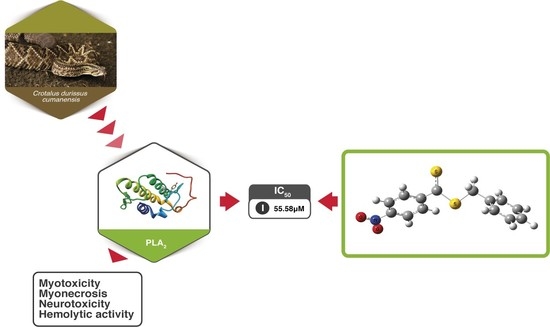
Sulfur Compounds as Inhibitors of Enzymatic Activity of a Snake Venom Phospholipase A2: Benzyl 4-nitrobenzenecarbodithioate as a Case of Study
Abstract
1. Introduction
2. Results
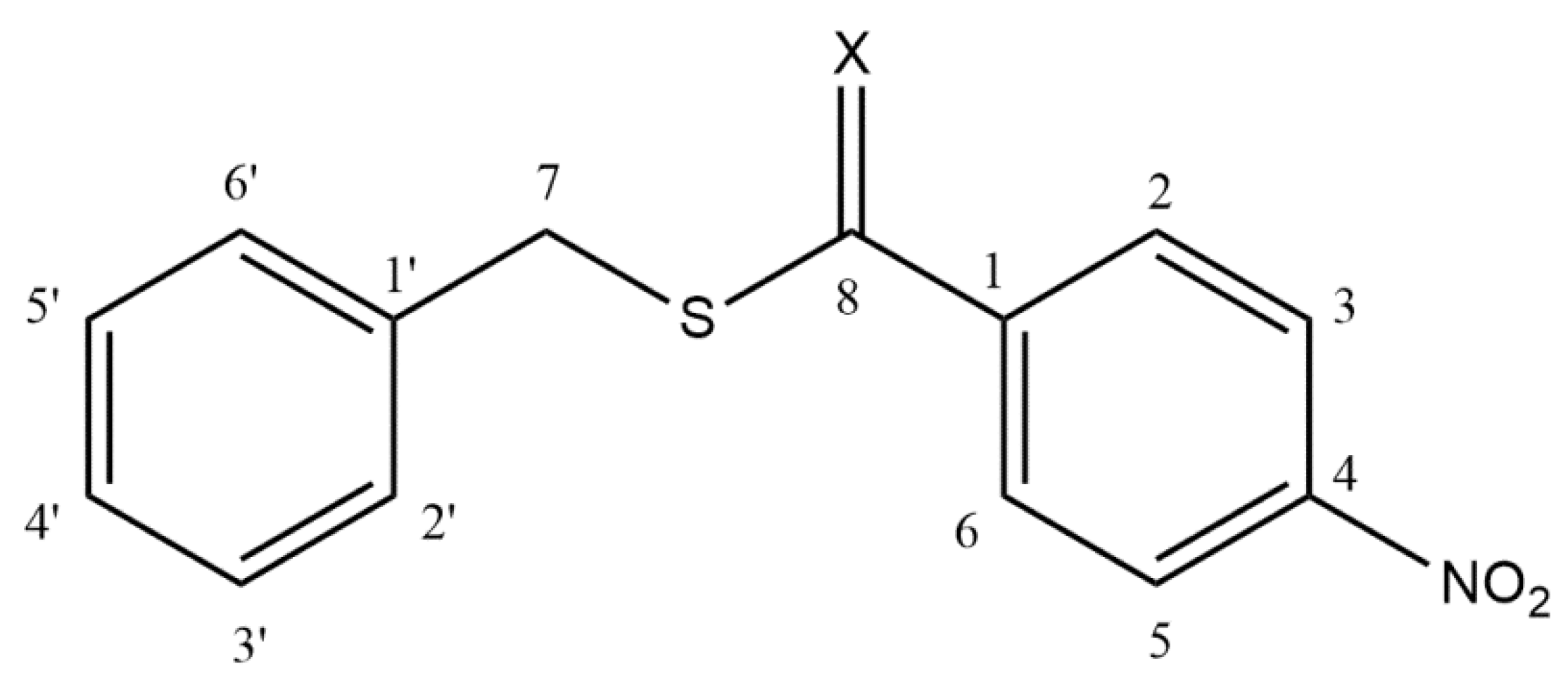
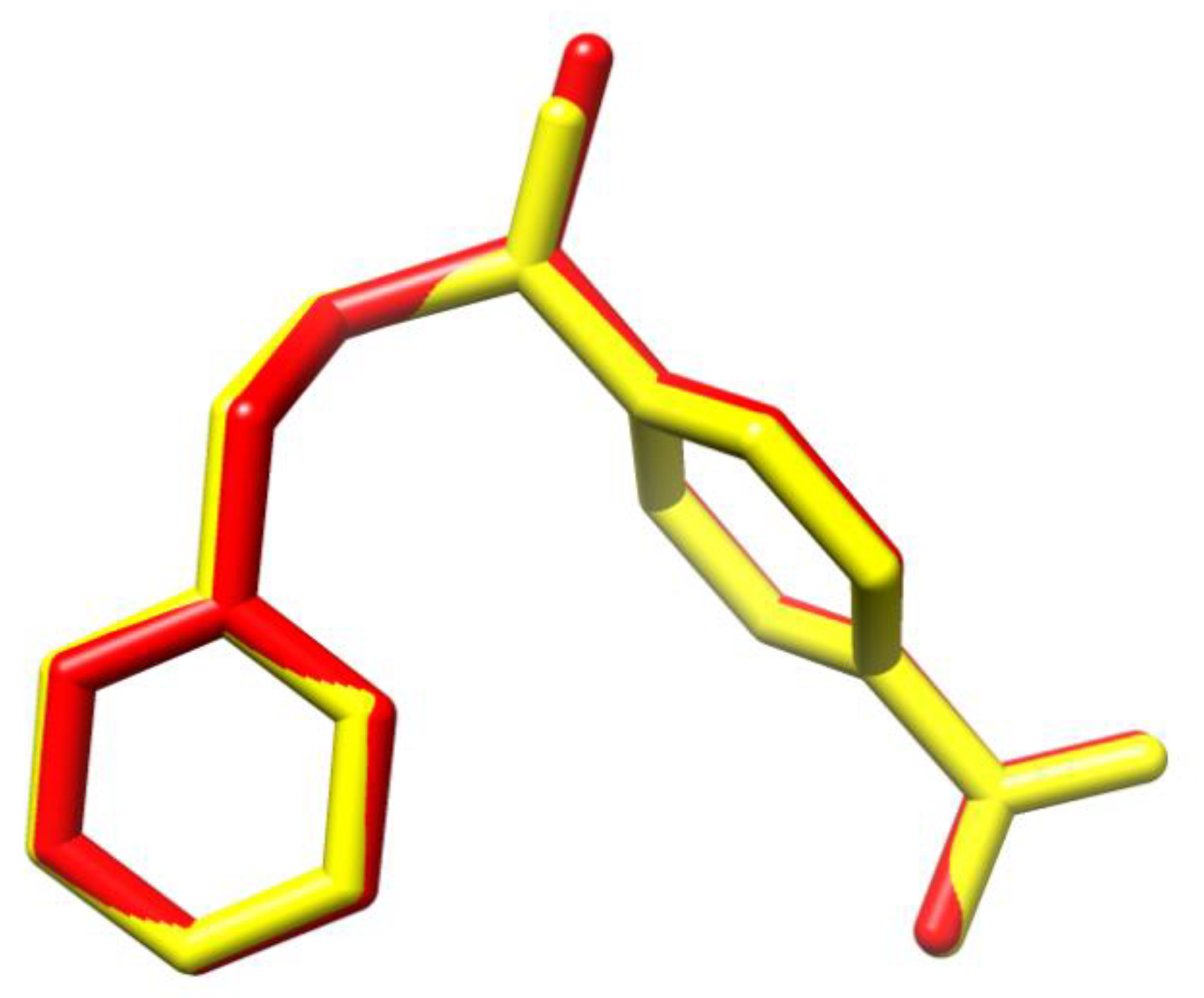
2.1. Conformational Analysis
2.2. Vibrational Analysis
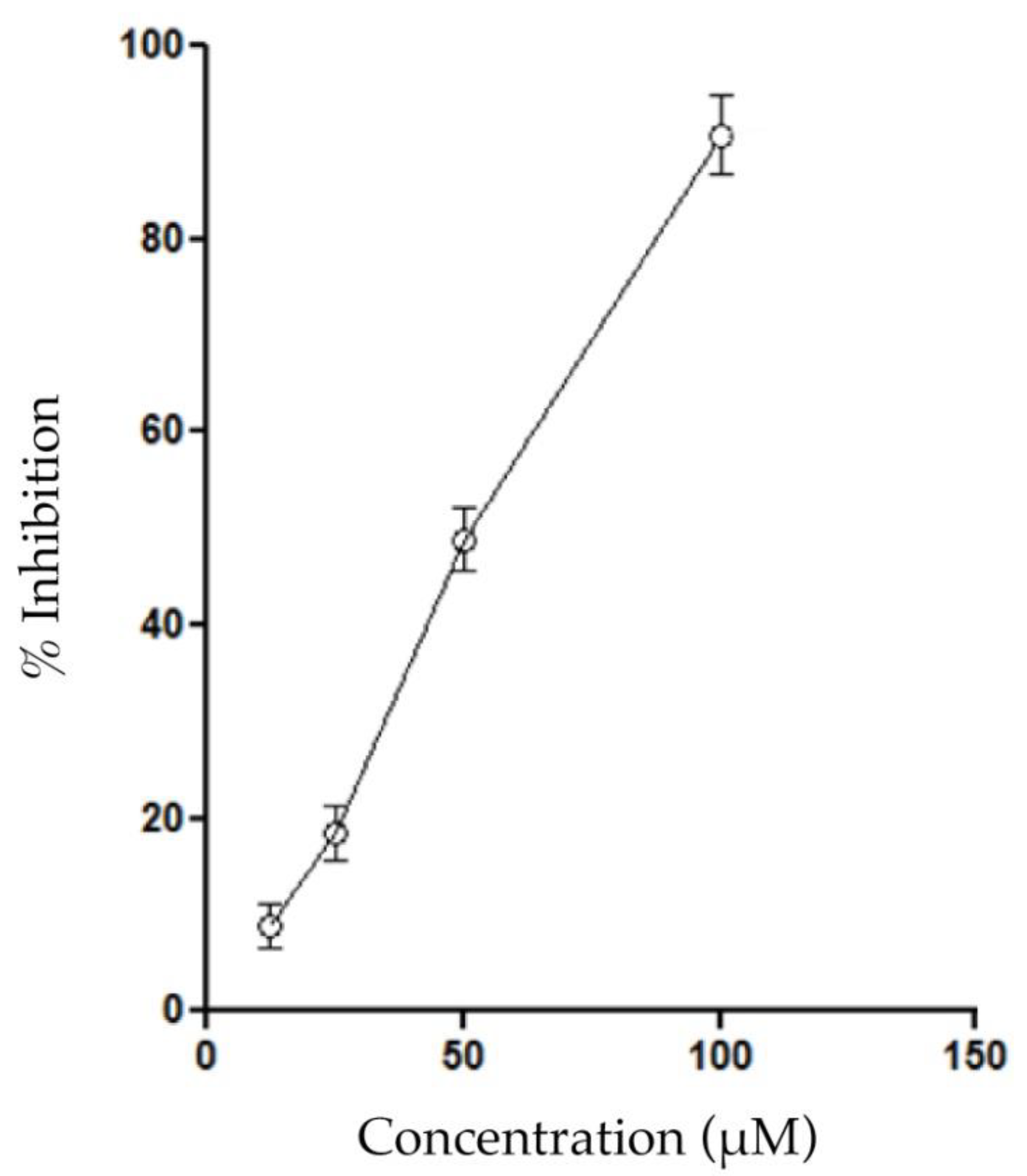
2.3. Biological Activity
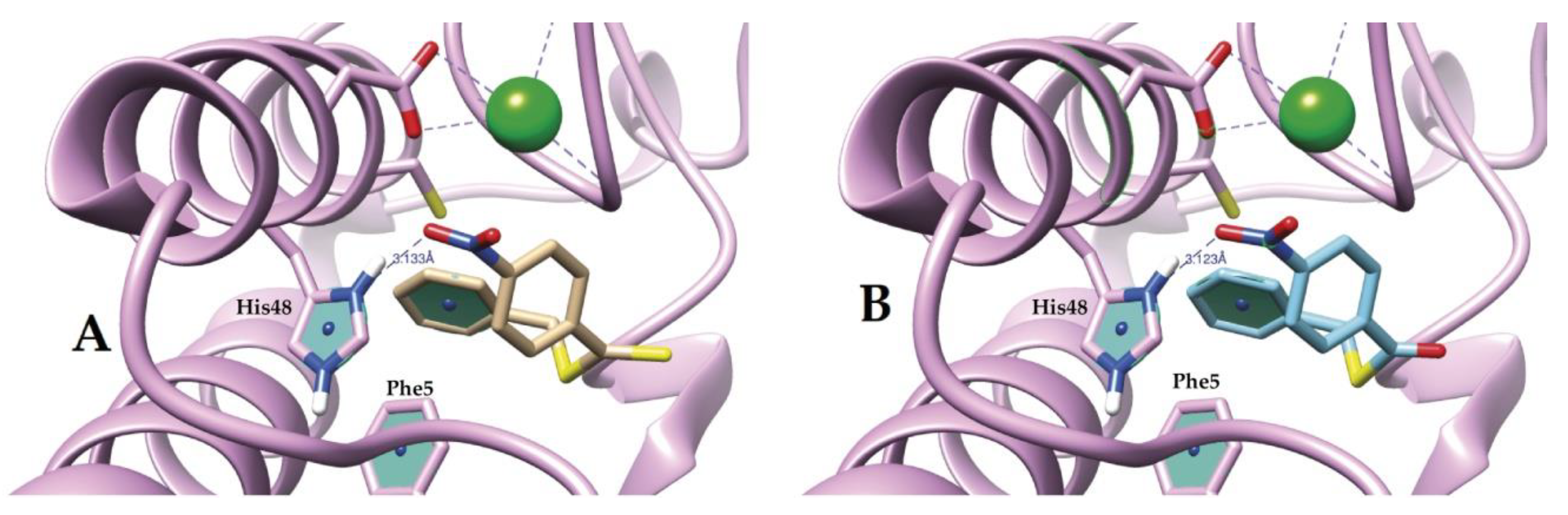
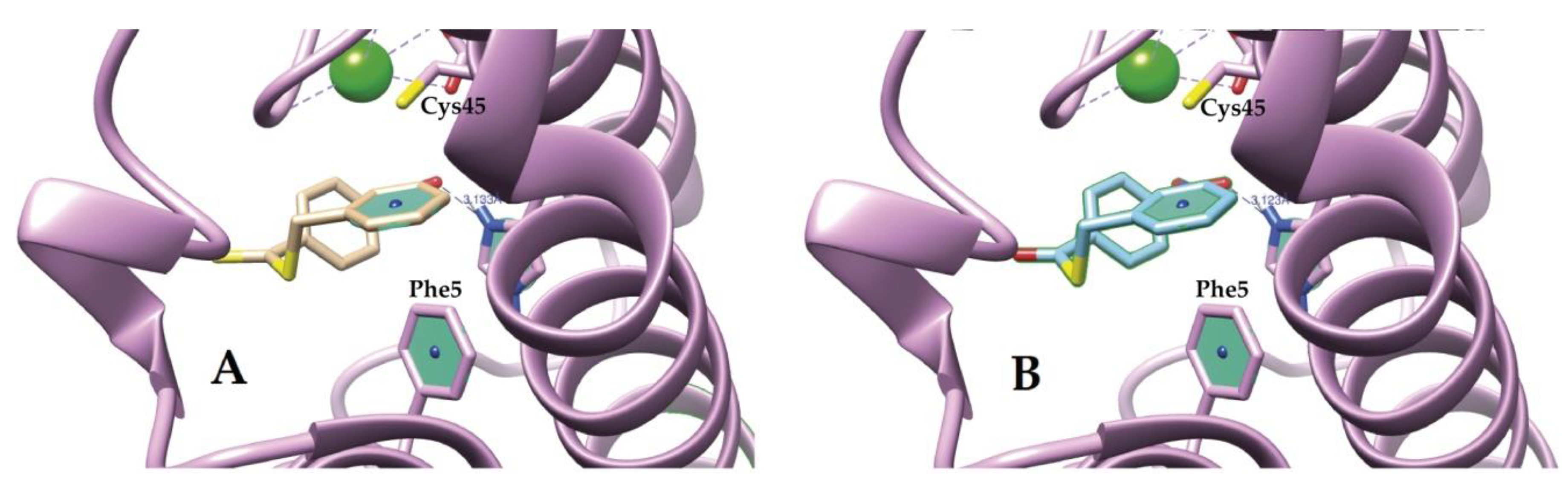
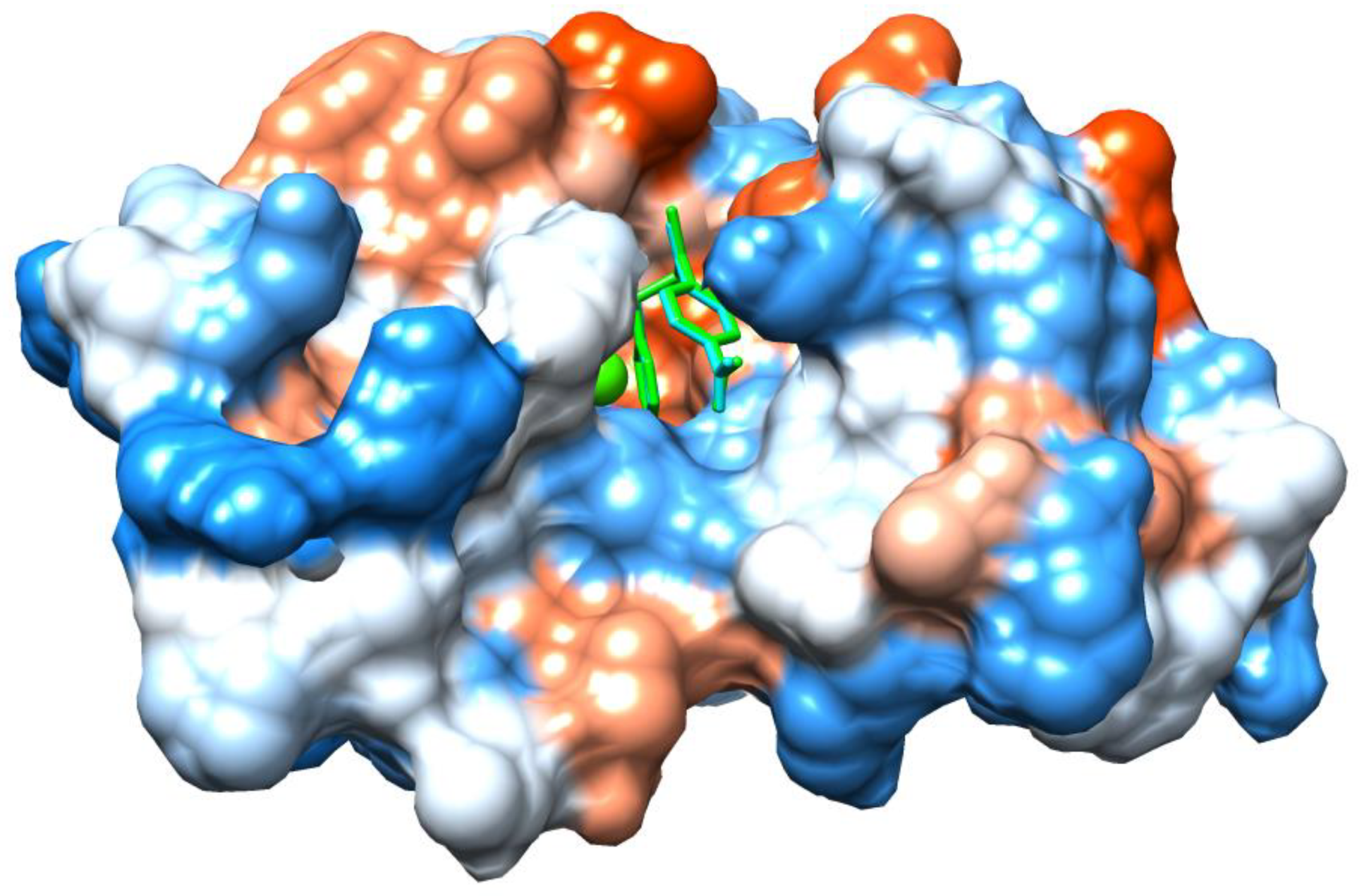
2.4. Molecular Docking Study of I and II
3. Discussion
3.1. Structural Details
3.2. Molecular Docking and Biological Test
4. Materials and Methods
4.1. Chemistry
4.2. Quantum Chemical Calculations
4.3. Molecular Docking
4.4. Toxin Isolation
4.5. Inhibition of Phospholipase A2 Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chippaux, J.-P. Snakebite envenomation turns again into a neglected tropical disease. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 38. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/snakebites/epidemiology/en (accessed on 18 December 2019).
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Salud. Available online: https://www.ins.gov.co/buscador-eventos/Informesdeevento/ACCIDENTE%20OF%C3%8DDICO_2018.pdf (accessed on 18 December 2019).
- Pereañez, J.A.; Patiño, A.C.; Castañeda, I.C.H. Toxinas provenientes de venenos de serpiente: Blancos terapéuticos, herramientas en investigación biomédica y agentes con potencial terapéutico. Curare 2014, 1, 49–60. [Google Scholar] [CrossRef][Green Version]
- Six, D.; A Dennis, E. The expanding superfamily of phospholipase A2 enzymes: Classification and characterization. Biochim. Biophys. Acta 2000, 1488, 1–19. [Google Scholar] [CrossRef]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef]
- Berg, O.G.; Gelb, M.H.; Tsai, M.-D.; Jain, M.K. Interfacial Enzymology: The Secreted Phospholipase A2-Paradigm. Chem. Rev. 2001, 101, 2613–2654. [Google Scholar] [CrossRef]
- Borges, R.; Lemke, N.; Fontes, M.R. PLA2-like proteins myotoxic mechanism: A dynamic model description. Sci. Rep. 2017, 7, 15514. [Google Scholar] [CrossRef] [PubMed]
- Squaiella-Baptistão, C.C.; Sant’Anna, O.A.; Marcelino, J.R.; Tambourgi, D.V. The history of antivenoms development: Beyond Calmette and Vital Brazil. Toxicon 2018, 150, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Herrera, C.; Fernandez, J.; Lomonte, B.; Fox, J.W. Unresolved issues in the understanding of the pathogenesis of local tissue damage induced by snake venoms. Toxicon 2018, 148, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, I.H.; Pereañez, J.; Jios, J.J. Substituted thiobenzoic acid S-benzyl esters as potential inhibitors of a snake venom phospholipase A2: Synthesis, spectroscopic and computational studies. J. Mol. Struct. 2012, 1028, 7–12. [Google Scholar] [CrossRef]
- Lättig, J.; Böhl, M.; Fischer, P.; Tischer, S.; Tietböhl, C.; Menschikowski, M.; Gutzeit, H.O.; Metz, P.; Pisabarro, M.T. Mechanism of inhibition of human secretory phospholipase A2 by flavonoids: Rationale for lead design. J. Comput. Mol. Des. 2007, 21, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Doley, R.; Saikia, D. Isolation of a snake venom phospholipase A2 (PLA2) inhibitor (AIPLAI) from leaves of Azadirachta indica (Neem): Mechanism of PLA2 inhibition by AIPLAI in vitro condition. Toxicon 2008, 51, 1548–1553. [Google Scholar] [CrossRef] [PubMed]
- Pereañez, J.A.; Núñez, V.; Patiño, A.C. Inhibitory Effects of Bile Acids on Enzymatic and Pharmacological Activities of a Snake Venom Phospholipase A2 from Group IIA. Protein J. 2011, 30, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.R.; Samuel, S.; Merkel, J.; E Bickler, P. Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation. Toxins 2016, 8, 248. [Google Scholar] [CrossRef]
- Gómez-Betancur, I.; Pereañez, J.A.; Patiño, A.C.; Benjumea, D. Inhibitory effect of pinostrobin from Renealmia alpinia, on the enzymatic and biological activities of a PLA2. Int. J. Boil. Macromol. 2016, 89, 35–42. [Google Scholar] [CrossRef]
- Pereañez, J.A.; Patiño, A.C.; Núñez, V.; Osorio, E. The biflavonoid morelloflavone inhibits the enzymatic and biological activities of a snake venom phospholipase A2. Chem. Interact. 2014, 220, 94–101. [Google Scholar] [CrossRef]
- Castañeda, I.C.H.; Pereañez, J.A.; Preciado, L.M. Synthetic Inhibitors of Snake Venom Enzymes: Thioesters Derived from 2-Sulfenyl Ethylacetate. Pharmaceuticals 2019, 12, 80. [Google Scholar] [CrossRef]
- Castañeda, I.H.; Jios, J.J.; Piro, O.; Tobón, G.; Della Védova, C.; Della Védova, C. Conformational and vibrational analysis of S-(2-methoxyphenyl)-4-substituted-benzenecarbothioates, using X-ray, infrared and Raman spectroscopy and theoretical calculations. J. Mol. Struct. 2007, 842, 46–54. [Google Scholar] [CrossRef]
- Castaneda, I.C.H.; Della Védova, C.; Piro, O.E.; Metzler-Nolte, N.; Jios, J.J. Synthesis of two new thioesters bearing ferrocene: Vibrational characterization and ab initio calculations. X-ray crystal structure of S-(2-methoxyphenyl)ferrocenecarbothioate. Polyhedron 2010, 29, 827–832. [Google Scholar] [CrossRef]
- Henao Castañeda, C.; Cadavid Vargas, J.F.; Jios, J.L.; Della Védova, C.O.; Echeverría, G.A.; Piro, O.E. CCDC 928055: Experimental Crystal Structure Determination. Available online: https://www.ccdc.cam.ac.uk/structures/search?id=doi:10.5517/ccdc.csd.cc104q84&sid=DataCite (accessed on 6 March 2013). [CrossRef]
- Cherkasov, R.A.; Kutyrev, G.A.; Pudovik, A.N. Tetrahedron report number 186: Organothiophosphorus reagents in organic synthesis. Tetrahedron 1985, 41, 2567–2624. [Google Scholar]
- Cava, M.P.; Levinson, M.I. Thionation reactions of lawesson’s reagents. Tetrahedron 1985, 41, 5061–5087. [Google Scholar] [CrossRef]
- Yousif, N.; Pedersen, U.; Yde, B.; Lawesson, S.-O. Studies on organophosphorus compounds XLVIII Synthesis of Dithioesters from P,S-Containing Reagents and Carboxylic Acids and Their Derivatives. Tetrahedron 1984, 40, 2663–2669. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R. Gaussian 09, Revision, B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Majed, H.; Johnston, T.; Kelso, C.; Monachino, E.; Jergic, S.; Dixon, N.E.; Mylonakis, E.; Kelso, M.J. Structure-activity relationships of pyrazole-4-carbodithioates as antibacterials against methicillin–resistant Staphylococcus aureus. Bioorganic Med. Chem. Lett. 2018, 28, 3526–3528. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, Y.; Badali, H.; Hashemi, S.M.; Ansari, M.; Fakhim, H.; Fallah, M.; Shokrzadeh, M.; Emami, S. New potent antifungal triazole alcohols containing N-benzylpiperazine carbodithioate moiety: Synthesis, in vitro evaluation and in silico study. Bioorganic Chem. 2019, 90, 103060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, C.-R.; Tang, X.; Cao, S.-L.; Ren, T.-T.; Gao, M.; Liao, J.; Xu, X. Synthesis and antitumor activity evaluation of quinazoline derivatives bearing piperazine-1-carbodithioate moiety at C4-position. Bioorganic Med. Chem. Lett. 2016, 26, 4666–4670. [Google Scholar] [CrossRef]
- Salvador, G.H.M.; Gomes, A.A.S.; Bryan-Quirós, W.; Fernández, J.; Lewin, M.R.; Gutiérrez, J.M.; Lomonte, B.; Fontes, M.R. Structural basis for phospholipase A2-like toxin inhibition by the synthetic compound Varespladib (LY315920). Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Jackman, L. Dynamic nuclear magnetic resonance spectroscopy. J. Mol. Struct. 1977, 36, 170. [Google Scholar] [CrossRef]
- Martin, J.; Dailey, B.P. Proton NMR Spectra of Disubstituted Benzenes. J. Chem. Phys. 1962, 37, 2594–2602. [Google Scholar] [CrossRef]
- Bergander, K.; He, R.; Chandrakumar, N.; Eppers, O.; Günther, H. NMR spectroscopy of organolithium compounds, Part XVI. Tetrahedron 1994, 50, 5861–5868. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.; Millam, J. Gauss View, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Trott, O.; Olson, A.J. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar]
- Salvador, G.; dos Santos, J.; Lomonte, B.; Fontes, M.R.M. Biochimie: Crystal structure of a phospholipase A2 from Bothrops asper venom: Insights into a new putative “myotoxic cluster”. Biochimie 2017, 133, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration cheminformatics. Choice Rev. Online 2006, 43, 43.
- Pereañez, J.A.; Núñez, V.; Huancahuire-Vega, S.; Marangoni, S.; Ponce-Soto, L.A. Biochemical and biological characterization of a PLA2 from crotoxin complex of Crotalus durissus cumanensis. Toxicon 2009, 53, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Mackessy, S.P. An aqueous endpoint assay of snake venom phospholipase A2. Toxicon 1996, 34, 1149–1155. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are or are not available from the authors. |
| Compound | X | MW a | nON a | nOHNH a | LogP(calc) a |
|---|---|---|---|---|---|
| I | S | 289.38 | 3 | 0 | 4.41 |
| II | O | 273.31 | 4 | 0 | 3.86 |
| Calculated a | IR a | Assignment b |
|---|---|---|
| 1647 m | 1561 w | δi.p dithiobenzoate ring+νas NO2 |
| 1589 s | 1516 vs | νas NO2 |
| 1382 vs | 1343 vs | νs NO2 |
| 1250 m | 1210 m | νC8-C1 |
| 1065 s | 1048 vs | νC8=S |
| 902 w | 895 w | νas S-C8-C1 |
| 852 m | 848 m | Scissor NO2 |
| 758 vw | 752 w | o.o.p. wag NO2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henao Castañeda, I.; Pereañez, J.A.; Preciado, L.M.; Jios, J. Sulfur Compounds as Inhibitors of Enzymatic Activity of a Snake Venom Phospholipase A2: Benzyl 4-nitrobenzenecarbodithioate as a Case of Study. Molecules 2020, 25, 1373. https://doi.org/10.3390/molecules25061373
Henao Castañeda I, Pereañez JA, Preciado LM, Jios J. Sulfur Compounds as Inhibitors of Enzymatic Activity of a Snake Venom Phospholipase A2: Benzyl 4-nitrobenzenecarbodithioate as a Case of Study. Molecules. 2020; 25(6):1373. https://doi.org/10.3390/molecules25061373
Chicago/Turabian StyleHenao Castañeda, Isabel, Jaime Andrés Pereañez, Lina María Preciado, and Jorge Jios. 2020. "Sulfur Compounds as Inhibitors of Enzymatic Activity of a Snake Venom Phospholipase A2: Benzyl 4-nitrobenzenecarbodithioate as a Case of Study" Molecules 25, no. 6: 1373. https://doi.org/10.3390/molecules25061373
APA StyleHenao Castañeda, I., Pereañez, J. A., Preciado, L. M., & Jios, J. (2020). Sulfur Compounds as Inhibitors of Enzymatic Activity of a Snake Venom Phospholipase A2: Benzyl 4-nitrobenzenecarbodithioate as a Case of Study. Molecules, 25(6), 1373. https://doi.org/10.3390/molecules25061373