Cellular Internalization of Beta-Carotene Loaded Polyelectrolyte Multilayer Capsules by Raman Mapping
Abstract
:1. Introduction
2. Results
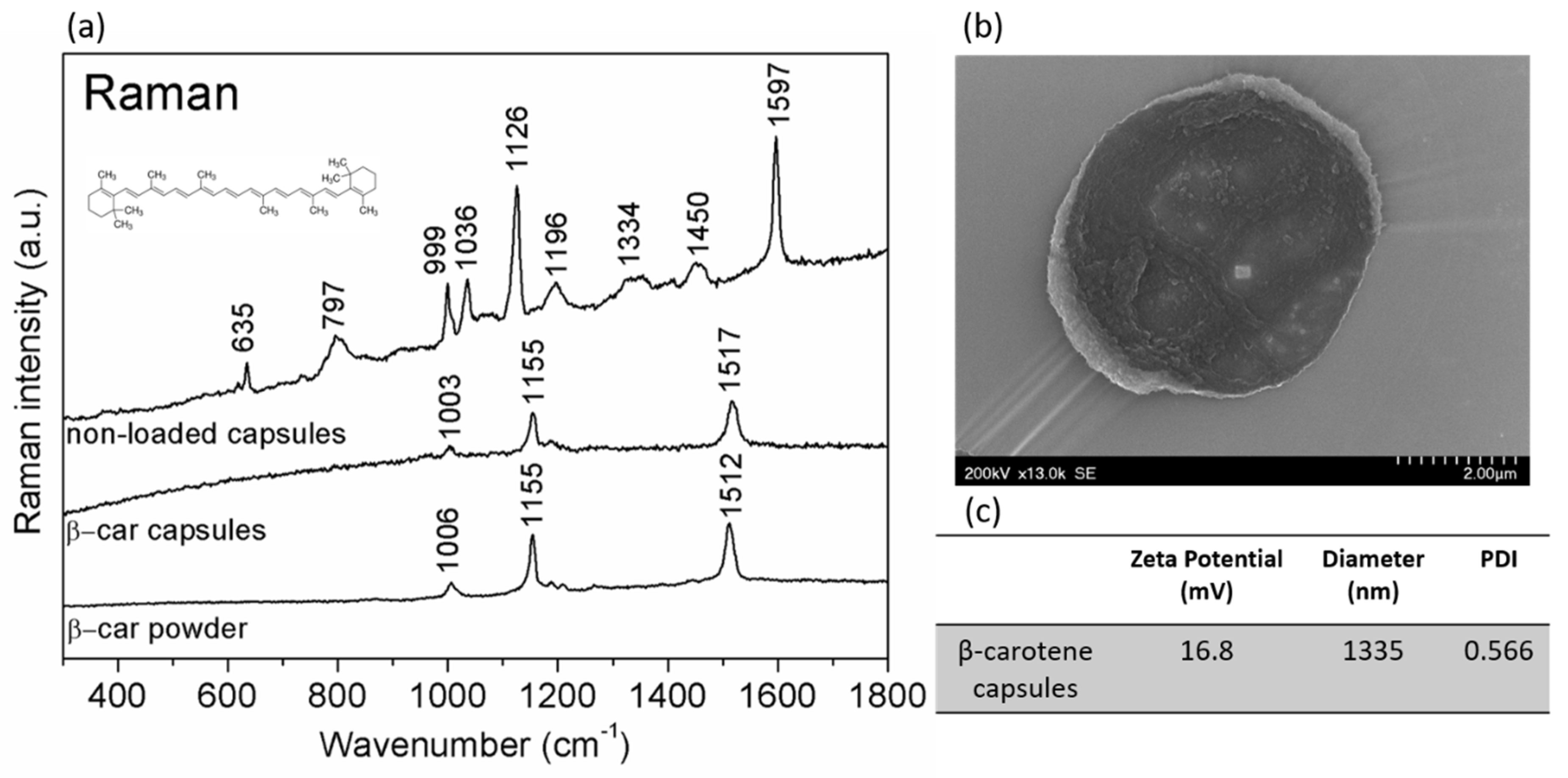
2.1. Capsules Characterization
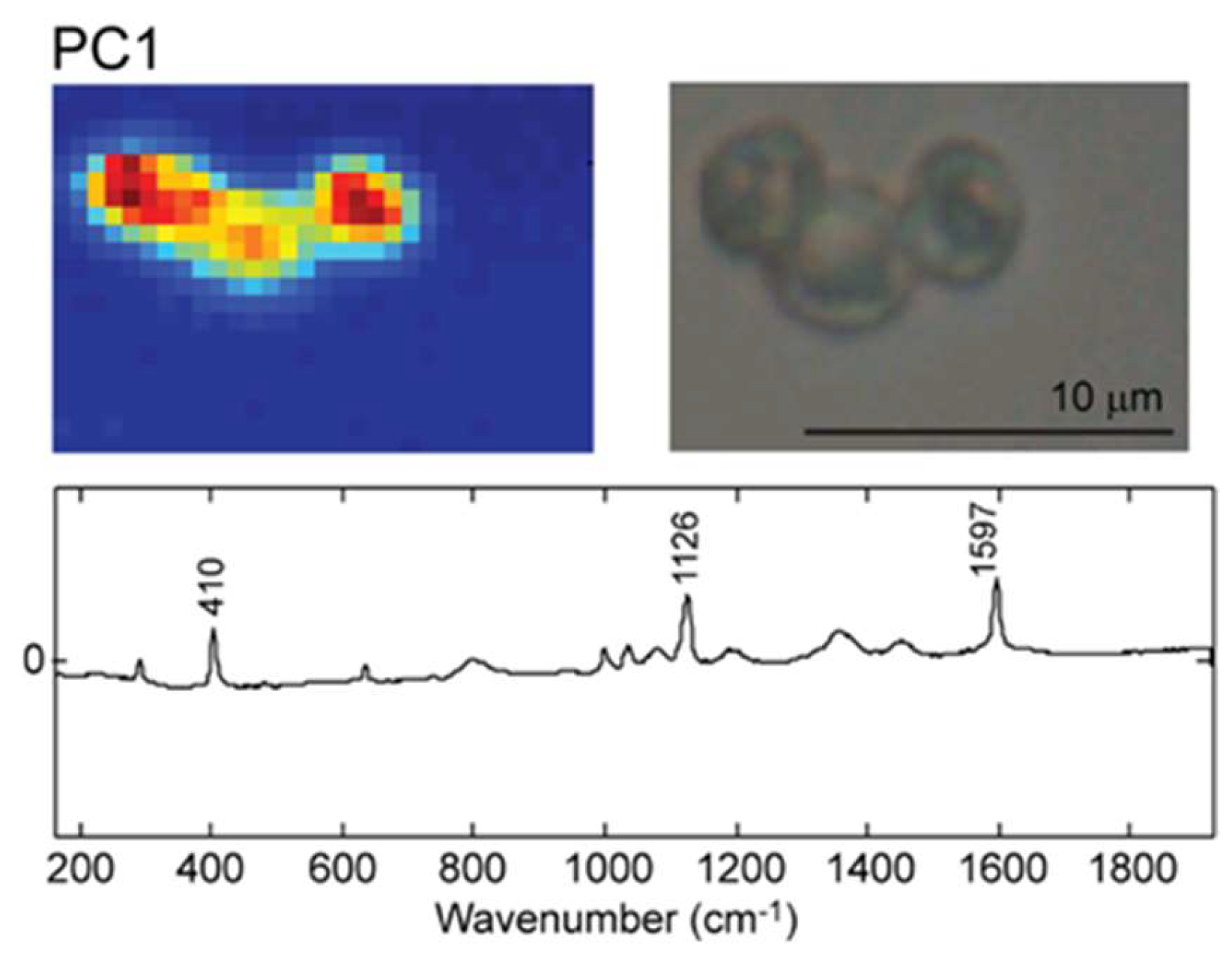
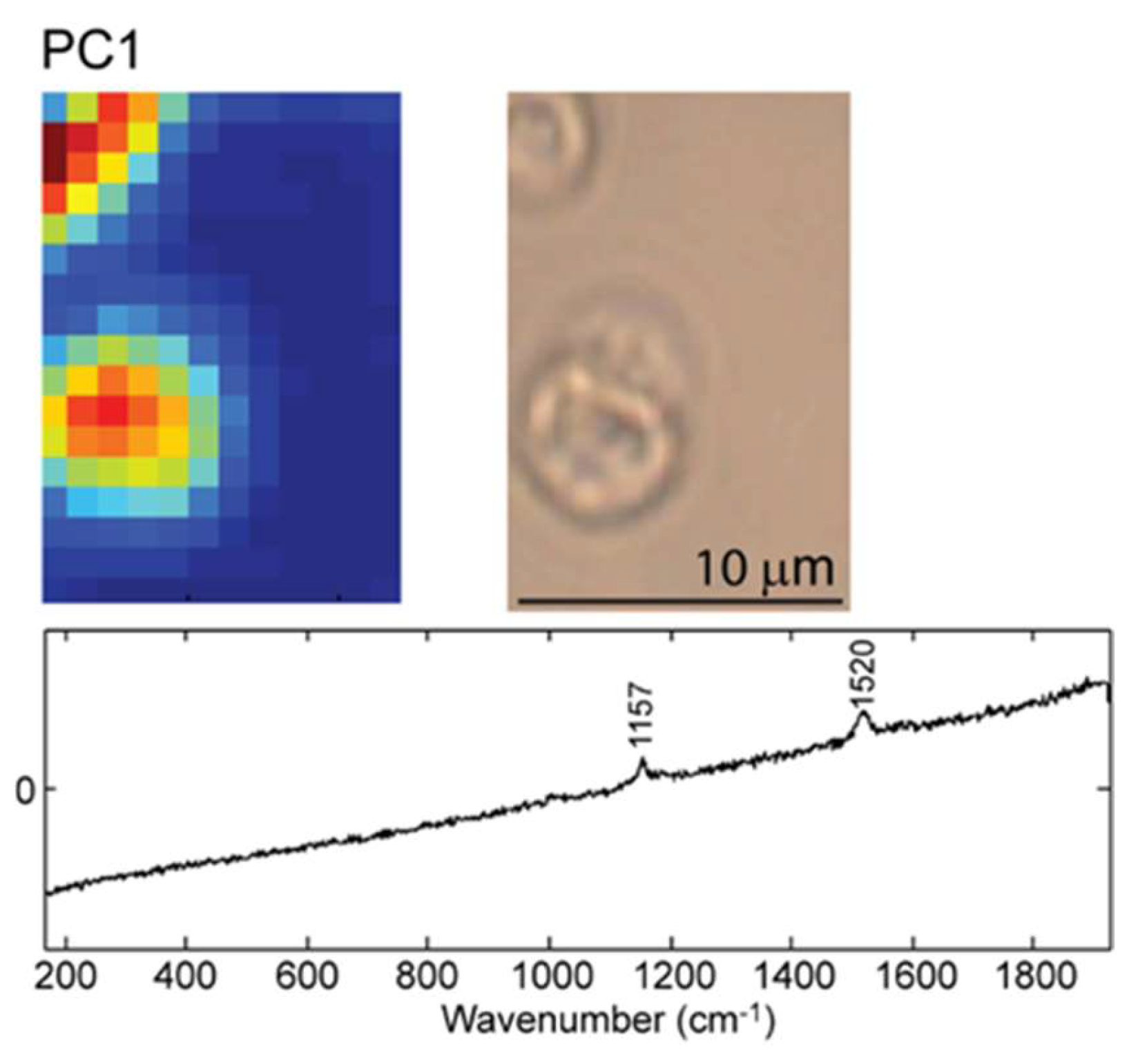
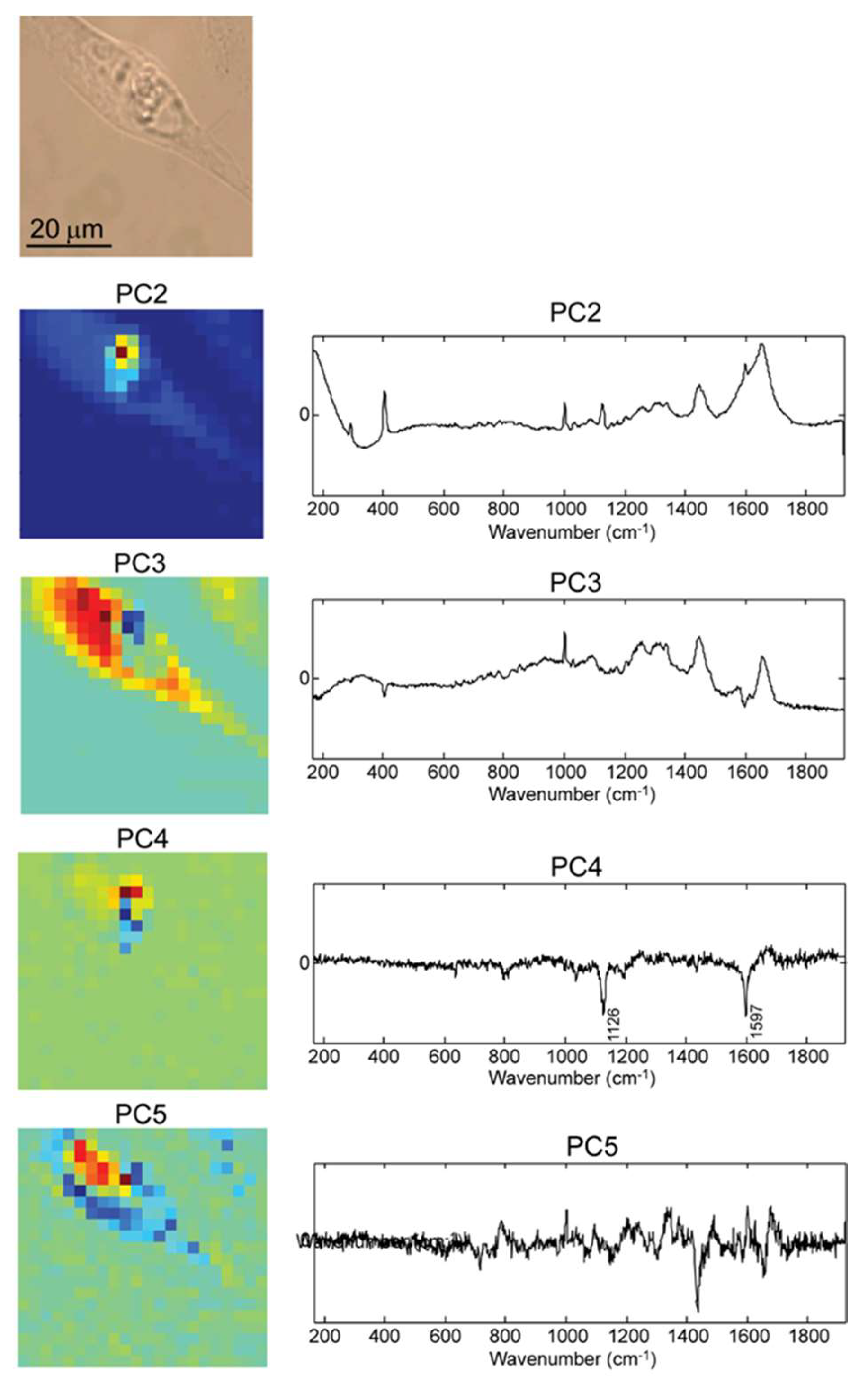
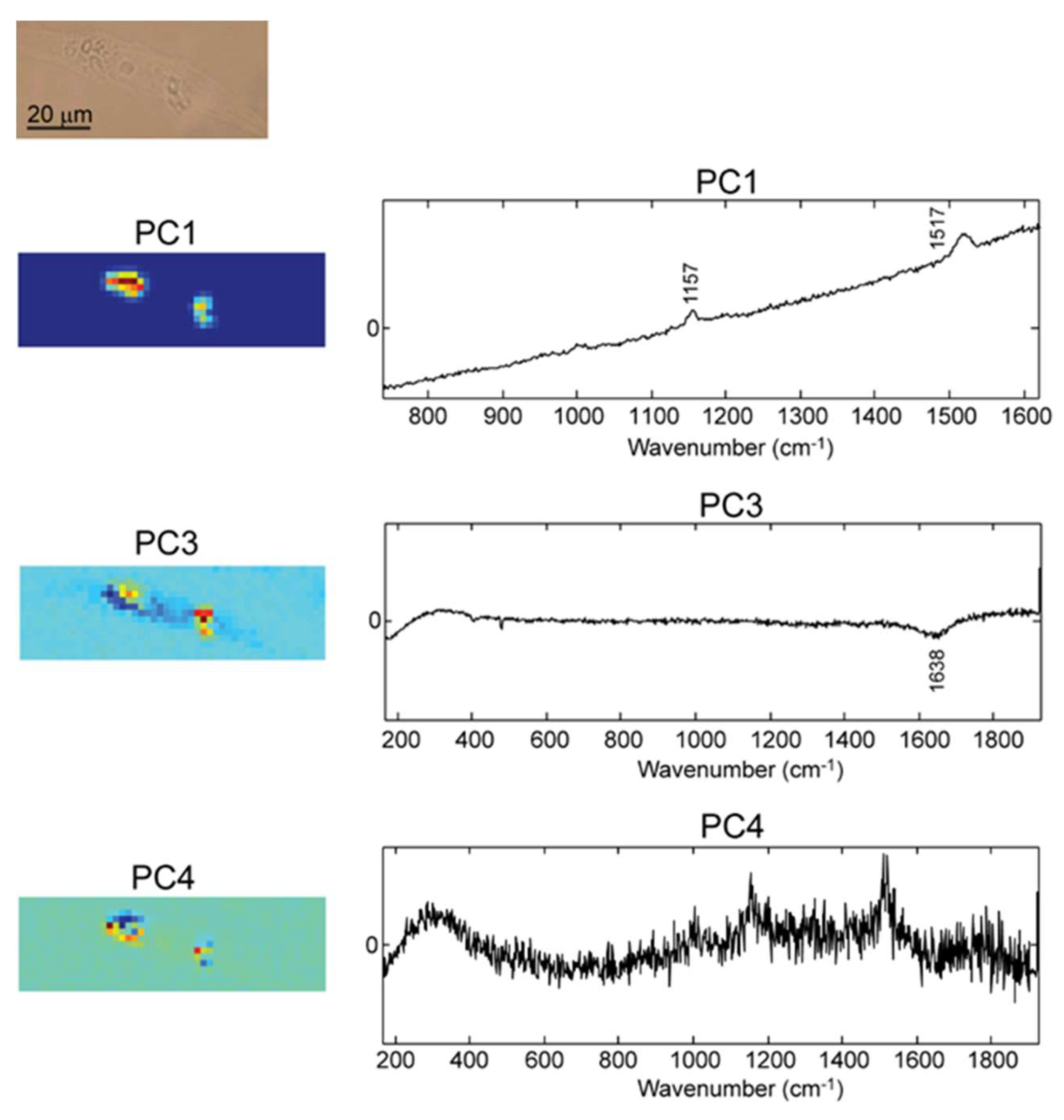
2.2. Raman Mapping
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of Polyelectrolyte Multilayer Capsules
3.3. Beta-Carotene Encapsulation Efficiency
3.4. Cell Culture and Treatment
3.5. Methods
3.5.1. Raman Spectroscopy and Mapping
3.5.2. Multivariate Data Analysis
3.5.3. Physical Characterization of PEM Capsules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De Koker, S.; Hoogenboom, R.; de Geest, B.G. Polymeric multilayer capsules for drug delivery. Chem. Soc. Rev. 2012, 41, 2867–2884. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.P.; Flôres, S.H.; de Oliveira Rios, A.; Chisté, R.C. Biodegradable polymers as wall materials to the synthesis of bioactive compound nanocapsules. Trends Food Sci. Technol. 2016, 53, 23–33. [Google Scholar] [CrossRef]
- Lee, W.L.; Widjaja, E.; Loo, S.C.J. Designing drug-loaded multi-layered polymeric microparticles. J. Mater. Sci. Mater. Med. 2012, 23, 81–88. [Google Scholar] [CrossRef]
- Yang, X.; Han, X.; Zhu, Y. (PAH/PSS)5 microcapsules templated on silica core: Encapsulation of anticancer drug DOX and controlled release study. Colloids Surf. A 2005, 264, 49–54. [Google Scholar] [CrossRef]
- Ariga, K.; Hill, J.P.; Ji, Q. Layer-by-layer assembly as a versatile bottom-up nanofabrication technique for exploratory research and realistic application. PCCP 2007, 9, 2319–2340. [Google Scholar] [CrossRef] [PubMed]
- Donhowe, E.G.; Kong, F. Beta-carotene: Digestion, Microencapsulation, and In Vitro Bioavailability. Food Bioorocess Techmol. 2014, 7, 338–354. [Google Scholar] [CrossRef]
- Singh, B.B.; Shakil, N.A.; Kumar, J.; Walia, S.; Kar, A. Development of slow release formulations of β-carotene employing amphiphilic polymers and their release kinetics study in water and different pH conditions. J. Food Sci. Technol. 2015, 52, 8068–8076. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Liang, R.; Williams, P.A.; Zhong, F. Factors affecting the bioaccessibility of β-carotene in lipid-based microcapsules: Digestive conditions, the composition, structure and physical state of microcapsules. Food Hydrocoll. 2018, 77, 187–203. [Google Scholar] [CrossRef]
- Mihalcea, L.; Turturică, M.; Ghinea, I.O.; Barbu, V.; Ioniţă, E.; Cotârleț, M.; Stănciuc, N. Encapsulation of carotenoids from sea buckthorn extracted by CO2 supercritical fluids method within whey proteins isolates matrices. Innov. Food Sci. Emerg. 2017, 42, 120–129. [Google Scholar] [CrossRef]
- Tan, C.; Xue, J.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Liposome as a delivery system for carotenoids: Comparative antioxidant activity of carotenoids as measured by ferric reducing antioxidant power, DPPH assay and lipid peroxidation. J. Agric. Food. Chem. 2014, 62, 6726–6735. [Google Scholar] [CrossRef]
- Brauchle, E.; Schenke-Layland, K. Raman spectroscopy in biomedicine—non-invasive in vitro analysis of cells and extracellular matrix components in tissues. Biotechnol. J. 2013, 8, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Krafft, C.; Knetschke, T.; Funk, R.H.W.; Salzer, R. Identification of organelles and vesicles in single cells by Raman microspectroscopic mapping. Vib. Spectrosc. 2005, 38, 85–93. [Google Scholar] [CrossRef]
- Kastl, L.; Sasse, D.; Wulf, V.; Hartmann, R.; Mircheski, J.; Ranke, C.; Carregal-Romero, S.; Martinez-Lopez, J.A.; Fernandez-Chacon, R.; Parak, W.J.; et al. Multiple Internalization Pathways of Polyelectrolyte Multilayer Capsules into Mammalian Cells. ACS Nano 2013, 7, 6605–6618. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, V.E.; Castro, H.V.; Edwards, H.G.M.; de Oliveiraa, L.F.C. Carotenes and carotenoids in natural biological samples: A Raman spectroscopic analysis. J. Raman Spectrosc. 2010, 41, 642–650. [Google Scholar] [CrossRef]
- Thomas, D.B.; McGoverin, C.M. The analytical niche for Raman spectroscopy in biological pigment research. Spectrosc. Eur. 2016, 28, 6–9. [Google Scholar]
- Pilát, Z.; Bernatová, S.; Ježek, J.; Šerý, M.; Samek, O.; Zemánek, P.; Nedbal, L.; Trtílek, M. Raman microspectroscopy of algal lipid bodies: β-carotene quantification. J. Appl. Phycol. 2012, 24, 541–546. [Google Scholar] [CrossRef]
- Tong, W.; Dong, W.; Gao, C.; Möhwald, H. Charge-controlled permeability of polyelectrolyte microcapsules. J. Phys. Chem. B 2005, 109, 13159–13165. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Brown, D.R.; Dale, J.A.; Plant, S. Raman spectroscopy of sulfonated polystyrene resins. Vib. Spectrosc. 2000, 24, 213–224. [Google Scholar] [CrossRef]
- Wang, F.; Feng, J.; Tong, W.; Gao, C. A facile pathway to fabricate microcapsules by in situ polyelectrolyte coacervation on poly(styrene sulfonate)-doped CaCO3 particles. J. Mater. Chem. 2007, 17, 670–676. [Google Scholar] [CrossRef]
- De, R.; Lee, H.; Das, B. Exploring the interactions in binary mixtures of polyelectrolytes: Influence of mixture composition, concentration, and temperature on counterion condensation. J. Mol. Liq. 2018, 251, 94–99. [Google Scholar] [CrossRef]
- Maslova, O.A.; Guimbretière, G.; Ammar, M.R.; Desgranges, L.; Jégou, C.; Canizarès, A.; Simon, P. Raman imaging and principal component analysis-based data processing on uranium oxide ceramics. Mater. Charact. 2017, 129, 260–269. [Google Scholar] [CrossRef]
- Notingher, I.; Verrier, S.; Romanska, H.; Bishop, A.E.; Polak, J.M.; Hench, L.L. In situ characterisation of living cells by Raman spectroscopy. Spectroscopy 2002, 16, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Leopold, L.F.; Coman, C.; Todor, T.S.; Szabo, L.; Sorițău, O.; Virag, P.; Mihu, C.M.; Moisoiu, V.; Leopold, N. Raman Imaging of Dental Follicle Mesenchymal Stem Cells. Studia UBB Phys. 2015, 60, 69–76. [Google Scholar]
- Notingher, I.; Hench, L.L. Raman microspectroscopy: A noninvasive tool for studies of individual living cells in vitro. Expert Rev. Med. Devices 2006, 3, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Del Mercato, L.L.; Gonzalez, E.; Abbasi, A.Z.; Parak, W.J.; Puntes, V. Synthesis and evaluation of gold nanoparticle-modified polyelectrolyte capsules under microwave irradiation for remotely controlled release for cargo. J. Mater. Chem. 2011, 21, 11468–11471. [Google Scholar] [CrossRef]
- Pintea, A. HPLC analysis of carotenoids in four varieties of Calendula officinalis L. flowers. Acta Biol. Szeged. 2003, 47, 37–40. [Google Scholar]
- Bonnier, F.; Byrne, H.J. Understanding the molecular information contained in principal component analysis of vibrational spectra of biological systems. Analyst 2012, 137, 322–332. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Not available. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leopold, L.F.; Marișca, O.; Oprea, I.; Rugină, D.; Suciu, M.; Nistor, M.; Tofană, M.; Leopold, N.; Coman, C. Cellular Internalization of Beta-Carotene Loaded Polyelectrolyte Multilayer Capsules by Raman Mapping. Molecules 2020, 25, 1477. https://doi.org/10.3390/molecules25071477
Leopold LF, Marișca O, Oprea I, Rugină D, Suciu M, Nistor M, Tofană M, Leopold N, Coman C. Cellular Internalization of Beta-Carotene Loaded Polyelectrolyte Multilayer Capsules by Raman Mapping. Molecules. 2020; 25(7):1477. https://doi.org/10.3390/molecules25071477
Chicago/Turabian StyleLeopold, Loredana F., Oana Marișca, Ioana Oprea, Dumitrița Rugină, Maria Suciu, Mădălina Nistor, Maria Tofană, Nicolae Leopold, and Cristina Coman. 2020. "Cellular Internalization of Beta-Carotene Loaded Polyelectrolyte Multilayer Capsules by Raman Mapping" Molecules 25, no. 7: 1477. https://doi.org/10.3390/molecules25071477
APA StyleLeopold, L. F., Marișca, O., Oprea, I., Rugină, D., Suciu, M., Nistor, M., Tofană, M., Leopold, N., & Coman, C. (2020). Cellular Internalization of Beta-Carotene Loaded Polyelectrolyte Multilayer Capsules by Raman Mapping. Molecules, 25(7), 1477. https://doi.org/10.3390/molecules25071477





