In Vitro Activities of MMV Malaria Box Compounds against the Apicomplexan Parasite Neospora caninum, the Causative Agent of Neosporosis in Animals
Abstract
1. Introduction
2. Results
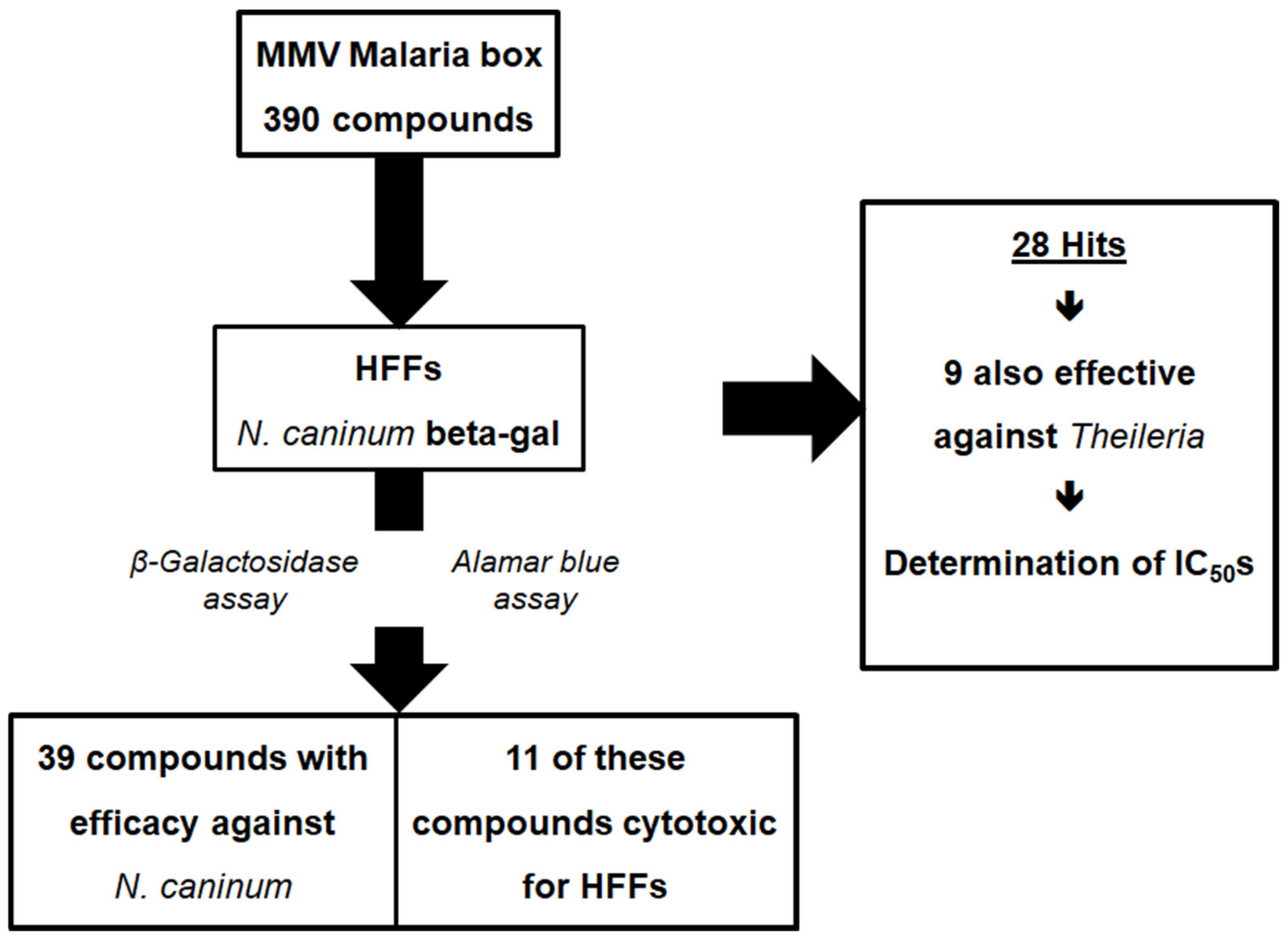
2.1. Differential Screening
2.2. Inhibition Curves
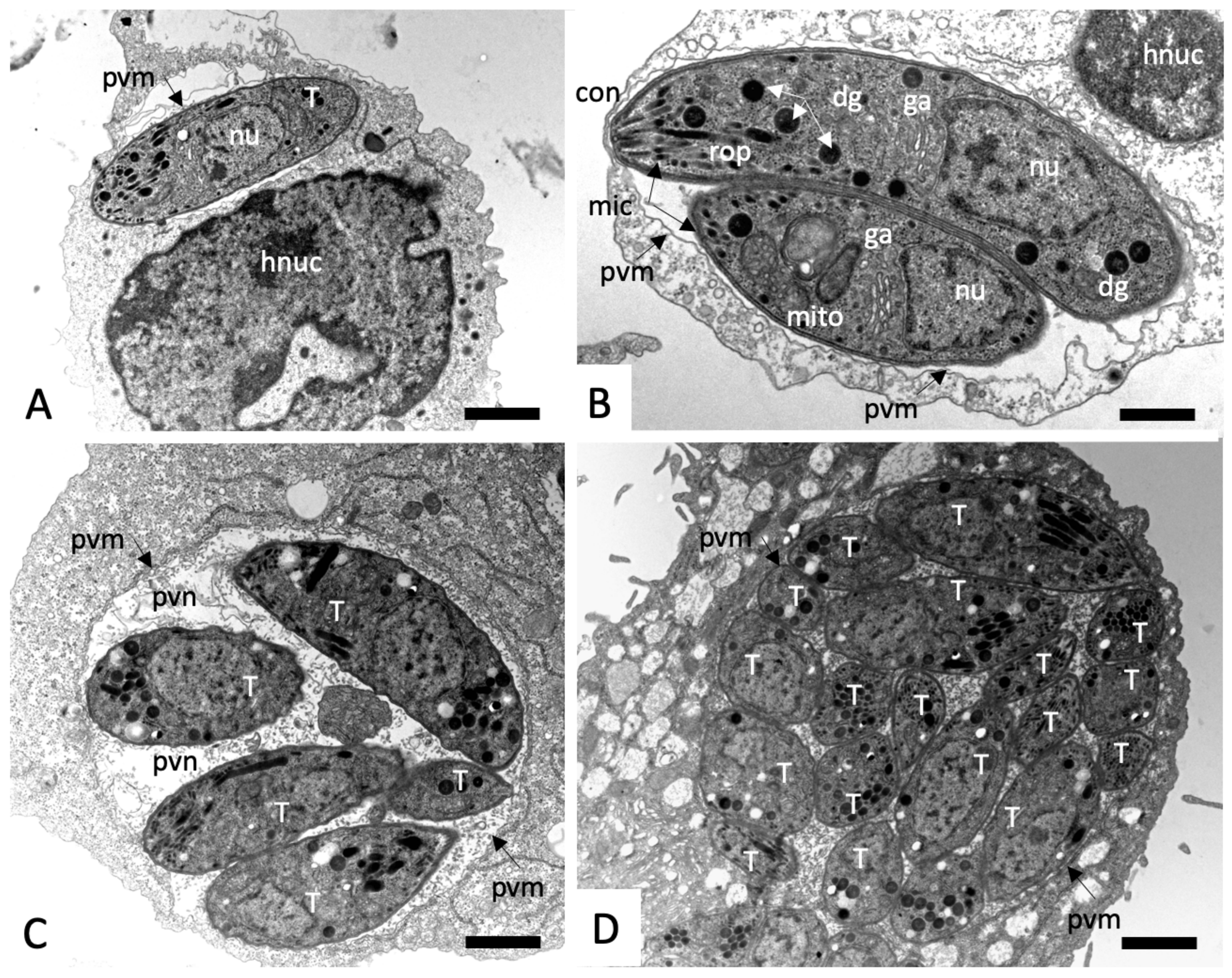
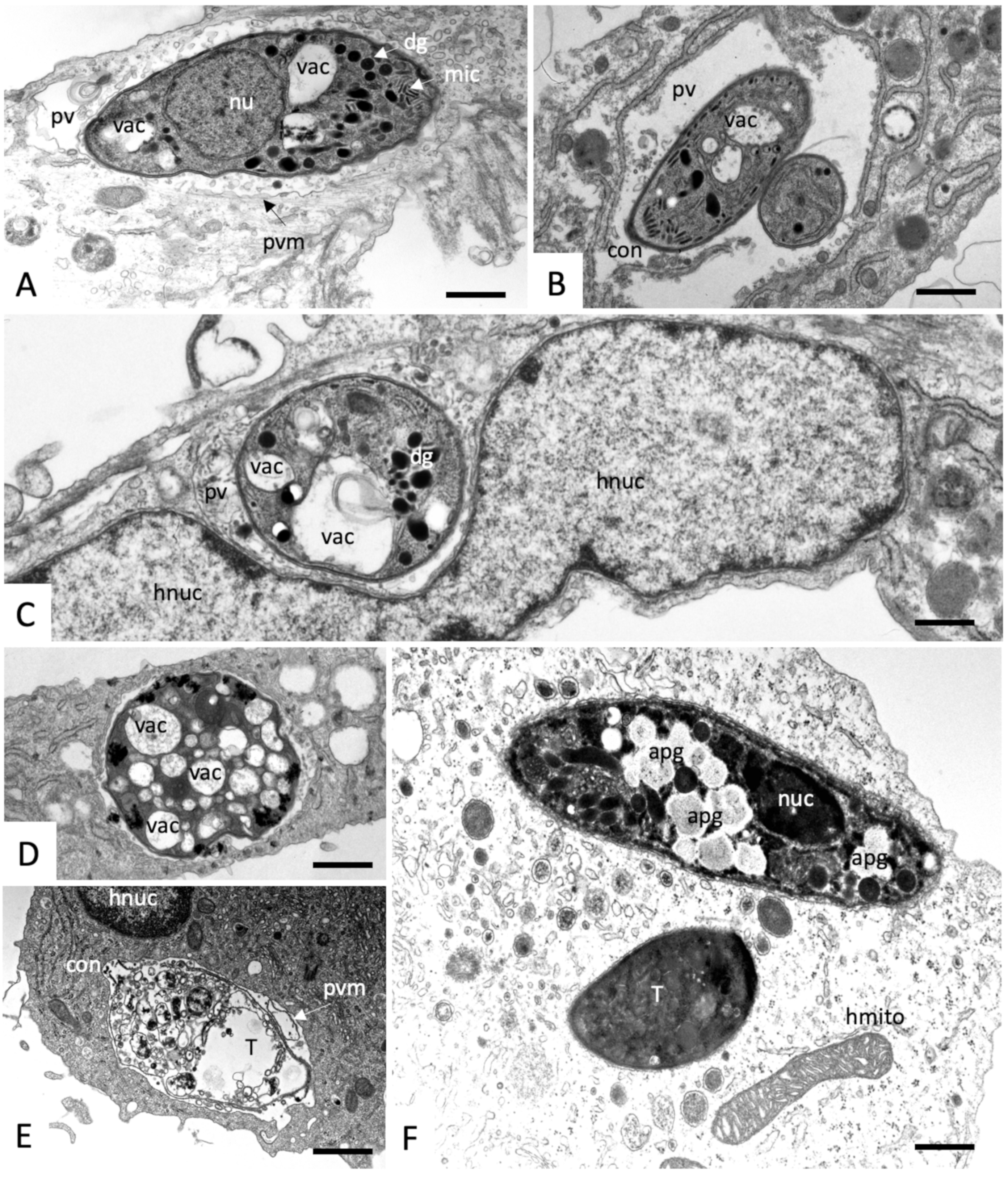
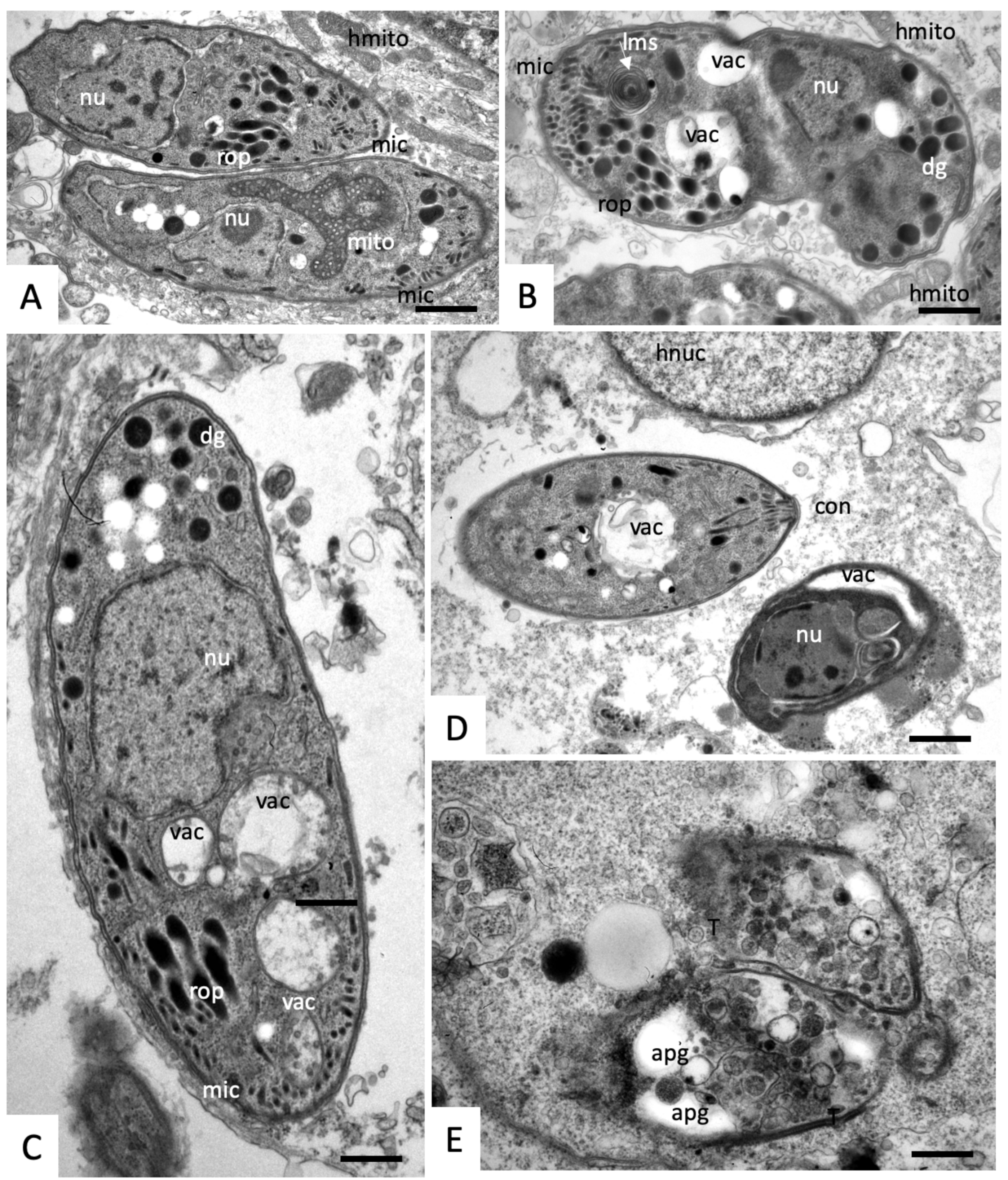
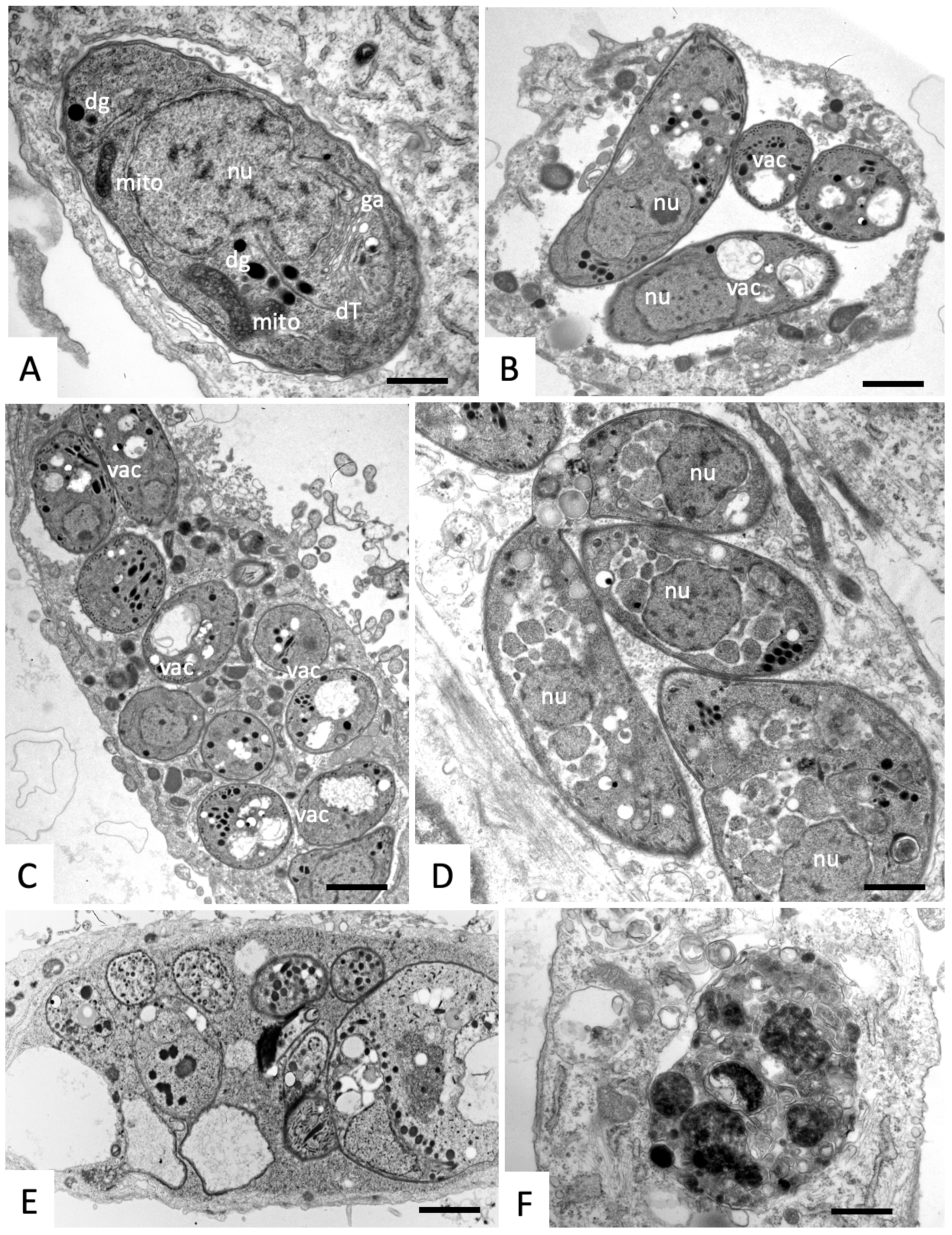
2.3. Effects on the Ultrastructure of N. caninum Tachyzoites
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Differential Screening and Inhibition Curves
4.4. Transmission Electron Microscopy
4.5. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| MMV-N° | MMV-N° | MMV-N° | MMV-N° |
|---|---|---|---|
| 000445 p | 006172 p | 019670 d | 665923 p |
| 000699 p | 006309 p | 019918 d | 665940 d |
| 000787 p | 006389 p | 084434 p | 665941 p |
| 000788 d | 006522 p | 085203 p | 666054 p |
| 000911 d | 008416 p | 665807 d | 666080 d |
| 001246 p | 009085 p | 665830 p | 666687 p |
| 001318 d | 019555 p | 665852 p | 666693 d |
| Parameter | Compound (1) | Compound (2) | Compound (5) |
|---|---|---|---|
| MMV-N° | 665941 | 665807 | 009085 |
| Administered dose p. o. (µmol/kg) | 114 | 119 | 114 |
| Cmax (µM) | 1.09 | 0.062 | 0.308 |
| Tmax (h) | 5.00 | 6.33 | 0.444 |
| T1/2 (h) | NR | NR | 1.48 |
| Tlast (h) | 9.00 | 9.00 | 7.00 |
| AUC0-last (M*h) | 27.2 | 0.264 | 0.475 |
| AUC0-inf (M*h) | NR | NR | 0.486 |
| MRT0-last (h) | 4.80 | 5.24 | 1.55 |
| MRT0-inf (h) | NR | NR | 1.80 |
References
- Goodswen, S.J.; Kennedy, P.J.; Ellis, J.T. A review of the infection, genetics, and evolution of Neospora caninum: From the past to the present. Infect. Genet. Evol. 2013, 13, 133–150. [Google Scholar] [CrossRef]
- Anvari, D.; Saberi, R.; Sharif, M.; Sarvi, S.; Hosseini, S.A.; Moosazadeh, M.; Hosseininejad, Z.; Chegeni, T.N.; Daryani, A. Seroprevalence of Neospora caninum infection in dog population worldwide: A systematic review and meta-analysis. Acta Parasitol. Witold Stefanski Inst. Parasitol. Warszawa Pol. 2020. [Google Scholar] [CrossRef]
- Donahoe, S.L.; Lindsay, S.A.; Krockenberger, M.; Phalen, D.; Slapeta, J. A review of neosporosis and pathologic findings of Neospora caninum infection in wildlife. Int. J. Parasitol. Parasites Wildl. 2015, 4, 216–238. [Google Scholar] [CrossRef] [PubMed]
- Reichel, M.P.; Alejandra Ayanegui-Alcerreca, M.; Gondim, L.F.; Ellis, J.T. What is the global economic impact of Neospora caninum in cattle-the billion dollar question. Int. J. Parasitol. 2013, 43, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Reichel, M.P.; McAllister, M.M.; Pomroy, W.E.; Campero, C.; Ortega-Mora, L.M.; Ellis, J.T. Control options for Neospora caninum–is there anything new or are we going backwards? Parasitology 2014, 141, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, A.; Aguado-Martinez, A.; Müller, J. Approaches for the vaccination and treatment of Neospora caninum infections in mice and ruminant models. Parasitology 2016, 143, 245–259. [Google Scholar] [CrossRef] [PubMed]
- McHardy, N.; Wekesa, L.S.; Hudson, A.T.; Randall, A.W. Antitheilerial activity of BW720C (buparvaquone): A comparison with parvaquone. Res. Vet. Sci. 1985, 39, 29–33. [Google Scholar] [CrossRef]
- Müller, J.; Aguado-Martinez, A.; Manser, V.; Balmer, V.; Winzer, P.; Ritler, D.; Hostettler, I.; Arranz-Solis, D.; Ortega-Mora, L.; Hemphill, A. Buparvaquone is active against Neospora caninum in vitro and in experimentally infected mice. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 16–25. [Google Scholar] [CrossRef]
- Müller, J.; Aguado-Martinez, A.; Manser, V.; Wong, H.N.; Haynes, R.K.; Hemphill, A. Repurposing of antiparasitic drugs: The hydroxy-naphthoquinone buparvaquone inhibits vertical transmission in the pregnant neosporosis mouse model. Vet. Res. 2016, 47, 32. [Google Scholar] [CrossRef]
- Müller, J.; Aguado-Martinez, A.; Ortega-Mora, L.M.; Moreno-Gonzalo, J.; Ferre, I.; Hulverson, M.A.; Choi, R.; McCloskey, M.C.; Barrett, L.K.; Maly, D.J.; et al. Development of a murine vertical transmission model for Toxoplasma gondii oocyst infection and studies on the efficacy of bumped kinase inhibitor (BKI)-1294 and the naphthoquinone buparvaquone against congenital toxoplasmosis. J. Antimicrob. Chemother. 2017, 72, 2334–2341. [Google Scholar] [CrossRef]
- Guiguemde, W.A.; Shelat, A.A.; Bouck, D.; Duffy, S.; Crowther, G.J.; Davis, P.H.; Smithson, D.C.; Connelly, M.; Clark, J.; Zhu, F.; et al. Chemical genetics of Plasmodium falciparum. Nature 2010, 465, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Anderson, M.; Weisman, J.L.; Lu, M.; Choy, C.J.; Boyd, V.A.; Price, J.; Sigal, M.; Clark, J.; Connelly, M.; et al. Evaluation of diarylureas for activity against Plasmodium falciparum. ACS Med. Chem. Lett. 2010, 1, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Gamo, F.J.; Sanz, L.M.; Vidal, J.; de Cozar, C.; Alvarez, E.; Lavandera, J.L.; Vanderwall, D.E.; Green, D.V.; Kumar, V.; Hasan, S.; et al. Thousands of chemical starting points for antimalarial lead identification. Nature 2010, 465, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, D.; Brinker, A.; McNamara, C.; Henson, K.; Kato, N.; Kuhen, K.; Nagle, A.; Adrian, F.; Matzen, J.T.; Anderson, P.; et al. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc. Natl. Acad. Sci. USA 2008, 105, 9059–9064. [Google Scholar] [CrossRef]
- Meister, S.; Plouffe, D.M.; Kuhen, K.L.; Bonamy, G.M.; Wu, T.; Barnes, S.W.; Bopp, S.E.; Borboa, R.; Bright, A.T.; Che, J.; et al. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 2011, 334, 1372–1377. [Google Scholar] [CrossRef]
- Spangenberg, T.; Burrows, J.N.; Kowalczyk, P.; McDonald, S.; Wells, T.N.; Willis, P. The open access malaria box: A drug discovery catalyst for neglected diseases. PLoS ONE 2013, 8, e62906. [Google Scholar] [CrossRef]
- Van Voorhis, W.C.; Adams, J.H.; Adelfio, R.; Ahyong, V.; Akabas, M.H.; Alano, P.; Alday, A.; Aleman Resto, Y.; Alsibaee, A.; Alzualde, A.; et al. Open source drug discovery with the Malaria Box compound collection for neglected diseases and beyond. PLoS Pathogens 2016, 12, e1005763. [Google Scholar] [CrossRef]
- Hostettler, I.; Müller, J.; Hemphill, A. In vitro screening of the open-source Medicines for Malaria Venture Malaria Box reveals novel compounds with profound activities against Theileria annulata schizonts. Antimicrob. Agents Chemother. 2016, 60, 3301–3308. [Google Scholar] [CrossRef]
- Bessoff, K.; Spangenberg, T.; Foderaro, J.E.; Jumani, R.S.; Ward, G.E.; Huston, C.D. Identification of Cryptosporidium parvum active chemical series by repurposing the open access malaria box. Antimicrob. Agents Chemother. 2014, 58, 2731–2739. [Google Scholar] [CrossRef]
- Ullah, I.; Sharma, R.; Mete, A.; Biagini, G.A.; Wetzel, D.M.; Horrocks, P.D. The relative rate of kill of the MMV Malaria Box compounds provides links to the mode of antimalarial action and highlights scaffolds of medicinal chemistry interest. J. Antimicrob. Chemother. 2020, 75, 362–370. [Google Scholar] [CrossRef]
- Dutt, M.K. Staining of depolymerised DNA in mammalian tissues with methyl violet 6B and crystal violet. Folia Histochem. Cytochem. (Krakow) 1980, 18, 79–83. [Google Scholar] [PubMed]
- Djelad, A.; Mokhtar, A.; Khelifa, A.; Bengueddach, A.; Sassi, M. Alginate-whey an effective and green adsorbent for crystal violet removal: Kinetic, thermodynamic and mechanism studies. Int. J. Biol. Macromol. 2019, 139, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, F.R.; Moreno, S.N.; De Souza, W.; Cruz, F.S.; Docampo, R. The mitochondrion of Trypanosoma cruzi is a target of crystal violet toxicity. Mol. Biochem. Parasitol. 1989, 34, 117–126. [Google Scholar] [CrossRef]
- Anghel, N.; Balmer, V.; Müller, J.; Winzer, P.; Aguado-Martinez, A.; Roozbehani, M.; Pou, S.; Nilsen, A.; Riscoe, M.; Doggett, J.S.; et al. Endochin-like quinolones exhibit promising efficacy against Neospora caninum in vitro and in experimentally infected pregnant mice. Front. Vet. Sci. 2018, 5, 285. [Google Scholar] [CrossRef] [PubMed]
- Basto, A.P.; Müller, J.; Rubbiani, R.; Stibal, D.; Giannini, F.; Suss-Fink, G.; Balmer, V.; Hemphill, A.; Gasser, G.; Furrer, J. Characterization of the activities of dinuclear thiolato-bridged arene ruthenium complexes against Toxoplasma gondii. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Basto, A.P.; Anghel, N.; Rubbiani, R.; Müller, J.; Stibal, D.; Giannini, F.; Suss-Fink, G.; Balmer, V.; Gasser, G.; Furrer, J.; et al. Targeting of the mitochondrion by dinuclear thiolato-bridged arene ruthenium complexes in cancer cells and in the apicomplexan parasite Neospora caninum. Metallomics 2019, 11, 462–474. [Google Scholar] [CrossRef]
- Jelk, J.; Balmer, V.; Stibal, D.; Giannini, F.; Süss-Fink, G.; Butikofer, P.; Furrer, J.; Hemphill, A. Anti-parasitic dinuclear thiolato-bridged arene ruthenium complexes alter the mitochondrial ultrastructure and membrane potential in Trypanosoma brucei bloodstream forms. Exp. Parasitol. 2019, 205, 107753. [Google Scholar] [CrossRef]
- Allman, E.L.; Painter, H.J.; Samra, J.; Carrasquilla, M.; Llinas, M. Metabolomic profiling of the Malaria Box reveals antimalarial target pathways. Antimicrob. Agents Chemother. 2016, 60, 6635–6649. [Google Scholar] [CrossRef]
- Lehane, A.M.; Ridgway, M.C.; Baker, E.; Kirk, K. Diverse chemotypes disrupt ion homeostasis in the Malaria parasite. Mol. Microbiol. 2014, 94, 327–339. [Google Scholar] [CrossRef]
- Ortiz, D.; Guiguemde, W.A.; Johnson, A.; Elya, C.; Anderson, J.; Clark, J.; Connelly, M.; Yang, L.; Min, J.; Sato, Y.; et al. Identification of selective inhibitors of the Plasmodium falciparum hexose transporter PfHT by screening focused libraries of anti-malarial compounds. PLoS ONE 2015, 10, e0123598. [Google Scholar] [CrossRef]
- Blume, M.; Rodriguez-Contreras, D.; Landfear, S.; Fleige, T.; Soldati-Favre, D.; Lucius, R.; Gupta, N. Host-derived glucose and its transporter in the obligate intracellular pathogen Toxoplasma gondii are dispensable by glutaminolysis. Proc. Natl. Acad. Sci. USA 2009, 106, 12998–13003. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Aguado, A.; Laleu, B.; Balmer, V.; Ritler, D.; Hemphill, A. In vitro screening of the open source Pathogen Box identifies novel compounds with profound activities against Neospora caninum. Int. J. Parasitol. 2017, 47, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Hemphill, A. New approaches for the identification of drug targets in protozoan parasites. Int. Rev. Cell Mol. Biol. 2013, 301, 359–401. [Google Scholar] [CrossRef] [PubMed]
- Debache, K.; Hemphill, A. Effects of miltefosine treatment in fibroblast cell cultures and in mice experimentally infected with Neospora caninum tachyzoites. Parasitology 2012, 139, 934–944. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds (1) to (9) are available from the Medicines for Malaria Venture. |
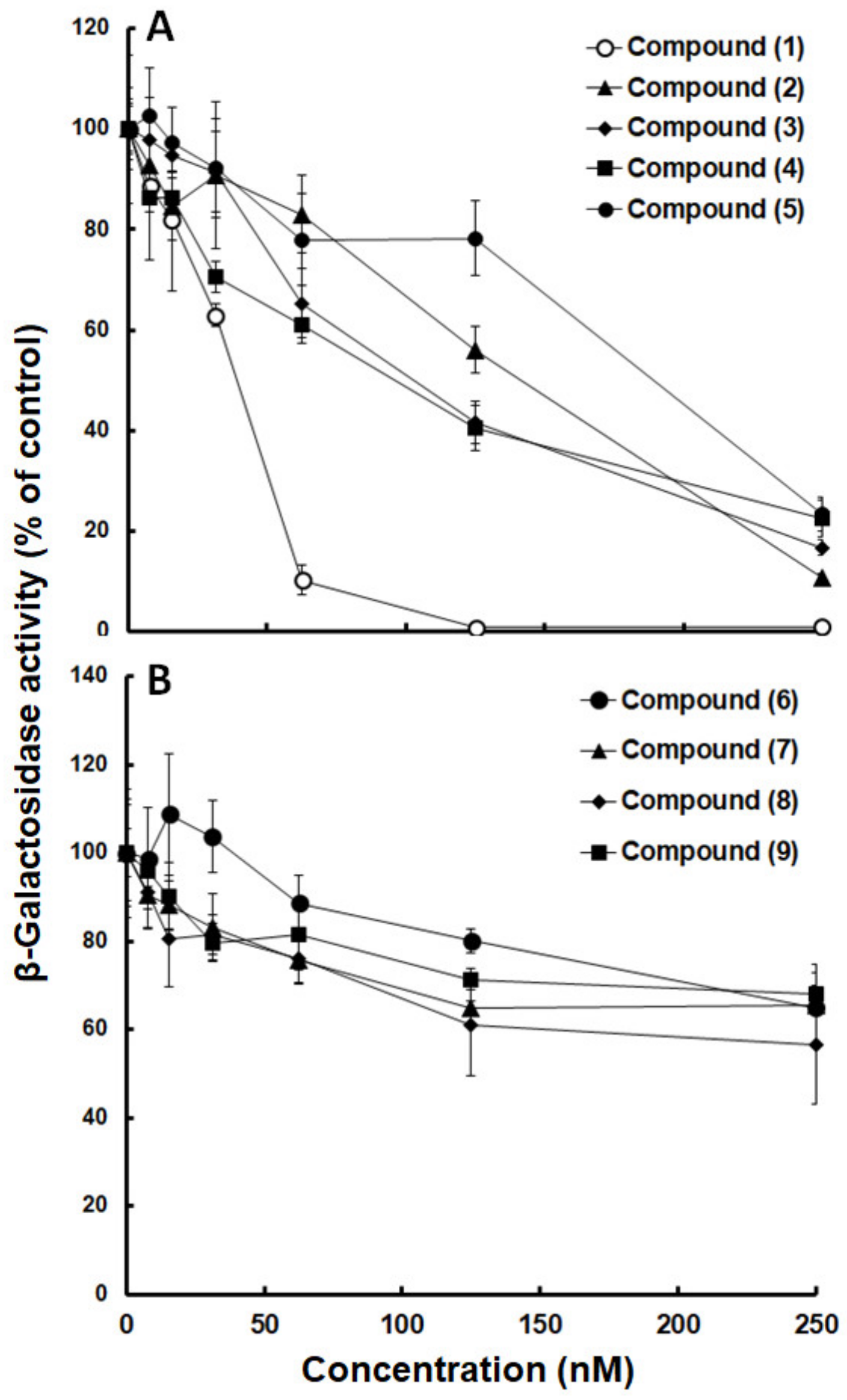

| Compound | MMV-N° | ChEMBL_NTD_ID | Source | LogP | IC50 (nM) |
|---|---|---|---|---|---|
| (1) | 665941 | SJ000140980 | SJ | 4.76 | 25 ± 5 |
| (2) | 665807 | SJ000010289 | SJ | 6.06 | 71 ± 30 |
| (3) | 000787 | SJ000117451 | SJ | 4.71 | 99 ± 10 |
| (4) | 666054 | TCMDC-125549 | G | 6.15 | 79 ± 10 |
| (5) | 009085 | GNF-Pf-3184 | N | −1.30 | 150 ± 30 |
| (6) | 000788 | SJ000117455 | SJ | 4.53 | 280 ± 80 * |
| (7) | 666080 | TCMDC-123701 | G | 4.64 | 550 ± 120 * |
| (8) | 006522 | GNF-Pf-2807 | N | 6.06 | 310 ± 110 * |
| (9) | 006172 | TCMDC-123912 | G | 3.50 | 430 ± 100 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, J.; Winzer, P.A.; Samby, K.; Hemphill, A. In Vitro Activities of MMV Malaria Box Compounds against the Apicomplexan Parasite Neospora caninum, the Causative Agent of Neosporosis in Animals. Molecules 2020, 25, 1460. https://doi.org/10.3390/molecules25061460
Müller J, Winzer PA, Samby K, Hemphill A. In Vitro Activities of MMV Malaria Box Compounds against the Apicomplexan Parasite Neospora caninum, the Causative Agent of Neosporosis in Animals. Molecules. 2020; 25(6):1460. https://doi.org/10.3390/molecules25061460
Chicago/Turabian StyleMüller, Joachim, Pablo A. Winzer, Kirandeep Samby, and Andrew Hemphill. 2020. "In Vitro Activities of MMV Malaria Box Compounds against the Apicomplexan Parasite Neospora caninum, the Causative Agent of Neosporosis in Animals" Molecules 25, no. 6: 1460. https://doi.org/10.3390/molecules25061460
APA StyleMüller, J., Winzer, P. A., Samby, K., & Hemphill, A. (2020). In Vitro Activities of MMV Malaria Box Compounds against the Apicomplexan Parasite Neospora caninum, the Causative Agent of Neosporosis in Animals. Molecules, 25(6), 1460. https://doi.org/10.3390/molecules25061460








