A Powdered Simulant of Triacetone Triperoxide (TATP) for Safe Testing of X-ray Transmission Screening Equipment
Abstract
1. Introduction
2. Results and Discussion
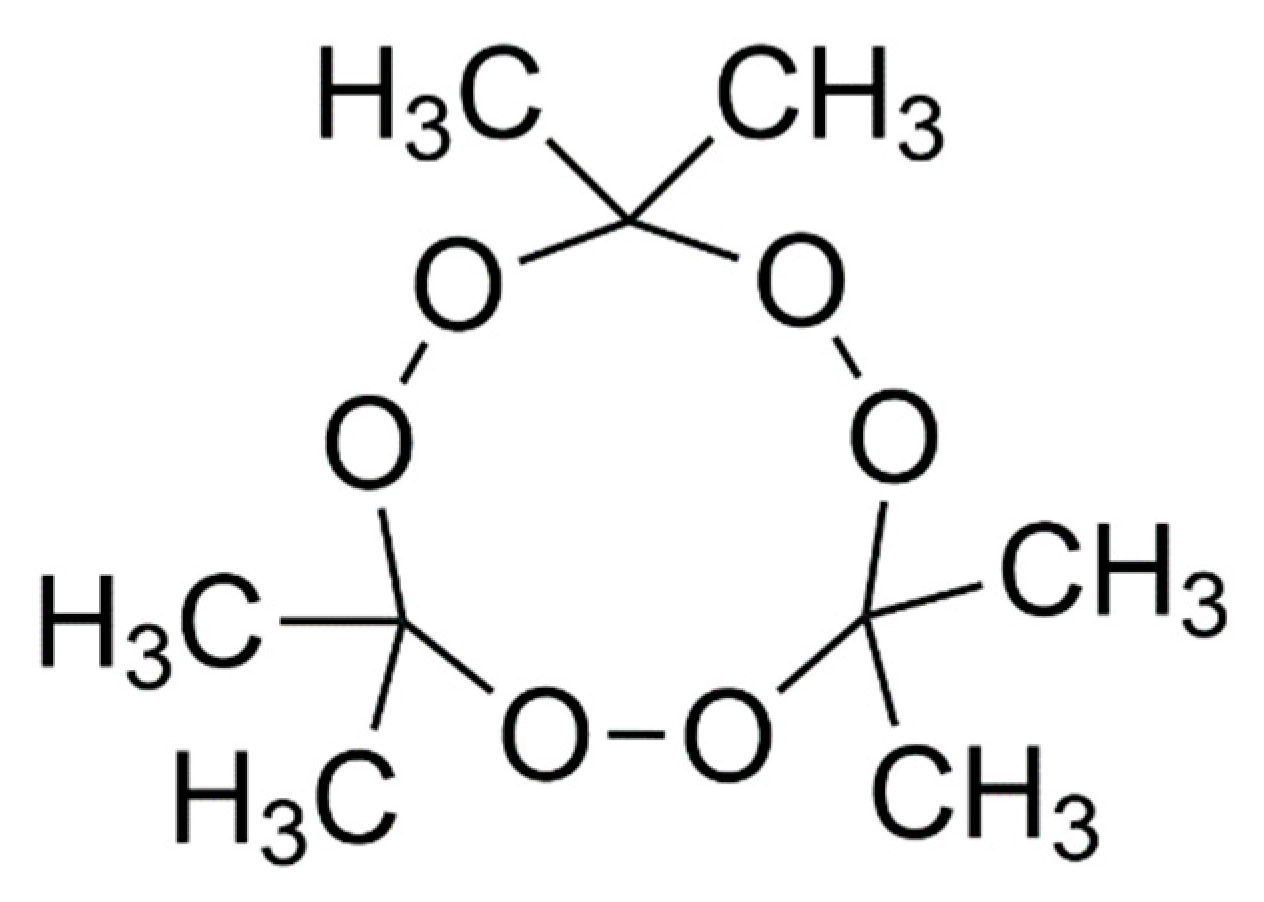
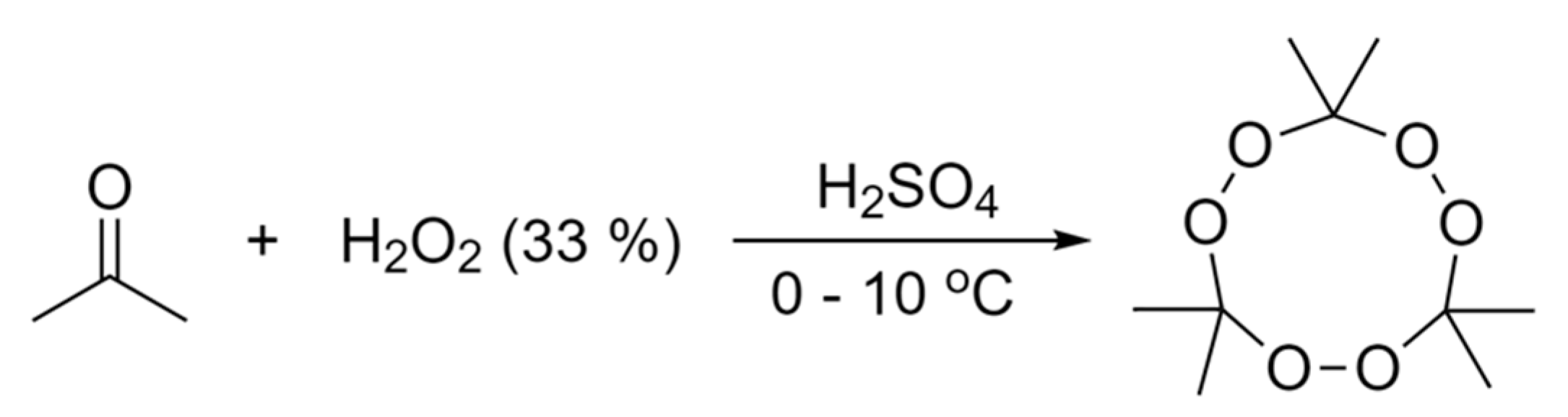
2.1. Production of TATP Simulants
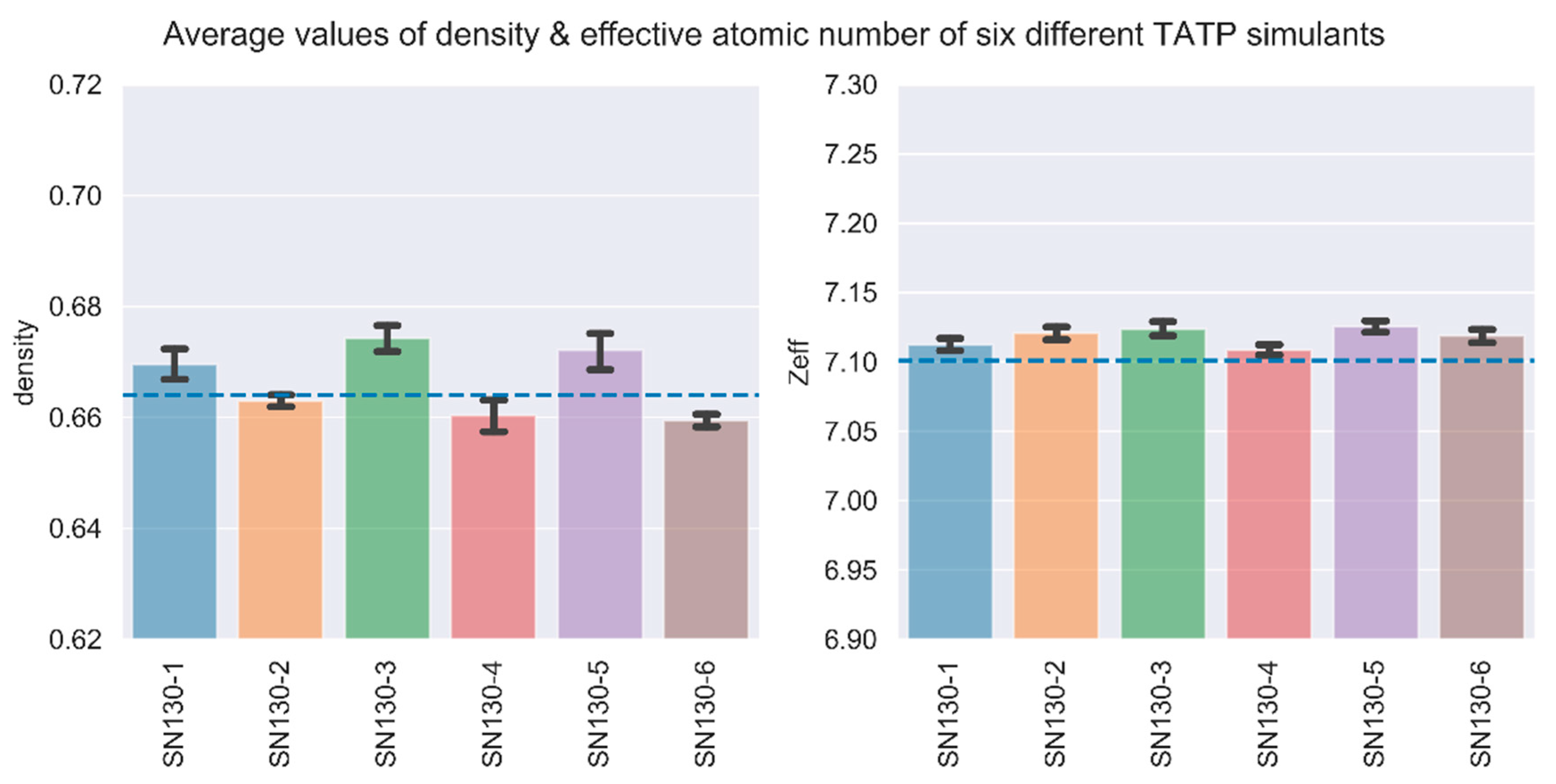
2.2. Accuracy of TATP Simulants
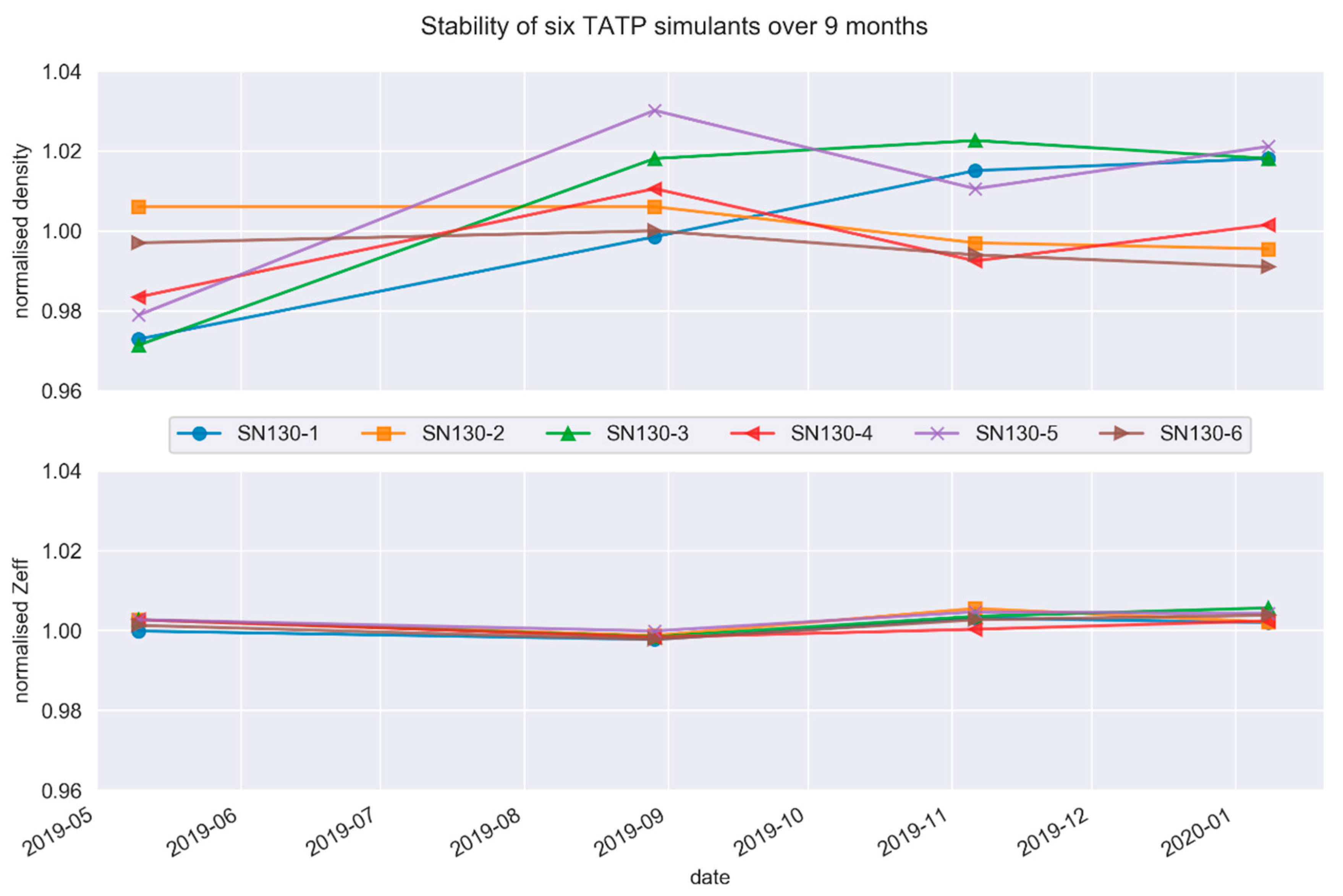
2.3. Stability of Simulants
2.4. Measurement Uncertainty
3. Materials and Methods
3.1. Explosives Detection Systems (EDS)
3.2. TATP Synthesis and Characterisation
3.2.1. In Situ Synthesis
3.2.2. Handling and Transport
3.2.3. Characterisation Testing
3.2.4. Destruction
3.3. Selection of Simulant Ingredients
3.4. Materials
3.5. Equipment for Simulant Production
3.6. Material Processing for Simulant Production
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Regulation (EC) No 300/2008 of the European Parliament and of the Council of 11 March 2008 on Common Rules in the Field of Civil Aviation Security and Repealing Regulation (EC) No 2320/2002 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32008R0300 (accessed on 23 October 2019).
- European Commission—Aviation Security—Legislation. Available online: https://ec.europa.eu/transport/modes/air/security/legislation_en (accessed on 23 October 2019).
- Commission Implementing Regulation (EU) 2015/1998 of 5 November 2015 Laying Down Detailed Measures for the Implementation of the Common Basic Standards on Aviation Security (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32015R1998 (accessed on 23 October 2019).
- ECAC Common Evaluation Process of Security Equipment. Available online: https://www.ecac-ceac.org/cep-main (accessed on 23 October 2019).
- Transportation Security Laboratory. Available online: https://www.dhs.gov/science-and-technology/transportation-security-laboratory (accessed on 23 October 2019).
- Sceibler, H. Memoriam: Richard Wolffenstein. Angew. Chem. 1929, 42, 1149–1151. [Google Scholar] [CrossRef]
- Stiasny, B.W. Investigation of Organic Peroxides and Their Properties as Energetic Materials. Ph.D. Dissertation, Ludwig Maximilian University of Munich, Munich, Germany, 2016. [Google Scholar]
- Vahčič, M.; Anderson, D.; Osés, M.R.; Rarata, G.; Diaconu, G. Development of Inert, Polymer-Bonded Simulants for Explosives Detection Systems Based on Transmission X-ray. Molcules 2019, 24, 4330. [Google Scholar] [CrossRef] [PubMed]
- Bureau International des Poids et Mesures. GUM: Guide to the Expression of Uncertainty in Measurement, Evaluation of Measurement Data; JCGM: Sèvres, France, 2008. [Google Scholar]
- Matyáš, R.; Pachman, J. Study of TATP: Influence of Reaction Conditions on Product Composition. Propellants Explos. Pyrotech. 2009, 35, 31–37. [Google Scholar] [CrossRef]
- Oxley, J.C.; Smith, J.L.; Bowden, P.R.; Rettinger, R.C. Factors Influencing Triacetone Triperoxide (TATP) and Diacetone Diperoxide (DADP) Formation: Part I. Propellants Explos. Pyrotech. 2013, 38, 244–254. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Bilusich, D. Sulfuric, Hydrochloric, and Nitric Acid-Catalyzed Triacetone Triperoxide (TATP) Reaction Mixtures: An Aging Study. J. Forensic Sci. 2011, 56, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Fuentes, E.A.; Peña-Quevedo, A.J.; Pacheco-Londoño, L.C.; Infante-Castillo, R.; Hernández-Rivera, S.P. A review of peroxide based homemade explosives. In Characterization and Detection. Explosive Materials: Classification, Composition and Properties; Janssen, T.J., Ed.; Publisher: Hauppauge, NY, USA, 2011; pp. 259–282. ISBN 978-161761188-9. [Google Scholar]
- Hudson, L.T.; Bateman, F.; Bergstrom, P.; Cerra, F.; Glover, J.; Minniti, R.; Seltzer, S.; Tosh, R. Measurements and standards for bulk-explosives detection. Appl. Radiat. Isot. 2012, 70, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Capua, E.; Cao, R.; Sukenik, C.N.; Naaman, R. Detection of triacetone triperoxide (TATP) with an array of sensors based on non-specific interactions. Sens. Actuators B Chem. 2009, 140, 122–127. [Google Scholar] [CrossRef]
- Wackerbarth, H.; Salb, C.; Gundrum, L.; Niederkrüger, M.; Christou, K.; Beushausen, V.; Viöl, W. Detection of explosives based on surface-enhanced Raman spectroscopy. Appl. Opt. 2010, 49, 4362–4366. [Google Scholar] [CrossRef] [PubMed]
- Haroune, N.; Crowson, A.; Campbell, B. Characterization of triacetone triperoxide (TATP) conformers using LC-NMR. Sci. Justice 2011, 51, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Oxley, J.C.; Smith, J.L.; Shinde, K.; Moran, J. Determination of the Vapor Density of Triacetone Triperoxide (TATP) Using a Gas Chromatography Headspace Tecnhnique. Propellants Explos. Pyrotech. 2005, 30, 127–130. [Google Scholar] [CrossRef]
- Schulte-Ladbeck, R.; Kolla, P.; Karst, U. Trace Analysis of Peroxide-Based Explosives. Anal. Chem. 2003, 75, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Suslick, K.S. A Colorimetric Sensor Array for Detection of Triacetone Triperoxide Vapor. J. Am. Chem. Soc. 2010, 132, 15519–15521. [Google Scholar] [CrossRef] [PubMed]
- Oxley, J.C.; Smith, J.L.; Brady, J.E.; Steinkamp, L. Factors Influencing Destruction of Triacetone Triperoxide (TATP). Propellants Explos. Pyrotech. 2013, 39, 289–298. [Google Scholar] [CrossRef]
- Bellamy, A.J. Triacetone Triperoxide: Its Chemical Destruction. J. Forensic Sci. 1999, 44, 603–608. [Google Scholar] [CrossRef]
- Costantini, M. Destruction of Acetone Peroxides. U.S. Patent 5,003,109, 26 March 1991. [Google Scholar]
- Matyas, R. Chemical Decomposition of Triacetone Triperoxide and Hexamethylene triperoxide diamide. In Proceedings of the NTREM Symposium, Pardubice, Czech Republic, 20 April 2004; pp. 241–247. [Google Scholar]
- Oxley, J.C.; Smith, J.L.; Huang, J.; Luo, W. Destruction of Peroxide Explosives. J. Forensic Sci. 2009, 54, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.; Riches, B.; Rowan, A.; Logan, M. Expedient destruction of organic peroxides including triacetone triperoxide (TATP) in emergency situations. J. Chem. Heal. Saf. 2018, 25, 22–27. [Google Scholar] [CrossRef]
- Bonnin, A.; Duvauchelle, P.; Kaftandjian, V.; Ponard, P. Concept of effective atomic number and effective mass density in dual-energy X-ray computed tomography. Nucl. Instrum. Methods Phys. Res. 2014, 318, 223–231. [Google Scholar] [CrossRef]
- Smith, J.A.; Martz, H.E.; Kallman, J.S. Case for an Improved Effective-Atomic-Number for the Electronic Baggage Scanning Program; LLNL-TR-609327; Lawrence Livermore National Laboratory: Livermore, CA, USA, 2011.
- Bond, K.C.; Smith, J.A.; Treuer, J.N.; Azevedo, S.G.; Kallman, J.S.; Martz, H.E. ZeCalc Algorithm Details; LLNL-TR-609327; Lawrence Livermore National Laboratory: Livermore, CA, USA, 2013.
- Anderson, D. Calculation of Effective Atomic Number, Ze, for the Production of Explosives Simulants for X-Ray Screening Equipment; JRC Technical Report 100571; European Commission Joint Research Centre: Geel, Belgium, 2016. [Google Scholar]
Sample Availability: Samples of the TATP simulants are not available from the authors. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vahčič, M.; Anderson, D.; Seghers, J.; Leys, H.; Ruiz Oses, M.; Rarata, G.; Fernández García, M.; Prados Román, R.; Pellico Escudero, D. A Powdered Simulant of Triacetone Triperoxide (TATP) for Safe Testing of X-ray Transmission Screening Equipment. Molecules 2020, 25, 1473. https://doi.org/10.3390/molecules25061473
Vahčič M, Anderson D, Seghers J, Leys H, Ruiz Oses M, Rarata G, Fernández García M, Prados Román R, Pellico Escudero D. A Powdered Simulant of Triacetone Triperoxide (TATP) for Safe Testing of X-ray Transmission Screening Equipment. Molecules. 2020; 25(6):1473. https://doi.org/10.3390/molecules25061473
Chicago/Turabian StyleVahčič, Mitja, David Anderson, John Seghers, Hanne Leys, Miguel Ruiz Oses, Grzegorz Rarata, Maximino Fernández García, Rosana Prados Román, and Daniel Pellico Escudero. 2020. "A Powdered Simulant of Triacetone Triperoxide (TATP) for Safe Testing of X-ray Transmission Screening Equipment" Molecules 25, no. 6: 1473. https://doi.org/10.3390/molecules25061473
APA StyleVahčič, M., Anderson, D., Seghers, J., Leys, H., Ruiz Oses, M., Rarata, G., Fernández García, M., Prados Román, R., & Pellico Escudero, D. (2020). A Powdered Simulant of Triacetone Triperoxide (TATP) for Safe Testing of X-ray Transmission Screening Equipment. Molecules, 25(6), 1473. https://doi.org/10.3390/molecules25061473





