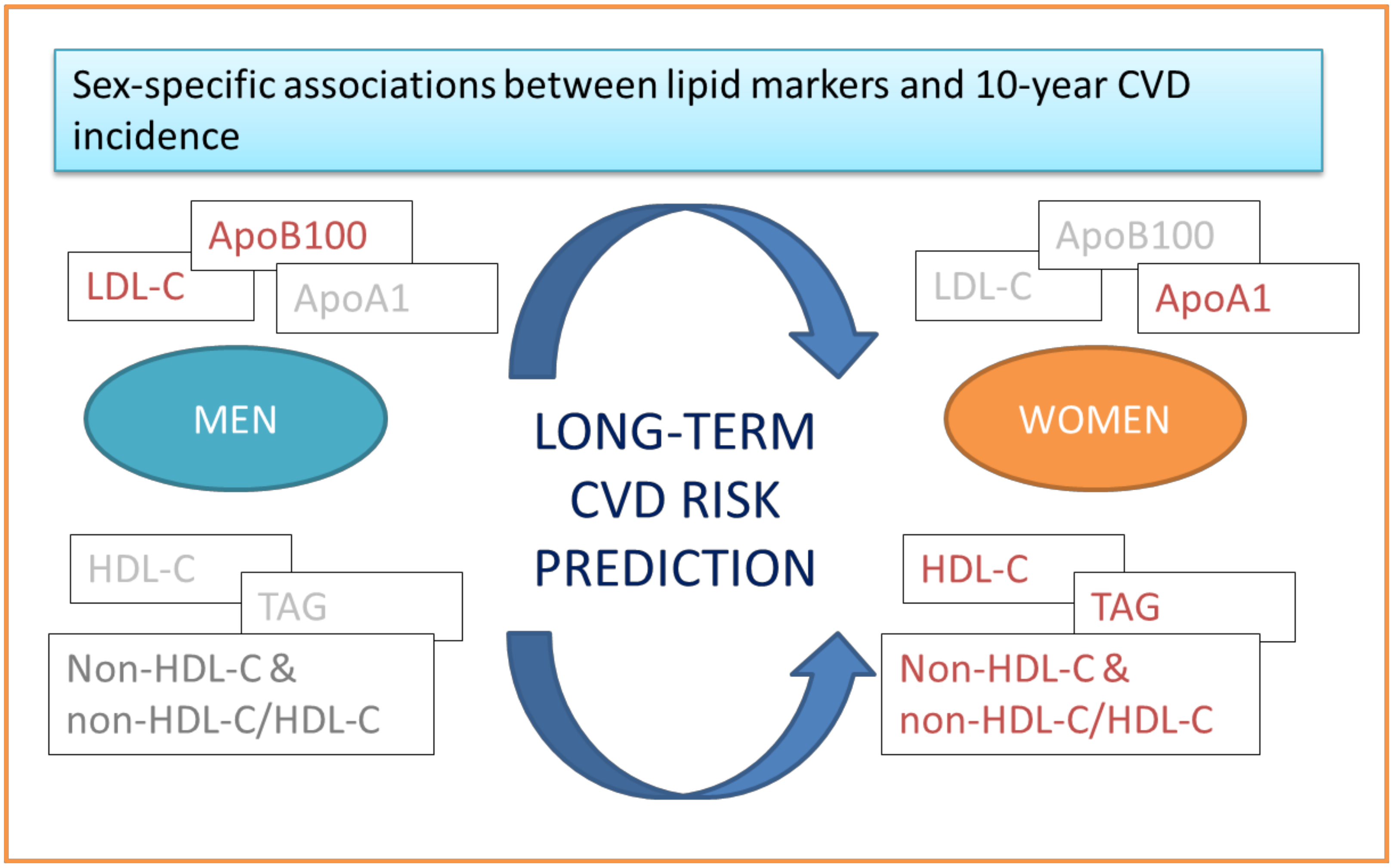
2.1. Conventional Lipid-Related Biomarkers, Sex, and CVD
2.2. Apolipoproteins, Sex, and CVD
2.3. Alternative Lipid-Related Biomarkers, Sex, and CVD
2.4. Sex-Specific Discrimination Ability of Lipid-Related Biomarkers Against CVD
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Study Sample
4.2. Bioethics
4.3. Lipid-Related Markers Measurements at Baseline Examination
4.4. Other Baseline Measurements
4.5. Endpoint and Follow-Up Evaluation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Hear. J. 2016, 37, 3232–3245. [Google Scholar]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.; Lloyd-Jones, D.M.; Newby, L.K.; Piña, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: A guideline from the American Heart Association. J. Am. Coll. Cardiol. 2011, 123, 1243–1262. [Google Scholar]
- Kouvari, M.; Yannakoulia, M.; Souliotis, K.; Panagiotakos, D. Challenges in Sex- and Gender-Centered Prevention and Management of Cardiovascular Disease: Implications of Genetic, Metabolic, and Environmental Paths. Angiology 2018, 69, 843–853. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, A.B.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Hear. J. 2019, 41, 111–188. [Google Scholar]
- Collaborators CTT; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, E.L.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar]
- Emerging Risk Factors Collaboration; Di Angelantonio, E.; Gao, P.; Pennells, L.; Kaptoge, S.; Caslake, M.; Thompson, A.; Butterworth, A.S.; Sarwar, N.; Wormser, D.; et al. Lipid-related markers and cardiovascular disease prediction. JAMA 2012, 307, 2499–2506. [Google Scholar]
- Estrada-Luna, D.; Rodríguez, M.A.O.; Medina-Briseño, L.; Carreón-Torres, E.; Vega, J.A.I.; Sharma, A.; Cancino-Diaz, J.C.; Pérez-Méndez, O.; Belefant-Miller, H.; Betanzos-Cabrera, G. Current Therapies Focused on High-Density Lipoproteins Associated with Cardiovascular Disease. Molecules 2018, 23, 2730. [Google Scholar]
- Albrektsen, G.; Heuch, I.; Løchen, M.-L.; Thelle, D.S.; Wilsgaard, T.; Njølstad, I.; Bønaa, K.H. Risk of incident myocardial infarction by gender: Interactions with serum lipids, blood pressure and smoking. The Tromsø Study 1979–2012. Atherosclerosis 2017, 261, 52–59. [Google Scholar]
- Lu, Y.; Zhou, S.; Dreyer, R.P.; Caulfield, M.; Spatz, E.S.; Geda, M.; Lorenze, N.P.; Herbert, P.; D’Onofrio, G.; Jackson, E.A.; et al. Sex differences in lipid profiles and treatment utilization among young adults with acute myocardial infarction: Results from the VIRGO study. Am. Hear. J. 2016, 183, 74–84. [Google Scholar]
- Madssen, E.; Laugsand, L.E.; Wiseth, R.; Mørkedal, B.; Platou, C.; Vatten, L.; Janszky, I. Risk of Acute Myocardial Infarction. Epidemiology 2013, 24, 637–642. [Google Scholar]
- Mehta, L.S.; Beckie, T.M.; DeVon, H.A.; Grines, C.L.; Krumholz, H.M.; Johnson, M.N.; Lindley, K.J.; Vaccarino, V.; Wang, T.Y.; Watson, K.E.; et al. Acute Myocardial Infarction in Women: A Scientific Statement from the American Heart Association. Circulation 2016, 133, 916–947. [Google Scholar]
- Manson, J.E.; Bassuk, S.S. Biomarkers of cardiovascular disease risk in women. Metabolism 2014, 64, S33–S39. [Google Scholar]
- Calling, S.; Johansson, S.-E.; Wolff, M.; Sundquist, J.; Sundquist, K. The ratio of total cholesterol to high density lipoprotein cholesterol and myocardial infarction in Women’s health in the Lund area (WHILA): A 17-year follow-up cohort study. BMC Cardiovasc. Disord. 2019, 19, 239. [Google Scholar]
- Matthan, N.R.; Zhu, L.; Pencina, M.; D’Agostino, R.B.; Schaefer, E.J.; Lichtenstein, A.H. Sex-Specific Differences in the Predictive Value of Cholesterol Homeostasis Markers and 10-Year Cardiovascular Disease Event Rate in Framingham Offspring Study Participants. J. Am. Hear. Assoc. 2013, 2, e005066. [Google Scholar]
- Brunner, F.J.; Waldeyer, C.; Ojeda, F.; Salomaa, V.; Kee, F.; Sans, S.; Thorand, B.; Giampaoli, S.; Brambilla, P.; Tunstall-Pedoe, H.; et al. Application of non-HDL cholesterol for population-based cardiovascular risk stratification: Results from the Multinational Cardiovascular Risk Consortium. Lancet 2019, 394, 2173–2183. [Google Scholar]
- Drexel, H.; Aczel, S.; Marte, T.; Vonbank, A.; Saely, U.-P.D.C.H. Factors predicting cardiovascular events in statin-treated diabetic and non-diabetic patients with coronary atherosclerosis. Atherosclerosis 2010, 208, 484–489. [Google Scholar]
- Rader, D.J.; Hovingh, G.K. HDL and cardiovascular disease. Lancet 2014, 384, 618–625. [Google Scholar]
- Toth, P.P.; Barter, P.J.; Rosenson, R.S.; Boden, W.E.; Chapman, M.J.; Cuchel, M.; D’Agostino, R.B.; Davidson, M.H.; Davidson, W.S.; Heinecke, J.W.; et al. High-density lipoproteins: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 484–525. [Google Scholar]
- Carr, S.S.; Hooper, A.J.; Sullivan, D.; Burnett, J.R. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology 2019, 51, 148–154. [Google Scholar]
- Mora, S.; Buring, J.E.; Ridker, P.M.; Cui, Y. Association of High-Density Lipoprotein Cholesterol with Incident Cardiovascular Events in Women, by Low-Density Lipoprotein Cholesterol and Apolipoprotein B100 Levels. Ann. Intern. Med. 2011, 155, 742–750. [Google Scholar]
- Cooney, M.T.; Dudina, A.; De Bacquer, D.; Fitzgerald, A.; Conroy, R.; Sans, S.; Menotti, A.; De Backer, G.; Jousilahti, P.; Keil, U.; et al. How much does HDL cholesterol add to risk estimation? A report from the SCORE investigators. Eur. J. Cardiovasc. Prev. Rehabilit. 2009, 16, 304–314. [Google Scholar]
- Von Mühlen, D.; Langer, R.D.; Barrett-Connor, E. Sex and time differences in the associations of non–high-density lipoprotein cholesterol versus other lipid and lipoprotein factors in the prediction of cardiovascular death (The Rancho Bernardo Study). Am. J. Cardiol. 2003, 91, 1311–1315. [Google Scholar]
- Liu, J.; Zeng, F.-F.; Liu, Z.; Zhang, C.-X.; Ling, W.; Chen, Y.-M. Effects of blood triglycerides on cardiovascular and all-cause mortality: A systematic review and meta-analysis of 61 prospective studies. Lipids Heal. Dis. 2013, 12, 159. [Google Scholar]
- Paul, T.K.; Chen, W.; Srinivasan, S.R.; Toprak, A.; He, J.; Berenson, G.S. Gender Divergence on the Impact of Multiple Cardiovascular Risk Factors on the Femoral Artery Intima-Media Thickness in Asymptomatic Young Adults: The Bogalusa Heart Study. Am. J. Med Sci. 2012, 343, 40–45. [Google Scholar]
- Qin, G.; Tu, J.; Zhang, C.; Tang, X.; Luo, L.; Wu, J.; Liu, L.; Lu, W.; Tao, L.; Shen, S.; et al. The value of the apoB/apoAΙ ratio and the non-HDL-C/HDL-C ratio in predicting carotid atherosclerosis among Chinese individuals with metabolic syndrome: A cross-sectional study. Lipids Heal. Dis. 2015, 14, 24. [Google Scholar]
- Shaw, L.J.; BaireyMerz, C.N.; Pepine, C.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Mankad, S.; Sharaf, B.L.; et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. Am. J. Cardiol. 2006, 47, S4–S20. [Google Scholar]
- Yusufali, A.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar]
- Katsouyanni, K.; Rimm, E.B.; Gnardellis, C.; Trichopoulos, D.; Polychronopoulos, E.; Trichopoulou, A. Reproducibility and relative validity of an extensive semi-quantitative food frequency questionnaire using dietary records and biochemical markers among Greek schoolteachers. Int. J. Epidemiol. 1997, 26, 118–127. [Google Scholar]
- Panagiotakos, D.; Milias, G.A.; Pitsavos, C.; Stefanadis, C. MedDietScore: A computer program that evaluates the adherence to the Mediterranean dietary pattern and its relation to cardiovascular disease risk. Comput. Methods Programs Biomed. 2006, 83, 73–77. [Google Scholar]
- Pitsavos, C.; Panagiotakos, D.; Chrysohoou, C.; Stefanadis, C. Epidemiology of cardiovascular risk factors in Greece: Aims, design and baseline characteristics of the ATTICA study. BMC Public Heal. 2003, 3, 32. [Google Scholar]
- Nomikos, T.; Panagiotakos, D.; Georgousopoulou, E.; Metaxa, V.; Chrysohoou, C.; Skoumas, I.; Antonopoulou, S.; Tousoulis, D.; Stefanadis, C.; Pitsavos, C.; et al. Hierarchical modelling of blood lipids’ profile and 10-year (2002-2012) all cause mortality and incidence of cardiovascular disease: The ATTICA study. Lipids Heal. Dis. 2015, 14, 108. [Google Scholar]
- Kouvari, M.; Panagiotakos, D.; Chrysohoou, C.; Georgousopoulou, E.N.; Yannakoulia, M.; Tousoulis, D.; Pitsavos, C. Lipoprotein (a) and 10-year Cardiovascular Disease Incidence in Apparently Healthy Individuals: A Sex-based Sensitivity Analysis from ATTICA Cohort Study. Angiology 2019, 70, 819–829. [Google Scholar]
| Men | With 10-Year CVD Event | Without 10-Year CVD Event | p-value |
| N | 198 | 808 | |
| Age, years | 56 (13) | 43 (12) | <0.001 |
| Body mass index, kg/m2 | 28.3 (4.0) | 27.1 (3.9) | 0.001 |
| Waist circumference, cm | 101.5 (11.3) | 97.0 (12.9) | <0.001 |
| Current smoking, % | 28 | 38 | <0.001 |
| MedDietScore, range 0–55 | 22.4 (6.4) | 24.5 (5.2) | <0.001 |
| History of hypertension, % | 51 | 36 | <0.001 |
| History of diabetes mellitus, % | 22 | 5 | <0.001 |
| History of hypercholesterolemia, % | 58 | 44 | <0.001 |
| TC, mg/dL | 206 (43) | 195 (42) | 0.001 |
| LDL-C, mg/dL | 135 (42) | 125 (37) | 0.01 |
| HDL-C, mg/dL | 44 (13) | 41 (10) | 0.01 |
| TAG, mg/dL | 182 (63) | 133 (86) | <0.001 |
| non-HDL-C, mg/dL | 164 (44) | 151 (43) | <0.001 |
| TC/HDL-C | 5.22 (1.81) | 4.70 (1.52) | 0.001 |
| non-HDL-C/HDL-C | 4.22 (1.81) | 3.70 (1.52) | 0.001 |
| ApoA1, mg/dL | 144 (23) | 147 (25) | 0.13 |
| ApoB100, mg/dL | 124 (27) | 112 (29) | <0.001 |
| Family history of cardiovascular disease, % | 29 | 26 | <0.001 |
| Women | With 10-Year CVD Event | Without 10-Year CVD Event | p-value |
| N | 119 | 895 | |
| Age, years | 59 (12) | 42 (13) | <0.001 |
| Body mass index, kg/m2 | 27.3 (5.1) | 24.9 (4.7) | <0.001 |
| Waist circumference, cm | 89.7 (14.1) | 82.4 (13.3) | <0.001 |
| Current smoking, % | 39 | 45 | <0.001 |
| MedDietScore, range 0–55 | 23.4 (6.4) | 28.1 (6.6) | <0.001 |
| History of hypertension, % | 49 | 20 | <0.001 |
| History of diabetes mellitus, % | 19 | 3 | <0.001 |
| History of hypercholesterolemia, % | 55 | 36 | <0.001 |
| TC, mg/dL | 208 (41) | 189 (40) | <0.001 |
| LDL-C, mg/dL | 131 (36) | 117 (36) | 0.08 |
| HDL-C, mg/dL | 45 (12) | 53 (14) | 0.006 |
| TAG, mg/dL | 126 (66) | 94 (54) | <0.001 |
| non-HDL-C, mg/dL | 156 (40) | 135 (41) | <0.001 |
| TC/HDL-C | 4.47 (2.05) | 3.75 (1.25) | 0.001 |
| non-HDL-C/HDL-C | 3.47 (2.05) | 2.75 (1.25) | 0.001 |
| ApoA1, mg/dL | 158 (23) | 163 (26) | 0.03 |
| ApoB100, mg/dL | 108 (29) | 101 (49) | 0.08 |
| Family history of cardiovascular disease, % | 37 | 29 | <0.001 |
| Men (n = 1006/n = 198 CVD cases) | |||
| Unadjusted (Crude) Model | Age-Adjusted model | Fully Adjusted Model | |
| Model for TC | |||
| TC, per 10 mg/dL increase | 1.10 (1.00, 1.21) | 1.00 (0.90, 1.10) | 1.00 (0.90, 1.10) |
| TC (>200 vs. ≤200 mg/dL)) | 1.75 (1.28, 2.40) | 1.24 (0.87, 1.75) | 1.21 (0.85, 1.73) |
| Model for LDL | |||
| LDL, per 10 mg/dL increase | 1.00 (0.90, 1.10) | 1.00 (0.90, 1.10) | 1.00 (0.90, 1.10) |
| LDL status (>100 vs. ≤100 mg/dL) | 1.57 (1.00, 2.50) | 0.90 (0.54, 1.52) | 1.10 (0.57, 2.13) |
| Model for HDL | |||
| HDL, per 10 mg/dL increase | 0.81 (0.66, 0.90) | 0.81 (0.66, 0.90) | 0.81 (0.66, 1.21) |
| HDL status (<50 vs. ≥50 mg/dL) | 1.42 (0.91, 2.21) | 1.44 (0.87, 2.46) | 1.36 (0.81, 2.31) |
| Model for TAG | |||
| TAG, per 10 mg/dL increase | 1.10 (1.00, 1.21) | 1.00 (0.90, 1.10) | 1.00 (0.90, 1.10) |
| TAG status (>150 vs. ≤150 mg/dL) | 2.29 (1.39, 3.77) | 1.10 (0.63, 1.92) | 1.60 (0.24, 1.49) |
| Women (n = 1014/n = 119 CVD cases) | |||
| Unadjusted (Crude) Model | Age-Adjusted Model | Multi-Adjusted Model | |
| Model for TC | |||
| TC, per 10 mg/dL increase | 1.10 (1.00, 1.21) | 1.00 (0.90, 1.10) | 1.00 (0.90, 1.10) |
| TC (>200 vs. ≤200 mg/dL)) | 2.19 (1.49, 3.23) | 0.96 (0.42, 1.69) | 0.91 (0.58, 1.43) |
| Model for LDL | |||
| LDL, per 10 mg/dL increase | 1.10 (1.00, 1.21) | 1.00 (0.90, 1.10) | 1.00 (0.90, 1.10) |
| LDL status (>100 vs. ≤100 mg/dL) | 2.66 (1.50, 4.72) | 1.19 (0.63, 2.25) | 2.10 (0.72, 2.57) |
| Model for HDL | |||
| HDL, per 10 mg/dL increase | 0.73 (0.66, 0.90) | 0.73 (0.66, 0.90) | 0.73 (0.53, 1.00) |
| HDL status (<40 vs. ≥40 mg/dL) | 1.53 (1.07, 2.17) | 1.65 (1.12, 2.43) | 1.44 (1.17, 2.14) |
| Model for TAG | |||
| TAG, per 10 mg/dL increase | 1.10 (1.00, 1.21) | 1.10 (1.00, 1.21) | 1.10 (1.00, 1.21) |
| TAG status (>150 vs. ≤150 mg/dL) | 2.14 (1.52, 3.03) | 1.60 (1.09, 2.34) | 1.31 (1.01, 2.12) |
| Men (n = 1006/n = 198 CVD cases) | |||
| Unadjusted (Crude) Model | Age-Adjusted Model | Multi-Adjusted Model | |
| Model for ApoB100 | |||
| ApoB100, per 10 mg/dL increase | 1.21 (1.10, 1.34) | 1.10 (1.00, 1.21) | 1.10 (1.00, 1.21) |
| Model for ApoA1 | |||
| ApoA1, per 10 mg/dL increase | 0.81 (0.66, 0.90) | 0.81 (0.66, 0.90) | 0.81 (0.66, 1.21) |
| Model for ApoB100/ApoA1 | |||
| ApoB100/ApoA1, per 1 unit increase | 1.63 (1.03, 2.57) | 1.18 (0.73, 1.89) | 0.93 (0.56, 1.54) |
| Women (n = 1014/n = 119 CVD cases) | |||
| Unadjusted (Crude) Model | Age-Adjusted Model | Multi-Adjusted Model | |
| Model for ApoB100 | |||
| ApoB100, per 10 mg/dL increase | 1.10 (1.00, 1.21) | 1.00 (0.90, 1.10) | 1.00 (0.90, 1.10) |
| Model for ApoA1 | |||
| ApoA1, per 10 mg/dL increase | 0.81 (0.66, 0.90) | 0.90 (0.81, 0.99) | 0.90 (0.81, 0.99) |
| Model for ApoB100/ApoA1 | |||
| ApoB100/ApoA1, per 1 unit increase | 1.40 (0.89, 2.22) | 0.83 (0.34, 2.00) | 0.69 (0.25, 1.88) |
| Total (N/Cases) | Men (N/Cases) | Women (N/Cases) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2020/317 | 1006/198 | 1014/119 | ||||||
| Non-HDL-C | CVD incidence, % | HR (95% CI) | Non-HDL-C | CVD incidence, % | HR (95%CI) | Non-HDL-C | CVD incidence, % | HR (95% CI) |
| per 10 mg/dL | - | 1.10 (1.00, 1.21) | per 10 mg/dL | - | 1.00 (0.90, 1.10) | per 10 mg/dL | - | 1.10 (1.00, 1.21) |
| <100 mg/dL | 5.3 | ref | <100 mg/dL | 7.6 | ref | <100 mg/dL | 4.2 | ref |
| 100–<145 mg/dL | 13.3 | 1.18 (0.61, 2.27) | 100–<145 mg/dL | 18.2 | 2.69 (0.91, 6.50) | 100–<145 mg/dL | 9.4 | 2.32 (0.89, 5.34) |
| 145–<185 mg/dL | 18.0 | 1.16 (0.60, 2.24) | 145–<185 mg/dL | 20.4 | 3.12 (0.89, 4.50) | 145–<185 mg/dL | 14.8 | 3.45 (1.09, 7.43) |
| 185–<220 mg/dL | 20.0 | 2.10 (1.54, 3.26) | 185–<220 mg/dL | 21.6 | 3.04 (1.32, 4.44) | 185–<220 mg/dL | 17.3 | 3.64 (1.10, 5.96) |
| >220 mg/dL | 28.9 | 2.95 (1.24, 4.32) | >220 mg/dL | 33.9 | 3.14 (1.26, 5.10) | >220 mg/dL | 20.6 | 3.79 (1.20, 6.20) |
| Non-HDL-C/HDL-C | CVD incidence, % | HR (95% CI) | Non-HDL-C/HDL-C | CVD incidence, % | HR (95% CI) | Non-HDL-C/HDL-C | CVD incidence, % | HR (95% CI) |
| per 1 unit | - | 1.15 (1.06, 1.26) | per 1 unit | - | 1.13 (1.02, 1.26) | per 1 unit | - | 1.18 (1.02, 1.36) |
| <2.49 | 8.4 | ref | <2.49 | 13.2 | ref | <2.49 | 6.5 | ref |
| 2.49–3.71 | 15.2 | 1.27 (0.85, 1.90) | 2.49–3.71 | 17.7 | 0.95 (0.53, 1.71) | 2.49–3.71 | 12.4 | 1.25 (0.70, 2.21) |
| >3.71 | 22.0 | 1.96 (1.34, 2.86) | >3.71 | 24.4 | 1.35 (0.78, 2.33) | >3.71 | 18.9 | 1.72 (1.10, 3.07) |
| TC/HDL-C | CVD incidence, % | HR (95% CI) | TC/HDL-C | CVD incidence, % | HR (95% CI) | TC/HDL-C | CVD incidence, % | HR (95% CI) |
| per 1 unit | - | 1.15 (1.06, 1.26) | per 1 unit | - | 1.13 (1.02, 1.26) | per 1 unit | - | 1.18 (1.02, 1.36) |
| <3.49 | 8.4 | ref | <3.49 | 13.2 | ref | <3.49 | 6.5 | ref |
| 3.49–4.71 | 15.2 | 1.10 (0.73, 1.66) | 3.49–4.71 | 17.7 | 0.95 (0.53, 1.71) | 3.49–4.71 | 12.4 | 1.25 (0.70, 2.21) |
| >4.71 | 22.0 | 1.54 (1.04, 2.29) | >4.71 | 23.4 | 1.35 (0.78, 2.33) | >4.71 | 18.9 | 1.72 (1.01, 3.07) |
| Models | Model Adjustment Description | C-Index (95% CI) | Correct Classification Rate, % (Total) | Correct Classification Rate, % (Cases) |
|---|---|---|---|---|
| Model 1 | Standard model * adjusted for conventional lipid markers⸠ | Men | ||
| 0.772 (0.713, 0.831) | 83.6 | 33.3 | ||
| Women | ||||
| 0.831 (0.777, 0.886) | 89.6 | 19.6 | ||
| Model 2 | Standard model * adjusted for LDL-C | Men | ||
| 0.830 (0.789, 0.872) | 88.6 | 21.9 | ||
| Women | ||||
| 0.772 (0.728, 0.816) | 82.8 | 10.9 | ||
| Model 3 | Standard model * adjusted for HDL-C & TAG | Men | ||
| 0.784 (0.741, 0.827) | 83.0 | 18.3 | ||
| Women | ||||
| 0.829 (0.795, 0.877) | 89.3 | 24.4 | ||
| Model 4 | Standard model * adjusted for ApoB100/ApoA1 | Men | ||
| 0.833 (0.792, 0.874) | 88.6 | 23.9 | ||
| Women | ||||
| 0.776 (0.734, 0.818) | 82.4 | 13.3 | ||
| Model 5 | Standard model * adjusted for non-HDL-C | Men | ||
| 0.769 (0.729, 0.809) | 82.4 | 13.0 | ||
| Women | ||||
| 0.836 (0.790, 0.869) | 89.1 | 23.3 | ||
| Model 6 | Standard model * adjusted for non-HDL-C/HDL-C | Men | ||
| 0.772 (0.732, 0.812) | 82.2 | 15.0 | ||
| Women | ||||
| 0.833 (0.793, 0.873) | 89.1 | 26.2 | ||
| Model 7 | Standard model * adjusted for TC/HDL-C | Men | ||
| 0.772 (0.732, 0.812) | 82.2 | 15.0 | ||
| Women | ||||
| 0.836 (0.793, 0.873) | 89.1 | 26.2 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouvari, M.; Panagiotakos, D.B.; Chrysohoou, C.; Georgousopoulou, E.N.; Tousoulis, D.; Pitsavos, C. Sex-Related Differences of the Effect of Lipoproteins and Apolipoproteins on 10-Year Cardiovascular Disease Risk; Insights from the ATTICA Study (2002–2012). Molecules 2020, 25, 1506. https://doi.org/10.3390/molecules25071506
Kouvari M, Panagiotakos DB, Chrysohoou C, Georgousopoulou EN, Tousoulis D, Pitsavos C. Sex-Related Differences of the Effect of Lipoproteins and Apolipoproteins on 10-Year Cardiovascular Disease Risk; Insights from the ATTICA Study (2002–2012). Molecules. 2020; 25(7):1506. https://doi.org/10.3390/molecules25071506
Chicago/Turabian StyleKouvari, Matina, Demosthenes B. Panagiotakos, Christina Chrysohoou, Ekavi N. Georgousopoulou, Dimitrios Tousoulis, and Christos Pitsavos. 2020. "Sex-Related Differences of the Effect of Lipoproteins and Apolipoproteins on 10-Year Cardiovascular Disease Risk; Insights from the ATTICA Study (2002–2012)" Molecules 25, no. 7: 1506. https://doi.org/10.3390/molecules25071506
APA StyleKouvari, M., Panagiotakos, D. B., Chrysohoou, C., Georgousopoulou, E. N., Tousoulis, D., & Pitsavos, C. (2020). Sex-Related Differences of the Effect of Lipoproteins and Apolipoproteins on 10-Year Cardiovascular Disease Risk; Insights from the ATTICA Study (2002–2012). Molecules, 25(7), 1506. https://doi.org/10.3390/molecules25071506






